Abstract
Cav2.1 Ca2+ channels (P/Q-type), which participate in various key roles in the central nervous systems by mediating calcium influx, are extensively spliced. One of its alternatively-spliced exon is 37, which forms part of the EF hand. The expression of exon 37a (EFa form), but not exon 37b (EFb form), confers the channel an activity-dependent enhancement of channel opening known as Ca2+-dependent facilitation (CDF). In this study, we analyzed the trend of EF hand splice variant distributions in mouse, rat and human brain tissues. We observed a developmental switch in rodents, as well as an age and gender bias in human brain tissues, suggestive of a possible role of these EF hand splice variants in neurophysiological specialization. A parallel study performed on rodent brains showed that the data drawn from human and rodent tissues may not necessarily correlate in the process of aging.
Keywords: Calcium channel, EF hand, mutually-exclusive exons, developmental switch, aging
Cav2.1 (P/Q type) voltage-gated calcium channels (VGCCs) play key roles in the trigger of neurotransmitter release in the central and peripheral nervous systems (Takahashi and Momiyama, 1993, Dunlap et al., 1995) regulation of the neuroarchitecture of developing cerebellar Purkinje cells (Miyazaki et al., 2004), and optimization of presynaptic function (Piedras-Renteria et al., 2004). Mutations in the principal α1A subunit have been associated with a number of neurological conditions including cerebellar or episodic ataxia, familial hemiplegic migraine (FHM-1) (Ophoff et al., 1996, Zhuchenko et al., 1997), epilepsy (Fletcher et al., 1996) while auto-antibodies directed against the same channel leads to myasthenic syndrome with particular features (Flink and Atchison, 2003). The α1A subunit is highly expressed in the brain, particularly in the soma and dendrites of the Purkinje neurons of the cerebellum (Stea et al., 1994), and the presynaptic terminals of synapses. Conceivably, this class of channels plays a major role in determining the electrical excitability of these neurons as well as regulating neurotransmitter release.
The α1A subunit is subjected to extensive alternative splicing (Mori et al., 1991, Starr et al., 1991, Ophoff et al., 1996, Zhuchenko et al., 1997, Bourinet et al., 1999, Soong et al., 2002, Tsunemi et al., 2002), in which 7 and 9 loci of variation had been characterized to date in human (Soong et al., 2002) and in rat cerebellum (Kanumilli et al., 2006) respectively. Likewise, Cav2.1 channel splice variants had also been reported in mice (Vigues et al., 1998, Tsunemi et al., 2002), including some novel C-terminii that were detected in mutant mouse (Fletcher et al., 1996).
At the carboxyl terminal, exons 36 and 37 encode the EF hand-like domain. Exon 37 is encoded by two mutually-exclusive exons 37a and 37b, hence producing two variants of the EF hand, EFa and EFb. Channels lacking exon 37 had also been reported in rodents (Ligon et al., 1998, Vigues et al., 1998). The EF hand plays a role in the transduction of the IQ-motif-bound Ca2+/calmodulin (CaM) into channel modulations (Peterson et al., 2000, Kim et al., 2004). CaM exerts two opposing effects, calcium-dependent facilitation (CDF), an activity-dependent enhancement of channel opening and a subsequent slower process, calcium-dependent inactivation (CDI) – a process of channel inactivation following Ca2+ influx. Interestingly, in both heterologous systems expressing recombinant channels and in cerebellar Purkinje neurons, the alternative splicing of exon 37 acts as a molecular switch for CDF without affecting CDI (Chaudhuri et al., 2004, Chaudhuri et al., 2005). EFa expression confers this facilitative property to the channel. We also demonstrated a developmental activation of CDF due to enhanced postnatal EFa expression (Chaudhuri et al., 2005). This correlates with the differential expression of the two exons previously reported in development (Vigues et al., 2002, Chaudhuri et al., 2004) or in different regions of the human brain (Chaudhuri et al., 2004). Distinct and separate subcellular localization of the two splice variants observed in Purkinje cells strongly suggested specialized neurophysiological roles such as synaptic plasticity, gene transcription and remodelling (Chaudhuri et al., 2005). To date, the Cav2.1 (P/Q-type) calcium channels are the only channels in the Cav1-2 family to exhibit calcium-dependent facilitation (CDF) (Liang et al., 2003, Inchauspe et al., 2004), although calmodulin kinase-mediated facilitation had been earlier established in Cav1.2 (L-type) calcium channels (Dzhura et al., 2000). With these physiological bases, this report investigates the splice variant distribution within individual mouse and rat brains during development. Parallel analyses of human cerebellar samples provided valuable information on age and gender bias of EF hand expression. For a broader perspective, we also obtained data from other human brain regions.
EXPERIMENTAL PROCEDURES
RNA extraction and cDNA synthesis
Swiss albino mice, F344 rats of 1 to 30 months of age (NIA, USA), early postnatal Wistar rats were sacrificed and the cerebellar/ whole brain tissues were harvested and immediately stored in RNAlater reagent [Ambion® (Europe) Ltd, Cambridgeshire, UK]. Two to five brains from rodents of each time point were pooled together for analyses, except for the three individual female mouse brains shown in Fig. 2C. Postmortem human brain samples were obtained from the brain tissue repository of the Johns Hopkins Medical Institutions, and the National Neuroscience Institute. Prior to RNA extraction by the RNAeasy kit (QIAGEN GmbH, Hilden, Germany), the RNAlater reagent was removed and homogenization was performed. The protocols used in this study conformed to our institution's IACUC and IRB regulations and guidelines (NUS-IRB 05-034E; IACUC 859/05).
