Abstract
Fear conditioning and fear extinction play key roles in the development and treatment of anxiety-related disorders, yet there is little information concerning experiential variables that modulate these processes. Here we examined the impact of exposure to a stressor in a different environment on subsequent fear conditioning and extinction, and whether the degree of behavioral control that the subject has over the stressor is of importance. Rats received a session of either escapable (controllable) tailshock (ES), yoked inescapable (uncontrollable) tailshock (IS), or control treatment (HC) 7 days before fear conditioning in which a tone and footshock were paired. Conditioning was measured 24 h later. In a second experiment rats received ES, IS or HC 24 h after contextual fear conditioning. Extinction then occurred every day beginning 7 days later until a criterion was reached. Spontaneous recovery of fear was assessed 14 days after extinction. IS potentiated fear conditioning when given before fear conditioning, and potentiated fear responding during extinction when given after conditioning. Importantly, ES potently interfered with later fear conditioning, decreased fear responding during fear extinction, and prevented spontaneous recovery of fear. Additionally, we examined if the activation of the ventral medial prefrontal cortex (mPFCv) by ES is critical for the protective effects of ES on later fear conditioning. Inactivation of the mPFCv with muscimol at the time of the initial experience with control prevented ES-induced reductions in later contextual and auditory fear conditioning.
Finally, we explored if the protective effects of ES extended to an unconditioned fear stimulus, ferret odor. Unlike conditioned fear, prior ES increased the fear response to ferret odor to the same degree as did IS.
Keywords: stressor controllability, medial prefrontal cortex, fear conditioning, fear extinction, spontaneous recovery, PTSD
The phenomena of Pavlovian fear conditioning and fear extinction have come to be viewed as key processes involved in the development (Mineka and Zinbarg, 2006) and treatment (Rothbaum and Davis, 2003) of anxiety disorders, respectively. In addition to the obvious procedural similarity between fear conditioning/extinction and the conditions that foster anxiety and that are used in treatment (Foa, 2000), there is extensive overlap between the neurocircuitry that underlies fear conditioning/extinction and anxiety disorders. A large body of evidence from animal studies implicates the amygdala as a key site for the development, expression, and extinction of conditioned fear (Fendt and Fanselow, 1999; Davis et al., 2003; Maren and Quirk, 2004; Sotres-Bayon et al., 2004). The predominant view is that the association between an aversive event (the US, e.g., a footshock) and a neutral stimulus (the CS, e.g., a tone) forms in the lateral nucleus of the amygdala (LA), and that the LA sends input to the central nucleus (CeA), either directly or via the basal nucleus (BA). The CeA, in turn, projects to the regions of the brain that are the proximate mediators of the different aspects of fear responding. The mechanisms are actually more complex (Maren, 2005; Kim and Jung, 2006; Wilensky et al., 2006) but this organization nevertheless captures much of the data. Analogously, human neuroimaging studies demonstrate amygdala activation during the development of conditioned fear (Knight et al., 2003). Anxiety disorders also appear to involve amygdala activation, or perhaps exaggerated activation (Tanev, 2003).
Despite the wealth of data concerning the neurocircuitry of fear conditioning and extinction as well as the conditioning/extinction parameters that modulate the speed and strength of fear conditioning/extinction, there is very little known about experiential variables that modulate fear conditioning and/or extinction. Not all individuals who experience a traumatic event develop an anxiety disorder, and so an understanding of circumstances that might facilitate or retard fear conditioning and/or extinction is of some importance.
Recently, there has been considerable interest in medial prefrontal cortex (mPFC) regulation of amygdala function, fear conditioning/extinction, and anxiety (Quirk and Beer, 2006). Both infralimbic (IL) and prelimbic (PL) regions of the mPFC project to the amygdala (Vertes, 2006). Although the literature regarding mPFC regulation of the amygdala and fear is seemingly inconsistent (see Discussion), stimulation within the mPFC has been reported to inhibit CeA function (Berretta et al., 2005) and to both interfere with the acquisition (Rosenkranz et al., 2003) and expression of conditioned fear responses (Milad et al., 2004). These and other data (see Discussion) suggest that regions of the mPFC can exert inhibitory control over the amygdala and conditioned fear. This is noted here because recent work suggests that exposure to a stressor over which the organism has behavioral control activates mPFC output to stress-responsive brainstem nuclei, and that this mPFC output activation is responsible for the protective effects of behavioral control on stress-induced brainstem activity and behavioral changes controlled by these nuclei (Amat et al., 2005). Furthermore, an initial experience of behavioral control over a stressor appears to alter the mPFC in such a way that a subsequent uncontrollable stressor, which would not normally activate mPFC output since it is not controllable, now does so, resulting in protection against the neurochemical and behavioral impacts of the uncontrollable stressor (Amat et al., 2006). More specifically, prior exposure to escapable shock (ES) blocked the dorsal raphe nucleus (DRN) serotonergic activation and escape learning deficits produced by inescapable shock (IS) administered 7 days later.
If exposure to control over a stressor alters the mPFC in such a way that later stressors that would not normally induce mPFC output to the brainstem now do so, then perhaps a previous experience of control would also lead later to increased mPFC output to the amygdala during fear conditioning. If the mPFC exerts inhibitory control over amygdala function, then the development of fear, the extinction of fear, and/or the expression of fear might be expected to be reduced by experiences with control. Furthermore, any effect of experiencing control on later conditioned fear should be dependent on mPFC activation during the experience of control. Finally, the effects of prior stressor control on subsequent fear responses might be expected to be restricted to conditioned fear. Prior control might not impact on unconditioned fear processes because the same mPFC inactivation that modulates conditioned fear has been shown to have no effect on unconditioned fear responses evoked by a cat (Corcoran and Quirk, 2007). This specificity of mPFC regulation to conditioned fear may occur because unconditioned fear responses do not depend on the CeA (Fendt et al., 2003), the target of inhibitory control from the mPFC. The present experiments examined these possibilities.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats (300–325 g; Harlan, Indianapolis, IN) were used in all experiments. Rats were housed in pairs on a 12-h light/dark cycle (lights on at 7:00 A.M.). However, in Experiment 4 the animals were kept on a reverse light/dark cycle (lights on at 9:00 P.M. and off at 9:00 A.M.). Standard lab chow and water were available ad libitum. All rats were allowed to acclimate to colony conditions for 7–10 days prior to experimentation. All experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder.
