Abstract
Following anaphase, the segregated chromosomes are sequestered by cytokinesis into two separate daughter cells by a cleavage furrow formed by the actomyosin-based contractile ring. Failure to properly position the contractile ring between the segregated chromosomes can result in aneuploidy. In both C. elegans embryos and human cells, the central spindle regulates division plane positioning in parallel with a second pathway that involves astral microtubules. Here we combine genetic and pharmacological manipulations with live cell imaging to spatially separate the two division cues in a single cell. We demonstrate that the two pathways are mechanistically and genetically distinct. By following the distribution of gfp-tagged non-muscle myosin, we have found that the astral pathway for furrow formation involves negative regulation of cortical myosin recruitment. An asymmetrically positioned spindle induces the asymmetric cortical accumulation of myosin. This cortical myosin behaves as a coherent contractile network. If the cortical network is non-uniform over the cell, the cortical contractile elements coalesce into a single furrow. This coalescence requires interconnections among contractile elements. These results provide direct evidence that spindle cues negatively regulate myosin distribution.
Introduction
Cell division in animal cells is mediated by an actomyosin-based contractile ring that forms at the site of the presumptive cleavage furrow, and separates the two nascent daughter cells in a process called cytokinesis. If the two daughter cells are to inherit a complete copy of the genome, cytokinesis must be spatially and temporally coordinated with chromosome segregation. Spatial regulation of cytokinesis is also critical for asymmetric cell division as the cleavage furrow must be precisely positioned in order for the two daughter cells to receive appropriate dowries of cell fate determinants. Although it has been established that the mitotic spindle directs positioning of the division plane, the underlying molecular mechanisms remain incompletely understood.
Positioning and assembly of the contractile ring are critical early steps in cytokinesis. The contractile ring contains large numbers of actin filaments and minifilaments of non-muscle myosin. Contractile ring assembly requires the small GTPase RhoA and its guanine nucleotide exchange factor, ECT-2, myosin regulatory light chain (rMLC) and myosin heavy chain (MHC, NMY-2). These proteins constitute a regulatory cascade required for local activation of actomyosin-based contractility during cytokinesis (see (Glotzer, 2005) for review). At anaphase onset, localized accumulation of an active form of the small GTPase RhoA prefigures contractile ring assembly (Bement et al., 2005, Yuce et al., 2005). RhoA regulates rMLC phosphorylation to promote myosin polymerization and contractile ring assembly. RhoA also promotes formin-mediated actin polymerization in the cleavage furrow (see (Piekny et al., 2005) for review). Myosin II motor molecules assemble into minifilaments that move actin filaments relative to one another to provide the driving force for constriction.
The position of the division plane is determined by the position of the spindle during anaphase. In several cell types, two parts of the spindle function redundantly to establish the division plane: the central spindle and the astral arrays of microtubules that surround the two spindle poles. The central spindle is composed of antiparallel microtubule bundles that lie between the spindle poles during anaphase. A core component of the central spindle is centralspindlin, a complex containing RhoGAP and kinesin subunits (CYK-4 and ZEN-4/MKLP1, respectively). In C. elegans embryos, the central spindle is dispensable for furrow initiation since depletion of either of its core components or the microtubule associated protein SPD-1 disrupts the central spindle but does not prevent cleavage furrow ingression (Powers et al., 1998, Raich et al., 1998, Jantsch-Plunger et al., 2000, Verbrugghe and White, 2004). Conversely, cells of Drosophila asterless mutants are largely depleted of astral microtubules yet form cleavage furrows (Bonaccorsi et al., 1998), suggesting that the central spindle is sufficient to direct cleavage furrow formation. This apparent contradiction was resolved by genetic and laser manipulation experiments that indicate that parallel pathways can act redundantly to induce furrow formation (Dechant and Glotzer, 2003, Bringmann and Hyman, 2005). Thus, the central spindle is usually dispensable for furrow formation in C. elegans embryos, but it nevertheless has furrow-inducing capacity. In the absence of a functional central spindle, the position of the division plane is determined by the position of the spindle, such that the furrow forms between the two asters (Dechant and Glotzer, 2003, Bringmann and Hyman, 2005). While cleavage furrow formation is usually directed by a bipolar spindle, cultured mammalian cells form furrows in the absence of a bipolar spindle (Canman et al., 2003). In the more extreme case, cells largely depleted of microtubules, exhibit dramatic albeit highly disorganized contractions for a limited time period following anaphase onset (Canman et al., 2000, Kurz et al., 2002). Thus cortical contractility does not appear to require microtubules, but rather microtubule-based structures coordinate contractility so that it occurs at the appropriate site.
A molecular pathway has been defined that appears sufficient to explain how the central spindle induces furrow formation. HsCYK-4 has been shown to directly recruit the RhoGEF ECT2 (Somers and Saint, 2003, Yuce et al., 2005, Nishimura and Yonemura, 2006). This interaction is critical for RhoA activation and contractile ring assembly. Furthermore, in human cells, HsCYK-4 is required for contractile ring assembly, but its partner protein, MKLP1, is not, indicating that HsCYK-4 can promote RhoA activation without being localized to the central spindle (Yuce et al., 2005). Therefore, in human cells, as in C. elegans embryos, furrow formation does not strictly require the central spindle..
Whereas this molecular pathway can account for furrow induction by the central spindle, less information is available to explain the aster-regulated pathway (Bringmann et al., 2007). To analyze how the central spindle-independent, astral, pathway can lead to formation of a cleavage furrow, we monitored the cortical recruitment of GFP-tagged non muscle myosin (NMY-2) at high spatial and temporal resolution during the first cell division of the C. elegans embryo. We demonstrate that anaphase onset triggers the RhoA-dependent recruitment of cortical myosin. Using genetics or chemical treatments to mis-position the mitotic spindle within the embryo, we demonstrate that the astral pathway functions by locally inhibiting myosin recruitment and generating anisotropy within the cell cortex. Subsequent coordinated reorganization of the anisotropic actomyosin network generates a contractile structure that drives furrowing.
Results
Dynamic redistribution of cortical myosin upon anaphase onset in the early C. elegans embryo
To investigate the molecular mechanisms underlying the redundant pathways for cleavage furrow positioning, we followed the distribution of GFP-tagged non-muscle myosin (NMY-2) (Munro et al., 2004) before and during the initial stages of cytokinesis. In order to precisely time key transitions in myosin organization and dynamics relative to other cell cycle events, we simultaneously visualized NMY-2:GFP and GFP-Histone (Fig 1A). During prophase, as the centrosomes and nuclei migrate towards the center of the zygote, the cortex is polarized, and small particles of myosin are enriched in the anterior portion of the embryo forming a structure we will refer to as an anterior cap. This anterior cap persists until metaphase, when myosin largely dissociates from the cortex. During the first 30 seconds after anaphase onset, myosin re-accumulates on the cell cortex, primarily in bright foci. Hereafter, the term foci will refer to this population of myosin that accumulates upon anaphase onset. The initial recruitment of anaphase foci is broadly biased to the anterior 2/3 of the embryo, with strongest accumulation in a broad equatorial band (centered midway between the spindle poles) and at the extreme anterior. During the next 60 seconds, myosin foci and smaller myosin-containing particles within the equatorial band move towards the center as a cleavage furrow forms and begins to ingress (Fig. 1A, supp. movie 1). Similar distributions of myosin were observed in fixed specimens co-stained with an antibody specific for the serine-19-phosphorylated form of myosin regulatory light chain and with fluorescent phalloidin to label F-actin (Fig. 1B). Interestingly, the foci of myosin colocalize with regions of high local F-actin density. The accumulation of foci near the equator prior to furrow initiation correlates with the emergence of aligned circumferential filament bundles that connect neighboring foci.
Figure 1.
