Abstract
PURPOSE
To examine the effect of a period of continuous darkness on the refractive state and vitreous chamber depth of normal light-reared juvenile tree shrew eyes, and to learn whether eyes that developed myopia in response to monocular minus-lens wear will recover in darkness.
METHODS
Starting at 16 days of visual experience (VE), the refractive state of five dark-treatment tree shrews was measured daily to confirm that it was stable and nearly emmetropic. After corneal and ocular component dimension measures, the animals were placed into continuous darkness for 10 days. On removal of the animals from darkness, corneal and ocular component measures were repeated, and daily refractive measures were resumed. The refractive state of the dark-treatment group was compared with that of a normal-lighting group (n = 5) that received standard colony lighting throughout the measurement period. Five dark-recovery animals wore a monocular -5-D lens for 11 days to induce myopia before they were placed into continuous darkness for 10 days.
RESULTS
The animals in the normal-lighting group completed the emmetropization process, stabilizing at approximately (mean ± SEM) 0.7 ± 0.3 D of hyperopia (noncycloplegic refraction, corrected for the small eye artifact) at 60 days of VE. Dark-treatment group eyes shifted toward myopia (mean ± SEM, -4.3 ± 0.5 D) in the dark. The vitreous chamber became elongated by 0.09 ± 0.02 mm relative to normal eyes. Corneal power showed a small, near-normal decrease (1.4 ± 0.3 D). Four of five myopic eyes in the dark-recovery group became more myopic (-2.2 ± 0.9D) in darkness, and all the fellow control eyes shifted toward myopia (-2.8 ± 0.5 D).
CONCLUSIONS
Maintaining emmetropia is an active process. After eyes have achieved emmetropia or have compensated for a minus lens, continued visual guidance is necessary to maintain a match between the axial length and the focal plane or for recovery to occur. Absence of light is myopiagenic in tree shrews that have developed with normal diurnal lighting. This result contrasts with the apparent absence of a darkness effect in tree shrews reared in the dark from before normal eye opening.
Studies of animal models of human juvenile-onset myopia have shown that the axial length of the eye is actively matched to the eye’s optical power through a visual feedback mechanism (for recent reviews, see Refs. 1-5). When normal tree shrews open their eyes at about 3 weeks of age, they are over 20 D hyperopic, and the axial length is approximately 85% of the adult value.6 As the vitreous chamber depth increases rapidly over the first 15 days of visual experience (VE) the hyperopia decreases and, by 60 days of VE, the eyes stabilize close to emmetropia.7
This process is visually guided. If a concave (minus-power) lens is placed in front of an eye that has nearly completed the emmetropization process, the emmetropization mechanism causes the eye to increase its elongation rate until the retina is moved to the appropriate location so that it again receives focused images. After “minus lens compensation” occurs, the elongation rate returns to normal for that stage of postnatal development.8
Another way to induce axial elongation and myopia is to use a translucent diffuser (form deprivation) to eliminate high-spatial-frequency images and drastically reduce the contrast of all retinal images. In this instance, the axial elongation rate continues to be elevated if the diffuser is left in place because, in contrast to the situation with a minus lens, there is never a signal to indicate that the retina has reached a shifted focal plane.8,9 Form deprivation is ineffective in very young tree shrews, less than approximately 15 days of VE, possibly because the retinal signaling mechanisms are immature.6,9,10
After myopia has been induced, if the diffuser or minus lens is then removed, a “recovery” process begins; the eye remains nearly the same length but the optics mature, moving focused images back to the retina.8,10,11 This process has been reported to be visually guided (Amedo AO et al. IOVS 2005;46:ARVO E-Abstract 1977).12,13 However, McFadden et al.14 reported recently that, after 3 days in complete darkness, guinea pigs recovered from myopia induced either with form deprivation or a minus lens. This raised the possibility that recovery may not require visual cues and might be guided by another signal, related in some way to the eye’s being elongated beyond its normal axial length.
The effect of removing all visual stimuli by placing young animals in the dark has been found to affect refractive development in some species more than in others. Raising monkeys in the dark for many months had no apparent effect on refractive development in one study.15 In another study in which shorter periods were used, darkness prevented normal emmetropization, so that the animals generally remained hyperopic.16 In tree shrews, a long-duration study (McKanna JA et al. IOVS 1983;24:ARVO Abstract 56, p 226) in which dark rearing began before eye opening and continued to adulthood (6-8 months) found no effect of darkness on refraction and axial lengths. In chicks, however, rearing from near hatching in the dark has consistently produced elongated, hyperopic eyes.17-22 The increased axial length is due to vitreous chamber elongation; the hyperopia occurs because there is substantial flattening of the cornea.
In the present study, the effect of darkness was examined after tree shrews had nearly completed the emmetropization process in the standard lighting of an animal colony. Their refractive state was measured for several days to establish that their refractions were stabilizing near emmetropia before they were placed in darkness for 10 days and then remeasured. In addition, a group of animals was made myopic in one eye by wearing a monocular -5-D lens for 11 days. They were then placed into darkness for 10 days to learn whether recovery would occur without visual guidance.
METHODS
The subjects in this study were juvenile tree shrews (Tupaia glis belangeri) raised by their mothers in our breeding colony on a 14/10-hour light-dark cycle. All procedures in this study were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Tree shrew pups open their eyes about 3 weeks after birth. The first day both eyes are open is defined as day 1 of visual experience (VE).
Experimental Groups
There were three groups, each comprising five animals: a normal-lighting group, a dark-treatment group, and a dark-recovery group. Males and females were included in each group with a ratio of three males to two females.
