The ammonium transporter Amt-1 from the cytoplasmic membrane of the hyperthermophilic archaeon A. fulgidus has been purified and crystallized.
Keywords: ammonium transporters, Amt-1, archaeal membrane proteins
Abstract
Ammonium transporters (Amts) are a class of membrane-integral transport proteins found in organisms from all kingdoms of life. Their key function is the transport of nitrogen in its reduced bioavailable form, ammonia, across cellular membranes, a crucial step in nitrogen assimilation for biosynthetic purposes. The genome of the hyperthermophilic archaeon Archaeoglobus fulgidus has been annotated with three individual genes for ammonium transporters, amt1–3, the roles of which are as yet unknown. The amt1 gene product has been produced by heterologous overexpression in Escherichia coli and the resulting protein has been purified to electrophoretic homogeneity. Crystals of Amt-1 have been obtained by sitting-drop vapour diffusion and diffraction data have been collected.
1. Introduction
The element nitrogen is an essential constituent of all classes of biological macromolecules. Although dinitrogen gas (N2) makes up around 80% of the earth’s atmosphere, this reservoir cannot easily be accessed by organisms owing to the extraordinary stability of the N2 triple bond. To date, the only known method for the reductive fixation of dinitrogen by living organisms is the reaction of the enzyme nitrogenase, which cleaves the triple bond and reduces N2 to two molecules of ammonia (NH3; Rees & Howard, 2000 ▶). Assimilation of nitrogen for biosynthetic purposes occurs exclusively in the form of ammonia (or, in solution, ammonium ion; NH ) and the crucial function of ammonia or ammonium uptake across biological membranes is the domain of the Amt/Mep family of transport proteins.
) and the crucial function of ammonia or ammonium uptake across biological membranes is the domain of the Amt/Mep family of transport proteins.
Ammonium transporters are found in archaea and bacteria as well as in eukaryotes; organisms are frequently found to possess multiple Amt homologues. Despite their high physiological relevance, essential mechanistic questions remain, including that concerning the nature of the transported substrate, NH3
versus NH and the type of transport, passive versus active. Proposals range from a bidirectional facilitated diffusion of gaseous NH3 (Soupene et al., 2002 ▶) to an energy-dependent uptake of NH
and the type of transport, passive versus active. Proposals range from a bidirectional facilitated diffusion of gaseous NH3 (Soupene et al., 2002 ▶) to an energy-dependent uptake of NH or a potential-driven NH
or a potential-driven NH uniport (Ludewig et al., 2002 ▶; Kleiner, 1985 ▶) to a proton-coupled symport mechanism (Wang et al., 1994 ▶).
uniport (Ludewig et al., 2002 ▶; Kleiner, 1985 ▶) to a proton-coupled symport mechanism (Wang et al., 1994 ▶).
In addition to their transport function, ammonium transporters may also function as ammonium sensors or may combine both functions, as has been shown for Mep2 from Saccharomyces cerevisiae (Lorenz & Heitman, 1998 ▶) and AmtY from Rhodobacter capsulatus (Yakunin & Hallenbeck, 2002 ▶). In some cases, Amt proteins have also been found to transport methylammonium (Mep family). Several paralogues, including the Rhesus blood-type proteins of mammalian erythrocytes (Marini et al., 2000 ▶), have recently been suggested to function as exporters of ammonium ions (Hemker et al., 2003 ▶).
Indirect participation of Amt proteins in the global nitrogen regulation of the cell has been demonstrated for the Escherichia coli system, in which a direct interaction with GlnK was found (Blauwkamp & Ninfa, 2003 ▶; Javelle et al., 2004 ▶; Coutts et al., 2002 ▶). This trimeric regulatory protein belongs to the PII family of signal transducers that play an essential role in the control of the cellular nitrogen metabolism. Functional studies indicating a trimeric structure for Amt proteins (Blakey et al., 2002 ▶) were recently confirmed by high-resolution structures of E. coli AmtB (Khademi et al., 2004 ▶; Zheng et al., 2004 ▶) as well as by atomic force microscopy (Conroy et al., 2004 ▶). AmtB contains 11 membrane-spanning helices per monomer. An additional twelfth N-terminal helix is part of a leader peptide and is cleaved upon insertion of the protein into the membrane. The N-terminus is located in the periplasm, while the C-terminus is cytoplasmatic.
The genome of the hyperthermophilic archaeon Archaeoglobus fulgidus has been annotated with three genes coding for homologues of ammonium transporters, designated amt1, amt2 and amt3. Secondary-structure predictions indicate that amt2 contains 12 transmembrane helices with a cleavable leader peptide, while both amt1 and amt3 lack the first helix and do not show a leader peptide-cleavage site. In order to understand the structural and functional differences between these proteins and to provide new insight into the transport mechanism, we have overproduced and purified A. fulgidus Amt-1, which shows significant sequence identity (41.6%) with E. coli AmtB, but does not contain a cleavable N-terminal leader sequence. The protein was crystallized and diffraction data have been collected.
