Abstract
Obese individuals often suffer from depression. The olfactory bulbectomy (OBX) model is an animal model of depression that produces behavioral, physiological, and neurochemical alterations resembling clinical depression. The OBX model was employed to assess depression-related changes in food intake in obesity-prone, Osborne-Mendel (OM) rats and obesity-resistant, S5B/Pl rats. OBX increased food intake in OM rats beginning 7 days following surgery, however, OBX did not alter food intake in S5B/Pl rats at any time point. Fourteen days following surgery, OBX significantly increased locomotor activity (total lines crossed and rears) in the openfield test in OM and S5B/Pl rats. Fifteen days following surgery, prepro-neuropeptide Y (NPY) mRNA levels were significantly increased in the hypothalamus of bulbectomized OM rats and in the medial nucleus of the amygdala of bulbectomized OM and S5B/Pl rats. OBX decreased NPY Y2 receptor mRNA levels in the hypothalamus and medial nucleus of the amygdala in OM rats, while increasing NPY Y2 receptor mRNA levels in the medial nucleus of the amygdala of S5B/Pl rats. These data indicate that though both obesity-prone and obesity-resistant strains were susceptible to the locomotor effects of OBX, food intake and hypothalamic prepro-NPY mRNA were only increased in OM rats. Therefore, strain specific alterations in hypothalamic NPY may account for increased food intake in the obesity-prone rats following OBX, and suggests a potential mechanism to explain the comorbidity of obesity and depression.
Keywords: Olfactory Bulbectomy, Depression, Food Intake, Neuropeptide Y, Obesity-prone, Hypothalamus, Amygdala
Introduction
Obesity is an increasingly prevalent medical condition associated with a variety of health conditions and mood disorders, including depression. Higher BMIs (Body Mass Index) have been linked to depressive symptoms in adults and adolescents. The DSM IV (1994) reports symptoms of major depression as changes in appetite or weight, sleep, psychomotor activity, irritability, anxiety, loss of interest in previously pleasurable activities, impaired ability to concentrate and depressed mood. To be diagnosed with major depressive disorder, an individual must have either depressed mood or loss of interest or pleasure and four of the other symptoms. According to the DSM IV, major depressive disorder with atypical symptoms is characterized by increased food intake and body weight. Twins with atypical depression are more likely to be obese (BMI>28.6) than those with other forms of depression [18,40,54,63].
The olfactory bulbectomy (OBX) animal model of depression produces a syndrome characterized by behavioral, physiological and neurochemical changes that resemble clinical depression. This syndrome is behaviorally characterized by increased locomotor activity in a novel openfield [17,36,41,43,61], deficits in aversively-motivated behaviors [29, 41, 42, 43] and deficits in appetitively-motivated behaviors [4, 30,31,43]. Alterations in feeding patterns and circadian rhythms have been seen in bulbectomized Sprague Dawley rats [27,30,31,39]. Though total food intake was not altered, these rats eat smaller, but more frequent meals [30,31]. Kelly et al. [16] reported a significant increase in food intake at a single time point (11 days) following OBX.
There are many neurochemical similarities between obesity and depression, [2,7,28,60]. Specifically, the 36 amino acid neuropeptide, neuropeptide Y (NPY), has been implicated in both disorders [8,9,23,33,42,58]. Animal models indicate that the orexigenic effects of NPY are mediated through the hypothalamus, in particular by NPY neurons that project from the arcuate nucleus to the paraventricular nucleus [1,50,51]. Both intracerebroventricular and intra-paraventricular nucleus administration of NPY increases food intake [13,32,56,58] with the macronutrient specificity of the response being somewhat dependent on the rat's original preference for carbohydrate or fat [6,55,57,62]. Chronic central administration of NPY and NPY Y1 receptor agonists increases body weight and adiposity and produces significant hyperphagia [9].
In addition to it's important role in ingestive behaviors, the role of NPY has been investigated in a number of animal models of anxiety and depression [3,10,12,19,52,59,66]. Though no studies have fully investigated NPY alterations in the hypothalamus of bulbectomized rats, increased NPY mRNA levels and peptide levels in the piriform cortex and medial nucleus of the amygdala have been found in the OBX animal model of depression [10,42,48]. The medial nucleus of the amygdala is an important brain region involved in both ingestive behaviors and emotional behaviors, such as anxiety and depression [5,26,38,64]. Lesions of the medial nucleus of the amygdala lead to increased food intake and body weight [21,22].