Normal Lighting
Animals in the normal-lighting group were housed in the animal colony throughout the study. On day 15 of VE, they received a dental acrylic pedestal that facilitated refractive measures, as described later. Starting on day 16 of VE (measurement day 1), daily autorefractor measures were made for 22 days, followed by less-frequent measures until measurement day 44 ± 2. These measures established the refractive development of normal colony-reared animals.
Dark-Treatment Group
Animals in the dark-treatment group had normal visual experience in the animal colony from day 1 of VE until they were placed in darkness for 10 days. On day 15 of VE, they received a dental acrylic pedestal. Starting on day 16 of VE (measurement day 1), daily autorefractor measures were made for 5 days, 12 days, or 19 days before dark treatment began. These measures established that the refractive state of the animals had stabilized near emmetropia. Three of the five animals began dark treatment on day 27 of VE (measurement day 12). To examine whether age was an important factor in the effects of dark treatment, one animal began dark treatment earlier, at day 20 of VE (measurement day 5), and another began it later, at day 34 of VE (measurement day 19). Just before the start of dark treatment, measures were made of corneal topography and the animals were anesthetized (90 mg/kg ketamine, 10 mg/kg xylazine) for measurement of axial component dimensions. To begin to learn how quickly darkness has an effect on refractive state, one tree shrew in the group was transported to the laboratory after 5 days in a darkened box, and its refractive state was measured in a very dimly lit room. It was then returned to darkness for the rest of the 10 day period.
On return to the light, noncycloplegic autorefractor measures were made, corneal topography was remeasured, and the animals were anesthetized for another measure of axial component dimensions. On recovery from anesthesia, the animals were returned to cages in the animal colony. Daily noncycloplegic refractive measures were made for the first 10 days after the end of the dark treatment, followed by less-frequent measures until measurement day 60.
Dark-Recovery Group
This group was also raised in the animal colony and also received a pedestal on day 15 of VE. Starting on day 16 VE (treatment day 1), myopia induction was begun in one eye by clipping to the pedestal a goggle frame containing a monocular -5 D lens for 11 days. The eye that was treated was balanced between left and right eyes. Daily autorefractor measurements were taken on all animals with no lens in place. Periodically, measures also were made with the -5-D lens in place.
After 11 days of lens wear (treatment day 12), immediately after the autorefractor measure, the goggle frame was removed, and the animals were placed in complete darkness for 10 days. Measuring axial length requires anesthetizing the animals and using atropine cycloplegia, both of which may interfere with recovery from induced myopia. To avoid any possible interference with recovery, the animals were not anesthetized at the start of recovery, and no measures were made of axial component dimensions or corneal topography. This protocol was also used so that these animals could be compared with those in a large study of recovery involving other groups of animals, which also were not anesthetized at the start of recovery (Amedo AO et al. IOVS 2005;46:E-Abstract 1977).
Based on previous studies of recovery in the light,8,23,24 the 10-day dark-treatment period was long enough for recovery to become evident, if it occurred. At the end of the dark period, the noncycloplegic refractive state was immediately remeasured with the autorefractor, and the animals were returned to their home cages in the colony. Daily refractive measures were made for the first 10 days after the end of the dark treatment, followed by less-frequent measures until treatment day 54 ± 2.
Procedures and Measurements
Pedestal Surgery
On day 15 of VE, a dental acrylic pedestal25 was mounted on the skull while the animal was anesthetized (90 mg/kg ketamine, 10 mg/kg xylazine, supplemented with 0.5% to 2.0% halothane as needed). For the dark-recovery group, the pedestal held the goggle frame containing the -5-D lens. For the normal-lighting and dark-treatment groups, it provided a “handle” that facilitated holding the animals steadily in front of the autorefractor for the daily refractive measurements. When the animals recovered from anesthesia, they were then housed individually in cages in the animal colony in well-lit cages (156-548 lux).
Ocular Component Dimension Measures
While the animals were anesthetized for the pedestal installation, the ocular component dimensions (anterior segment, lens thickness, vitreous chamber depth, and axial length) were measured with A-scan ultrasonography.6 This ensured that the right and left eyes were in the normal range and did not differ significantly in axial length. The A-scan was repeated under anesthesia on the animals in the dark-treatment group just before and after dark treatment. A final measure of ocular component dimensions was made on all animals in all groups with atropine cycloplegia on day 60 ± 2.
Corneal Measures
Just before and immediately after animals in the dark-treatment group were placed in darkness, corneal measures were made on the awake animals with a corneal topography system (Optikon 2000; Keratron, Rome, Italy), except there was no predarkness measure in one animal (0517). Three stored images of each eye were analyzed with the provided software, and the average spherical equivalent corneal power and radius were calculated using the Maloney best-fit26 3-mm zone. The postdarkness measures were then compared with the predarkness measures.
Refractive Measures
Refractive measures were made in a darkened room with an autorefractor (ARK 700A; Nidek, Gamagori, Japan) between 9 and 10 AM. The lighted target in the autorefractor was turned off to avoid presenting images to the eyes while they were being measured. Except for a final measure with atropine cycloplegia on day 60 ± 2, all measures were made without atropine cycloplegia because atropine treatment has been found to reduce the development of induced myopia.27,28 Previous studies that compared autorefractor measures with streak retinoscopy (Norton TT et al. IOVS 2000;41: ARVO Abstract 2990)29,30 have found that both measure similar amounts of induced myopia. Moreover, autorefractor measures can be made on awake animals while streak retinoscopy has required anesthesia, precluding daily measurements. The autorefractor measures were entered into a spreadsheet that calculated the spherical equivalent corrected to the corneal plane. Previous studies31 have found that an autorefractor measure in tree shrews of 4 D is approximately emmetropic, due to the “small eye artifact.”31
Lens-Related Procedures
In the dark-recovery group, the -5-D lens was cleaned twice daily, in the morning at the time of the autorefractor measures and in the afternoon between 4 and 5 PM During lens cleaning, the animals were placed in their nest box in a dimly lit room during the brief (1-3 minutes) procedure. If a lens became severely scratched, it was replaced with a new one. The lens replacement procedure took 10 to 20 minutes and was necessary approximately every 5 to 10 days. During lens replacement, the animal was kept in its nest box in the dark, to ensure that it received no visual signals while the lens was off.