Fig. 2.

Quantitation of EF hand splice variants in mouse brain samples. (A) Restriction profiles of the RT-PCR amplicons in 1.5% agarose showing the developmental switch between postnatal day 5 and 7. Not I and Sph I restrict exon 37a and 37b respectively as represented in the two uppermost cDNA diagrams. The sizes are compared against the 100bp ladder (i) Not I digestion showing increasing amounts of digested EFa fragments after P5. (ii) Sph I digestion showing the corresponding decreasing amounts of digested EFb fragments. (B) Quantitation results by colony PCR screening are presented as bar graphs. The percentage of EFa expression in bar graphs is expressed in white while that of EFb is expressed in black. The numbers in parenthesises above each bar indicate the number of colonies screened. (C) Restriction profiles of the RT-PCR amplicons of female mouse cerebellum tissues shows no gender bias in EFa predominant expression in adult mice. U: uncut fragment; N: Not I digestion; S: Sph I digestion
Reverse transcription (RT) was performed using 5 μg of total RNA, Superscript II™ reverse transcriptase (Invitrogen, California, USA), 0.5mM dNTPs, 1X 1st strand buffer (Invitrogen, California, USA) and 0.1M dithiothreitol (DTT) (Invitrogen, California, USA). For mouse brain tissues, mouse exon 38 reverse primer (5'-TATCAAGGGCGGTTCGGATCA-3') was used for cDNA synthesis. RT was performed in the following sequence: a 5-min denaturation step at 80°C and rapid cooling, a 75-min 42°C reverse transcription step, and a final 15-min inactivation step at 70°C. Reverse transcription was performed similarly with extracted rat and human brain RNA except for the use of 18-mer oligo-dT in place of a specific reverse primer and a human exon 38 reverse primer (5'-GCTGTGCGGATCAGA-3') respectively.
Screening using polymerase chain reaction
As a control, DNA of full-length human EFa and EFb clones (Soong et al., 2002) were linearized by Hind III (New England Biolabs, Inc., Massachusetts, USA), quantified by spectophotometry and the following ratios of each were mixed: 1:10; 1:1; 10:1. After serial dilutions, ten nanograms of these DNA mixtures were used for subsequent PCR and colony screening.
For the brain samples, the cDNA synthesized in section 2.1 was used for PCR amplification of the EF hand. A control reaction consisting of all the components but omitting the reverse transcriptase was also run in parallel. The mouse exon 35 forward primer (5'-CGGCAGACTGCGGCAACGAG-3') (5'-GGGAAACCGTGTGATAAGAA-3') and exon 38 primer (for sequence see section 2.1) were used for the amplification of a 384-bp fragment from the mouse brain samples. The reaction mix consisted of 200μM dNTPs, 100nM of primers, 2.5U of Taq DNA polymerase (Promega Corp., Wisconsin, USA), 1X Mg2+-free Buffer (Promega Corp., Wisconsin, USA), Mg2+ concentration of 1.5mM and 2μl of cDNA in a final volume of 25 μl. The cycling profile was set as follows: initial denaturation of template DNA at 94°C for 2 min, 5 touch-down cycles of denaturation at 94°C for 30 sec, annealing at 62°C to 58°C for 40 sec, and extension at 72°C for 1 min, followed by 30 cycles in which the annealing temperature was maintained at 57°C. The final extension was performed at 72°C for 10 min. Control reactions in which water and product from the control RT experiment were used in place of the cDNA were included. For rat tissues, the exon 35 primer (5'-CACAGGGGAAGCGTGGCAC-3') and exon 38 primer (5'-GGGCGGTTCGGATCAG-3') were used to amplify a 351-bp fragment. A final annealing temperature of 50°C was used. For human templates, the exon 35 primer (5'-GGGAAACCGTGTGATAAGAA-3') and exon 38 primer (5'-GCTGTGCGGATCAGA -3') were used to amplify a 410-bp fragment. The PCR reaction mix required a Mg2+ concentration of 2 mM, and a final annealing temperature of 53°C was used.
These amplicons represented the pool of both EFa and EFb cDNA forms. The fragment was gel-purified (QIAGEN GmbH, Hilden, Germany), cloned into pDrive (QIAGEN GmbH, Hilden, Germany) or pGEM T Easy (Promega Corp., Wisconsin, USA) and transformed into E. coli DH10B cells by electroporation. The clones, each carrying an insert of either splice variant, were identified by blue-white selection and cultured in Luria-Bertani broth with 100 μg/ml of ampicillin in 96-well round-bottomed microtitre plates. A minimum of two plates of white transformants were collected from each sample.