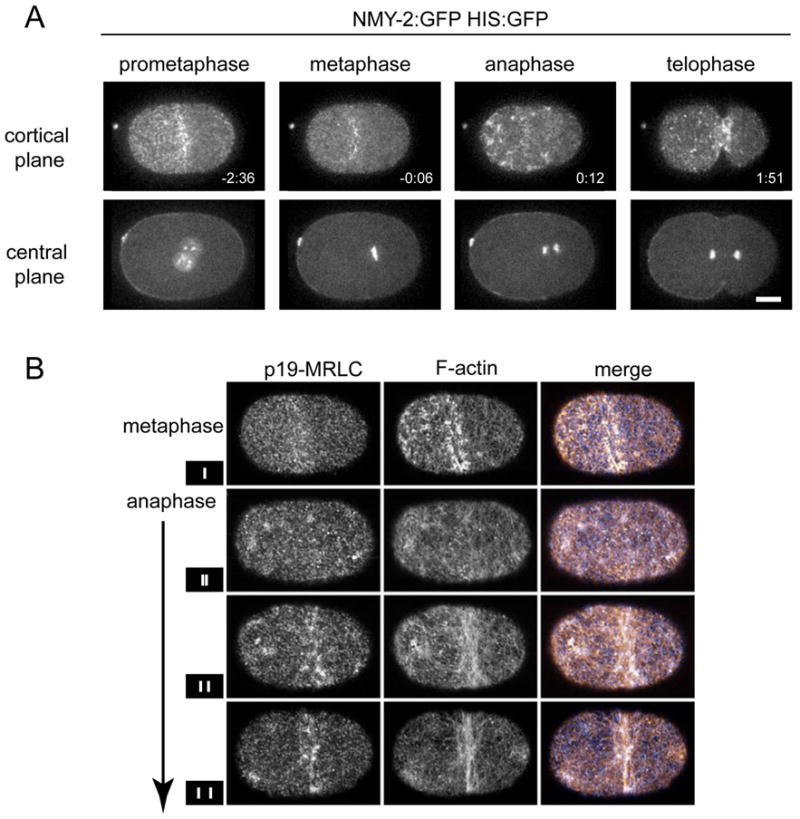
Cortical myosin undergoes cell cycle regulated changes in its localization. A strain expressing both NMY-2:GFP and HIS:GFP was imaged during the first cell division. (A). In all figures, anterior is to the left and scale bars are 10 μm. (B) Spatial distribution of cortical F-actin and active myosin II during early stages of cytokinesis. Fixed whole mount embryos stained with an antibody to serine-19-phosphorylated myosin regulatory light chain (p19-mRLC) (left) and stained with phalloidin to reveal filamentous actin and visualized by a single grazing plane using confocal microscopy. Vertical bars in the small boxes to the left indicate the approximate separation of chromosomes in each embryo.
To test whether the loss of cortical myosin in metaphase and the sudden accumulation of cortical myosin foci at anaphase onset are coordinated with cell cycle progression, we delayed mitotic exit by depleting embryos of the proteasome regulatory subunit RPT-6. Control and RPT-6-depleted embryos required the same time to align their chromosomes on a metaphase plate following nuclear envelope breakdown (NEBD), but the time from NEBD to anaphase onset is increased in RPT-6-depleted embryos ((Gonczy et al., 2000), McCarthy and Goldstein, personal communication). We examined the timing of myosin recruitment in these embryos and observed that it too was delayed relative to NEBD in precise correlation with the delay in anaphase onset (Supplemental Fig. 1A). We conclude that cortical recruitment of myosin requires initiation of mitotic exit which is triggered by activation of the anaphase promoting complex.
The molecular requirements for cortical myosin recruitment
RhoA is a critical regulator of cytokinesis. RhoA depletion prevents furrow formation and inhibits cortical contractility and formation of the pseudocleavage furrow (Jantsch-Plunger et al., 2000, Motegi et al., 2006, Schonegg and Hyman, 2006) in part through its regulation of myosin (Piekny et al., 2005). We investigated how depletion of RhoA affects myosin recruitment during cytokinesis. As shown previously, embryos severely depleted of RhoA lack an organized meshwork of cortical myosin during early interphase; loosely distributed small spots of myosin are observed, but the focal contractions that accompany polarization are absent. As in the wild type, a cortical cap of smaller myosin-containing particles forms during prophase, although it is often smaller and displaced. The anterior cap starts to disappear on schedule during metaphase, but myosin remains at low levels throughout anaphase (Fig. 2A; Supp. Fig. 1B), instead of recruiting as intense foci to the anterior cortex and subsequently accumulating at the equatorial region. To assess whether this correlates with RhoA activation, we depleted the Rho guanine exchange factor (GEF) ECT-2. ECT2 depletion abrogates furrow formation in worms, flies and human cells (Prokopenko et al., 1999, Dechant and Glotzer, 2003, Yuce et al., 2005). Depletion of ECT-2 prevented cortical recruitment of myosin during early interphase and anaphase, but did not abolish the anterior cap of myosin in prophase (Fig. 2A, and supplemental Fig. 1B). Thus, significant recruitment of myosin to the cortex and myosin-driven contraction during anaphase require active RhoA.
Figure 2.
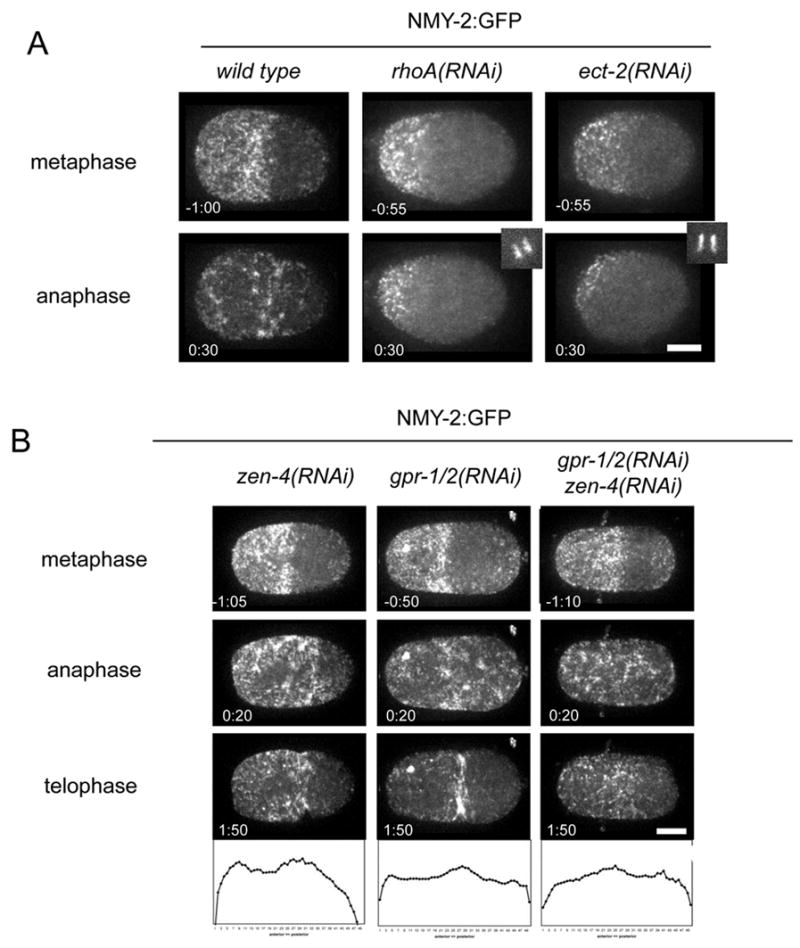
Cortical myosin accumulation upon anaphase onset is dependent on active RhoA. (A) Formation of the anterior cap in prometaphase occurs in wild type and rhoA(RNAi) or ect-2(RNAi) embryos but depletion of these factors blocks cortical re-accumulation upon anaphase onset. (B) Myosin accumulates at the anterior and equatorial cortex in zen-4(RNAi) embryos; and accumulates throughout the entire cortex in early anaphase in gpr-1/2(RNAi) and zen-4(RNAi);gpr-1/2(RNAi). Also shown are normalized profiles of myosin distribution (averages of >7 embryos) along the A/P axis at early anaphase.
Spatial and functional separation of two mechanisms for furrow ingression
Central spindle-dependent and independent mechanisms act redundantly to regulate formation of the cleavage furrow (Dechant and Glotzer, 2003, Bringmann and Hyman, 2005). Embryos defective in either central spindle assembly (e.g. zen-4(RNAi)) or spindle elongation (e.g. gpr-1/2(RNAi)) form cleavage furrows, but embryos in which both spindle elongation and central spindle assembly are defective do not furrow. To determine why these embryos fail to furrow, we compared cortical distributions of NMY-2:GFP in zen-4(RNAi) (n=13), gpr-1/2(RNAi)(n=20), and zen-4(RNAi); gpr-1/2(RNAi) (n=9) embryos. During prophase and metaphase, all embryos exhibited wild-type myosin dynamics. At anaphase onset, in ZEN-4-depleted embryos, the initial pattern of anaphase myosin recruitment was indistinguishable from the wild-type; foci were enriched at the equator and in the anterior of the embryo. By contrast, in GPR-1/2-depleted embryos, the broad anterior bias in initial recruitment of cortical myosin foci was lost, but not the slight enrichment at the equator. In embryos defective in both central spindle assembly and spindle elongation, myosin foci were recruited to the cortex uniformly, however, there was no equatorial bias, and in this case furrow formation was not observed (Fig. 2B). Since cortical recruitment of intense myosin foci requires RhoA and its activator ECT2, we conclude that the failure of these embryos to furrow is not due to a failure to activate RhoA and that the initial recruitment of cortical myosin is central spindle independent. We quantified the distribution of myosin at anaphase onset under these conditions, by measuring average intensity values along the A-P axis. These data confirm that myosin is uniform in GPR-1/2; ZEN-4-depleted embryos (Fig. 2B). Instead, the comparison between zen-4((RNAi) embryos and zen-4((RNAi); gpr1/2(RNAi) embryos suggests that the asymmetric accumulation of myosin requires asymmetric spindle positioning.