Wheel-turn escape/yoked inescapable tailshock procedure
Each rat was placed in a Plexiglas box (14 × 11 × 17 cm) with a wheel mounted in the front and a Plexiglas rod protruding from the rear. The rat’s tail was secured to the rod with tape and affixed with copper electrodes. Rats were run in yoked pairs (ES and IS) and each session consisted of 100 trials of tailshock (30 × 1.0 mA, 30 × 1.3 mA, and 40 × 1.6 mA, a procedure that maintains good escape behavior in ES subjects) with an average 60 s intertrial interval (ITI). Tailshock was terminated for both rats when the ES rat met the escape requirement. Thus, the duration and the intensity of the tailshocks were identical for each rat in the pair. The following procedure was used to insure that the ES rat learned the operant response to terminate the tailshock. Initially, the shock was terminated by a one-quarter turn of the wheel. The response requirements were increased by a one-quarter turn when three consecutive trials were completed in less than 5 s. Subsequent latencies under 5 s increased the requirement by 50% up to a maximum of 4 full turns. The requirement was reduced if the trial was not completed in less than 5 s. If the requirement was not reached in less than 30 s, the shock was terminated and the requirement was reduced to one-quarter turn of the wheel. Non-shocked home cage (HC) rats remained undisturbed in the colony.
Fear conditioning apparatus
Conditioning occurred in two identical clear plastic chambers (26 L × 21 W × 24 H cm) that were placed in two sound-attenuating Igloo ice chests with white interiors. The ice chests each had a speaker and a 6 W clear light bulb mounted to the ceiling. The conditioning chambers rested on a removable floor of 22 stainless-steel rods (0.5 cm diameter) and spaced 1.75 cm center to center (Coulbourn Instruments, Allentown, PA; model E63-23-MOD001). Each rod was wired to a shock generator and scrambler (Coulbourn Instruments; model H13-16) for the delivery of the footshock US. The mounted speaker delivered a 76 dB, 2000 Hz tone that served as the auditory CS. The rods and floor of each chamber were cleaned with water before each animal was trained or tested.
Experiment 1: Effect of escapable and yoked inescapable tailshock on subsequent contextual and auditory-cued fear conditioning occurring 7 days later
Rats were randomly assigned to ES, IS, or HC and administered tailshock between 8:00 A.M. and 12:00 P.M. One week following the tailshock procedure, rats were subjected to a single contextual and auditory-cued fear conditioning session between 10:00 A.M. and 2:00 P.M. Each rat was taken from its home cage and transported to a conditioning chamber in an illuminated isolated room. After exploration of the context chamber for 2 min, rats were presented with a 15 s tone. Immediately following the termination of the tone, a 2 s footshock (1.0 mA) was delivered through the shock grids. Rats were then immediately removed from the conditioning chamber and returned to the colony.
The following day all rats were returned to the conditioning chamber to determine the freezing response to the context and to the tone. The rats were counterbalanced so that half received the context test first and the other half received the tone test first. The two tests were separated by 4 h. Using a sampling procedure, each subject’s behavior was scored every 10 s as either being freezing or not. Freezing was defined as the absence of all movement except that required for respiration, including vibrissae. The observer was blind with regard to treatment condition.
To test for fear of the context, rats were placed in the original conditioning context and levels of freezing were assessed for 5 min. To test for fear of the tone the context was altered. In the novel test environment the shock grid was removed from the chamber and the original test chamber was replaced with a novel test chamber (26 L × 21 W × 10 H cm) that was altered by placing a Plexiglas plate (34 L × 10 H cm) between two diagonally opposite corners, forming a triangular chamber. The novel chamber sat on a clear Plexiglas floor rather than grids, and the brightly lit enclosure was replaced with a 7 W red light bulb. The intensity of the light in the observation room was reduced to the minimum level by which the observer could still score the behavior. During the initial 3 min (pretone condition) the subject’s freezing to the novel environment was scored. This was followed by presentation of the conditioned tone for 3 min. Freezing behavior was scored for the full 6 min using the sampling procedure described above.
Experiment 2: Effect of escapable and yoked inescapable tailshock administered 24 h after fear conditioning on the extinction of fear beginning 7 days later
In Experiment 2, rats received contextual fear conditioning 24 h prior to the tailshock procedure. Each rat was taken from their home cage and transported to a conditioning chamber in an illuminated isolated room. A single session of contextual fear conditioning occurred between 10:00 A.M. and 12:00 P.M. and consisted of two, 2 s footshocks (1.0 mA) with the first footshock occurring 120 s after the rat was placed in the conditioning chamber. The ITI was 120 s and immediately following the termination of the second footshock, rats were removed from the conditioning chambers and returned to the colony. Auditory-cue conditioning was eliminated for simplicity of extinction, and two shocks rather than one was used to increase conditioning and so prolong extinction.
The next day rats were randomly assigned to one of the three conditions in the tailshock paradigm (IS, ES, or HC). Following tailshock, rats were brought back to the colony where they remained for 7 days until extinction testing. During extinction, rats were re-exposed to the previously conditioned context daily for 5 min. As in Experiment 1, freezing behavior was scored using a sampling procedure. Extinction tests continued for each group until the group met a criterion of 10 percent or less freezing for a given session.
To assess for spontaneous recovery of fear, groups were tested 14 days after reaching the extinction criterion. Assessment of freezing behavior consisted of a single 5 min observation period.
Experiment 3: Effect of intra-mPFC muscimol microinjection during IS/ES/HC on how these treatments modulate fear conditioning occurring 7 days later
Rats were surgically implanted with dual cannula guides for microinjections (26 gauge, 1 mm center-to-center, Plastics One, Roanoke, VA). Surgery was performed under halothane anesthesia (Halocarbon Laboratories, River Edge, NJ). The cannula tips were aimed at the PL/IL junction of the mPFCv: 2.6 mm rostral to bregma, 3.3 mm ventral from the dura mater and 0.5 mm relative to the midline. Some rats were implanted with cannula guides for microinjection 2 mm rostral (ventral orbital cortex, VO) to the PL/IL junction as site-specificity controls. Rats were allowed to recover for 2 weeks before experimentation.