2. Materials and methods
2.1. Cloning and recombinant protein production
The 1176 bp amt1 gene was amplified from genomic DNA of A. fulgidus by PCR. Oligonucleotide primers were designed to contain restriction sites for NdeI in the forward primer and XhoI in the reverse primer. The amplified fragment was then cloned into the ampicillin-resistant expression vector pET-21a (Novagen), allowing overexpression of the entire native protein sequence (amino acids 1–391) plus a C-terminal His6 tag connected through a Leu-Glu linker (the XhoI site) and under the control of a T7 promoter. The correctness of the sequence was confirmed by DNA sequencing of the construct.
For heterologous expression of the Amt1 protein, the plasmid was transformed into E. coli C43(DE3), a strain reported to be favourable for the expression of membrane proteins (Miroux & Walker, 1996 ▶). Cells were grown at 303 K in Luria–Bertani medium supplemented with 100 µg ml−1 ampicillin and expression was induced with 0.5 mM IPTG after the culture reached an optical density at 600 nm of 0.6–0.8 absorbance units. 4–6 h after induction, cells were harvested by centrifugation for 15 min at 6000g at 277 K. Prior to disruption in a microfluidizer (Microfluidics), cells were resuspended in buffer A (50 mM Tris–HCl pH 8.0, 500 mM NaCl and 10% glycerol) supplemented with a protease-inhibitor cocktail (Complete Plus EDTA-free, Roche). Cell debris was removed by centrifugation for 20 min at 20 000g at 277 K. The membrane fraction was obtained after a second centrifugation for 2 h at 108 800g and 277 K. All subsequent procedures were carried out at room temperature.
The membrane fraction was resuspended in buffer B (20 mM Tris–HCl pH 8.0, 300 mM NaCl and 10% glycerol). Solubilization of membrane proteins was achieved by stirring the membrane fraction with a 1%(w/v) final concentration of n-dodecyl-β-d-maltopyranoside (DDM; Anatrace). A final centrifugation step of 45 min at 108 800g and 277 K allowed separation of the non-solubilized material.
2.2. Protein purification
The solubilized membrane fraction was loaded onto a 5 ml His-Trap affinity column (Amersham Biosciences) pre-equilibrated with buffer B supplemented with 0.03% DDM. After injection, the column was washed with buffer B with 0.03% DDM and contaminants were progressively eliminated by two linear gradients of 0–10% and 10–15% buffer C (20 mM Tris–HCl pH 8.0, 300 mM NaCl, 10% glycerol, 0.5 M imidazole and 0.03% DDM). The protein was finally eluted from the column at an imidazole concentration of 250 mM (50% buffer C).
The fractions containing Amt-1 were pooled, concentrated using YM-50 concentrators (50 kDa molecular-weight cutoff, Amicon) and loaded onto a Hi-Trap Desalting 26/10 column (Amersham Biosciences) pre-equilibrated with buffer D (20 mM Tris–HCl pH 8.0, 100 mM NaCl, 10% glycerol, 0.03% DDM), in which it was used for all subsequent experiments.
At various stages of the work, from protein expression to purification, the presence and purity of Amt-1 was confirmed by SDS–PAGE and Western blotting using His-tag-specific antibodies.
2.3. Crystallization and preliminary data collection
Crystallization experiments were performed using the sitting-drop vapour-diffusion method at 293 K. Initial crystals were obtained with protein purified as described above and diffracted to about 6 Å. Better quality crystals were obtained with protein purified using 0.05%(w/v) n-dodecyl-N,N-dimethylamine-N-oxide (LDAO; Anatrace) in place of DDM, but otherwise following the exact same protocol. Drops were prepared by mixing 1 µl Amt-1 solution (10 mg ml−1) with 1 µl reservoir solution and were equilibrated against 200 µl reservoir solution. Initial crystallization conditions were obtained from a home-made sparse-matrix screen and were subsequently further optimized.
Diffraction experiments were carried out on a Rigaku MicroMax007 rotating-anode X-ray generator (MSC) with a MAR345dtb image-plate detector (MAR Research) and an X-stream 2000 cryosystem (MSC) using Cu Kα radiation with a wavelength of 1.5418 Å.