To further characterize the mechanism of furrow formation, we sought to spatially separate these two pathways. Genetic or chemical perturbations of the microtubule cytoskeleton in the early embryo is known to cause misplacement of the spindle to the posterior and the subsequent formation of two cleavage furrows, one in the anterior of the embryo and a second in the posterior (Hird and White, 1993). The posterior furrow initiates near the misplaced mitotic spindle, bisecting it. The anterior furrow forms at a distance from the mitotic spindle (Fig. 3A). Multiple perturbations elicit the same effect, microtubule destabilization with nocodazole, a conditional allele of β-tubulin, tbb-2(or3620ts), depletion or mutation of the microtubule-associated protein (MAP) ZYG-9, or mutations or depletion of MEL-26, thereby stabilizing the microtubule severing complex katanin. To assess how myosin distribution during anaphase is altered in response to perturbed spindle location and geometry we simultaneously monitored the distribution of cortical myosin and cytoplasmic microtubules using confocal microscopy. Until anaphase, embryos with posterior spindles exhibited myosin dynamics indistinguishable from wild type. At anaphase onset, bright myosin foci formed with size, intensity, and local spacing similar to the anaphase foci seen in the wild type. However, the initial appearance of these foci was biased to the anterior, and there was no preferential accumulation at the equator (Fig. 3B). Within seconds of their formation, foci began to move towards one another and towards the anterior through a coherent flow, consistent with the coordinated contraction of the entire anterior myosin-rich domain (Fig. 4A). A furrow subsequently formed at the edge of the domain enriched in myosin foci. Following recruitment of anterior foci, cortical myosin accumulated in the posterior cortex between the spindle poles, forming a second furrow (Fig. 3B, supp. movie 2). Cortical myosin accumulation and formation of both furrows required ECT2 (data not shown). Thus, two furrows can form in a single embryo with a posterior spindle, and these furrows have distinct positions relative to the spindle and display distinct modes of myosin recruitment and reorganization.
Figure 3.
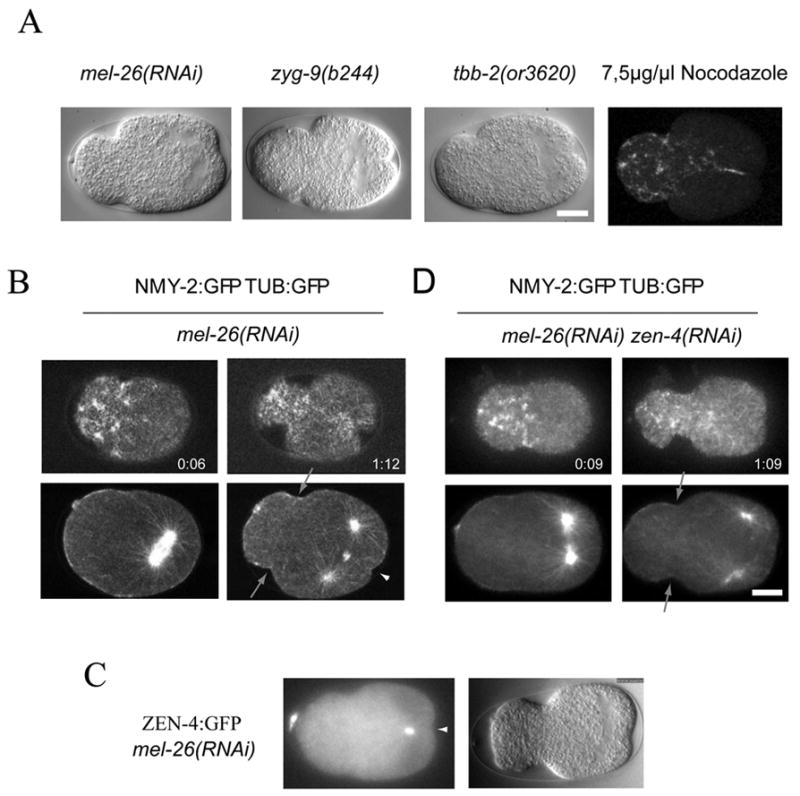
Genetic and spatial separation of two redundant pathways for furrow formation within one embryo. (A) DIC images of embryos with perturbed microtubule stability form a small posterior spindle and two cleavage furrows. (B) Embryos with a posterior spindle accumulate myosin in the anterior and form an anterior furrow (arrow). A second, central spindle dependent furrow forms in the posterior (arrowhead). NMY-2:GFP and TUB:GFP were visualized in the same embryo. Myosin can be observed in cortical planes (top) whereas tubulin is visualized in the central plane of the embryo (bottom) representative images before and after anaphase onset are shown. (C) ZEN-4:GFP localizes to the central spindle in embryos with posterior spindles. (D) The anterior furrow (arrows) but not the posterior furrow forms when central spindle assembly is prevented.
Figure 4.
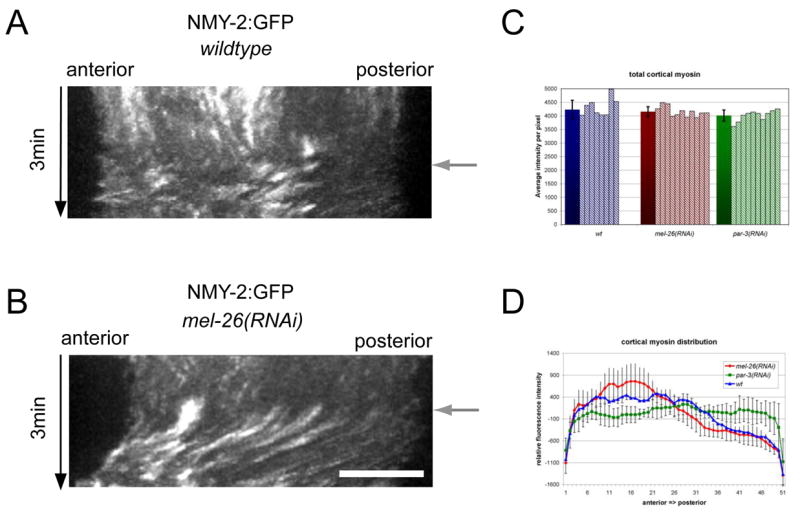
The position of the mitotic spindle controls cortical myosin distribution. Kymographs of cortical myosin distribution in wild type embryos (A) and embryos with a posterior spindle (B). Anaphase onset (black arrow) was determined by the appearance of cortical myosin foci. (C) The cortical myosin distribution after anaphase onset was determined by measuring the average cortical intensity of NMY-2:GFP over the first 20 seconds after anaphase onset representing 8 time points. Measurements for wild type embryos (blue), embryos with posterior positioned mitotic spindles, mel-26(RNAi), (red) and embryos with symmetrically position spindles, par-3(RNAi), green are shown. The data from at least 8 individual recordings were normalized for the length of the embryo. (D) Average total cortical myosin after anaphase onset was measured during the first 20 sec after anaphase onset. The graph represents average total intensity per pixel values (solid bars) for at least 8 individual recordings as well as the values for the individual recordings (stripes).
Next, we examined whether these two furrows are differentially dependent on the central spindle. In MEL-26-depleted embryos, ZEN-4:GFP localizes to the spindle midzone during anaphase, confirming that a central spindle forms (Fig. 3C). We depleted MEL-26 by RNAi either alone or in combination with zen-4(RNAi). During anaphase, in both mel-26(RNAi) and mel-26(RNAi); zen-4(RNAi) embryos, the mitotic spindle formed in the posterior of the embryo and, upon anaphase onset, cortical myosin accumulated and reorganized to create an anterior furrow. Thus, as shown above for myosin recruitment and furrow initiation in the wild type, the anterior mode of furrow formation is independent of the central spindle. By contrast, in zen-4(RNAi); mel-26(RNAi) embryos, myosin did not accumulate in the posterior cortex over the spindle and no posterior furrow appeared (n=8) (Fig. 3D). We observed identical results when we produced posterior spindles by other methods or when a temperature sensitive allele of zen-4 was used to inhibit central spindle assembly (n=6). In summary, central spindle-dependent and independent mechanisms of patterning cortical myosin recruitment and furrow initiation can be analyzed in the same embryo. Furthermore, the anterior furrow and the central-spindle-independent furrow have similar genetic requirements, both positive and negative, and display similar patterns of myosin accumulation. In contrast the anterior and posterior furrows can be distinguished by these criteria
Spindle positioning spatially biases myosin accumulation through local inhibition, but the total accumulation is constant
To gain insight into the patterning of Rho-dependent myosin recruitment in anaphase, we quantitatively compared the accumulation of cortical myosin in control embryos and embryos with posterior spindles. Each 2.5 seconds, we acquired five confocal sections, generated projections, and created kymographs from line scans along the A-P axis. In embryos with posterior spindles, as in wild type embryos, foci appeared within 5 seconds of anaphase onset. In the anterior half of the embryo, the size, intensity, and local spacing of foci were the same as in the wild type. By contrast, only small foci accumulated in the posterior cortex, as in wild type embryos (Fig. 4A, B). Thus, the initial recruitment of myosin at anaphase is inhibited in the neighborhood of the posterior mitotic spindle, likely due to a failure to recruit myosin, rather than its recruitment and subsequent displacement.