The Osborne-Mendel (OM) rat and the S5B/Pl rat are animal models used to assess sensitivity toward developing obesity. When compared to obesity-resistant, S5B/Pl rats, obesity-prone OM rats become obese when given access to a high fat diet (55% of energy from fat) and consume more calories from fat than carbohydrates when given a choice [34]. In addition to the OM rat's propensity toward obesity, these animals have increased hypothalamic prepro-NPY, NPY Y1 and Y2 receptor mRNA when compared to obesity-resistant S5B/Pl rats [20,49,53]. The goal of the current experiment was to examine the effects of OBX on body weight, food intake, locomotor activity, and NPY gene expression in obesity-prone and obesity-resistant rats. We hypothesized that OBX would increase locomotor activity in both strains of rats, but would selectively increase food intake in obesity-prone OM rats. Increased food intake in bulbectomized OM rats would suggest that these rats are representative of individuals with major depression with atypical symptoms, which includes increased food intake. Similar to what was previously reported in Sprague-Dawley rats [27,30,31,37], we do not expect to find OBX-induced increases in food intake in S5B/Pl rats. NPY and NPY receptor gene expression was assessed in the hypothalamus and medial nucleus of the amygdala following OBX by Real-Time PCR. We predict that OBX will increase prepro-NPY mRNA levels in the medial nucleus of the amygdala in both strains, though we expect differential expression of NPY and NPY receptor subtypes in the hypothalamus.
Materials and Methods
Subjects
Eight week old male OM and S5B/Pl rats, bred in the Pennington Biomedical Research Center vivarium, were used in this experiment. Rats were individually housed (beginning at 7 weeks of age) on a 12/12 LD cycle (lights on at 0700) with food/water available ad libitum. Rats were fed a standard laboratory chow diet (Rodent Diet 5001, LabDiet; 28/12/60: % calories from protein/fat/carbohydrates). All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee and followed the Principles for Care and Use of Laboratory Animals. This facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Olfactory Bulbectomy
OBX surgery was performed as previously described [10,41]. Briefly, rats were anesthetized with sodium pentobarbital (Nembutal, Abbot Laboratories, 55mg/kg, i.p.) and the scalp was shaved. Following a midline incision, two 2mm diameter burr holes were drilled 6mm rostral to bregma and 1mm to the right and left of the midline. The olfactory bulbs were aspirated in the bulbectomized rats with a 2mm diameter plastic pipette tip and the cavity was filled with gel-foam (Upjohn) to control bleeding. Special care was taken to avoid damaging the frontal cortex. Carprofen (Rimadyl, Pfizer, 1mg/kg, s.c.) was administered immediately following surgery to control pain. Sham-operated controls were treated identically except the olfactory bulbs were not aspirated. Immediate post-operative care consisted of fluid replacement (0.9% saline, i.p.) and thermoregulatory measures. Rats were monitored daily following surgery for any signs of distress.
Food Intake & Body Weight
Twenty-four hour food intake was measured for two days prior to OBX surgery to determine the average 24h baseline food intake for each strain. Following OBX, twenty-four hour food intake was measured daily beginning 6 days following surgery and continuing through Day 15. Body weight was also measured daily following surgery.
Openfield Testing
Fourteen days following surgery, all animals were tested in the openfield test. The openfield apparatus is a four-sided 50 × 50 × 50cm black Plexiglas chamber, with a stainless steel floor. The floor of the openfield was divided into 9 equal squares (16.67cm). Rats were placed into the middle of the openfield apparatus, initially. Each rat was given 5 minutes to explore the openfield, during which time they were videotaped. The number of rears and the number of lines crossed in the openfield were used as measures of spontaneous locomotor activity and manually assessed by the experimenter. The experimenter was blind to the treatment condition during testing and during the measurement of activity. The openfield was cleaned with a 20% chlorine bleach solution between each animal in an attempt to eliminate odors.
Real-time Polymerase Chain Reaction (PCR)