Mouse EF hand screening were performed with primer pairs, exon 35 primer and exon 37a primer (5'-GTACATGTCCTTATAGTGAA-3') or exon 37b primer (5'-ATACATGTCCGGGTAAGGCA-3'), both generating amplicon sizes of 224 bp. The reaction mix consisted of 50 μM of dNTPs, 50 nM of each primer, 0.3 U of Taq polymerase (Promega Corp., Wisconsin, USA), 1X PCR Buffer (Promega Corp., Wisconsin, USA), a Mg2+ concentration of 1 μM and 1 μl of culture in a final volume of 12.5 μl. Similar touch-down cycling profiles were used in which the final annealing temperature was maintained at 51°C (for EFa screening) or 54°C (for EFb screening). Rat EFa screening was performed with primer pairs, exon 35 primer (5'-CACAGGGGAAGCGTGGCAC-3') and exon 37a primer (5'-GCAAGCAACCCTATGAGGAC-3'), while EFb screening was performed with exon 35 primer (5'-TCCAAAAACCAGAGTGTG-3') and exon 37b primer (5'-CATGTGTCTCAGCATCTGA-3'). The amplicon sizes were 367 bp and 248 bp respectively. The reaction mix was similar to that for mouse EF screening, except that a Mg2+ concentration of 2 μM was used and that a final annealing temperature of 50°C was adopted. Human nested PCR was performed directly on the clones using an exon 36 primer (5'-CGTCATCATGGACAACTT-3') and either the exon 37a primer (5'-ATATTACTCGTAATAAACTG-3'), or the exon 37b primer (5'-GGGCGGAGACATGTGTCTCA-3'). The amplicons were 154 bp and 175 bp respectively. The reaction mix was similar except that the Mg2+ concentration used was 2 μM (for EFa screening) or 1 μM (for EFb screening). The final annealing temperature was maintained at 54°C for both EFa and EFb screening. Appropriate controls were performed for all samples at both the RT and PCR steps, as mentioned previously.
All PCR products were visualized in 1.5% agarose gel. Only clones that are positive for EFa and negative for EFb, and vice versa, were taken to be confirmative results. All ambiguous results were disregarded. We analysed at least one hundred clones per tissue sample to ensure unbiased quantification. For confirmation, identified clones were randomly selected for DNA sequencing.
Quantitative restriction analyses
Equal amounts of PCR amplicons from each set of RT reactions were pooled together and digested with Not I (Roche Diagnostics GmbH, Mannheim, Germany) and Sph I (Roche Diagnostics GmbH, Mannheim, Germany), which diagnostically cut rat and mouse exons 37a and 37b respectively. For human exon 37a, Bsr GI (New England Biolabs, Inc., Massachusetts, USA) was used. Digestions were performed in total reaction volumes of 20μl at 37°C for 4 hrs. The products were visualized in 1.5% agarose gel.
RESULTS
Our objective was to quantify the relative amounts of the two splice variants of the EF hand. To ensure that the final quantitative method of PCR colony screening was unbiased, we first validated the PCR amplification method used by performing relevant controls.
Implementation of stringent controls and elimination of PCR bias
To eliminate the possibility of skewed results due to contamination, proper controls were implemented during the RT-PCR step. For every RT step, we included a duplicate reaction mix whereby the reverse transcriptase was omitted. Both were then used as templates for the subsequent PCR reactions, together with a third PCR mix with water as the template as a check for reagent contamination (see Suppl. Fig. 1).
Prior to analyzing brain tissue samples, we also needed to ensure that there is no PCR bias during amplification, although this method of colony PCR screening had been adopted by various groups (Welling et al., 1997, Regan et al., 2000, Soong et al., 2002, Chaudhuri et al., 2004, Splawski et al., 2004, Fan et al., 2005). Our previous studies had also demonstrated good correlation between the colony screening data from clones containing human cerebellum full-length (Soong et al., 2002) or smaller amplicons (Chaudhuri et al., 2004) indicating that PCR bias is minimal or unlikely. We performed controls using designated amounts of plasmids measured by spectrophotometry and inspected visually on agarose gel. The PCR amplicons were cloned into TA vector and colonies were picked, grown in 96-well plates, and screened by colony PCR. Positive EFa and EFb clones were included in every 96-well plate as controls. Fig. 1 shows that the screening data corresponds to the relative proportion of EFa/b splice isoforms used. When the EFa:EFb ratio was 1:10, 9.6% of the 218 clones screened were EFa clones. Similarly, 9.6% of the 167 clones screened were EFb clones when the EFa/EFb ratio was 10:1. The percentage of the EFa and EFb clones were 47.6% and 52.4% respectively when a 1:1 ratio of both templates was used.
Fig. 1.
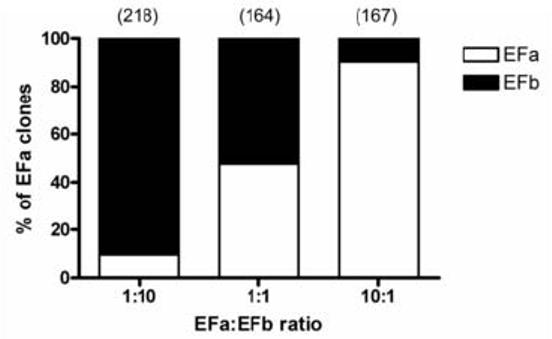
Validation of colony PCR screening method. Using a mixture of defined amounts of linearized EFa and EFb DNA as the PCR template, colony PCR screening was performed. The bar graphs (of tabulated clones) validated the reliability of the colony screening method with expected relative percentages of EFa and EFb clones.