We quantified the distribution of these foci, as they first appear after the onset of anaphase. In wild-type embryos, the initial anaphase accumulation of myosin foci is weakly biased in favor of the anterior, with many embryos showing an early peak of accumulation at the equator and the extreme anterior, with minima between these peaks and the extreme posterior (Fig. 4D). Strikingly, embryos with a posterior localized spindle (e.g. mel-26(RNAi)) exhibit a stronger bias in the accumulation of myosin foci, and lack an equatorial peak. This anterior bias is induced by the asymmetrically positioned spindle because it was suppressed when the spindle was symmetrically positioned due to depletion of PAR-3 or PAR-2 (Fig. 4D and data not shown, respectively).
We next analyzed the total accumulation of myosin at anaphase onset in embryos of various genotypes to determine whether the local inhibition of myosin recruitment near the spindle causes an overall reduction in total myosin accumulation, or a shift in myosin accumulation away from the spindle. Total cortical myosin accumulation was not significantly affected by the position of the spindle (Fig. 4C).
Although myosin is polarized prior to metaphase and occupies the same region of the embryo as in anaphase, these patterns are independent. Kymograph analysis shows that distribution of the anaphase foci does not directly correlate with the position or extent of the anterior cap (Fig. 4A, B). In addition, zen-4(or153);gpr-1/2(RNAi) embryos exhibit an anterior cap of myosin in prophase but in anaphase, myosin is uniformly distributed to the entire cell cortex (Fig. 2C).
The anterior furrow emerges from the coherence of contractile elements in the cortex
After the initial, polarized recruitment of myosin in embryos with posterior spindles, cortical myosin foci move collectively towards one another and towards the anterior, revealing a large scale contraction away from the zone of myosin depletion at the posterior (Fig. 4B). The anterior furrow then forms at the edge of the domain enriched in myosin foci. As this appears to involve coordinated behavior of myosin foci, we how the anterior furrow responds to perturbation of the actomyosin network. The formin-homology domain containing protein CYK-1 is required for normal F-actin assembly in the early embryo and furrow initiation at cytokinesis. Although allelic combinations of CYK-1 can be created that block furrow initiation, maternal effect lethal mutations and RNAi-mediated partial depletion of CYK-1 results in cleavage furrow ingression and regression (Severson et al., 2002). Similarly, we observed that cyk-1(RNAi) embryos reproducibly form furrows over a five hour window of RNAi depletion (n=9). The furrow that forms in embryos partially depleted of formin activity requires the central spindle, as cyk-1(or36); zen-4(or153ts) double mutant embryos do not furrow (Severson et al., 2000). Likewise, we find that zen-4(or153ts); cyk-1(RNAi) consistently fail to furrow (n=8). Next, we examined whether the anterior and posterior furrows in zyg-9(b244) mutant embryos are differentially sensitive to partial formin depletion. CYK-1 was partially depleted in zyg-9(b244) mutant embryos using the above conditions. All zyg-9(b244); cyk-1(RNAi) embryos failed to form an anterior furrow, yet a majority of these same embryos (63%) formed a deeply ingressing posterior furrow (n=11) (Fig. 5A). Thus, the anterior and posterior furrows are differentially sensitive to CYK-1 depletion. Moreover, the anterior furrow shares genetic and cytological similarities with the equatorial furrow in embryos lacking a central spindle.
Figure 5.
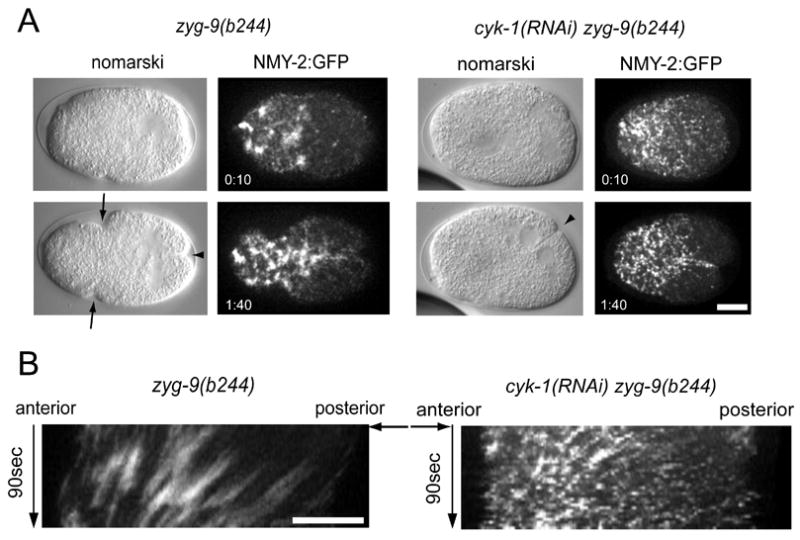
Coordinated cortical myosin contractions are required for the formation of the anterior furrow. Nomarski images and images from embryos expressing NMY-2:GFP after anaphase onset and in telophase. Embryos with a posterior spindle but unperturbed cortical actomyosin (zyg-9(b244)) show anterior myosin localization and formation of an anterior and a posterior furrow (A) whereas embryos with perturbed cortical actomyosin (zyg-9(b244); cyk-1(RNAi)) only form a posterior furrow although myosin still localizes to the anterior. Nomarski and NMY:2:GFP images are representative images from different recordings. Kymographs from corresponding NMY-2:GFP recordings (B). Anaphase onset is indicated by a black arrow.
To determine why the anterior cleavage furrow fails to form under these conditions, we examined NMY-2:GFP in cyk-1(RNAi); zyg-9(b244) embryos. In these embryos, as in the zyg-9 single mutant, NMY-2 accumulates at anaphase onset with similar kinetics and a strong anterior bias. However, in cyk-1(RNAi); zyg-9(b244) embryos, as anaphase proceeds, the myosin foci are smaller and more dispersed. Instead of the coherent flows of myosin seen in zyg-9(b244) embryos, we observed short range flows in a variety of directions (Fig. 5B). A single furrow failed to form at the edge of the anterior myosin-rich domain; instead, we observed small cortical ruffles throughout the anterior (supp. movies 2, 3). To characterize the defect further, we quantified the relative movements of individual pairs of myosin foci (supplemental Fig. 2B). In wild-type embryos and embryos with posterior spindles, pairs of foci most often move towards one another at rates less than 4 μm/min. However, when CYK-1 is depleted, particle movements are greatly exaggerated and pairs of foci frequently move away from each other at rates that often exceed 4 μm/min. Together these data suggest that when CYK-1 is limiting, mechanical coupling of neighboring contractile elements is diminished or lost; they suggest, furthermore, that this mechanical coupling is necessary to produce a globally coordinated contraction and to resolve a single deeply ingressing furrow, instead of many weak and transient invaginations. Thus, the posterior spindle promotes the biased accumulation of contractile elements that then assemble into a globally coupled, contractile network that creates a single furrow as an emergent behavior.
Do γ-tubulin-nucleated microtubules positively regulate furrowing?
In contrast to the results presented here, it has recently been proposed that γ-tubulin nucleated microtubules promote the initial accumulation of contractile ring components (Motegi et al., 2006). The data that led to this proposal are (i) AIR-1-depleted embryos form a γ-tubulin-dependent precocious furrow shortly after NEBD and (ii) furrowing often fails in γ-tubulin-depleted embryos.
The conclusion that furrows form precociously in AIR-1-depleted embryos was based on a comparison of the timing of furrow initiation relative to NEBD. We confirmed the basic finding, measuring a delay between NEBD and furrow initiation of ~2 minutes in AIR-1-depleted embryos as compared to ~4.5 min in wild-type embryos (116±47, 265±45 seconds respectively). However, we and others have observed enhanced chromosome condensation at NEBD in AIR-1-depleted embryos as compared to wild-type embryos (Hannak et al., 2001). We therefore quantified the timing of chromosome condensation, NEBD, and the onset of furrowing in wild-type and AIR-1-depleted embryos and found no significant difference in the interval between chromosome condensation and furrow formation (Fig. 6A). However, in wild-type embryos, NEBD was roughly coincident with half-maximal chromosome condensation, whereas in AIR-1-depleted embryos NEBD occurred ~4 min after half-maximal chromosome condensation (0±30 sec; 240±90 sec respectively)(Fig. 6A). We conclude that AIR-1 depletion results in delay of NEBD, and similar conclusions were recently published (Hachet et al., 2007, Portier et al., 2007). Thus precocious furrowing does not occur in AIR-1 depleted embryos.