One day following openfield testing, S5B/Pl rats and OM rats were killed by rapid decapitation. Dissected brains were frozen on dry ice, and stored at −80°C until processing. RNA was isolated from bilateral punches of the hypothalamus and medial nucleus of the amygdala using Tri-Reagent (Molecular Research Ctr, Cincinnati, OH USA) and RNeasy Minikit procedures (Qiagen, Valencia, CA USA) and based on previous experiments by Primeaux et al. [44]. Briefly, thawed tissue was homogenized in Tri-Reagent using a motorized tissue homogenizer, chloroform (200μl) was added to the lysate, and the mixture was centrifuged (12,000×g) in phase lock tubes to separate RNA. Ethanol (600 μl) was added to the upper aqueous phase, which was filtered by centrifugation (8000×g). Following three washes, the samples were subjected to an elution step using RNAase-free water. Reverse transcriptase (RT) was conducted using M-MLV procedures (Promega, Madison, WI, USA). For RT, 2μg of RNA from each sample was added to random primers (Promega) and incubated in a thermal cycler (PTC-100, MJ Research, Inc. Watertown, MA, USA) for 5 min at 70°C. Tubes were removed, placed on ice and a mixture of 5x M-MLV (Moloney Murine Leukemia Virus), 10mM dNTP (solution containing sodium salts of dATP, dCTP, dGTP and dTTP) and RT buffer (250mM Tris-HCl (pH 8.3 at 25°C), 375mM KCl, 15mM MgCl2, 50mM DTT) was added and tubes were returned to the thermal cycler for 60 min at 37°C and then 15min at 70°C. Primers were designed using Primer Express (Applied Biosystems, Foster City, CA, USA). The following primers were used for NPY: 5'-CCGCTCTGCGACACTACATC-3' and 5'-AATCAGTGTCTCAGGGCTGGAT-3'; NPY Y1 receptor: 5'-TTCTTCTCTGCCCTTCGTGATC-3' and 5'-GAACGCCGCAAGTGATACATT-3'; NPY Y2 receptor: 5'-AGCCATGTCCTGGACCTGAA-3' and 5'-GGTGGAGCACATCGCAATAATAA-3' and cyclophilin: 5'-CCCACCGTGTTCTTCGACAT -3' AND 5'-CTGTCTTTGGAACTTTGTCCTGCAA-3'. For Real Time PCR, SYBR Green 2x Master Mix (Applied Biosystems), forward and reverse primers (10μM), and RT product (10ng) were added to 384 well plates. The cycling parameters consisted of an initial 2 min incubation at 50°C, followed by 10 min at 95°C, then 15 sec at 95°C, and a 1 min annealing step at 60°C (40 cycles). A dissociation step (15 sec at 95°C) was added following 40 cycles to determine specificity of primers. Quantity of prepro-NPY, NPY Y1 receptor, and NPY Y2 receptor mRNA was based on a standard curve and normalized to cyclophilin mRNA (ABI Prism 7900 Sequence Detection System, Applied Biosystems).
Data Analysis
Upon sacrifice, lesions were confirmed. The criterion for a complete lesion of the olfactory bulb was a tissue weight of less than 30% of the tissue weight of an intact olfactory bulb [10,41]. Three S5B/Pl rats and 6 OM rats were removed from analyses due to incomplete lesions, therefore, data were analyzed with OM-OBX n=19; OM-SHAM n=16; S5B/Pl-OBX n=12; S5B/Pl-SHAM n=13. At the time of sacrifice, retroperitoneal and epididymal fat pads were weighed as a measure of body adiposity. Pre-surgery food intake was subjected to a between subjects t-test. Food intake (grams) following surgery was subjected to a mixed ANOVA, in which days post-surgery were considered the repeated measure, while strain (OM, S5B/Pl) and surgery (OBX, SHAM) were considered the between subject factors. Body weight was analyzed as the percentage of pre-surgery body weight and was subjected to a mixed ANOVA in which weeks were considered the repeated measure, while strain (OM, S5B/Pl) and surgery (OBX, SHAM) were considered the between subject factors. Openfield behavior (rears and total lines crossed) and percent body fat ((retroperitoneal fat + epididymal fat (grams)/body weight (grams)) *100) were subjected to a 2×2 ANOVA with strain (OM, S5B/Pl) and surgery (OBX, SHAM) as the independent variables. For Real-Time PCR data, fold-changes of prepro-NPY, NPY Y1 and NPY Y2 receptor mRNA (normalized to cyclophilin) at 15 days following OBX were calculated based on the sham-operated control's average mRNA level (for each strain). Fold-changes were analyzed with a between subjects t-test. Bonferroni post-hoc tests were used to further explore differences between groups if significant main effects or interactions are found in the initial analyses. A significance level of p<.05 was set for all measures.
Results
Food Intake
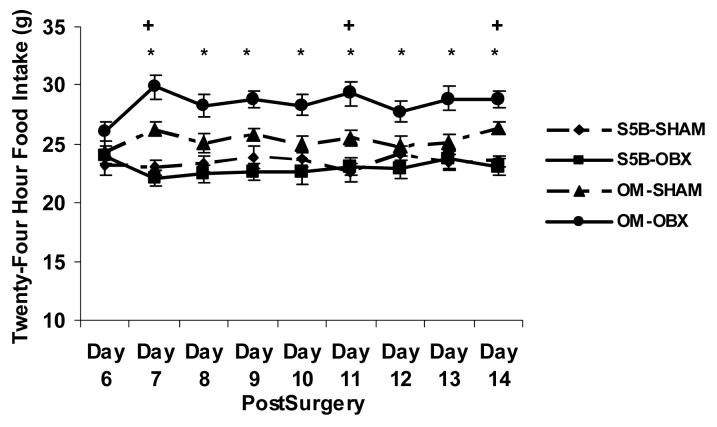
Baseline pre-surgery 24h food intake was significantly higher in OM rats (25.28 ± 0.4g, means ± SEM) compared to S5B/Pl rats (23.85 ± 0.4g, means ± SEM) (t(57)=2.21, p<.05; effect size .28). Following OBX surgery, a significant interaction was found between strain and surgery on 24h food intake (F(1,51) = 10.64, p<.01; effect size .17; See Figure 1). Post-hoc analyses revealed a significant increase in food intake in bulbectomized OM rats, compared to sham-operated OM rats, beginning 6 days following surgery and ending at 15 days following surgery ( p<.05). Sham-operated OM rats ate significantly more than sham-operated S5B/Pl rats 7, 11 and 14 days following surgery (p<.05). There were no differences in food intake between sham-operated S5B/Pl rats and bulbectomized S5B/Pl rats (p>.05).