Developmental switch from predominant EFb to EFa splice variants in mouse whole brain and cerebellum
We had previously looked at the EF hand splice variation in human whole and different brain regions (Chaudhuri et al., 2004). To assess the profiles in rodents, we assayed mouse cerebellum samples of different developmental ages. Due to the immature development of the brain during the embryonal and early postnatal stages, we used whole brains for screening. We first adopted the method of restriction endonuclease assays of the RT-PCR products. Digestions of exon 37a by Not I and exon 37b by Sph I provided a semi-quantitative profile of the postnatal developmental switch from predominant EFb to EFa splice variant expression, specifically between 5 to 7 days postnatal (P5-P7) (Fig. 2A). The colony PCR screening method, which is quantitative, clearly confirmed this postnatal developmental switch at exactly the same time point (Fig. 2B), with a drastic decrease in EFb expression from 92.1% on P5 to 35.3% on P7. Notably, EFa expression predominates at 97.8% even at an older age of 9 months (Fig. 2B). To investigate if gender bias existed, we also analyzed two 8-week old and one 11-week old female mouse cerebellum samples. From Fig. 2C, the extensive digestion by Not I (N) and much less digestion by Sph I (S) indicated that EFa expression was predominant in adult female mouse brain. Hence, we can deduce that gender differences do not affect EF-hand alternative splicing in mice.
Rat and mouse exhibit similar EF hand developmental switch during the early postnatal days; EFa predominant expression extends to old age
While the data from mice provided an interesting insight into the developmental expression profile, we were also interested to investigate the expression profile during aging. To cover a sufficient spectrum that reflects upon all the developmental stages extending till old age (Coleman et al., 2004, Coleman, 2004), we harvested cerebellum tissues ranging from postnatal Wistar rats to 30-month old F344 rats, the latter being an established model for aging study. Likewise, we quantified the amount the EFa and EFb transcripts in rat cerebellar tissues by adopting a combination of restriction analyses and PCR colony screening (Fig. 3). The early postnatal samples (P1, P3, P5, P7, P9) express predominantly EFb splice forms (Fig. 3A). The reverse occurs from P21 onwards whereby EFa expression is much more elevated over EFb expression. This is consistent with previous observation made by two previous studies (Vigues et al., 2002, Chaudhuri et al., 2005). The colony PCR method confirmed EFa as the predominant splice form in all adult age groups of F344 rats from 1 month to 30 months, including a 2-month old female rat cerebellum (Fig. 3B). EFa expression ranges from 81% to 92.9%. Therefore, both rat and mice exhibit a significant EFa switch within the first two weeks of their lives and high EFa expression persisted throughout adulthood. Like mice, gender differences do not seemingly affect the predominant EFa expression during adulthood.
Fig. 3.

Quantitation of EF hand splice variants in rat cerebellar samples. (A) Restriction profile of the RT-PCR amplicons showing the developmental switch. Not I and Sph I restrict exon 37a and 37b respectively as represented in the two uppermost cDNA diagrams. (i) Not I digestion showing increasing amounts of digested EFa fragments over development. (ii) Sph I digestion showing the corresponding decreasing amounts of digested EFb fragments over development. (B) The percentage of EFa expression in bar graphs is expressed in white while that of EFb is expressed in black. The numbers in parenthesises above each bar indicate the number of colonies screened. The inclusion of the data of one adult female rat revealed no gender bias in EF hand expression.
Gender-specific differential expression of EF hand splice variants in the human cerebellum; poor correlation between human and rat data in aging
Having validated the colony PCR screening method, we adopted it for the analysis of the relative expression of either splice variant in individual human cerebellum tissues, an extension of our previous work on human brain (Chaudhuri et al., 2004). As our previous data was based on pooled cDNA collected from individuals over a range of ages, it only provided an overall spatial expression profile. As such we were unable to analyze the EF splice variant expression temporally and especially in human aging. Here, the human male cerebellar tissues (Fig. 4A) demonstrated a switch from predominant EFa expression at 16 and 30 years of age, to a predominant EFb expression after 40 years of age. This display of EFb predominance extended even to the age of 93. In the female cerebellar tissues (Fig. 4B), EFb was the predominant splice isoform in the female cerebellum samples from ages 24 to 93, ranging from 71.2 to 98.1%. Therefore, an obvious phenomenon of the aging human cerebellum was sustained elevated EFb expression. This is distinctly different from the rodent data, whereby EFa expression remained predominant at old age, and gender bias did not seemingly exist. It is noteworthy to point out that our previous work has shown that human fetal brains expressed high levels of EFb, although we could not determine the point in time in early human development for the switch to high EFa expression.
Fig. 4.

Quantitation of exon 37 splice variants in human male (A) and female (B) cerebellar samples. The percentage of EFa expression in bar graphs is expressed in white while that of EFb is expressed in black. The numbers in parenthesises above each bar indicate the number of colonies screened. A switch from predominant EFa to predominant EFb expression was observed in males between 30 to 40 years old. Such a switch was absent in the females, whereby EFb expression predominated from 24 to 93 years of age.