Dark Treatment
Dark treatment occurred in a photographic darkroom into which a tree shrew cage was placed. Food and water were available continuously and were supplemented with daily fresh fruit. A circular darkroom door allowed entry without allowing light exposure. Undisturbed tape over the light switch and restricted access to the darkroom provided evidence that darkness was continuous. In addition, a latching photodiode device was used while some animals were receiving dark exposure to monitor that the lights in the darkroom were not accidentally turned on. Tree shrews grew normally in the dark, usually gaining ∼30 g, a typical weight gain for this age range.
Statistical Analysis
Measures of refractive state, axial component dimensions, and corneal power were entered into spreadsheets (Excel; Microsoft, Redmond, WA). Repeated-measures ANOVA was used to assess whether the refraction of the normal-lighting group changed over time. For the dark-normal and dark-recovery groups, because of daily variability in the refractive state measures and because of concerns that measures taken immediately after a return to the light might be affected by changes in tonic accommodation that might occur during 10 days in darkness, the first two postdark measures were averaged. For symmetry, the last two predarknessrefractive measures also were averaged. Paired t-tests were applied to the averaged values to compare the pre- and postdark treatment values of each group.
RESULTS
Normal-Lighting Group
On day 16 of VE (measurement day 1), the initial >20 D of hyperopia found at eye-opening in tree shrews6 had decreased to a measured refraction of (mean ± SEM) 6.3 ± 0.6 D. Similar to previous reports6 and as shown in Figure 1, refractive measures in the right and left eyes of the normal animals were similar to each other throughout the period. The refractive measures of the normal-lighting group showed a gradual, significant decrease (Fig. 1B) between measurement days 1 and 44 (repeated-measures ANOVA on averaged left and right eye data; P < 0.001), stabilizing at 4.7 ± 0.3 D on measurement day 44. The evoked potential study of Norton et al.30 concluded that a Nidek autorefractor value of +4 D corresponds approximately to emmetropia in tree shrews because the autorefractor reflex measures the location of the vitreous-retinal boundary rather than the photoreceptors, as first suggested by Glickstein and Millodot.31 Thus, the normal animals had an average hyperopic refraction of approximately 2.3 D at the start of the measurement period and were slightly hyperopic (0.7 D) at the end of the measurement period.
FIGURE 1.
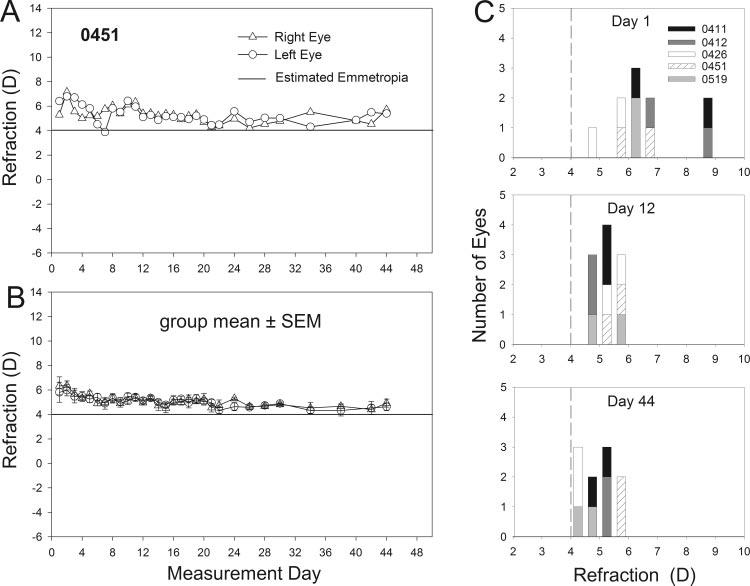
(A) Refractive measures in the left and right eyes of a normal tree shrew (0451) starting at 16 days of VE (measurement day 1). The horizontal line at 4 D indicates estimated emmetropia as measured with the autorefractor. (B) The mean ± SEM refractive values for the left and right eyes of the normal-lighting group. (C) Distribution of refractive values for each eye of the five normal-lighting animals on measurement days 1, 12 and 44. Each shade of gray represents the two eyes of an individual animal. Bin width is 0.5 D. Vertical dashed line: estimated emmetropia as measured with the autorefractor.
In addition to the progression from hyperopia to emmetropia, the distribution of the refractive measures narrowed between measurement days 1 and 44, and the left and right eye refractions became more closely correlated over the measurement period. As shown in Figure 1C, on measurement day 1, refractive measures ranged from 4.6 to 8.5 D, and the right and left eyes differed from each other by an average of 1.3 D. By measurement day 12, the range of refractive values narrowed to 3.9 to 5.7 D, and the average difference between the left and right eyes had declined to 0.3 D. The distribution of refractive measures remained about the same at measurement day 44, and the average left-right difference was still 0.3 D. These changes in juvenile tree shrews resemble the tightening of the refractive distribution seen in human children.32-36
Dark-Treatment Group
As shown in Figure 2, the dark-treatment group eyes followed the same pattern as the normal-lighting group until dark treatment began. As shown in Figure 2F, the refractions, (average of the last 2 days in the light) were very near to emmetropia (4.7 ± 0.2 D) and the left and right eyes of all animals were closely coordinated.