Figure 1.
Twenty-four hour food intake was measured in OM and S5B/Pl rats following OBX surgery. Beginning 7 days following surgery, OBX increased food intake in OM rats compared to sham-operated OM rats. Sham-operated OM rats ate more than sham-operated S5B/Pl rats 7, 11, and 14 days after surgery. Data are shown as means ± SEM. * indicates p<.05 between OM-OBX and OM-SHAM, + indicates p<.05 between OM-SHAM and S5B-SHAM.
Body Weight
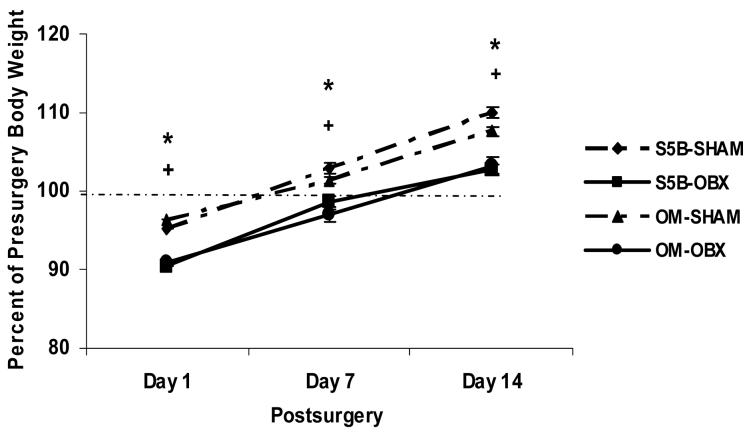
Body weight was measured immediately prior to OBX or sham surgery. Prior to OBX, OM rats weighed (354.64 ± 7.5g; mean ± SEM) significantly more than S5B/Pl rats (263.92 ± 4.4g, means + SEM) (t(56) = 9.58, p<.001; effect size .80). Following OBX surgery, a significant main effect of surgery was found for the percentage of pre-surgery body weight (F(1,54) = 42.78, p<.0001; effect size .44; See Figure 2). Bulbectomized rats lost significantly more weight at 1, 7, and 14 days following surgery than those receiving sham surgeries (p<.05). Body fat was expressed as a percentage of body weight. A main effect of strain was found (F(1,43) = 26.99, p<.001; effect size .39), OM rats had a greater percentage of body fat than S5B/Pl rats (p<.05), however, OBX did not alter percentage body fat (data not shown).
Figure 2.
The percentage of pre-surgery body weight was measured in OM and S5B/Pl rats. The percentage of pre-surgery body weight was significantly decreased in OM and S5B/Pl rats following OBX compared to their sham-operated controls at 1 day, 7 days and 14 days following OBX. Data are shown as means ± SEM. * indicates p<.05 between OM-OBX and OM-SHAM, + indicates p<.05 between S5B-OBX and S5B-SHAM.
Openfield Behavior
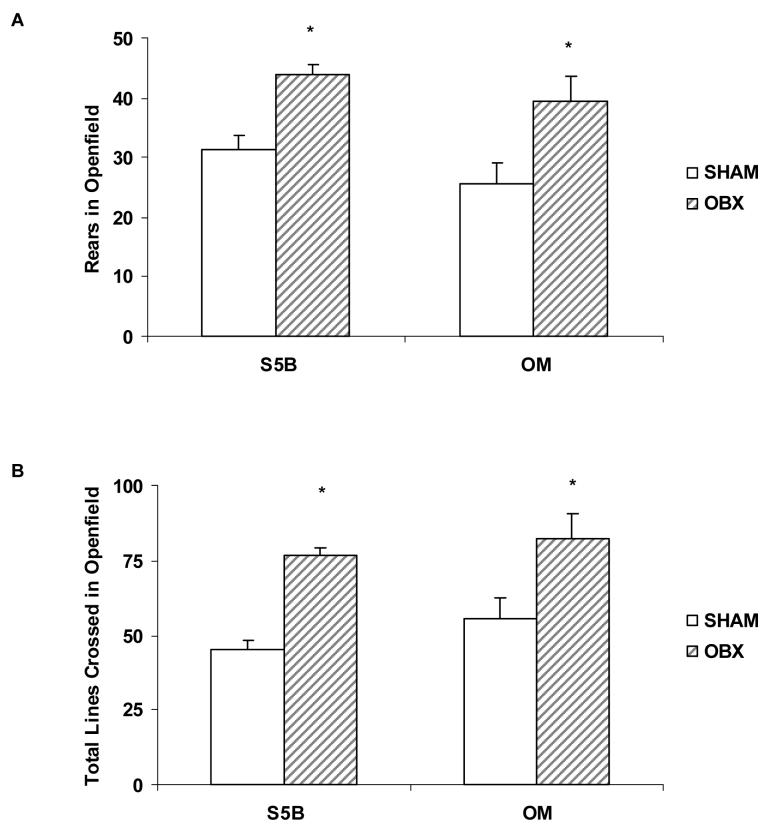
A 2×2 ANOVA indicated a significant increase in rearing behavior in the openfield test following OBX (F(1,53) = 17.12, p<.001; effect size .24; See Figure 3A). A main effect of surgery was also found in the total number of lines crossed in the openfield test (F(1,51) = 23.38, p<.001; effect size .31; See Figure 3B). Bulbectomized rats of both strains made more rears and line crossings than sham-operated rats (p<.05).