Rats were randomly assigned to one of 6 groups in a 2 (muscimol or vehicle) × 3 (ES, IS or HC) design. Rats were injected bilaterally with 0.5 μl of either 50 ng muscimol (Sigma) or 0.9% saline vehicle over a 30 s period. The injector was left in place an additional 2 min to allow for diffusion. One hour after injection, tailshock was administered according to group assignment as described above. At the end of the tailshock session, all rats returned to the colony.
One week later, contextual and auditory fear conditioning procedures were conducted as described above in Experiment 1. At the end of the experiment, rats received a lethal dose of sodium pentobarbital (65 mg/kg), their brains were then removed and flash frozen in −60 °C isopentane. Frozen sections (35 μm) were cut in a −20 °C cryostat and mounted onto glass slides. Sections were then stained with cresyl violet and visualized under a light microscope for cannula placement.
Experiment 4: Effect of escapable and yoked inescapable tailshock on subsequent unconditioned fear responses to ferret odor
Experiments 1–3 examined the effects of shocks varying in controllability on conditioned fear responses. Experiment 4 was conducted to determine if stressor controllability modulates the impact of a stressor on responses to an unconditioned fear stimulus, ferret odor, in a defensive withdrawal apparatus. In this experiment rats were kept on a reverse light/dark cycle and all behavioral testing was conducted during the rat’s dark phase. First, rats were individually acclimated to the defensive withdrawal apparatus (no ferret odor) for 10 min on two consecutive days. The apparatus was a 58 × 58 × 39 cm (L × W × H) black acrylic open field chamber previously described by Masini et al (2006). A metal chamber (29 × 20 × 14 cm) with an opening (9 × 8 cm) was placed in one corner of the open field. The floor and sides of the open field chamber were black, and white tape was used to delineate 16 equal sized squares on the floor. The room that contained the open field chamber was dimly lit by a 60 W red light and ambient noise was masked by white noise (60 dB sound pressure level).
Following habituation to the open field chamber, the next day rats were randomly assigned to ES, yoked IS, or HC as described in the experiments above. Beginning 24 h after tailshock, rats were placed in the defensive withdrawal chamber that now contained a piece of towel with ferret odor (5 × 5 cm square) for 10 min. Ferret odor was collected by housing an adult ferret with a bath towel for approximately one month (courtesy of The Mile High Ferret Club, Thornton, CO). The piece of ferret odor towel was taped to the floor of the apparatus in the diagonal corner opposite the small metal chamber. A different towel piece was used for each rat and the apparatus was cleaned with a 5% bleach solution after each rat. Rats were exposed to the ferret odor in the defensive withdrawal chamber for a total of 7 days. Repeated tests were used because it seemed possible that stressor controllability might influence habituation to the ferret odor even if it did not alter behavior on the first day. After the 7 days of exposure to ferret odor a further test was conducted on Day 8 in which a piece of towel with strawberry (100 μl/towel) odor was used instead of the ferret odor. This was done to examine specificity to the ferret odor.
Two observers blind to the stress treatment independently analyzed the videotaped behavior. Behaviors analyzed included number of rears and time spent with the towel.
Data analysis
Percentage freezing was determined by dividing the number of observations of freezing by the total number of observations and multiplying by 100. Data were analyzed by either between-subjects ANOVA or repeated measures ANOVA followed by post-hoc comparisons (Fisher’s protected least significant difference, PLSD). All group differences were considered statistically significant if p < 0.05.
RESULTS
Experiment 1: Effect of escapable and yoked inescapable tailshock on subsequent contextual and auditory-cued fear conditioning occurring 7 days later
This experiment investigated whether exposure to ES or yoked IS administered in small wheel-turn boxes would alter fear conditioning occurring 7 days later. Thus, rats (n=8/group) were exposed to ES, yoked IS, or HC treatment and 7 days later all subjects received fear conditioning. Fear to the context and to the tone was tested 24 h later. Prior exposure to ES reduced subsequent contextual fear conditioning, whereas prior IS led to a potentiation. Percentage freezing scores to the context are shown in Fig. 1A. An ANOVA indicated a significant effect of group (F (2, 21) = 20.528, p < 0.001) and post-hoc Fisher’s PLSD tests revealed that all three groups were significantly different from each other (ps < 0.001), with the ES group exhibiting significantly less freezing than both the HC and IS groups.
Fig. 1.
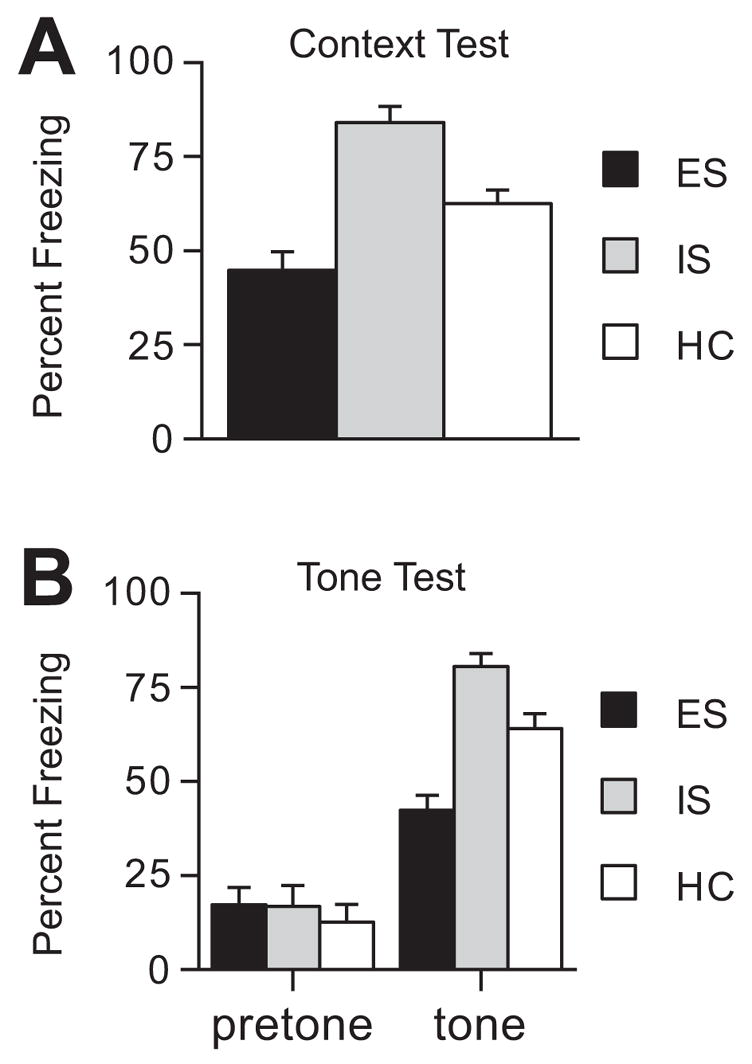
(A) Mean percent freezing in the conditioning context for groups given escapable (ES), yoked inescapable (IS), or no shock (HC) before fear conditioning. (B) Mean percent freezing to the altered experimental context (pretone) and to the tone CS for groups given ES, IS, or HC before fear conditioning.