FIGURE 2.
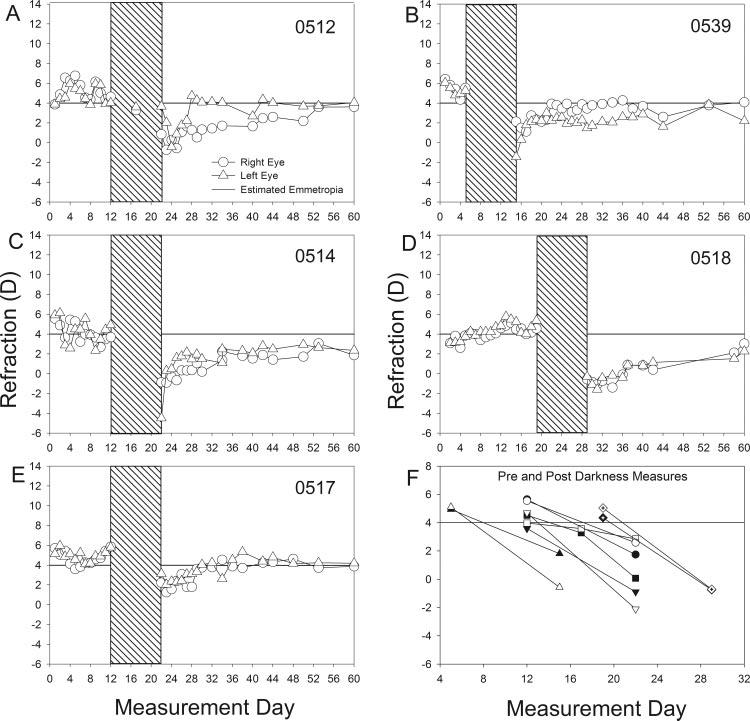
(A-E) Refractive measures of the left and right eyes of each animal in the dark-treatment group starting at 16 days of VE (measurement day 1). All daily measures are shown without averaging. Hatched region: the timing of the 10-day dark-recovery period. (F) Refractive measures of the left and right eyes of each animal before and after dark treatment. Lines connect the pre- and postdarkness measures. Each data point is the mean of the last 2 days before or the first 2 days after darkness. A different symbol is used for each animal. Filled symbols: right eye; open symbols: left eye. Horizontal line at 4 D: estimated emmetropia as measured with the autorefractor.
After 10 days in the dark, all 10 dark-treatment eyes shifted in the direction of myopia by -4.3 ± 0.5 D. This change from the predarkness values was statistically significant (t = 8.34, P < 0.01). As shown in Figure 2F, the refractive state of the left and right eyes was less well-coordinated after dark treatment than they had been before dark treatment. The mean ± SEM difference in refractive state was 0.5 ± 0.2 D before and 1.4 ± 0.5 D after dark treatment. The one tree shrew that was measured after 5 days in the dark had developed a small myopic shift in both the right (-1.2 D) and left (-0.4 D) eyes that was intermediate to the starting and ending refractions.
Ocular Component Dimensions
Examination of ocular component dimensions and corneal power (Fig. 3 and Fig. 4) showed that the myopic shift was due to elongation of the vitreous chamber. The vitreous chamber depth increased in all animals. The mean ± SEM increase was 0.09 ± 0.02 mm. This result is very similar to the 20- to 25-μm/D relationship when myopia is induced with a diffuser or a minus lens.8-10,37 As originally found by Norton and McBrien,6 the vitreous chamber depth in the age range studied (20-45 days of VE) remained nearly constant and then decreased slightly in older normal animals (Fig. 3; thick solid line) due to the continued thickening of the lens and the slowed increase in axial length. The predarkness measures generally followed this trend. The increase in vitreous chamber depth in the dark-treatment animals contrasted with the lack of change in normal values. There were also small increases in anterior segment depth (0.02 ± 0.01) mm and lens thickness (0.07 ± 0.01 mm), in keeping with normal growth at this age.6
FIGURE 3.
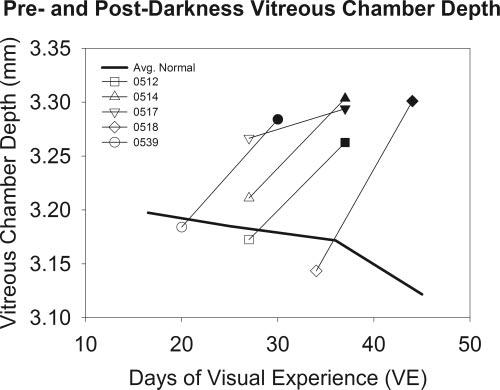
Vitreous chamber depths in dark-treatment animals (mean of left and right eyes) compared with normal vitreous chamber depth at comparable ages. Open symbols: predarkness measures; filled symbols: postdarkness measures. A different symbol is used for each animal. Normal data (solid line) are the average of values from previous reports.6,10,23,24
FIGURE 4.