3. Results and discussion
3.1. Protein purification
A. fulgidus Amt-1 was purified to electrophoretic homogeneity and the extraordinary stability of the trimer that has been described for the E. coli homologue AmtB (Blakey et al., 2002 ▶) was obvious on SDS–PAGE (Fig. 1 ▶). Minor bands were visible at lower molecular weights corresponding to small amounts of monomer and dimer, which can be attributed to partial dissociation in buffers containing SDS. The apparent size of the protein relative to the size markers was smaller than the value estimated from the amino-acid sequence, a common finding for membrane proteins and presumably arising from incomplete unfolding of the protein chain even in the presence of SDS. Therefore, the Amt-1 monomer, with a calculated molecular weight of 40.1 kDa, migrates in SDS–PAGE at around 33 kDa, while the trimer of predicted 120.3 kDa migrates at around 90 kDa (Fig. 1 ▶).
Figure 1.
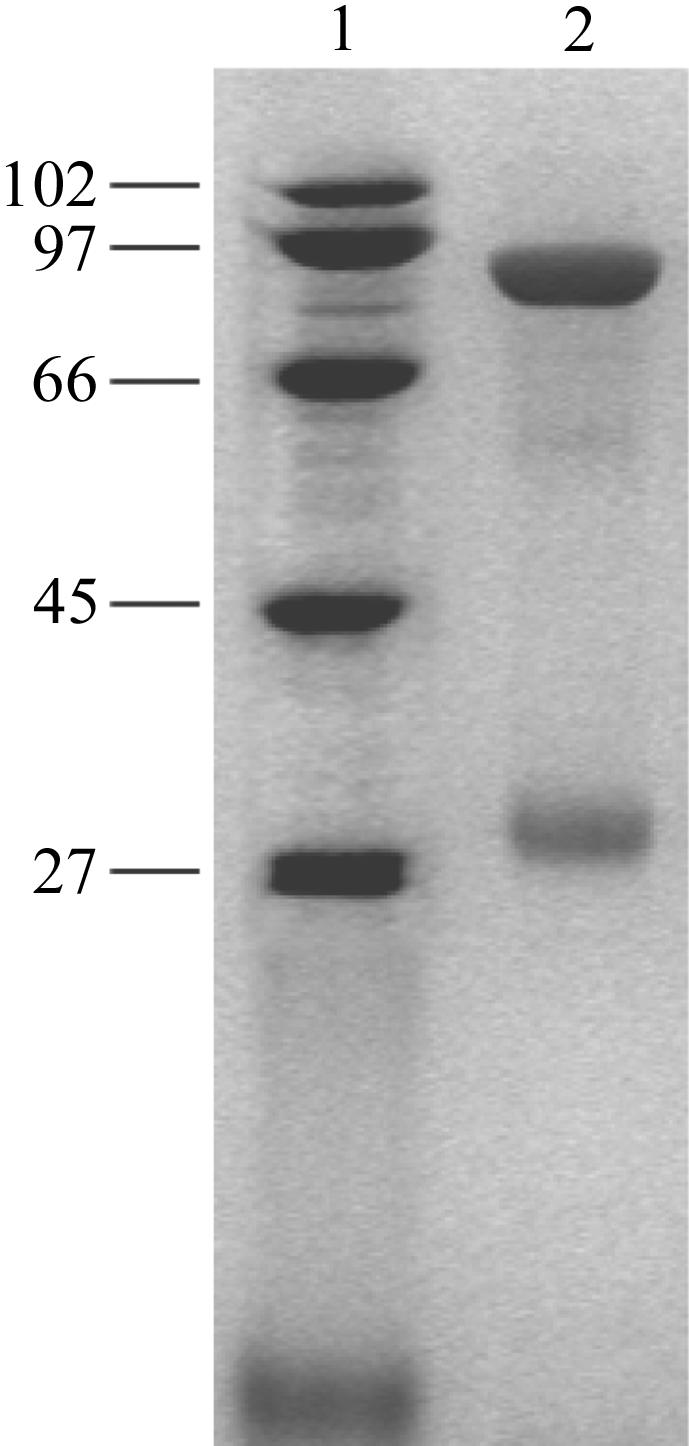
SDS–PAGE of purified A. fulgidus Amt-1 stained with Coomassie Brillant Blue. Lane 1 shows electrophoresis markers (kDa); lane 2 contains purified Amt-1. Although the gel was made under denaturing conditions, the main band in the gel corresponds to the Amt-1 trimer (90 kDa), while the weaker bands show the dimer (60 kDa) and monomer (33 kDa). All protein bands run approximately 25% lower than expected from the sequence, most likely as a consequence of incomplete unfolding by SDS.
3.2. Crystallization
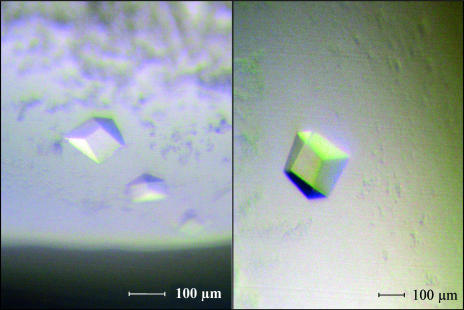
Single crystals were obtained using a reservoir solution composed of 28–33% PEG 400, 100 mM Tris–HCl pH 8.5 or bicine pH 9.0, 100 mM MgCl2 and 100–150 mM NaCl. They appeared after 1–2 d and reached average dimensions of up to 300 × 300 × 200 µm within about two to three weeks (Fig. 2 ▶).