In order to eliminate the contribution of contextual fear conditioning to freezing to the tone, the tone test was conducted in a novel environment. There were no significant differences between the three groups in freezing to the novel environment (pretone, Fig. 1B). However, presentation of the conditioned tone produced the same pattern of freezing scores that was observed in response to the conditioned context (tone, Fig. 1B). An ANOVA showed a significant main effect of group (F (2, 21) = 9.104, p < .001) and post-hoc Fisher’s PLSD tests revealed that all three groups were significantly different from one another (ps < 0.001). ES subjects showed significantly less freezing to the cue compared to HC and IS groups.
Experiment 2: Effect of escapable and yoked inescapable tailshock administered 24 h after fear conditioning on the extinction of fear beginning 7 days later
Experiment 2 explored whether IS or ES given 24 h after fear conditioning would modulate later extinction. IS/ES or HC treatments (n=8/group) were given after fear conditioning so that any effects of these conditions could not be attributed to modulation of conditioning rather than extinction. For simplicity, this experiment employed only context conditioning. Daily 5 min extinction sessions began 7 days after IS/ES or HC and continued until an extinction criterion had been reached (see Methods). Fear responses that have been extinguished often undergo spontaneous recovery (i.e., conditioned fear returns with the passage of time), and this phenomenon can make fear difficult to extinguish (Bouton, 2004). Thus, spontaneous recovery was assessed 14 days after the extinction criterion had been reached. Extinction to a criterion was used, rather than a fixed number of days of extinction, so that any differences in spontaneous recovery could not be readily attributed to group differences in the degree of extinction. Fig. 2A shows freezing levels of the three groups across days of extinction testing. The extinction criterion was reached on Day 4 for ES, Day 6 for HC, and Day 7 for IS rats. A repeated measures ANOVA was performed on freezing data for the first 4 days of extinction testing when all groups were represented. There was a significant main effect of group (F (2, 21) = 23.444, p < 0.0001) and time (F (19, 399) = 22.838, p < 0.0001), but no significant interaction between group and time (F (38, 399) = 1.028, p < 0.4276). The overall freezing levels were reduced in ES subjects compared to IS and HC subjects.
Fig. 2.
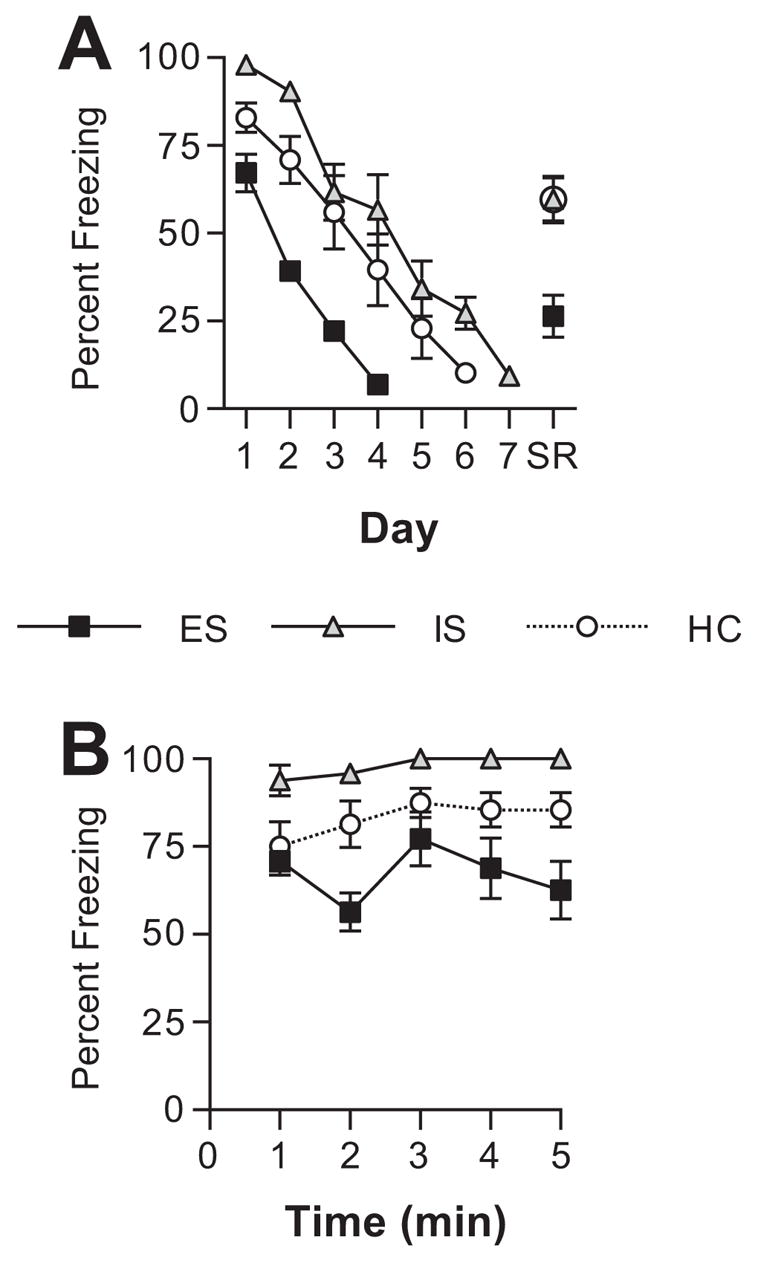
(A) Mean percent freezing to the conditioning context on each day of extinction, and 2 weeks after meeting the extinction criterion (spontaneous recovery, SR). Groups received escapable (ES), yoked inescapable (IS), or no shock (HC) 24 h after fear conditioning. (B) Mean percent freezing to the conditioning context during each minute of the first day of extinction.