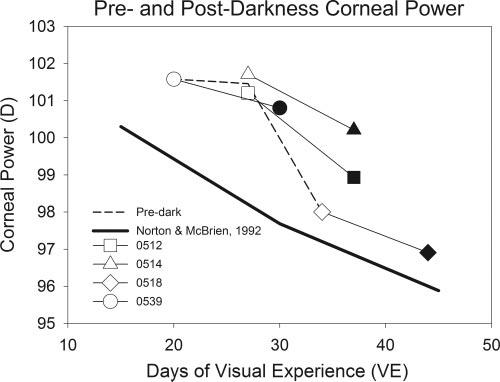
Corneal power measures in dark-treatment animals (average of left and right eyes) compared with normal corneal power at comparable ages and average predarkness values. Open symbols: predarkness measures; filled symbols: postdarkness measures. A different symbol is used for each animal. Normal data are the values from a previous study in which a different keratometer was used.6 Dashed line: average pretreatment corneal powers.
Corneal Power
A previous study of normal development6 found that corneal power decreased slightly as a function of age in this age period (Fig. 4, solid line). Corneal flattening also occurred during the 10-day dark-treatment period in the four animals with pre- and post-darkness measures. The left and right eye averaged decrease was 1.4 ± 0.3 D. Because the dark-treatment animals began dark treatment at different ages, it was possible to plot the predarkness decrease in corneal power (dashed line) and show that it followed a course similar to that in the previous study.6 The difference in values between the two studies presumably occurred because investigators in the previous study used a modified keratometer rather than the corneal topography system used in the present study. Thus, it appears that the corneal changes in this group were normal and did not contribute to the change in refractive status of the animals. Any possible effect would have been to reduce the amount of myopia by an average of 1.4 D.
Recovery from Dark-Induced Myopia
On returning to normal colony lighting, some of the eyes (e.g., in animal 0517) recovered fully and quickly from the dark-induced myopia. Others (0514, 0518) exhibited slow recovery that was incomplete at the end of the measurement period. In two animals (0512, 0539) one eye recovered more rapidly than the other eye, suggesting that recovery is mediated independently in the two eyes.
Cycloplegic autorefraction (mean ± SEM, 3.7 ± 0.4 D) and vitreous chamber measurements (2.8 ± 0.01 mm; average of right and left eyes) in the animals in this group on measurement day 60 ± 2 were compared with the refractive (5.1 ± 0.3 D) and vitreous chamber (2.7 ± 0.04 mm) measures in the normal-lighting group. The two animals (0514 and 0518) with the least refractive recovery had vitreous chamber depths that were within the range of the normal group, but were above the average, suggesting that the recovery was largely related to recovery of the vitreous chamber.
Dark-Recovery Group
As shown in Figure 5, all the treated eyes in this group showed compensation for the -5 D lens. Refractive measures made while the treated eyes wore the -5 D lens show that the treated eye moved from an initial hyperopia to a refractive value that was nearly the same as in the untreated control eye. The induced myopic shift (treated eye - control eye) for this group was -4.7 ± 0.6 D after 11 days of -5-D lens wear, measured with no lens in place. One animal (Fig 5E, 0515) developed considerably less myopia than the other animals in the group. Before dark treatment, the untreated control eyes closely resembled the normal-lighting and dark-treatment eyes at the same time point.
FIGURE 5.
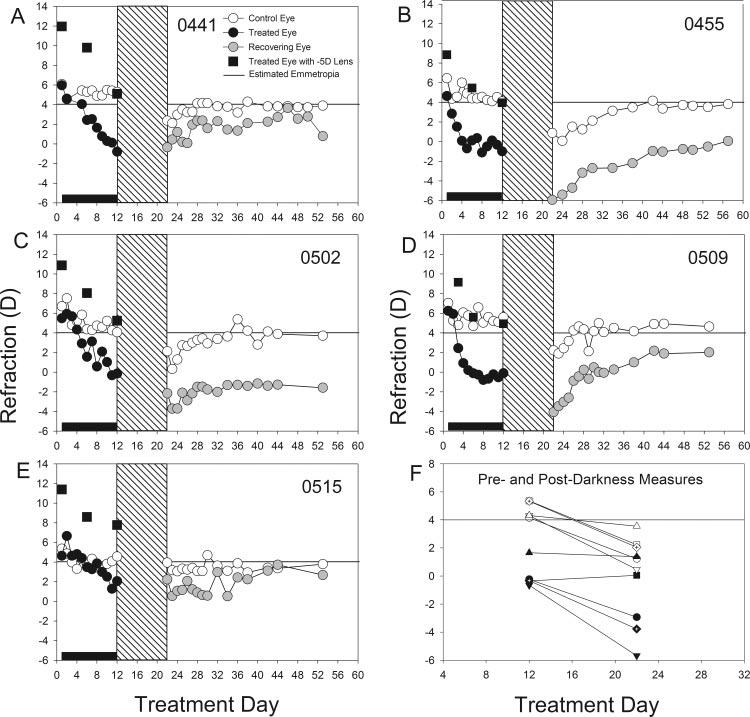
(A-E) Refractive measures of the treated and control eyes of each animal in the dark-recovery group starting at 16 days of VE (treatment day 1). Filled squares: refractive measures while the animals were wearing the -5-D lens. (F) Refractive measures of the treated and control eyes of each animal before, and after, dark treatment. Each data point is the average of the last 2 days before, or the first 2 days after darkness. Horizontal line at 4 D: estimated emmetropia as measured with the autorefractor.
When measured immediately after removal from darkness, none of the treated eyes had recovered from the induced myopia (Fig. 5F). Rather, three of the five treated eyes had become more myopic. The other two had maintained nearly the same amount of myopia as when dark treatment was begun. Overall, the treated eyes were -2.2 ± 0.9 D more myopic than before dark treatment. In addition, four of the control eyes shifted in the myopic direction, whereas a fifth remained nearly unchanged, with an overall shift toward myopia of -2.8 ± 0.5 D.