Figure 6.
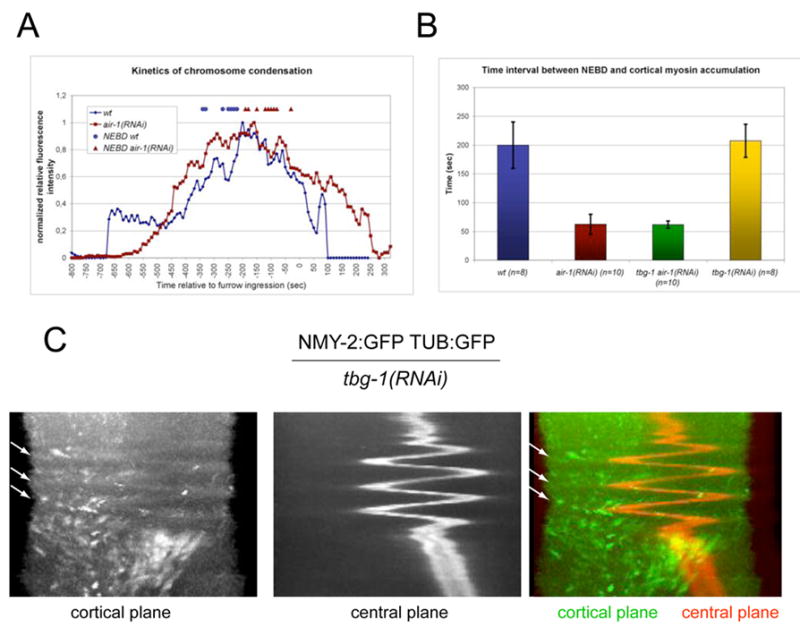
(A) Timing of chromosome condensation relative to furrow ingression in wild type and air-1(RNAi) embryos. The measurements shown are averages for at least 8 individual recordings of embryos expressing both NMY-2:GFP and HIS:GFP. Time of NEBD of each individual recording is indicated for wild type (□) and air-1(RNAi) (·) embryos. (B) Timing of cortical myosin accumulation relative to NEBD. (C) Overlay of kymographs taken along the same line of a maximum projection of 5 cortical planes (green) and one central plane (red) of a movie of an embryo expressing NMY-2:GFP and TUB:GFP. Arrows indicate a subset of foci that accumulate when the spindle is at a distance and dissociate as it returns.
We next investigated whether γ-tubulin regulates the timing of anaphase recruitment of cortical myosin. We assayed NEBD and cortical myosin recruitment in wild-type, air-1(RNAi), tbg-1(RNAi), and tbg-1(RNAi); air-1(RNAi) embryos. The time interval between NEBD and cortical myosin accumulation at anaphase was ~3.5 min. in both wild-type and tbg-1(RNAi) embryos (Fig. 6B). In contrast, this time interval was only ~1 min. in both air-1(RNAi) and tbg-1(RNAi); air-1(RNAi) embryos (Fig. 6B). Thus, γ-tubulin does not regulate the onset of cortical myosin accumulation in anaphase.
Although myosin recruitment occurs on schedule in γ-tubulin-depleted embryos, furrow formation is frequently blocked in these embryos. One interpretation of these data is that γ-tubulin nucleated microtubules promote recruitment of contractile ring components as suggested (Motegi et al., 2006). If this recruitment occurs and is independent of the central spindle as proposed, posterior spindles like those in mel-26(RNAi); zen-4(RNAi) embryos would be predicted to induce a posterior furrow adjacent to the source of the positive signal. However, such furrows do not form (Fig 3D), rather myosin is enriched exclusively in the anterior and only an anterior furrow forms. To examine the requirement for gamma tubulin more closely, we simultaneously visualized the distribution of NMY-2 and microtubules, in γ-tubulin-depleted embryos and discovered a remarkable phenotype. γ-tubulin depletion results in a monopolar spindle that undergoes rapid oscillations along the A-P axis beginning shortly after anaphase onset (Fig. 6C). Each oscillation covered 20–25 μm and required 20–30 seconds; the spindle moved at equal rates in each direction. As the spindle oscillated, cortical myosin transiently accumulated distal to the position of the spindle. As the spindle approached the newly recruited cortical myosin, it was displaced. Once the oscillations ceased, the monopolar spindle remained in the vicinity of the posterior pole of the embryo and furrow formation occurred in a fraction of the embryos. This furrow appeared to arise from a pool of myosin that accumulates in the anterior cortex, distal to the final position of the spindle. These data emphasize that high microtubule density inhibits myosin accumulation and that microtubules can reduce the cortical residence time of myosin foci.
4D visualization of myosin dynamics
To further understand the relationship between the distribution of microtubules and the recruitment of cortical myosin, we collected high resolution z-series through the top half of embryos expressing GFP-Tubulin and GFP-NMY2 during a short time interval surrounding furrow formation. These data sets were reconstructed into 4D images. Three-dimensional reconstructions of MEL-26-depleted embryos highlights the peripheral accumulation of myosin forming a cortical shell (Fig. 7A, supp. movie 4, 5). These images permit visualization of the extent of the spindle, and reveal that the bright foci of myosin are excluded from the portion of the cortex that is directly contacted by spindle microtubules. Analogous reconstructions of wild-type embryos demonstrate that cortical myosin recruitment is strongly inhibited in the vicinity of the posterior centrosome (Fig. 7B, supp. movie 6). The inhibition was somewhat less striking and penetrant over the anterior centrosome. Most foci do not appear to specifically interact with astral microtubules. In a brief interval, ~20 seconds, the foci converge into an incomplete ring in the spindle midplane. The cortex begins to contract locally even before the ring is complete. Microtubule bundles are deformed as the furrow ingresses, ultimately forming the midbody which sets the stage for cell separation.
Figure 7.
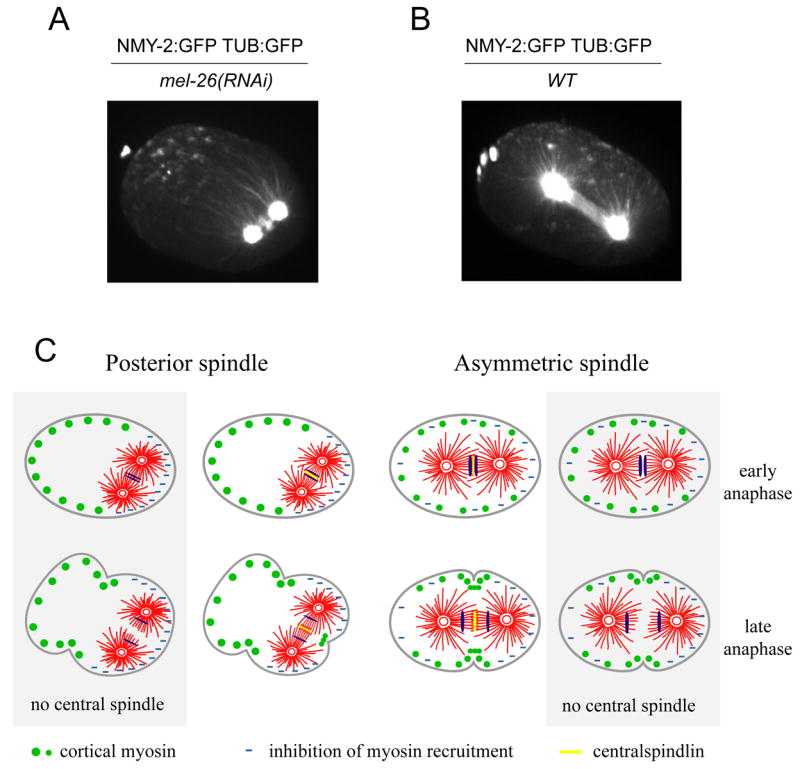
The relative positions of myosin and tubulin in wild-type embryos and embryos with posterior spindles. 3D reconstructions of mel-26(RNAi) (A) and wild type (B) embryos expressing NMY-2:GFP and TUB:GFP are shown shortly after anaphase onset demonstrating reduced myosin accumulation in regions of high microtubule density. (C) A model describing how the central spindle-dependent and central spindle-independent pathways regulate myosin localization and cooperate in furrow formation (see text for details).
Discussion
Here we have presented an analysis of the mechanism of cleavage furrow positioning in the early C. elegans embryo based on high temporal and spatial resolution microscopy. Previous studies established that central spindle dependent and central spindle independent mechanisms are each sufficient to direct cleavage furrow formation (Dechant and Glotzer, 2003, Bringmann and Hyman, 2005). Here, we have focused primarily on the aster-mediated pathway for furrow initiation. Our analysis points to a two-step mechanism for aster-mediated furrowing: first, a signal associated with astral microtubules locally inhibits cortical myosin recruitment at anaphase onset, biasing myosin accumulation. Subsequently, these contractile elements coordinately contract to form a single cleavage furrow.