Because the groups differed on Day 1 of extinction, even though ES/IS/HC treatment did not occur until 24 h after fear conditioning, freezing for each min of Day 1 of extinction is presented in Fig. 2B to assess whether the groups differed at the very beginning of extinction. As is evident, IS subjects froze more than did HC even during the first minute. ES did not reduce freezing during the first minute, but a difference developed by the second minute. A repeated measure ANOVA indicated a significant effect of group (F (2,21) = 15.01, p < .0001) and time (F (4,84) = 3.09, p = .02). Fisher’s PLSD indicated that ES had significantly lower freezing levels than HC and IS (ps < .05).
Spontaneous recovery was assessed 2 weeks after the extinction criterion was met. The data for spontaneous recovery (SR) are also presented in Fig. 2A. IS and HC groups showed similar levels of spontaneous recovery, but ES substantially reduced this increase in fear. An ANOVA revealed a significant main effect of group (F (2, 21) = 9.365, p = 0.0012) and post-hoc Fisher’s PLSD showed that ES subjects showed significantly less spontaneous recovery than did HC and IS. Spontaneous recovery in HC and IS did not differ from one another.
Experiment 3: Effect of intra-mPFC muscimol microinjection during IS/ES/HC on how these treatments modulate fear conditioning occurring 7 days later
If activation of the mPFCv by ES is essential to the proactive effects of ES on fear conditioning, then inactivation of the mPFCv during ES should prevent ES-induced reductions in later fear conditioning. The locations of the cannula placements within the mPFCv are shown in Fig. 3. Indeed, inactivation of the mPFCv during ES blocked the reduction in subsequent contextual and auditory-cue conditioned fear observed after ES (Fig. 4). Muscimol injected into the mPFCv during IS or HC had no detectable effect on subsequent freezing. Percentage freezing scores (n=6–8/group) to the context are shown in Fig. 4A. An ANOVA revealed a significant main effect for drug (F (1,48) = 4.145, p = .047), group (F (3,48) = 31.314, p < .0001), and an interaction between drug and group (F (3,48) = 12.297, p < .0001). The main effect for group reflects the same pattern as Experiment 1 in which prior IS increased, and ES decreased freezing relative to HC (Fisher’s PLSD, ps < .05). The main effect for drug and the drug by group interaction reflect a selective effect of muscimol on freezing in the ES group. The muscimol-ES treated group increased freezing to the context compared to the vehicle-ES treated group (Fisher’s PLSD, p < .05).
Fig. 3.
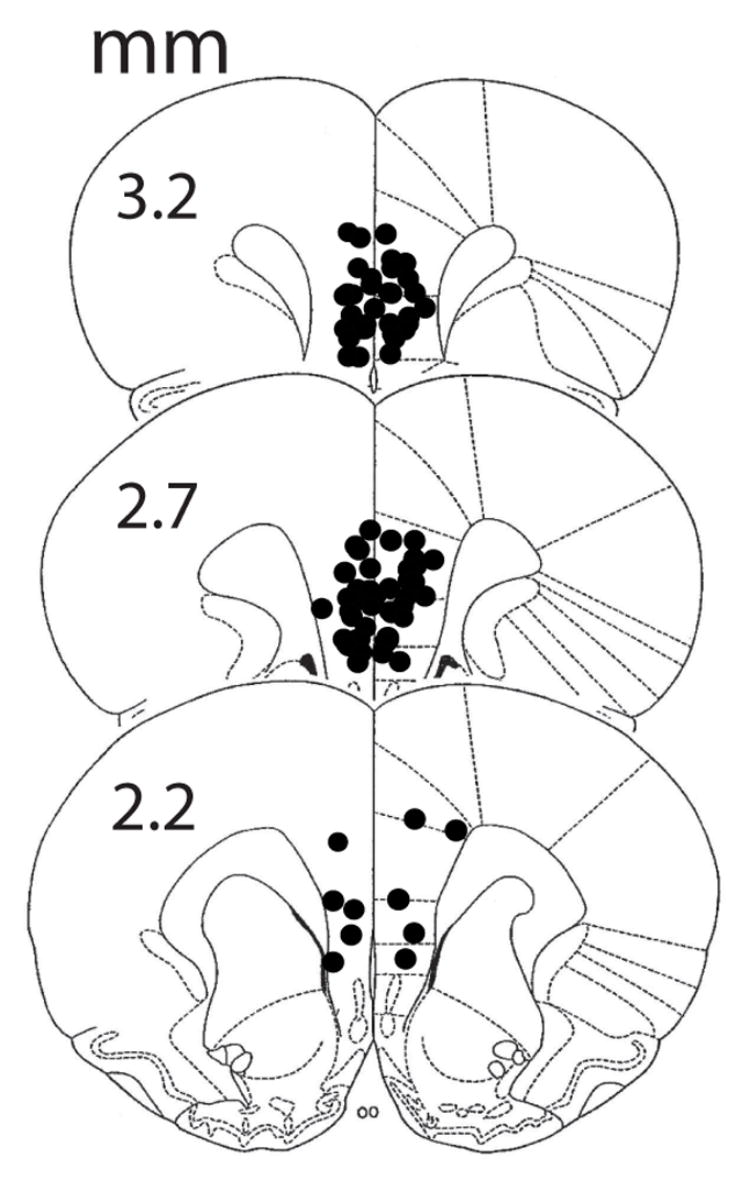
Microinjection cannula placements in the mPFCv. The black circles represent the sites of the injection cannula tips. Numerals indicate distance from bregma (mm).
Fig. 4.