Recovery in the Light
After their return to normal colony lighting, all the control eyes returned to near their predarkness refractive state. The treated eyes exhibited a variety of responses. Three (0441, 0455, 0509) exhibited slow recovery that was incomplete in two of them by treatment day 54. In another animal (0502), the treated eye did not recover by the end of the measurement period. The fifth animal (0515) had developed much less myopia than was typical of animals that wore a -5-D lens for 11 days and recovered nearly fully by the end of the recovery period.
Examination of the differences (treated eye - control eye) in cycloplegic autorefraction and vitreous chamber depth in the individual animals on treatment day 60 ± 2 suggested that the recovery was largely axial. The two animals (0502 and 0455) with the least treated-eye refractive recovery (-4.0 and -2.5 D) had the largest residual differences in vitreous chamber depth (0.1 and 0.9 mm) in the group of five animals.
DISCUSSION
The results of this study show that the emmetropization mechanism uses visual signals, not only to establish emmetropia but also to maintain it in the juvenile period. As shown by the normal-lighting group, tree shrew eyes progress from hyperopia until they are nearly emmetropic when measured without cycloplegia. However, the eyes are not static at this point. During the late stage of emmetropization (approximately 28-60 days of VE), the axial length continues to increase slowly6 but the refractive state remains nearly constant. The dark-treatment animals and the control eyes of the dark-recovery group showed that this refractive stability depends on the continued presence of visual signals that are associated with being nearly emmetropic. Without these signals, eyes elongate, and become myopic. Moreover, the coordination between left and right eyes appears to decrease.
The treated eyes of the dark-recovery group showed that visual signals also are necessary in tree shrews for recovery from an induced myopia That the treated eyes were elongated compared with their fellow control eyes at the start of the dark treatment was not sufficient to cause recovery. Indeed, most of the already myopic eyes became more myopic during the period of darkness.
This result contrasts with the finding of McFadden et al.14 in guinea pigs that recovery occurred after 3 days in the dark. The reason for the different results is unknown. One possibility may involve where the animals were in the emmetropization process at the time dark treatment was imposed. McFadden et al. found that a guinea pig eye could recover half-way from a-6-D induced myopia in 24 hours. For this to occur suggests that normal growth is very rapid at this age (13 days after birth). In contrast, the tree shrews in this study had nearly completed the emmetropization process, and the axial elongation rate had greatly slowed. A second factor, having an elongated eye, also has been found to play a role in recovery such that elongated eyes slow their elongation rate more readily than normal-sized eyes.38 It may be that this nonvisual “shape-sensitive mechanism”39 plays a greater role in the guinea pig than in tree shrew.
Early-Dark Treatment
Soon after it was learned that eyelid-closure in young animals causes eyes to develop form deprivation-induced myopia, control studies were conducted to learn whether eyelid closure, per se, could produce axial elongation and myopia. These focused on whether the lidclosed eyes would elongate in the dark. In macaque monkeys15 and in tree shrews (McKanna et al. IOVS 1983;24:ARVO Abstract 56, p 226) it was found that the lid-sutured eyes of dark-reared animals did not elongate, relative to their fellow open control eyes and were not consistently myopic. In tree shrews, both the axial dimensions and interocular refractive differences were more variable in the dark-reared lid-sutured animals. The conclusion from these studies was that form deprivation in a lighted environment, rather than eyelid closure, is the cause of the induced myopia.
The control studies also provided information on whether dark rearing affects the untreated fellow control eyes. It was found that the dark-reared open control eyes did not differ from light-reared control eyes in either species. As shown in Figure 6, the axial length of five open control eyes of tree shrews raised to adulthood in continuous darkness from before the time of normal eye opening, seemed slightly more variable than the light-reared control eyes, but, on average, the two groups were not significantly different. Both groups of eyes had axial lengths comparable to those of normal adult tree shrews.6
FIGURE 6.
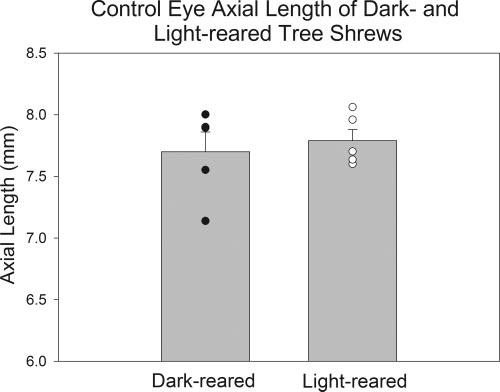
Axial lengths of open control eyes from two groups of tree shrews. Dark-reared animals were raised in continuous darkness from before the time the eyes opened. Light-reared animals were raised in normal colony lighting conditions. Histograms indicate mean axial lengths. Filled and open circles: axial lengths of individual animals. (Data from McKanna JA et al. IOVS 1983;24:ARVO Abstract 56, p 226, a collaborative study between this laboratory and Dr. McKanna’s.)