Figure 3.
Fourteen days following OBX or sham surgeries, locomotor activity was measured in the novel openfield test. A. OBX significantly increased the number of rears in the openfield test in both OM and S5B/Pl rats. B. OBX significantly increased the total number of lines crossed in the openfield test in both OM and S5B/Pl rats. Data are shown as means ± SEM. * indicates p<.05 between OBX and sham values.
Real-time PCR
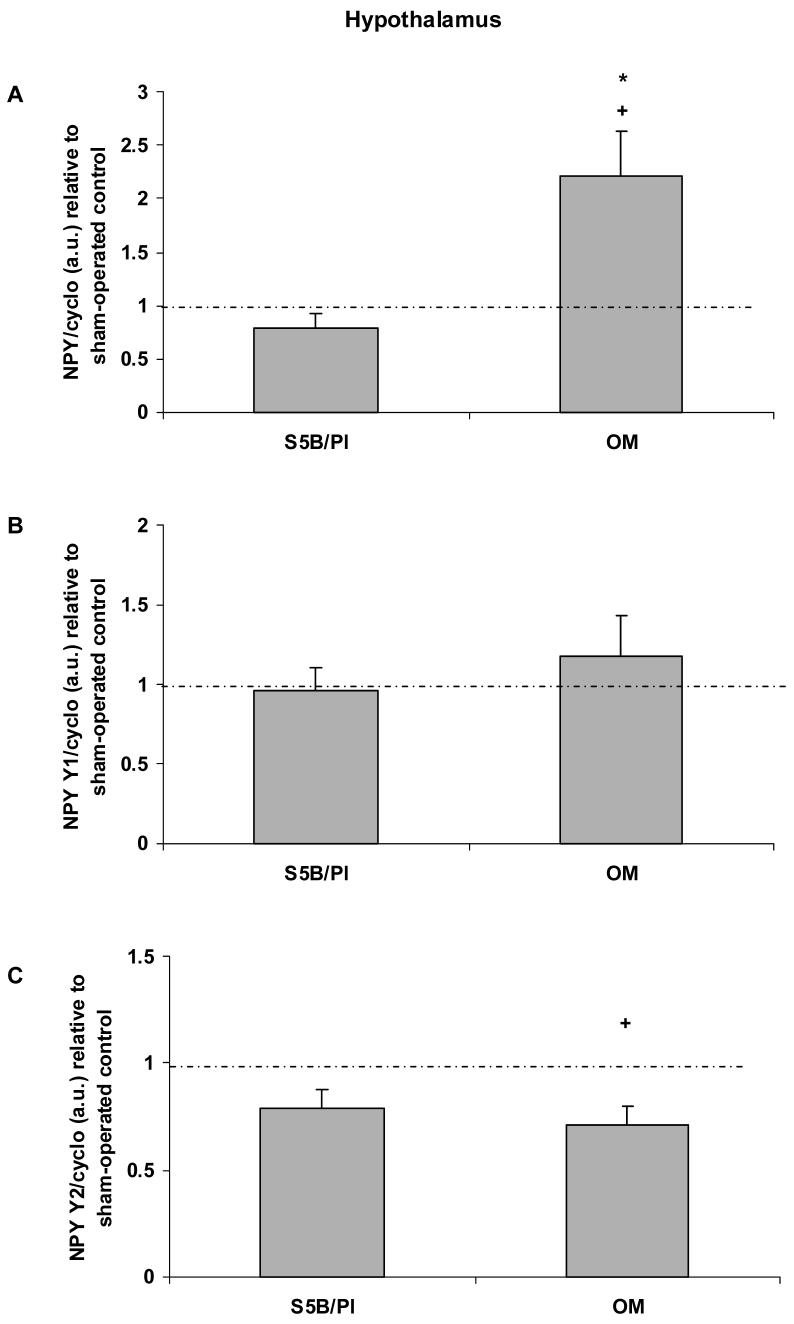
In the hypothalamus, OBX increased prepro-NPY mRNA levels in OM rats (t(14) = 2.89, p<.01; effect size .61; See Figure 4A). OBX did not alter hypothalamic prepro-NPY mRNA in S5B/Pl rats (p>.05). Prepro-NPY mRNA levels were significantly increased in OM compared to S5B/Pl rats following OBX (t(12) = 2.83, p<.02; effect size .63). OBX did not alter NPY Y1 receptor mRNA levels in either OM or S5B/Pl rats (p>.05; See Figure 4B). OBX significantly decreased NPY Y2 receptor mRNA of OM rats (t(12) = 3.08, p<.01; effect size .66; See Figure 4C), but did not alter NPY Y2 receptor mRNA in S5B/Pl rats (p>.05).