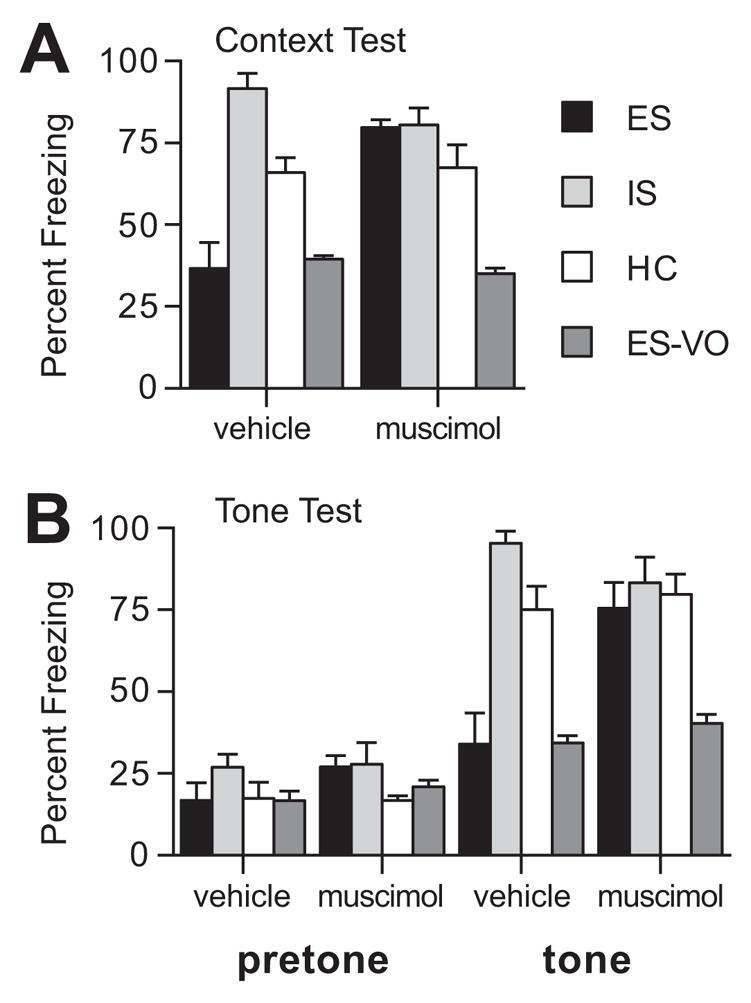
(A) Mean percent freezing in the conditioning context for groups given escapable (ES), yoked inescapable (IS), or no shock (HC) one week before fear conditioning. (B) Mean percent freezing to the altered experimental context (pretone) and to the tone CS for groups given ES, IS, HC one week before fear conditioning. Muscimol or vehicle saline was microinjected into the mPFCv 30 min before onset of tailshock. Site specificity controls for ES were injected with muscimol or vehicle saline into the ventral orbital cortex (ES-VO).
The pattern of freezing responses to the conditioned auditory-cue was identical to that observed above. Percentage freezing scores (n=6–8/group) to novel context (pretone) and to the conditioned auditory cue (tone) are shown in Figure 4B. A repeated measures ANOVA revealed a significant main effect for drug (F (1,48) = 4.776, p = .033), group (F (3,48) = 16.469, p < .0001), and tone (F (1,48) = 286.88, p < .0001), and significant interactions between drug and group (F (3,48) =4.778, p = .005) and drug, group, and tone (F (3,48) = 3.301, p = .028). These effects and interactions reflect, primarily, the selective effect of muscimol on freezing in the ES group. Fisher’s PLSD revealed significantly greater freezing in the muscimol-ES group relative to the vehicle-ES treated group, p < .05. Muscimol had no affect on subsequent freezing in the IS or HC conditions.
In addition, we added a site-specificity control group (VO) that was injected with muscimol (n=8) or vehicle (n=6) 2.0 mm rostral relative to the usual mPFC injection site. As shown in Fig. 4, muscimol injected at the control site did not alter the typical conditioned fear response in ES subjects.
Experiment 4: Effect of escapable and yoked inescapable tailshock on subsequent unconditioned fear responses to ferret odor
During the 7 days of ferret odor exposure, ES and IS subjects spent less time exploring the towel than did HC subjects (n=5–6/group). A repeated measures ANOVA revealed a nearly significant main effect for group (F (2,14) = 3.54, p = .056) and a significant main effect for day (F (6,84) = 4.89, p < .001). Post hoc comparisons revealed that ES and IS groups spent significantly less time with the towel, but ES and IS did not differ. In a similar manner, ES and IS groups spent less time engaged in non-defensive behaviors as shown by the number of rears on each day (Figure 4B). A repeated measures ANOVA revealed that the main effect for group only approached significance (F (2,14) =2.717, p = .101), a significant main effect for day (F (6,84) =9.974, p < .001), and a significant group by day interaction (F (12,84) =2.012, p = .033). To investigate the group by day interaction, pair-wise comparisons were conducted between groups on each day. The number of rears observed in ES (Days 1, 6, and 7) and IS (Days 1 and 6) was significantly lower than HC. Although not statistically significant, rearing behavior for the days not indicated above are generally lower in both ES and IS groups across the 7 days.
On Day 8, the ferret odor towel was replaced with a strawberry odor towel. Rats in all groups increased rearing in the open field and time spent with the towel. One-way ANOVAs revealed no significant effects for group on either rearing or time with towel in the strawberry condition.
DISCUSSION
In the present experiments exposure to tailshocks of differing controllability in one environment had bi-directional effects on fear conditioning 7 days later in a different environment. IS potentiated fear conditioning both to the tone and context. These results are consistent with those of Rau et al. (2005) who found that the administration of inescapable gridshocks potentiated later fear conditioning in a different environment. Rau et al. (2005) eliminated a number of interpretations of the facilitation including the generalization of fear conditioned during IS to the later test situation and concluded that the fear conditioning process itself had been sensitized. The inability of generalization to explain the effects of IS on later fear conditioning is consistent with the data reported here. Subjects that had been exposed to IS did not freeze when placed in the fear conditioning chamber, likely because there are few cues in common between the wheel-turn boxes and the conditioning chamber that could mediate generalization. In addition, even after fear conditioning, IS subjects did not display increased freezing when placed in the altered context used to test for fear to the tone (pretone condition). The argument that IS led to generalization of fear to the fear conditioning environment would have expected IS-induced freezing increases under both conditions, and this did not occur.