A Role for Visual Experience
The different effects of darkness when imposed from before eye-opening versus darkness imposed after tree shrews develop in a normal lighting environment suggests that exposure to a lighted environment must occur for a period before dark treatment becomes myopiagenic. It also has been found9,10 that form deprivation does not become an effective stimulus for axial elongation until eyes enter a susceptible period starting ∼2 weeks after normal eye opening. Eyes of animals raised with eyelid closure from the time of normal eye opening are not elongated compared with control eyes when measured after 15 days of form deprivation.9 Only after that point do the form-deprived eyes begin to elongate more rapidly than normal. It may be that, until that time, the eyes are growing rapidly under genetic control and are unable to increase the elongation rate.10 However, it also has been suggested that the onset of the susceptible period may be dependent on maturation of the retina.9
Behaviorally, tree shrew pups show little visual behavior immediately after eye opening. Visually-guided orienting responses and following a visual stimulus mature gradually over the first week of VE. Pups do not venture outside of their nest box until 2 to 3 weeks of VE.6,40 Although these behaviors involve the maturation of the entire visual system, they may also reflect maturation of retinal responses. To the extent that retinal maturation is a factor in the onset of the susceptible period, however, it is important to note that the retina need not have experience with focused images for form deprivation to become myopiagenic. This is shown by the fact that tree shrew eyes raised in the light with eyelid closure from before normal eye opening, and thus never exposed to normal images on the retina, developed form deprivation myopia once the susceptible period began.9
If there is a susceptible period for darkness as a myopiagenic stimulus, it is possible that its onset may coincide with the onset of the susceptible period for form deprivation.10 In the present study, measurement day 1 occurred at 16 days of VE, and the youngest dark treatment began when the animal was at 20 days of VE. By that age, or during the ensuing 10 days, darkness had become myopiagenic so that the amount of induced myopia was similar to that which occurred in the older animals. Thus, it remains unknown how much exposure to normal lighting must occur, or even whether normal images must occur on the retina, before darkness becomes myopiagenic.
The length of time that eyes must experience a lighted environment before darkness becomes myopiagenic may vary across species. Neither of the monkeys in the Raviola and Wiesel study15 showed any ocular or refractive effects of 10 to 12 months of dark rearing. However, one animal was not placed in the dark until after 12 weeks in a lighted environment. It appears, in this animal, that 3 months in a lighted environment was insufficient to transform darkness from an innocuous condition to a myopiagenic stimulus, even though Smith and Hung41 found form deprivation and minus lens wear to produce myopia in animals younger than 3 months. In another study of macaque monkeys, Guyton et al.16 examined the effect on refractive development of shorter (58-161 day) periods of darkness imposed immediately after birth. This is a period when the monkeys are normally hyperopic42 and early in the emmetropization process. Four of the monkeys remained hyperopic; a fifth became myopic over a 130-day period of darkness. Further study is needed to learn whether darkness reliably induces myopia in monkeys that have emmetropized in a normally lighted environment.
Chicks do not appear to require an initial posthatching period in the light before darkness induces axial elongation. Chicks raised for long periods in darkness, starting at hatching, develop an elongated vitreous chamber that would cause the eye to become myopic if there were not a dark-induced flattening of the cornea.17-22 Initially, both processes counterbalance each other so that after 14 days in darkness, the refractive state is very similar to that in normal eyes.18 In chicks that have had 14 days in normal lighting, darkness causes thinning of the choroid within 2 to 3 days after dark-treatment begins, possibly due to disruption of endogenous rhythms.43
Darkness and Form Deprivation
The data of the present study show that darkness, like form deprivation, is myopiagenic in visually experienced juvenile tree shrews. As noted in previous studies of chicks,18,43 both create an “open-loop” condition where there are no (or very degraded) visual images on the retina. That darkness (an absence of visual stimuli) produces myopia, suggests that the common denominator could be that a visual stimulus is necessary to restrain axial elongation. If so, it suggests that, in the tree shrew, as has been suggested in the chick,18,43 the “default” elongation rate is high and eyes are restrained from the default elongation rate by images present on the retina in a normally lighted environment. However, the two conditions do not appear to produce identical amounts of elongation and myopia. For example, tree shrews of a similar age that wore a diffuser for a similar length of time developed slightly more myopia (-6.58 or -9.0 D23) and axial elongation (0.168 or 0.19 mm23) than did the dark-treatment group (-4.3 D, 0.09 mm).
What it is about a “normally lighted environment” that is essential for establishing and maintaining emmetropia is, of course, an important unanswered question. Both dark treatment and form deprivation share the property that the retina does not receive focused, high-spatial frequency images. Studies that have examined the role of spatial frequencies in the visual environment on refractive development have generally found that mid-to-high spatial frequencies (relative to the animal’s acuity limit) are needed to achieve and maintain emmetropia.44,45 The reason for this is not known, but could involve the retinal activity that is produced by well-focused, high-spatial-frequency images that occur on the retina and change position as the eye shifts its direction of gaze. Such changing images would be expected to produce large changes in the membrane polarization of the nonspiking retinal cells and in the firing rates of cells that produce action potentials. It may be that the modulation of retinal activity produced by minimally defocused stimuli on the retina (of the sort that occur when an animal is completing the emmetropization process) is an important component of a retinal “stop” signal and that this is missing in form deprivation and in the dark.
It is as yet not known whether darkness acts through the same retinal mechanisms to produce myopia as do form deprivation or minus lens wear. There is evidence to suggest that form deprivation and minus lenses involve different retinal mechanisms.46,47 Whether darkness would produce elongation and myopia through one of those mechanisms, or though an entirely different one, remains to be discovered. Of note, in the tree shrew, darkness does not appear to affect corneal shape, in contrast to the corneal flattening found in the chick.17-22
In the chick, continuous darkness, along with form deprivation and lens wear, affects the diurnal cycles of choroidal thickness and axial elongation.43 Such cycles have been measured in a primate, the marmoset.48 It remains to be examined whether dark treatment affects these cycles in tree shrews or marmosets and whether they are part of a common vertebrate pathway controlling axial elongation.