Expression of EF hand splice variants in the frontal cortex, temporal lobe and substantia nigra
P/Q-type calcium channels are expressed in many brain regions. For a broader perspective, we quantified the expression of the two splice variants from the other brain regions, such as the frontal cortex, temporal lobe and substantia nigra (Fig. 5). In particular, the data of the frontal cortex of four human subjects were of special interest to us (Fig. 5A). EFa expression was increased as age increases in both male and female samples, a trend clearly in contrast to that observed in human cerebellum. This may be an area of interest for further investigations. As for the temporal lobe and substantia nigra (Fig. 5B, C), varying relative expression levels of either splice form were exhibited though there was a hint of higher EFa expression early in the first three decades of life. It is premature to deduce the trend across gender and age due to the small numbers of tissues available, except for the trend that EFb expression appeared to be higher in females.
Fig. 5.

Differential distribution of EF hand splice variants in human frontal cortex (A), temporal lobe (B) and substantia nigra (C). In contrast to the cerebellum, EFa expression in the frontal cortex increased with age in both the male and female samples. For both the temporal lobe and substantia nigra, there was seemingly higher EFa expression in the first three decades of life although varying relative expression levels were exhibited.
We cannot ensure that each human brain sample is an absolute representation of that specific age group and gender, and there are legitimate considerations on the variability of the conditions under which these human brains were harvested. Nonetheless, we strive here to reveal a general trend in which future studies can be designed for the elucidation of EF hand splice variant regulation in relation to the specific roles of their encoded channels.
DISCUSSION
This report highlights major differences in the spatial or temporal distribution of mutually-exclusive exons 37a/b of the Cav2.1 calcium channels in human and rodent brains. A conserved mechanism is the predominant EFb expression in early brain development. While a major switch to EFa expression occurs 1-2 weeks after birth in rodents, this switch could not be determined in early development of human because of the lack of brain tissues at the earlier ages. On the other hand, while there is no age- or gender-dependence of switch to predominant EFa expression later in life in rodents, a more complex regulation in the selection of exons 37a/b in the adult brain is evident in human. There is certainly a second phase switch from predominant EFa to EFb expression. This high EFb expression in the cerebellum persists to old age in both gender. The reverse trend of EFa up-regulation seems to occur in the frontal cortex of older humans. This report therefore exemplifies the importance of validating results obtained in animal model with the patterns of expression in human. It is also points to the complexity in the regulation of alternative splicing to diversify Cav2.1 channel function over time and in different brain regions.
The human male cerebellar tissues show an interesting switch from a non-facilitative to a facilitative form during adulthood, between thirty and forty years of age. Due to limitations of sample availability, we are not able to assess the developmental switch from fetal to adult form as demonstrated by our previous data derived from whole brain cDNA libraries (Chaudhuri et al., 2004). Nonetheless, based on existing data, there are possibly two EF hand switches in a human male's lifetime, the first during the fetal or postnatal stage, and the second during adulthood. Our study also concludes that EFb was the predominant splice isoform in the female cerebellum samples from ages 24 to 93. Collectively, our data in this study definitely shows the phenomenon of regulated differential splicing of the EF hand possibly tailored for specific physiological purposes. These archive tissues provided important information on the EFb form being highly expressed in the process of aging.
Alternative splicing events have been documented to occur more frequently in transcripts from genes expressed in functionally complex tissues with diverse cell types such as the brain (Modrek et al., 2001, Johnson et al., 2003, Yeo et al., 2004). Taking into consideration that Cav2.1 calcium channels are critically localized in various regions of the brain, especially in Purkinje neurons, their importance in mediating neurotransmitter release at presynaptic terminals, it is predictable that they may play a possible role in synaptic efficacy changes in the cerebellar cortex such as long-term potentiation (LTP) (Salin et al., 1996, Linden, 1997), long term depression (LTD) (Ito, 2001), and more recently, short-term potentiation (STP) (Goto et al., 2006). Since lobe-specific CaM-mediated CDF is a property that has been observed only in the Cav2.1 calcium channels so far (Liang et al., 2003, Inchauspe et al., 2004), the differential expression of the EF hand splice variants is likely to be significant. The repertoire of splice variants of the P/Q-type calcium channel have been shown to display subtle differences in electrophysiological properties (Bourinet et al., 1999, Soong et al., 2002), but coupled with a differential distribution profile across different brain regions or ages, we will be able to relate the expression of certain splice variants to the specific neurobiology of synaptic functions. Although the impact of lack of CDF in channels expressing EFb is currently unknown, there may be implications in the up-regulation of a predominantly facilitative EFa form and distinct subcellular localizations of each form (Chaudhuri et al., 2005) taking into account the following neurophysiological processes.
Key developments occur in the cerebellum in the first two to three weeks of life in rodents, a restricted time-frame of the critical period where activity-dependent synapse refinement takes place. In particular, by P14 to P21, pruning of multiple climbing fibres (CF) innervations leading to a one-to-one relationship of CF to PC (Purkinje cell) is attained (Kakizawa et al., 2000, Miyazaki et al., 2004, Scelfo and Strata, 2005), and Cav2.1 calcium channels have been recently found to regulate such synaptic competition on developing cerebellar PCs (Miyazaki et al., 2004). In the brain as a whole, the 2-3 week postnatal period coincides with the onset of obvious pathology in Cav2.1 α1A knockout mice (Jun et al., 1999, Fletcher et al., 2001, Urbano et al., 2003, Miyazaki et al., 2004, Piedras-Renteria et al., 2004) as well as the overall up-regulation of EFa channels. Another possibility is the increasing prominence in the role played by P/Q-type channels only in the second week after birth (Iwasaki and Takahashi, 1998). A later study also showed that the initial Ca2+ currents directly recorded from the auditory calyceal presynaptic terminals were N-, P/Q-, and R-types at postnatal day 7 (P7) to P10, but P/Q-type Ca2+ currents dominate at P13 (Iwasaki et al., 2000). Collectively, these observations may indicate a lack of need for more extensive signalling across developing synapse in the early postnatal days, or conversely, optimised adaptations for dendritic development and synaptogenesis after the first 2 to 3 weeks of life. The difference in the switching time points in mice and rat can possibly be correlated to the different time-courses in architectural development of the different rodent cerebellum (Altman, 1972, Inouye and Murakami, 1980).