Probing cytokinesis via spindle displacement and manipulation
We have used genetic and pharmacological perturbations to displace the spindle within the embryo, in order to study the properties of the two furrow inducing signals at the same time in the same embryo. Many insights into cytokinesis have been gleaned by manipulation of the size and position of the spindle. For example, physical micromanipulation experiments conclusively demonstrated a role for the mitotic spindle in cleavage furrow positioning and ingression (Rappaport, 1961, Rappaport, 1985). Likewise, pharmacological inhibition of centrosome separation revealed that a monoaster is sufficient to direct cleavage furrow formation (Canman et al., 2003). Here, we used multiple methods to reduce spindle size and allowed the endogenous pathway for asymmetric spindle positioning to reposition the spindle in the posterior. These perturbations consistently resulted in two distinct furrows in a single embryo (Fig 7C). The posterior furrow forms in close apposition to the central spindle and requires the centralspindlin component ZEN-4; it reflects the central spindle dependent pathway. The following criteria indicate that the anterior furrow reflects the central spindle independent (astral-induced) furrow. Like the authentic cleavage furrow, anterior furrow formation involves the rapid appearance, and coordinated contraction, of myosin foci. These foci are highly similar in size, spacing and timing of their appearance to those associated with furrow formation in wild-type embryos. The anterior furrow, like the furrow that forms in the embryos lacking a central spindle, is sensitive to partial depletion of the formin CYK-1. Finally, like an unperturbed cleavage furrow, the anterior furrow requires RhoA and its RhoGEF, ECT-2.
Nonuniform accumulation of cortical myosin and its coordinated contraction results in furrow formation
In all the embryos we examined, with the exception of embryos depleted of RhoA and ECT-2, myosin II accumulates in foci within seconds of anaphase onset. Delaying anaphase onset correspondingly delays their appearance. In contrast, the kinetics of appearance of these foci are independent of spindle size and position, and the presence/absence of a central spindle. In particular, in embryos doubly compromised for both ZEN-4 and GPR-1/2, foci appear with normally timing over the entire cortex, indicating that myosin can bind to the entire cortex upon exit from mitosis. Our data strongly suggest that the mitotic spindle patterns the accumulation of myosin foci by locally inhibiting their recruitment (Fig 7c).
Soon after their appearance during anaphase, in both wild type embryos and embryos with posterior spindles, myosin foci move towards one another and towards regions of the embryo that are enriched in myosin. In both situations, myosin foci move in a similar pattern and at similar speeds and respond similarly to depletion of CYK-1 (Fig. 5C, Supp. Fig 2B). Since previous work has shown that the movement of foci during pseudocleavage is driven by myosin-mediated contraction (Munro et al., 2004), the movements that occur during anaphase are probably also driven by myosin motor activity. Such movements are expected if contractile forces pulled each focus towards each of its neighbors. These foci may be functionally related to the nodes of contractile proteins that coalesce into the cleavage furrow in S. pombe (Wu et al., 2006). The mechanical coupling between neighboring foci is likely mediated by the prominent bundles of F-actin connect myosin foci. In embryos with a posterior spindle, the biased accumulation of myosin results in a furrow positioned near the border between regions of high and low myosin accumulation. Not only is formation of this furrow independent of the central spindle, we also do not detect centralspindlin components associating with it (data not shown). Furthermore, partial depletion of the formin CYK-1 prevents these foci from coalescing and concentrating in the anterior of the embryo and inhibits anterior furrow formation. Importantly, however, CYK-1 depletion does not significantly affect the initial asymmetry in myosin recruitment. Thus, formation of the anterior furrow involves two distinct steps: local inhibition produces biased accumulation of a cortical actomyosin network at anaphase onset; then coordinated contraction of the network enhances this bias and promotes the production of a single coherent furrow.
It must be emphasized that, although we have shown that inhibition of myosin recruitment follows the distribution of astral microtubules, we do not yet know whether microtubules themselves mediate the inhibition or whether microtubules shape the distribution of another factor that is directly responsible for the inhibitory effect. The elucidation of the exact molecular nature of the inhibitory effect will be a focus of future experiments. The DEP-domain containing protein, LET-99, has recently been suggested to be a specific regulator of the astral pathway (Bringmann et al., 2007). We examined whether LET-99 is required for furrow formation in embryos with posterior spindles and found that depletion of LET-99 delays the appearance of the anterior furrow in tbb-2(or3620ts) embryos and reduces the extent of its ingression. However, anterior furrow formation was not abolished (n=12 let-99(RNAi);tbb-2(or3620ts) and n=6 let-99(RNAi);zyg-9(b244)). Depletion of LET-99 in tbb-2(or3620ts) embryos did not dramatically affect recruitment of myosin foci upon anaphase onset, but as the cell cycle proceeded, the cortical residence time of myosin foci was reduced, and the anterior, myosin-enriched zone contracted farther towards the anterior pole (Supp. movie 7; Supp. Fig 3). Thus, LET-99 appears to modulate actomyosin-based cortical contractility. The absolute requirement for LET-99 in embryos lacking a spindle midzone likely reflects its dual roles in regulating cortical components and its ability to modulate spindle positioning.
The myosin dynamics we observe during cytokinesis is similar to that observed during polarity establishment in the C. elegans zygote (Munro et al., 2004). Initially myosin-rich foci are distributed throughout the cortex. The sperm centrosome breaks cortical symmetry by inhibiting focus formation near the future posterior pole. The remaining meshwork contracts towards the anterior pole, and the pseudocleavage furrow transiently forms at the edge of the myosin-rich domain. Movements of myosin foci measured during pseudocleavage are quantitatively similar to those we observed during anaphase (Fig. 5C). Moreover, partial depletion of the formin CYK-1 affects these two processes in a similar manner. However, embryo polarization is triggered by a microtubule-independent cue from the centrosome (Cowan and Hyman, 2004, Sonneville and Gonczy, 2004). Although different cues may bias actomyosin contractility during these processes, the actomyosin dynamics that govern the response to these cues appear similar.
The posterior furrow forms near the central spindle in a cortical region otherwise depleted of cortical myosin. The central spindle could act positively to locally recruit an activating factor, such as ECT2, or by local relieving the inhibitory activity of microtubules. At this juncture, we favor the former possibility because the central spindle can recruit the RhoGEF ECT2 in other organisms (Somers and Saint, 2003, Yuce et al., 2005). Interestingly, this furrow can form in embryos with reduced CYK-1. This suggests that posterior furrow formation does not require long-range coordination between contractile elements but rather local accumulation of myosin.
The role of γ-tubulin in cleavage furrow positioning
A previous study suggested that γ-tubulin-nucleated microtubules positively regulate furrow formation (Motegi et al., 2006), as embryos depleted of γ-tubulin were furrowing defective and failed to accumulate the contractile ring components, RHO-1 and f-actin. In addition, AIR-1-depleted embryos were found to furrow precociously, shortly after NEBD and formation of this early furrow required γ-tubulin. However, we have shown here that the effect of AIR-1 depletion on the timing of contractility reflects a role in AIR-1 in promoting NEBD; cortical contractility occurs at the appropriate time when compared to other markers of cell cycle progression. Further, since the microtubule array plays a crucial role in spatially regulating contractility, a single, distinct, furrow would not be expected to form in embryos depleted of both AIR-1 and TBG-1, which are largely depleted of microtubules. However, embryos depleted of both of these factors exhibit cortical myosin recruitment and disorganized cortical contractility ~2 min after NEBD, i.e. at the same time of furrow formation in AIR-1-depleted embryos. Thus, our data does not support the hypothesis that γ-tubulin nucleated microtubules have a specialized, positive role in furrow formation. Why is furrowing reduced in γ-tubulin-depleted embryos? First rapid oscillations of the monoaster delays stable accumulation of myosin by displacing myosin from cortical regions in close proximity to the monoaster. Second, γ-tubulin depletion sharply reduces microtubule number (Hannak et al., 2002). Modeling of a microtubule array in a closed system predicts that simply reducing microtubule nucleation permits more microtubules to approach the cell cortex, due to reduced competition for tubulin dimers (Gregoretti et al., 2006). Thus, γ-tubulin depletion could generate a centrally located monoaster that has increased capacity to inhibit myosin accumulation.
Models for cleavage furrow positioning
Cells depleted of most microtubules undergo dramatic and disorganized cortical contractions for a defined time window following anaphase onset (Canman et al., 2000, Kurz et al., 2002), suggesting that microtubules can inhibit cortical contractility. Extending this finding, we had proposed that the two pathways that regulate cytokinesis may act through a common mechanism that generates a local minimum of microtubule density at the cell equator (Glotzer, 2004). Subsequent work has indicated that concentration of the RhoA GEF, ECT2, to the central spindle promotes RhoA activation in a narrow zone overlying the central spindle (Yuce et al., 2005, Kamijo et al., 2006, Nishimura and Yonemura, 2006). By directly visualizing the distribution of microtubules and myosin II in live embryos we have shown that cortical accumulation of this major contractile ring component is negatively regulated by microtubules that emanate from the spindle. This behavior is conceptually similar to the astral relaxation pathway (Wolpert, 1960). Thus, cleavage furrow formation in the early C. elegans embryo is triggered by a combination of positive and negative pathways. Furthermore, both pathways appear to regulate cytokinesis in a variety of animal cells.