Figure 2.
Crystals of A. fulgidus Amt-1 obtained by sitting-drop vapour diffusion at 293 K.
The reservoir solution proved to be a usable cryocondition for carrying out diffraction experiments. Crystals were flash-cooled directly in liquid nitrogen and subsequently transferred onto the goniometer. They belonged to the rhombohedral space group R3, with unit-cell parameters a = b = 111.4, c = 136.6 Å, α = β = 90, γ = 120°, and diffracted to a resolution of 2 Å using radiation from a Rigaku MicroMax 007 rotating-anode generator (Table 1 ▶). This crystal form appears to be similar to the R3 form obtained for E. coli AmtB (Zheng et al., 2004 ▶), although the latter was obtained at the much lower pH value of 4.6.
Table 1. Data-collection statistics.
Values in parentheses are for the outer shell (2.06–2.00 Å).
| Wavelength (Å) | 1.5418 |
| Resolution limits (Å) | 50.0–2.0 |
| Unique reflections | 33221 |
| Completeness (%) | 99.8 (99.8) |
| Observation redundancy | 3.9 |
| Rsym | 0.108 (0.368) |
| I/σ(I) | 11.5 (2.3) |
| Space group | R3 |
| Unit-cell parameters (Å) | a = b = 111.4, c = 136.6 |
| Monomers per AU | 1 |
Acknowledgments
The authors would like to thank Ruth Schmitz-Streit and Linda Thöny-Meyer for stimulating discussions. This research was supported by a Marie Curie Intra-European Fellowship within the 6th European Community Framework Programme to SLAA.
References
- Blakey, D., Leech, A., Thomas, G. H., Coutts, G., Findlay, K. & Merrick, M. (2002). Biochem. J.364, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwkamp, T. A. & Ninfa, A. J. (2003). Mol. Microbiol.48, 1017–1028. [DOI] [PubMed] [Google Scholar]
- Conroy, M. J., Jamieson, S. J., Blakey, D., Kaufmann, T., Engel, A., Fotiadis, D., Merrick, M. & Bullough, P. A. (2004). EMBO Rep.5, 1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts, G., Thomas, G., Blakey, D. & Merrick, M. (2002). EMBO J.21, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemker, M. B., Cheroutre, G., van Zwieten, R., Maaskant-van Wijk, P. A., Roos, D., Loos, J. A., van der Schoot, C. E. & von dem Borne, A. E. G. (2003). Br. J. Haematol.122, 333–340. [DOI] [PubMed] [Google Scholar]
- Javelle, A., Severi, E., Thornton, J. & Merrick, M. (2004). J. Biol. Chem.279, 8530–8538. [DOI] [PubMed] [Google Scholar]
- Khademi, S., O’Connell, J., Remis, J., Robles-Colmenares, Y., Miercke, L. J. W. & Stroud, R. M. (2004). Science, 305, 1587–1594. [DOI] [PubMed] [Google Scholar]
- Kleiner, D. (1985). FEMS Microbiol. Rev.32, 87–100.
- Lorenz, M. C. & Heitman, J. (1998). EMBO J.17, 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig, U., von Wirén, N. & Frommer, W. B. (2002). J. Biol. Chem.277, 13548–13555. [DOI] [PubMed] [Google Scholar]
- Marini, A., Matassi, G., Raynal, V., André, B., Cartron, J. P. & Cherif-Zahar, B. (2000). Nature Genet.26, 341–344. [DOI] [PubMed] [Google Scholar]
- Miroux, B. & Walker, J. E. (1996). J. Mol. Biol.260, 289–298. [DOI] [PubMed] [Google Scholar]
- Rees, D. C. & Howard, J. B. (2000). Curr. Opin. Chem. Biol.4, 559–566. [DOI] [PubMed] [Google Scholar]
- Soupene, E., Chu, T., Corbin, R. W., Hunt, D. F. & Kustu, S. (2002). J. Bacteriol.184, 3396–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. Y., Glass, A. D. M., Shaff, J. E. & Kochian, L. V. (1994). Plant Physiol.104, 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunin, A. F. & Hallenbeck, P. C. (2002). J. Bacteriol.184, 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L., Kostrewa, D., Bernèche, S., Winkler, F. K. & Li, X.-D. (2004). Proc. Natl Acad. Sci. USA, 101, 17090–17095. [DOI] [PMC free article] [PubMed] [Google Scholar]