To focus on the differential expression of the EF hand, we have disregarded clones that do not carry either splice variant of exon 37. Some previous studies have documented the absence of exon 37 in rodents (Ligon et al., 1998, Vigues et al., 1998) and we have also detected such splice variation in human cerebellum (unpublished data). The physiological importance of regulating the expression of Cav2.1 channels lacking exon 37 in development or in normal neuronal function is unknown and requires further experiments.
While EFa channels have been defined as the facilitative form, it is also important to consider another level of regulation - the synergistic effects of expression of splice variants of two exons. Our previous study (Chaudhuri et al., 2004) demonstrated that in heterologous expression system, while CDF is abolished when exon 37b is co-expressed with exon 47, a modest level of facilitation can be observed when this exon is co-expressed within the truncated splice variant (Δ47). Thus, even at old age, these channels may still exhibit CDF if exon Δ47 is expressed with exon 37b. This provides another mode of regulation. However, while CDF manifested by EFa channels is modulated by local Ca2+ influx, CDF manifested by EFb/ Δ47 channels is driven by elevations in global Ca2+ influx. Hence, EFa channels are unlikely to be affected by a number of known interacting partners like Ca2+-sensing molecules such as calcium binding protein (CaBP) and Visinin-like protein-2 (VILIP-2) (Lee et al., 2002, Few et al., 2005, Lautermilch et al., 2005). In the rat and mouse cerebellar Cav2.1 channels, at least two exon 47 splice variants have each been identified other than the Δ47 splice variant (Tsunemi et al., 2002, Kanumilli et al., 2006). However, due to the limited amino acid homology of these rodent exon 47 splice variants to the reported human exon 47, it is difficult therefore to predict if the inclusion of these rodent exons 47 would affect CDF in the EFb channels.
In this study the data obtained from aged rodents did not correlate with the aged human data. A switch to a predominant expression of the EFb splice variant was not observed even in 30-month old rats. For both rats and mice, the EFa splice variant seemed to be the main isoform after the developmental switch within 10 days postnatal. This should be taken into account when aging studies are designed for knockout or transgenic mice experiments. In fact, a considerable number of mouse models had been shown not to replicate human disease phenotypes or histopathology related to aging, such as those constructed for the study of Parkinson's disease and Alzheimer's disease (Gotz et al., 2004, Fleming et al., 2005).
Differential expression levels of splice variants had been shown to hold key implications in the pathophysiology. In the Cav2.1 calcium channels, transcripts of splice variants α1A-a and α1A-b exhibit a complex and specific spatio-temporal pattern following induced seizures, indicating that such regulations may represent protective mechanisms against brain injury after epileptic seizure attacks (Vigues et al., 1999). Timothy syndrome, a recently described multi-organ dysfunction, is caused by a point mutation within the exons 8 and 8a of the Cav1.2 (L-type) α1C calcium channel gene (Splawski et al., 2004, Splawski et al., 2005). These two mutually-exclusive exons are necessary for the expression of a functional channel, and the disease severity in cardiac arrhythmias correlates with the expression levels of exon 8a carrying the mutation. From a developmental point of view, splice variant regulation is important in the tissue/ organ development and normal physiological processes (Brocke et al., 1995, Kuppers et al., 2000, Muro et al., 2003, Thakur and Mani, 2005). In the neuronal context, alternative splicing determines the cell-specific expression of ion channel splice isoforms in various voltage-gated ion channels (Fettiplace and Fuchs, 1999, Baranauskas et al., 2003, Bell et al., 2004, Kanumilli et al., 2006), modifies synaptic strength through differential expression of calcium channels, various neuronal receptors and synaptic proteins (Lipscombe, 2005).
Calcium is a key signalling molecule involved in a wide array of biological functions. The tight regulation revolving around neuronal calcium levels in presynaptic terminals and Ca2+ sensors such as CaM ensures a versatile and highly adaptable nervous system. With such conserved alternative splicing of exon 37 across mouse, rat and human brains and the likelihood of strongly enriched splicing regulatory elements (Yeo et al., 2005), the creation of the exonic EF hand knockout mouse models will be pivotal in the unveiling of the roles of the splice variants in relation to their differential localizations in the brain. With time, the elucidation of the regulation of these splice variants will lead to deeper understanding of the intricacies of synaptogenesis and synaptic signaling.
Supplementary Material
RT-PCR amplicons spanning the EF hands of (i) Swiss albino mouse cerebellar samples and the (ii) frontal cortex of 87 year-old human male in 1.5% agarose gel. No contamination was observed in the water and RT-omitted control reactions.