Acknowledgments
The authors thank Joel Robertson for excellent technical assistance.
Supported by National Eye Institute Grants R01 EY05922 and P30 EY03039 (CORE).
Footnotes
Disclosure: T. Norton, None; A.O. Amedo, None; J.T. Siegwart, Jr, None
References
- 1.Edwards MH. Animal models of myopia: a review. Acta Ophthalmol Scand. 1996;74:213–219. doi: 10.1111/j.1600-0420.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 2.Wildsoet CF. Active emmetropization: evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–290. [PubMed] [Google Scholar]
- 3.Smith EL., III . Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Butterworth-Heinemann; Oxford, UK: 1998. pp. 57–90. [Google Scholar]
- 4.Norton TT. Animal models of myopia: learning how vision controls the size of the eye. ILAR J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 5.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 7.Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 9.McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- 10.Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 11.Gentle A, McBrien NA. Modulation of scleral DNA synthesis in development of and recovery from induced axial myopia in the tree shrew. Exp Eye Res. 1999;68:155–163. doi: 10.1006/exer.1998.0587. [DOI] [PubMed] [Google Scholar]
- 12.Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 13.McBrien NA, Gentle A, Cottriall C. Optical correction of induced axial myopia in the tree shrew: implications for emmetropization. Optom Vis Sci. 1999;76:419–427. doi: 10.1097/00006324-199906000-00022. [DOI] [PubMed] [Google Scholar]
- 14.McFadden S, Hawkins N, Howlett MHC. Recovery from experimentally induced myopia in the guinea pig. Exp Eye Res. 2004;79:92. [Google Scholar]
- 15.Raviola E, Wiesel TN. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci. 1978;17:485–488. [PubMed] [Google Scholar]
- 16.Guyton DL, Greene PR, Scholz RT. Dark-rearing interference with emmetropization in the Rhesus monkey. Invest Ophthalmol Vis Sci. 1989;30:761–763. [PubMed] [Google Scholar]
- 17.Lauber JK. Review: avian models for experimental myopia. J Ocular Pharmacol. 1991;7:259–276. [PubMed] [Google Scholar]
- 18.Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28:1225–1235. [PubMed] [Google Scholar]
- 19.Oishi T, Lauber JK, Vriend J. Experimental myopia and glaucoma in chicks. Zool Sci. 1987;4:455–464. [Google Scholar]
- 20.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 21.Li T, Howland HC. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Invest Ophthalmol Vis Sci. 2003;44:3692–3697. doi: 10.1167/iovs.02-0990. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Howland HC, Troilo D. Diurnal illumination patterns affect the development of the chick eye. Vision Res. 2000;40:2387–2393. doi: 10.1016/s0042-6989(00)00098-5. [DOI] [PubMed] [Google Scholar]
- 23.Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43:2067–2075. [PMC free article] [PubMed] [Google Scholar]
- 24.Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Lab Anim Sci. 1994;44:292–294. [PubMed] [Google Scholar]
- 26.Maloney RK, Bogan SJ, Waring GO., III Determination of corneal image-forming properties from corneal topography. Am J Ophthalmol. 1993;115:31–41. doi: 10.1016/s0002-9394(14)73521-4. [DOI] [PubMed] [Google Scholar]
- 27.McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Doc Ophthalmol. 1981;28:187–192. [Google Scholar]
- 28.McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–215. [PubMed] [Google Scholar]
- 29.Amedo AO, Norton TT. Comparison of Infrared photoretinoscope and autorefractor in tree shrews with and without induced myopia. Optom Vis Sci. 2003;80(suppl):120. [Google Scholar]
- 30.Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80:623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 32.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–1413. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- 33.Kempf GA, Collins SD, Jarman BL. Refractive errors in the eyes of children as determined by retinoscopic examination with a cycloplegic: results of eye examinations of 1,860 white school children in Washington, D.C. United States Public Health Service. United States Government Printing Office; Washington, DC: 1928. pp. 1–56. [Google Scholar]
- 34.Mohindra I, Held R. Refractions in humans from birth to five years. Doc Ophthalmol. 1981;28:19–27. [Google Scholar]
- 35.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 36.Mutti DO, Mitchell GL, Jones LA, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 37.Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 38.Nickla DL, Sharda V, Troilo D. Temporal integration characteristics of the axial and choroidal responses to myopic defocus induced by prior form deprivation versus positive spectacle lens wear in chickens. Optom Vis Sci. 2005;82:318–327. doi: 10.1097/01.opx.0000159368.31481.de. [DOI] [PubMed] [Google Scholar]
- 39.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 40.Martin RD. Reproduction and ontogeny in tree-shrews (Tupaia belangeri), with reference to their general behaviour and taxonomic relationships. Z Tierpsychol. 1968;25:409–532. doi: 10.1111/j.1439-0310.1968.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 41.Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 42.Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40:214–229. [PubMed] [Google Scholar]
- 43.Nickla DL, Wildsoet CF, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: implications for ocular growth regulation. Invest Ophthalmol Vis Sci. 2001;42:584–588. [PubMed] [Google Scholar]
- 44.Schmid KL, Wildsoet CF. Contrast and spatial-frequency requirements for emmetropization in chicks. Vision Res. 1997;37:2011–2021. doi: 10.1016/s0042-6989(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 45.Hess RF, Schmid KL, Dumoulin SO, Field DJ, Brinkworth DR. What image properties regulate eye growth? Curr Biol. 2006;16:687–691. doi: 10.1016/j.cub.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 46.Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 47.Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42:575–583. [PubMed] [Google Scholar]
- 48.Nickla DL, Wildsoet CF, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]