The negative regulation of cortical contractility by microtubules may not be limited to cytokinesis. Rearrangements of microtubules precedes the cortical contractility that induces nuclear migration in neurons (Bellion et al., 2005, Schaar and McConnell, 2005). Similarly, in cultured cells, microtubules suppress contractility (Enomoto, 1996, Elbaum et al., 1999). Finally, although migration and polarization of fibroblasts ordinarily requires microtubules, fibroblasts lacking microtubules can be induced to migrate by local application of myosin inhibitors (Kaverina et al., 2000). Thus, local modulation of cortical contractility is of general biological importance.
Experimental Procedures
Strains and Alleles
The following strains and alleles were used in this study: Bristol N2 (wild-type), zen-4(or153ts) IV, xsEx6[zen-4:GFP rol-6], zyg-9(b244), tbb-2(or3620ts), zuIs45 [NMY-2:GFP] V, ojIs1[GFP:TBB-2], xsIs3[HIS-11:GFP], ltIs25 [pAZ132; pie-1/GFP::tba-2; unc-119 (+)]. Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
RNA Interference
RNAi was performed using the feeding method as described (Timmons and Fire, 1998), using double feeding vectors where indicated. L4 larvae were picked onto the plates and incubated at 25°C for at least 24 hr for all experiments. We ensured that RNAi depletion resulted in fully penetrant phenotypes, and where possible we reproduced the phenotypes with well characterized alleles.
Immunolocalization
To simultaneously visualize serine-19-phosphorylated rMLC and F-actin, we used removed the eggshell as described (Costa et al., 1997), followed by fixation for 25 minutes in 4% formaldehyde, 0.2% glutaraldehyde, 0.1mg/ml lysolecithin (Sigma), 60 mM Pipes, 25 mM Hepes, 10 mM EGTA, 2 mM MgCl2, 100 mM dextrose. Embryos were blocked in 5% goat serum, 1% bovine serum albumin for 30 minutes, then sequentially stained with rabbit anti-phospho-myosin regulatory light chain 2 (ser-19) 1:200 (Cell Signaling Technology) followed by a mixture of Alexa-568 anti-rabbit IgG 1:600 (Molecular Probes) and Alexa-488-conjugated phallicidin (1 unit/100μl; Molecular Probes). DNA was stained with 1 μg/ml DAPI. Embryos were mounted in fluoromount G (Southern Biotechnology) and cured overnight at RT before collecting images. These images were collected on a BioRad radiance confocal microscope.
Microscopy
Images were acquired with a 63X/1.4 NA objective on a Zeiss Axiovert 200M equipped with a Yokogawa CSU-10 spinning disk unit (McBain), illuminated with a 50 mW 473 nm DPSS laser (Cobolt). Images were captured on a Cascade 512B EM-CCD camera (Photometrics), using MetaMorph (Molecular Devices). To image cortical NMY-2:GFP, 5 cortical planes were acquired covering a Z-distance of 3 μm. Z-series were acquired every 2.5 seconds for high temporal resolution. To simultaneously image NMY-2:GFP and HIS:GFP or NMY-2:GFP and TUB:GFP with high time resolution, each 3 sec. we acquired 5 cortical planes covering a Z-distance of 3 μm and a single plane in the center of the embryo. To quantify chromosome condensation in embryos expressing NMY-2GFP and HIS:GFP, every ten seconds we acquired two stacks of 5 planes spanning either 3 μm on the cortex or 5 μm in the center of the embryo. The Z-stacks were projected using maximum intensity algorithm and the resulting images assembled into a movie. To image microtubules and myosin with high spatial resolution, Z-stacks of 30 planes spaced by 0.5 μm were acquired from embryos expressing both TUB:GFP and NMY-2:GFP. The Z stack was then converted to 8-bit tiffs using Metamorph and visualized using OsiriX (homepage.mac.com/rossetantoine/osirix/). Kymographs were assembled from maximum pixel intensities over 3 pixels on central linescan along the A-P axis of the embryo. All imaging was performed with a stage temperature of 24–25 C; all temperature sensitive alleles exhibited full penetrance under these conditions (n>30 embryos for each allele).
Data analysis
To analyze the distribution of cortical myosin, 5 cortical planes spanning a Z-distance of 3 μm were acquired every 2.5 seconds. The Z-stacks were projected using a maximum intensity projection. Eight time points (20 secs) directly after anaphase onset were selected by the appearance of cortical myosin foci and projected again using maximum projection, to accumulate foci over time. Intensity values of pixels perpendicular to the AP-axis were averaged using ImageJ and the measurements binned into 50 regions along the A-P axis, to allow comparison between embryos of variable length. Average values for at least 8 individual recordings were plotted. To determine the total cortical intensities the same projected images were used to determine the average intensities per pixel using Metamorph. Chromosome condensation was measured as described (Kaitna et al., 2002).
Supplementary Material
Supplemental Figure 1 (A) The cortical re-accumulation of myosin in anaphase is cell cycle regulated. Perturbation of proteasome degradation by rpt-6(RNAi) coordinately delays anaphase onset and cortical recruitment of myosin but does not affect the timing of other events during mitosis. Images were acquired every 3 seconds from embryos expressing both NMY:2GFP and HIS:GFP. The plotted values represent averages of individual recordings of 8 wild type and 12 rpt-6(RNAi) embryos; NEBD was scored by the reduction in diffuse nuclear HIS-GFP fluorescence, metaphase scored as the time of formation of a metaphase plate and anaphase onset scored by the onset of chromosome separation.
(B) Quantitation of cortical myosin levels in wild-type, rhoA(RNAi), and ect-2(RNAi) embryos. Data shown are averages ± S.D.
Supplemental Figure 2 (A) Myosin foci move anteriorly along the A-P axis at rates that vary according to position along the axis. Using kymographs assembled as in Figure 4, the rate of movement for foci were measured and plotted based upon their position within the embryo. Data represent measurements from three mel-26(RNAi) embryos.
(B) Measurements of the change in separation of individual pairs of foci during 15 second intervals in wild-type embryos during pseudocleavage (PC) and anaphase (ana). Similar measurements were made for embryos with posterior spindles during anaphase (zyg-9(b244)) as well as in embryos partially depleted of CYK-1 during pseudocleavage (PC) and anaphase (ana) and with a posterior spindle. If the movement causes the foci to move apart, the value is positive, if they move towards each other the value is negative. The change in distance between ~50 pairs of foci were rank ordered and plotted.
Supplemental Figure 3 Quantitative analysis of cortical dynamics of GFP:NMY-2 in let-99(RNAi);tbb-2(or3620ts) and tbb-2(or3620ts) embryos. The residence time represents an average of 50 foci from 5 individual recordings. The width of the cortical myosin domain is measured from at least 8 individual recordings. Myosin foci in 2 frames during the first minute post anaphase onset in 8 individual recordings were counted.
Movie 1 Cortical dynamics of GFP:NMY-2 in a wild-type C. elegans embryo.
Movie 2 Cortical dynamics of GFP:NMY-2 in an embryo depleted of MEL-26.
Movie 3 Cortical dynamics of GFP:NMY-2 in a zyg-9(b244) embryo depleted of the formin CYK-1.
Movie 4 4D visualization of GFP:NMY-2 and GFP-Tubulin in an embryo depleted of MEL-26.
Movie 5 3D visualization of GFP:NMY-2 and GFP-Tubulin in an embryo depleted of MEL-26 at anaphase onset.
Movie 6 4D visualization of GFP:NMY-2 and GFP-Tubulin in a wild-type embryo.
Movie 7 Comparison of cortical dynamics of GFP:NMY-2 in tbb-2(or3620ts)(left) and let-99(RNAi);tbb-2(or3620ts) embryos.