Acknowledgements
The study was funded by the Biomedical Research Council of Singapore (BMRC Grant 01/1/33/18/023). The Brain Resource Center of the Johns Hopkins Medical Institutions is supported by the Johns Hopkins Alzheimer's Disease Research Center Grant NIA AG 05146.
LIST OF ABBREVIATIONS
- CaBP
calcium binding protein
- CaM
calmodulin
- CDF
calcium-dependent facilitation
- CDI
calcium-dependent inactivation
- cDNA
complementary DNA
- CF
climbing fibre
- DNA
deoxyribonucleic acid
- dNTP
deoxynucleotide triphosphate
- DTT
dithiothreitol
- LTD
long-term depression
- LTP
long-term potentiation
- PC
Purkinje cell
- PCR
polymerase chain reaction
- RT
reverse transcription
- STP
short-term potentiation
- VGCC
voltage-gated calcium channel
- VILIP-2
Visinin-like protein-2
Footnotes
Disclosure
We have no existing or potential conflict of interest with any person or organization. The protocols used in animal work and with human tissues conformed to our institution's IACUC and IRB regulations and guidelines (NUS-IRB 05-034E; IACUC 859/05).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci. 2003;6:258–266. doi: 10.1038/nn1019. [DOI] [PubMed] [Google Scholar]
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- Brocke L, Srinivasan M, Schulman H. Developmental and regional expression of multifunctional Ca2+/calmodulin-dependent protein kinase isoforms in rat brain. J Neurosci. 1995;15:6797–6808. doi: 10.1523/JNEUROSCI.15-10-06797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D, Alseikhan BA, Chang SY, Soong TW, Yue DT. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J Neurosci. 2005;25:8282–8294. doi: 10.1523/JNEUROSCI.2253-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D, Chang SY, DeMaria CD, Alvania RS, Soong TW, Yue DT. Alternative splicing as a molecular switch for Ca2+/calmodulin-dependent facilitation of P/Q-type Ca2+ channels. J Neurosci. 2004;24:6334–6342. doi: 10.1523/JNEUROSCI.1712-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P, Finch C, Joseph J. The need for multiple time points in aging studies. Neurobiol Aging. 2004;25:3–4. doi: 10.1016/j.neurobiolaging.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Coleman PD. How old is old? Neurobiol Aging. 2004;25:1. doi: 10.1016/j.neurobiolaging.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- Fan QI, Vanderpool KM, Chung HS, Marsh JD. The L-type calcium channel alpha 1C subunit gene undergoes extensive, uncoordinated alternative splicing. Mol Cell Biochem. 2005;269:153–163. doi: 10.1007/s11010-005-3455-8. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Few AP, Lautermilch NJ, Westenbroek RE, Scheuer T, Catterall WA. Differential regulation of CaV2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation. J Neurosci. 2005;25:7071–7080. doi: 10.1523/JNEUROSCI.0452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JD, Jr., Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- Fletcher CF, Tottene A, Lennon VA, Wilson SM, Dubel SJ, Paylor R, Hosford DA, Tessarollo L, McEnery MW, Pietrobon D, Copeland NG, Jenkins NA. Dystonia and cerebellar atrophy in Cacna1a null mice lacking P/Q calcium channel activity. Faseb J. 2001;15:1288–1290. doi: 10.1096/fj.00-0562fje. [DOI] [PubMed] [Google Scholar]
- Flink MT, Atchison WD. Ca2+ channels as targets of neurological disease: Lambert-Eaton Syndrome and other Ca2+ channelopathies. J Bioenerg Biomembr. 2003;35:697–718. doi: 10.1023/b:jobb.0000008033.02320.10. [DOI] [PubMed] [Google Scholar]
- Goto JI, Inoue T, Kuruma A, Mikoshiba K. Short-term potentiation at the parallel fiber-Purkinje cell synapse. Neurosci Res. 2006 doi: 10.1016/j.neures.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gotz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L, Kurosinski P, Chen F. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- Inchauspe CG, Martini FJ, Forsythe ID, Uchitel OD. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of held presynaptic terminal. J Neurosci. 2004;24:10379–10383. doi: 10.1523/JNEUROSCI.2104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M, Murakami U. Temporal and spatial patterns of Purkinje cell formation in the mouse cerebellum. J Comp Neurol. 1980;194:499–503. doi: 10.1002/cne.901940302. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J Physiol. 1998;509(Pt 2):419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, Shoemaker DD. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, Shin HS. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci U S A. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanumilli S, Tringham EW, Payne CE, Dupere JR, Venkateswarlu K, Usowicz MM. Alternative splicing generates a smaller assortment of CaV2.1 transcripts in cerebellar Purkinje cells than in the cerebellum. Physiol Genomics. 2006;24:86–96. doi: 10.1152/physiolgenomics.00149.2005. [DOI] [PubMed] [Google Scholar]
- Kim J, Ghosh S, Nunziato DA, Pitt GS. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 2004;41:745–754. doi: 10.1016/s0896-6273(04)00081-9. [DOI] [PubMed] [Google Scholar]
- Kuppers E, Sabolek M, Anders U, Pilgrim C, Beyer C. Developmental regulation of glutamic acid decarboxylase mRNA expression and splicing in the rat striatum by dopamine. Brain Res Mol Brain Res. 2000;81:19–28. doi: 10.1016/s0169-328x(00)00156-x. [DOI] [PubMed] [Google Scholar]