Figure 4.
Prepro-NPY, NPY Y1 receptor, and NPY Y2 receptor mRNA were measured in the hypothalamus of OM and S5B/Pl rats following OBX or sham surgeries. A: OBX increased hypothalamic prepro-NPY mRNA levels in OM rats. OBX-induced changes in prepro-NPY mRNA levels in OM rats are significantly higher than those in S5B/Pl rats. B: OBX did not alter NPY Y1 receptor mRNA in OM or S5B/Pl rats. C: OBX significantly decreased NPY Y2 receptor mRNA in OM rats compared to their sham-operated controls. Data are shown as means ± SEM and are relative to sham-operated controls. Dotted lines represent a similar value for sham-operated controls and bulbectomized animals. + indicates p<.05 difference between OBX and sham-control values, * indicates p<.05 between OM-OBX and S5B-OBX.
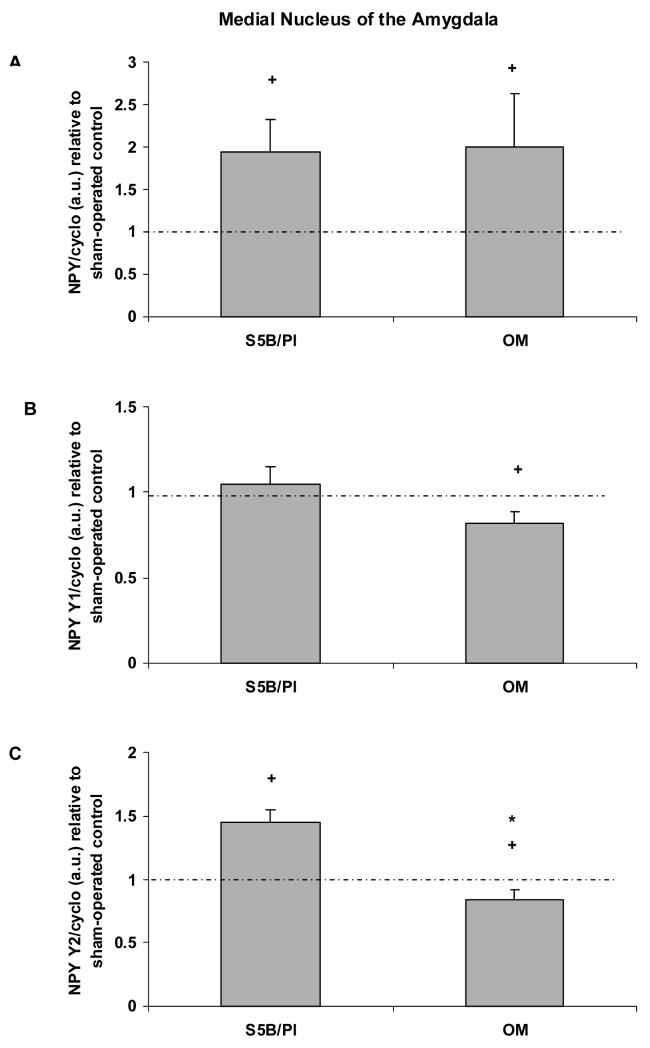
In the medial nucleus of the amygdala, OBX increased prepro-NPY mRNA in both OM rats (t(16) = 2.04, p<.05; effect size .45) and S5B/Pl rats (t(16) = 2.43, p<.05; effect size .52; See Figure 5A). NPY Y1 receptor mRNA was decreased in bulbectomized OM rats (t(14) = 2.56, p<.05; effect size .56; See Figure 5B), but not altered by OBX in S5B/Pl rats. NPY Y2 receptor mRNA levels were significantly increased in S5B/Pl rats compared to OM rats following OBX (t(16) = 4.65, p<.001; effect size .76; See Figure 5C). OBX significantly decreased NPY Y2 receptor mRNA in OM rats (t(15) = 2.07, p<.05; effect size .47), while NPY Y2 receptor mRNA levels were increased in S5B/Pl rats following OBX (t(18) = 4.65, p<.01; effect size .74).
Figure 5.