Here, we also found that IS given 24 h after fear conditioning increased fear responding assessed during fear extinction beginning 7 days after the IS. However, freezing was increased by IS from the very outset of the first extinction session. Since IS 24 h after fear conditioning would not be likely to be able to retroactively increase the strength of association between the contextual or tone CS and footshock, the data suggest that IS increases the expression of fear to stimuli that are associated with an aversive US. Thus, it is likely that the experience of IS increased the later expression of fear during extinction testing, rather than retarding the extinction process itself. These data also suggest that perhaps the experience of IS before fear conditioning may have increased fear expression rather than conditioning per se. That is, the experience of IS might not have increased that associative strength acquired by either the context or the tone that had been paired with footshock, but rather the level of fear responding produced by conditioned stimuli that have a given associative strength.
It is possible that IS exaggerates the expression of fear responses via action at the mPFC. Vidal-Gonzalez et al. (2006) have shown that microstimulation in the PL region potentiates the expression of conditioned fear responses. Thus, IS might have altered the mPFC in such a way that PL output is later increased when fear is experienced. The data that we obtained was ambiguous in this regard. Although intra-mPFC muscimol administration during IS reduced the difference between IS and HC groups, there was only a small and non-significant difference between IS groups that had received either muscimol or vehicle. In addition, IS potentiated fear responses to ferret odor, and inactivation of the PL has been reported to have no effect on unconditioned fear responses (Corcoran and Quirk, 2007). Thus, it may well be that IS increases fear responses via a mechanism not involving the mPFC. We do not believe that the data allow strong conclusions in this regard.
The focus of the present experiments was not, however, on the impact of IS or uncontrollable stress. Rather, the focus was on what effects the experience of controllable stress, ES, might have on subsequent fear conditioning. This has not previously been studied. ES administered 7 days before fear conditioning interfered with the development of fear responses to both the context and the tone. Clearly, this finding cannot be explained by the possibility of the generalization of fear. Even though ES may condition less fear to the wheel turn apparatus than does IS, it still conditions some fear (Mineka and Hendersen, 1985), certainly not “negative fear”. The present results are quite striking because ES exposes the subject to very aversive stimulation and is highly “stressful” as indicated by an asymptotic HPA axis response to ES (Maier et al., 1986; Helmreich et al., 1999), yet it reduced later fear conditioning. In addition, exposure to ES after fear conditioning facilitated the reduction of fear responses during extinction and eliminated spontaneous recovery of fear. As was true for the effects of IS, this reduction in fear occurred very quickly during extinction, by the second minute of testing. This is likely to be too rapid for any real extinction to have occurred, and so it would appear that the experience of ES reduces the expression of fear, rather than facilitates the extinction process itself. The fear response was reduced by ES and fear disappeared rapidly in these subjects, but this is likely caused by suppression of the fear response rather than by an enhancement of fear extinction learning.
Interestingly, ES did not reduce fear responses to the ferret odor, and instead facilitated fear, and did so to the same degree as did IS. That is, there was no effect of stressor controllability on fear responses to ferret odor. Thus, the reduction in fear expression produced by ES was specific to conditioned fear. It might be noted that we did not determine whether the reactions to the ferret odor were determined by the fact that ferret odor is a predator odor or other aspects of the stimulus such as novelty, although fear responses diminished and group differences disappeared when the odor was switched to strawberry at the end of testing. The purpose of the study was to examine fear-related responses to an unconditioned stimulus, and the issue of whether the stimulus was fearful because it derived from a predator was unimportant. However, Masini et al. (2005) and Masini et al. (2006) have shown that the behaviors measured here in the exact same apparatus to the exact same ferret odor are indeed related to the predatory nature of the odor.
The impact of intra-mPFC muscimol on ES subjects was clearcut. Inactivation of the mPFC during ES completely blocked the effects of ES on later fear conditioning, so that neither fear to the tone or to the context was reduced by ES. These data are consistent with those reported by Amat et al. (2005; 2006) and suggest that control over a stressor is protective because it engages the mPFC. The argument here would be that experiencing control during ES activates the mPFC and alters it in such a way that the occurrence of fear later now activates mPFC output to the amygdala to a greater extent than it otherwise would, thereby inhibiting fear responses controlled by the amygdala. The cannulae placements used here cannot discriminate whether the effects of muscimol on blunting the impact of ES were mediated in PL, IL, or both. However, the work of Quirk and colleagues (e.g., Vidal-Gonzalez et al., (2006) suggest that mPFC inhibition of the amygdala is mediated by the IL. The IL projects strongly to GABAergic cells in the lateral division of the CeA and within the intercalated cell mass (ITC) (Sesack et al., 1989; McDonald et al., 1996; Vertes, 2004). Since these GABAergic cells inhibit CeA output (Royer et al., 1999), the outcome of IL activation would be inhibition of the CeA outputs that produce conditioned fear responses. As would be expected from this argument, chemical stimulation of a region that included the IL increased Fos expression in the ITC (Berretta et al., 2005) and electrical stimulation of the IL reduced the responsiveness of the CeA to stimulation of the BA (Quirk et al., 2003).
Thus, the hypothesis is that the experience of ES alters the IL in such a way that conditioned fear stimuli activate IL output to the amygdala. Of course, PL output could also be increased, with the IL effect predominating. However, the present data do not implicate how the mPFC is altered by ES, but it can be noted that intra-mPFC anisomycin administration blocks the protective effects of ES on later escape learning deficits (Amat et al., 2006). Thus, the mPFC might be a site of plasticity that mediates the protective effects of behavioral control.