- Lautermilch NJ, Few AP, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci. 2005;25:7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA. Differential modulation of Ca(v)2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci. 2002;5:210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–960. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- Ligon B, Boyd AE, 3rd, Dunlap K. Class A calcium channel variants in pancreatic islets and their role in insulin secretion. J Biol Chem. 1998;273:13905–13911. doi: 10.1074/jbc.273.22.13905. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Long-term potentiation of glial synaptic currents in cerebellar culture. Neuron. 1997;18:983–994. doi: 10.1016/s0896-6273(00)80337-2. [DOI] [PubMed] [Google Scholar]
- Lipscombe D. Neuronal proteins custom designed by alternative splicing. Curr Opin Neurobiol. 2005;15:358–363. doi: 10.1016/j.conb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto K, Shin HS, Kano M, Watanabe M. P/Q-type Ca2+ channel alpha1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci. 2004;24:1734–1743. doi: 10.1523/JNEUROSCI.4208-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrek B, Resch A, Grasso C, Lee C. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res. 2001;29:2850–2859. doi: 10.1093/nar/29.13.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, et al. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, Lee JS, Mulle JG, Wang Y, de Leon M, Yue DT. Critical determinants of Ca(2+)-dependent inactivation within an EF-hand motif of L-type Ca(2+) channels. Biophys J. 2000;78:1906–1920. doi: 10.1016/S0006-3495(00)76739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedras-Renteria ES, Pyle JL, Diehn M, Glickfeld LL, Harata NC, Cao Y, Kavalali ET, Brown PO, Tsien RW. Presynaptic homeostasis at CNS nerve terminals compensates for lack of a key Ca2+ entry pathway. Proc Natl Acad Sci U S A. 2004;101:3609–3614. doi: 10.1073/pnas.0308188100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR, Emerick MC, Agnew WS. Full-length single-gene cDNA libraries: applications in splice variant analysis. Anal Biochem. 2000;286:265–276. doi: 10.1006/abio.2000.4819. [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Scelfo B, Strata P. Correlation between multiple climbing fibre regression and parallel fibre response development in the postnatal mouse cerebellum. Eur J Neurosci. 2005;21:971–978. doi: 10.1111/j.1460-9568.2005.03933.x. [DOI] [PubMed] [Google Scholar]
- Soong TW, DeMaria CD, Alvania RS, Zweifel LS, Liang MC, Mittman S, Agnew WS, Yue DT. Systematic identification of splice variants in human P/Q-type channel alpha1(2.1) subunits: implications for current density and Ca2+-dependent inactivation. J Neurosci. 2002;22:10142–10152. doi: 10.1523/JNEUROSCI.22-23-10142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. discussion 8086-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Starr TV, Prystay W, Snutch TP. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci U S A. 1991;88:5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain alpha 1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci U S A. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Thakur MK, Mani ST. Estradiol regulates APP mRNA alternative splicing in the mice brain cortex. Neurosci Lett. 2005;381:154–157. doi: 10.1016/j.neulet.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Tsunemi T, Saegusa H, Ishikawa K, Nagayama S, Murakoshi T, Mizusawa H, Tanabe T. Novel Cav2.1 splice variants isolated from Purkinje cells do not generate P-type Ca2+ current. J Biol Chem. 2002;277:7214–7221. doi: 10.1074/jbc.M108222200. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Piedras-Renteria ES, Jun K, Shin HS, Uchitel OD, Tsien RW. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci U S A. 2003;100:3491–3496. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigues S, Chabret C, Valentin S, Valmier J. Rat embryonic hippocampal neurons express a new class A calcium channel variant. Neurosci Lett. 1998;258:37–40. doi: 10.1016/s0304-3940(98)00842-8. [DOI] [PubMed] [Google Scholar]
- Vigues S, Gastaldi M, Chabret C, Massacrier A, Cau P, Valmier J. Regulation of calcium channel alpha(1A) subunit splice variant mRNAs in kainate-induced temporal lobe epilepsy. Neurobiol Dis. 1999;6:288–301. doi: 10.1006/nbdi.1999.0248. [DOI] [PubMed] [Google Scholar]
- Vigues S, Gastaldi M, Massacrier A, Cau P, Valmier J. The alpha(1A) subunits of rat brain calcium channels are developmentally regulated by alternative RNA splicing. Neuroscience. 2002;113:509–517. doi: 10.1016/s0306-4522(02)00213-0. [DOI] [PubMed] [Google Scholar]
- Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–532. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- Yeo G, Holste D, Kreiman G, Burge CB. Variation in alternative splicing across human tissues. Genome Biol. 2004;5:R74. doi: 10.1186/gb-2004-5-10-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GW, Van Nostrand E, Holste D, Poggio T, Burge CB. Identification and analysis of alternative splicing events conserved in human and mouse. Proc Natl Acad Sci U S A. 2005;102:2850–2855. doi: 10.1073/pnas.0409742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-PCR amplicons spanning the EF hands of (i) Swiss albino mouse cerebellar samples and the (ii) frontal cortex of 87 year-old human male in 1.5% agarose gel. No contamination was observed in the water and RT-omitted control reactions.