Prepro-NPY, NPY Y1 receptor, and NPY Y2 receptor mRNA were measured in the medial nucleus of the amygdala of OM and S5B/Pl rats following OBX or sham surgeries. A: OBX increased prepro-NPY mRNA levels in the medial nucleus of the amygdala of OM and S5B/PL rats relative to sham-operated controls. B: OBX decreased NPY Y1 receptor mRNA in OM rats compared to their sham-operated controls. C: OBX increased NPY Y2 receptor mRNA in S5B/Pl rats compared to their controls, while decreasing NPY Y2 mRNA in OM rats compared to their controls. Changes in NPY Y2 receptor mRNA were significantly decreased in OM vs. S5B/Pl rats following OBX. Data are shown as means ± SEM and are relative to sham-operated controls. Dotted lines represent a similar value for sham-operated controls and bulbectomized animals. + indicates p<.05 difference between OBX and sham-control values, * indicates p<.05 between OM-OBX and S5B-OBX.
Discussion
Obesity and depression are among the fastest growing health concerns in the United States. Recent clinical research has suggested a significant link between obesity and depression [15,24,35,37,45,46,47,63]. Therefore, the coexistence of obesity and depression is of immense concern and requires increasing attention. These concerns are particularly applicable to people suffering from major depression with atypical symptoms, such as increased food intake and weight gain. The OBX model is an animal model of depression that is characterized by behavioral, neurochemical and physiological symptoms resembling clinical depression. The goal of the current experiment was to examine the effects of OBX on food intake, body weight, and locomotor activity in an obesity-prone strain, the Osborne-Mendel (OM) rat and an obesity-resistant strain, the S5B/Pl rat.
As expected, OM rats ate more and weighed more than S5B/Pl rats prior to surgery. Following OBX surgery, bulbectomized OM rats ate significantly more food than sham-operated OM rats. The average increase in food intake of bulbectomized OM rats was 3.1g/day between 7 and 15 days following surgery, which is a cumulative increase of approximately 28g (see Figure 1). OBX did not increase food intake in obesity-resistant S5B/Pl rats at any time point following surgery, which is similar to the effects of OBX on Sprague Dawley rats [16, 30, 31]. Decreases in body weight have been reported following OBX surgery [16]. As expected, OBX decreased the percentage of pre-surgery body weight in both OM and S5B/Pl rats beginning immediately following surgery, and 1 and 2 weeks after surgery (See Figure 2). Increased locomotor activity in the novel openfield test, the behavioral marker of the OBX syndrome, was seen in both OM and S5B/Pl rats following OBX surgery (See Figure 3). These data suggest differential effects of OBX in OM and S5B/Pl rats with regards to food intake, but similar effects of OBX in OM and S5B/Pl rats with respect to locomotor activity.
Differences in the central nervous system of OM and S5B/Pl rats may contribute to OBX-induced differences in food intake. Previously, it has been reported that OM rats have increased hypothalamic prepro-NPY, NPY Y1 receptor, and Y2 receptor mRNA and decreased levels of hypothalamic serotonin and increased serotonin 2C receptors, when compared to S5B/Pl rats [20,49,53]. Additionally, studies have reported that OM rats were more sensitive to the orexigenic or anorectic effects of these peptides i.e. the orexigenic effects of NPY are greater in OM rats [25].
NPY is involved in both depression and food intake regulation [8,9,11,13,23,33,42,58]. The orexigenic effects of NPY are mediated through the hypothalamus [13,50,56,58], while the depressive effects may be mediated through the amygdala, specifically the medial nucleus of the amygdala [48]. Interestingly, lesions of the medial nucleus of the amygdala, but not other sub-regions of the amygdala, lead to hyperphagia and obesity [21,22]. Due to the importance of NPY in depression and food intake, this experiment investigated the effects of OBX on prepro-NPY, NPY Y1 receptor, and NPY Y2 receptor mRNA in the hypothalamus and medial nucleus of the amygdala of OM and S5B/Pl rats 15 days following OBX or sham surgery.
Following OBX, prepro-NPY mRNA levels were increased in the hypothalamus of bulbectomized OM rats. Hypothalamic prepro-NPY mRNA levels were not altered in S5B/Pl rats following OBX, suggesting a strain difference in the effects of OBX on hypothalamic NPY. NPY Y2 receptor mRNA was decreased in bulbectomized OM rats when compared to sham-operated OM rats, but not altered in S5B/Pl rats following OBX (See Figure 4). In the medial nucleus of the amygdala, OBX significantly increased prepro-NPY mRNA levels in OM and S5B/Pl rats compared to their sham-operated controls, while NPY Y1 receptor and NPY Y2 receptor mRNA were decreased in bulbectomized OM rats. NPY Y2 receptor mRNA was increased in bulbectomized S5B/Pl rats compared to sham-operated controls (See Figure 5). These data suggest that NPY and it's receptors, particularly NPY Y2 receptors, may play a role in regulating the effect of OBX on food intake in OM rats. The differential increase in prepro-NPY mRNA levels in the hypothalamus of bulbectomized OM rats may account for the increase in food intake seen following OBX. If this is the case, then OBX-induced increases in hypothalamic NPY levels may begin around 7 days after surgery, peak at 14 days after surgery and then begin to decrease, which is similar to the effects of OBX on NPY levels in the piriform cortex [10].