The results of the present experiments may help to understand a number of findings in the clinical literature. An inverse relationship between mPFC and amygdala activity has often been noted in humans (Kim et al., 2003; Urry et al., 2006). The amygdala plays a prominent role in human anxiety disorders (e.g., Rauch et al., (2006), and heightened amygdala activity in post-traumatic stress disorder (PTSD) during symptomatic states and the processing of trauma-related stimuli has often been reported (e.g., Bremner et al., (1999). Interestingly, a number of studies have found mPFC hypofunction in conjunction with amygdala activation, suggesting the possibility that the amygdala may show exaggerated responsivity in PTSD because there is a loss of inhibition from the mPFC (see review by Shin et al., (2006). Not all individuals that experience a traumatic event develop PTSD, and the importance of perceived behavioral control in determining resilience has been often noted (Charney, 2004). As specific examples, a careful analysis revealed that a high level of perceived control blunted the impact of the 1999 Marmara earthquake in Turkey (Sumer et al., 2005) and perceived control during a myocardial infarction (MI) reduced the likelihood of PTSD following the MI (Doerfler et al., 2005). Many other examples could be cited (e.g., Palyo and Beck, (2005). The experiments reported here provide a mechanism that could mediate the protective effects of perceived behavioral control on the development of fear/anxiety related disorders. They suggest that perceived control may strengthen mPFC inhibitory control over amygdala activity.
Fig. 5.
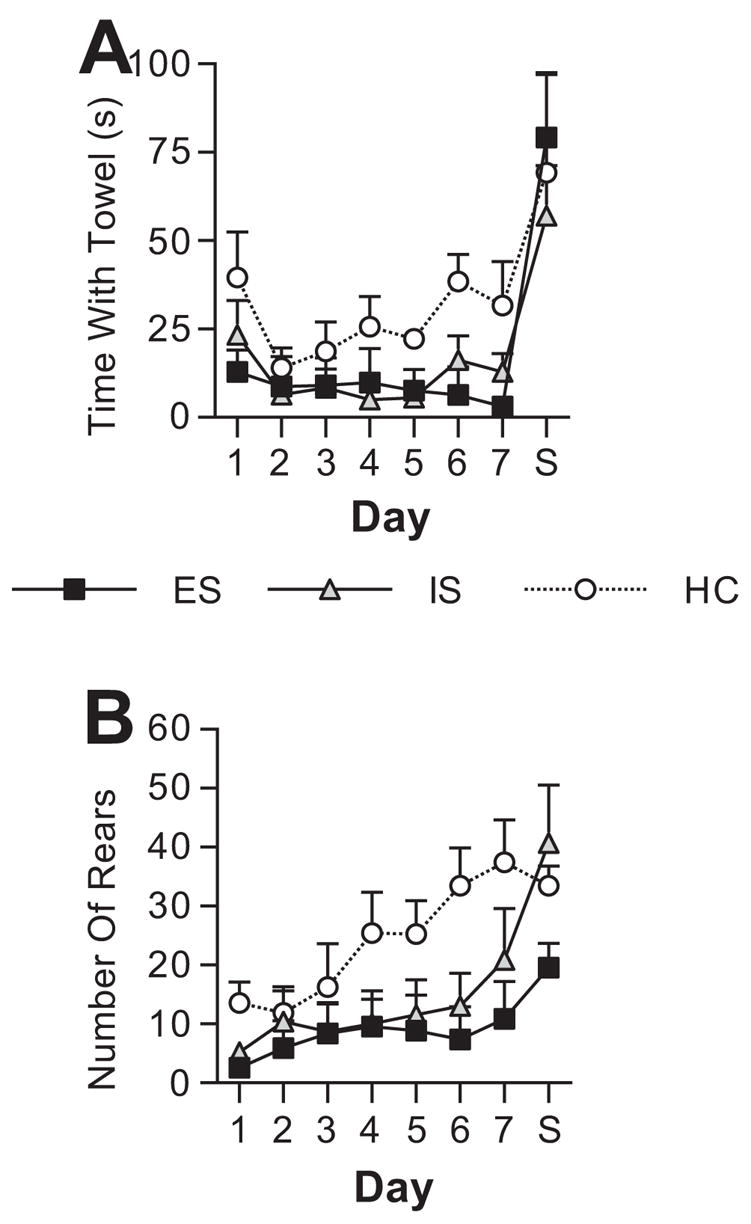
Graphs showing mean behavior in defensive withdrawal apparatus that contained a ferret odor towel (Days 1–7). On Day 8, the ferret odor towel was replaced with a strawberry (S) odor towel. (A) Group means for time (seconds, s) spent with the ferret odor towel for subjects given escapable (ES), yoked inescapable (IS), or no shock (HC) the previous day. (B) Group means for the number of rears for subjects given ES, IS, or HC the previous day.
Acknowledgments
The authors would like to thank Dr. Jerry Rudy for his insightful comments and discussion. This research was supported by grants MH 050479 (SFM) and MH 075213 (MVB) from the National Institute of Mental Health.
Abbreviations
- ES
escapable tailshock
- IS
inescapable tailshock
- LA
lateral nucleus of the amygdala
- CeA
central nucleus of the amygdala
- BA
basal nucleus of the amygdala
- mPFC
medial prefrontal cortex
- mPFCv
ventral medial prefrontal cortex
- IL
infralimbic cortex
- PL
prelimbic cortex
- DRN
dorsal raphe nucleus
- PTSD
post-traumatic stress disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza CM, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- Doerfler LA, Paraskos JA, Piniarski L. Relationship of quality of life and perceived control with posttraumatic stress disorder symptoms 3 to 6 months after myocardial infarction. J Cardiopulm Rehabil. 2005;25:166–172. doi: 10.1097/00008483-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 5):43–48. discussion 49–51. [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1999;11:121–128. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Curr Psychiatry Rep. 2003;5:369–383. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behav Neurosci. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sauer S, White J, Day HE, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Mineka S, Hendersen RW. Controllability and predictability in acquired motivation. Annu Rev Psychol. 1985;36:495–529. doi: 10.1146/annurev.ps.36.020185.002431. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. Am Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Palyo SA, Beck JG. Post-traumatic stress disorder symptoms, pain, and perceived life control: associations with psychosocial and physical functioning. Pain. 2005;117:121–127. doi: 10.1016/j.pain.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sumer N, Karanci AN, Berument SK, Gunes H. Personal resources, coping self-efficacy, and quake exposure as predictors of psychological distress following the 1999 earthquake in Turkey. J Trauma Stress. 2005;18:331–342. doi: 10.1002/jts.20032. [DOI] [PubMed] [Google Scholar]
- Tanev K. Neuroimaging and neurocircuitry in post-traumatic stress disorder: what is currently known? Curr Psychiatry Rep. 2003;5:369–383. doi: 10.1007/s11920-003-0072-7. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