Acknowledgments
The authors thank Erin McCarthy and Bob Goldstein for discussions concerning RPT-6 and Paul Maddox for providing a GFP:tubulin expressing strain. The Glotzer Lab is supported by NIGMS R01 GM074743. EM was supported by NIGMS 5P50 GM66050-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 2.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol Biol Cell. 1998;9:2037–2049. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers J, Bossinger O, Rose D, Strome S, et al. A nematode kinesin required for cleavage furrow advancement. Curr Biol. 1998;8:1133–1136. doi: 10.1016/s0960-9822(98)70470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jantsch-Plunger V, Gönczy P, Romano A, Schnabel H, et al. CYK-4: A Rho family GTPase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbrugghe KJ, White JG. SPD-1 Is Required for the Formation of the Spindle Midzone but Is Not Essential for the Completion of Cytokinesis in C elegans Embryos. Curr Biol. 2004;14:1755–1760. doi: 10.1016/j.cub.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 9.Bonaccorsi S, Giansanti MG, Gatti M. Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster. J Cell Biol. 1998;142:751–761. doi: 10.1083/jcb.142.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dechant R, Glotzer M. Centrosome Separation and Central Spindle Assembly Act in Redundant Pathways that Regulate Microtubule Density and Trigger Cleavage Furrow Formation. Dev Cell. 2003;4:333–344. doi: 10.1016/s1534-5807(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 11.Bringmann H, Hyman AA. A cytokinesis furrow is positioned by two consecutive signals. Nature. 2005;436:731–734. doi: 10.1038/nature03823. [DOI] [PubMed] [Google Scholar]
- 12.Canman JC, Cameron LA, Maddox PS, Straight A, et al. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- 13.Kurz T, Pintard L, Willis JH, Hamill DR, et al. Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science. 2002;295:1294–1298. doi: 10.1126/science.1067765. [DOI] [PubMed] [Google Scholar]
- 14.Canman JC, Hoffman DB, Salmon ED. The role of pre- and post-anaphase microtubules in the cytokinesis phase of the cell cycle. Curr Biol. 2000;10:611–614. doi: 10.1016/s0960-9822(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 16.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 17.Bringmann H, Cowan CR, Kong J, Hyman AA. LET-99, GOA-1/GPA-16, and GPR-1/2 are required for aster-positioned cytokinesis. Curr Biol. 2007;17:185–191. doi: 10.1016/j.cub.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 18.Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Gonczy P, Echeverri C, Oegema K, Coulson A, et al. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- 20.Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development. 2006;133:3507–3516. doi: 10.1242/dev.02527. [DOI] [PubMed] [Google Scholar]
- 21.Motegi F, Velarde NV, Piano F, Sugimoto A. Two phases of astral microtubule activity during cytokinesis in C. elegans embryos. Dev Cell. 2006;10:509–520. doi: 10.1016/j.devcel.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Prokopenko SN, Brumby A, O’Keefe L, Prior L, et al. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hird SN, White JG. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J Cell Biol. 1993;121:1343–1355. doi: 10.1083/jcb.121.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Severson AF, Baillie DL, Bowerman B. A Formin Homology Protein and a Profilin Are Required for Cytokinesis and Arp2/3-Independent Assembly of Cortical Microfilaments in C. elegans. Curr Biol. 2002;12:2066–2075. doi: 10.1016/s0960-9822(02)01355-6. [DOI] [PubMed] [Google Scholar]
- 25.Severson AF, Hamill DR, Carter JC, Schumacher J, et al. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Current Biology. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- 26.Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hachet V, Canard C, Gonczy P. Centrosomes Promote Timely Mitotic Entry in C. elegans Embryos. Dev Cell. 2007;12:531–541. doi: 10.1016/j.devcel.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Portier N, Audhya A, Maddox PS, Green RA, et al. A Microtubule-Independent Role for Centrosomes and Aurora A in Nuclear Envelope Breakdown. Dev Cell. 2007;12:515–529. doi: 10.1016/j.devcel.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rappaport R. Experiments concerning the cleavage stimulus in sand dollar eggs. J Exp Zool. 1961;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- 30.Rappaport R. Repeated furrow formation from a single mitotic apparatus in cylindrical sand dollar eggs. J Exp Zool. 1985;234:167–171. doi: 10.1002/jez.1402340120. [DOI] [PubMed] [Google Scholar]
- 31.Wu JQ, Sirotkin V, Kovar DR, Lord M, et al. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J Cell Biol. 2006;174:391–402. doi: 10.1083/jcb.200602032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–96. doi: 10.1038/nature02825. [DOI] [PubMed] [Google Scholar]
- 33.Sonneville R, Gonczy P. Zyg-11 and cul-2 regulate progression through meiosis II and polarity establishment in C. elegans. Development. 2004;131:3527–3543. doi: 10.1242/dev.01244. [DOI] [PubMed] [Google Scholar]
- 34.Hannak E, Oegema K, Kirkham M, Gonczy P, et al. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is gamma-tubulin dependent. J Cell Biol. 2002;157:591–602. doi: 10.1083/jcb.200202047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregoretti IV, Margolin G, Alber MS, Goodson HV. Insights into cytoskeletal behavior from computational modeling of dynamic microtubules in a cell-like environment. J Cell Sci. 2006;119:4781–4788. doi: 10.1242/jcs.03240. [DOI] [PubMed] [Google Scholar]
- 36.Glotzer M. Cleavage furrow positioning. J Cell Biol. 2004;164:347–351. doi: 10.1083/jcb.200310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamijo K, Ohara N, Abe M, Uchimura T, et al. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolpert L. The mechanics and mechanism of cleavage. Int Rev Cytol. 1960;10:163–216. [Google Scholar]
- 39.Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci U S A. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellion A, Baudoin JP, Alvarez C, Bornens M, et al. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci. 2005;25:5691–5699. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- 42.Elbaum M, Chausovsky A, Levy ET, Shtutman M, et al. Microtubule involvement in regulating cell contractility and adhesion-dependent signalling: a possible mechanism for polarization of cell motility. Biochem Soc Symp. 1999;65:147–172. [PubMed] [Google Scholar]
- 43.Kaverina I, Krylyshkina O, Gimona M, Beningo K, et al. Enforced polarisation and locomotion of fibroblasts lacking microtubules. Curr Biol. 2000;10:739–742. doi: 10.1016/s0960-9822(00)00544-3. [DOI] [PubMed] [Google Scholar]
- 44.Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- 45.Costa M, Draper BW, Priess JR. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev Biol. 1997;184:373–384. doi: 10.1006/dbio.1997.8530. [DOI] [PubMed] [Google Scholar]
- 46.Kaitna S, Pasierbek P, Jantsch M, Loidl J, et al. The Aurora B Kinase AIR-2 Regulates Kinetochores during Mitosis and Is Required for Separation of Homologous Chromosomes during Meiosis. Curr Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 (A) The cortical re-accumulation of myosin in anaphase is cell cycle regulated. Perturbation of proteasome degradation by rpt-6(RNAi) coordinately delays anaphase onset and cortical recruitment of myosin but does not affect the timing of other events during mitosis. Images were acquired every 3 seconds from embryos expressing both NMY:2GFP and HIS:GFP. The plotted values represent averages of individual recordings of 8 wild type and 12 rpt-6(RNAi) embryos; NEBD was scored by the reduction in diffuse nuclear HIS-GFP fluorescence, metaphase scored as the time of formation of a metaphase plate and anaphase onset scored by the onset of chromosome separation.
(B) Quantitation of cortical myosin levels in wild-type, rhoA(RNAi), and ect-2(RNAi) embryos. Data shown are averages ± S.D.
Supplemental Figure 2 (A) Myosin foci move anteriorly along the A-P axis at rates that vary according to position along the axis. Using kymographs assembled as in Figure 4, the rate of movement for foci were measured and plotted based upon their position within the embryo. Data represent measurements from three mel-26(RNAi) embryos.
(B) Measurements of the change in separation of individual pairs of foci during 15 second intervals in wild-type embryos during pseudocleavage (PC) and anaphase (ana). Similar measurements were made for embryos with posterior spindles during anaphase (zyg-9(b244)) as well as in embryos partially depleted of CYK-1 during pseudocleavage (PC) and anaphase (ana) and with a posterior spindle. If the movement causes the foci to move apart, the value is positive, if they move towards each other the value is negative. The change in distance between ~50 pairs of foci were rank ordered and plotted.
Supplemental Figure 3 Quantitative analysis of cortical dynamics of GFP:NMY-2 in let-99(RNAi);tbb-2(or3620ts) and tbb-2(or3620ts) embryos. The residence time represents an average of 50 foci from 5 individual recordings. The width of the cortical myosin domain is measured from at least 8 individual recordings. Myosin foci in 2 frames during the first minute post anaphase onset in 8 individual recordings were counted.
Movie 1 Cortical dynamics of GFP:NMY-2 in a wild-type C. elegans embryo.
Movie 2 Cortical dynamics of GFP:NMY-2 in an embryo depleted of MEL-26.
Movie 3 Cortical dynamics of GFP:NMY-2 in a zyg-9(b244) embryo depleted of the formin CYK-1.
Movie 4 4D visualization of GFP:NMY-2 and GFP-Tubulin in an embryo depleted of MEL-26.
Movie 5 3D visualization of GFP:NMY-2 and GFP-Tubulin in an embryo depleted of MEL-26 at anaphase onset.
Movie 6 4D visualization of GFP:NMY-2 and GFP-Tubulin in a wild-type embryo.
Movie 7 Comparison of cortical dynamics of GFP:NMY-2 in tbb-2(or3620ts)(left) and let-99(RNAi);tbb-2(or3620ts) embryos.