NPY Y2 receptor mRNA was decreased in the hypothalamus and medial nucleus of the amygdala of bulbectomized OM rats. In both brain regions, prepro-NPY mRNA levels were increased in OM rats. This is particularly interesting because NPY Y2 receptors are thought to act presynaptically as autoreceptors providing negative feedback to NPY containing nerve terminals, while NPY Y1 receptors are thought to act postsynaptically [14,65]. Therefore, when NPY levels are increased, NPY Y2 receptor levels would be expected to be increased in order to maintain proper negative feedback to NPY producing neurons. This is not the case in bulbectomized OM rats. The increase in NPY appears to coincide with a decrease in NPY regulation through NPY Y2 receptors, thereby potentially increasing the release of NPY. However, in S5B/Pl rats, OBX increased prepro-NPY mRNA in the medial nucleus of the amygdala and increased NPY Y2 receptor mRNA suggesting that S5B/Pl rats are able to compensate for the increase in NPY.
The current experiment provides promising evidence for a potential new animal model to study the comorbidity of obesity and depression. Specifically, bulbectomized OM rats appear to resemble individuals with major depression with atypical symptoms, which include increased food intake. Further investigations are needed to characterize this phenomenon.
Acknowledgements
This research was supported by: NIAAA F32AA014310 to S.D. Primeaux and NIDDK 32089 to G. A. Bray. The authors would like to thank C. Blackmon, H.D. Braymer and D.A. York for their contributions. We would also like to thank the Pennington Biomedical Research Center's Clinical Nutrition Research Unit Animal Models and Phenotyping Core and Molecular Mechanisms Core for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- 2.Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 3.Caberlotto L, Fuxe K, Overstreet DH, Gerrard P, Hurd YL. Alterations in neuropeptide Y and Y1 receptor mRNA expression in brains from an animal model of depression: region specific adaptation after fluoxetine treatment. Brain Res Mol Brain Res. 1998;59:58–65. doi: 10.1016/s0169-328x(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 4.Chambliss HO, Van Hoomissen JD, Holmes PV, Bunnell BN, Dishman RK. Effects of chronic activity wheel running and imipramine on masculine copulatory behavior after olfactory bulbectomy. Physiol Behav. 2004;82:593–600. doi: 10.1016/j.physbeh.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 5.Ebner K, Rupniak NM, Saria A, Singewald N. Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc.Natl.Acad.Sci.U.S.A. 2004;101:4280–4285. doi: 10.1073/pnas.0400794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass MJ, Cleary JP, Billington CJ, Levine AS. Role of carbohydrate type on diet selection in neuropeptide Y-stimulated rats. Am J Physiol Regul Integ Comp Physiol. 1997;273:2040–2045. doi: 10.1152/ajpregu.1997.273.6.R2040. [DOI] [PubMed] [Google Scholar]
- 7.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 8.Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjogren M, Asberg M, Ekman R, Wahlestedt C, Agren H. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psych Res. 2004;38:113–121. doi: 10.1016/s0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 9.Henry M, Ghibaudi L, Gao J, Hwa JJ. Energy metabolic profile of mice after chronic activation of central NPY Y1, Y2, or Y5 receptors. Obes Res. 2005;13:36–47. doi: 10.1038/oby.2005.6. [DOI] [PubMed] [Google Scholar]
- 10.Holmes PV, Davis RC, Masini CV, Primeaux SD. Effects of olfactory bulbectomy on neuropeptide gene expression in the rat olfactory/limbic system. Neurosci. 1998;86:587–596. doi: 10.1016/s0306-4522(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 11.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide-Y containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Vasquez PA, Diaz-Cabiale Z, Caberlotto L, Bellido I, Overstreet D, Fuxe K, Mathe AA. Electroconvulsive stimuli selectively effect behavior and neuropeptide Y (NPY) and NPY Y(1) receptor gene expressions in hippocampus and hypothalamus of Flinders Sensitive Line rat model of depression. Behav Brain Res. 2000;111:115–123. doi: 10.1016/j.euroneuro.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Kalra SP, Kalra PS. Neuropeptide Y: a physiological orexigen modulated by the feedback action of ghrelin and leptin. Endocrine. 2003;22:49–56. doi: 10.1385/ENDO:22:1:49. [DOI] [PubMed] [Google Scholar]
- 14.Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 15.Katon W, von Korff M, Ciechanowski P, Russo J, Lin E, Simon G, Ludman E, Walker E, Bush T, Young B. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 16.Kelly JP, Norman TR, O'Halloran A, Leonard BE. Home cage and open-field locomotor activity responses in singly housed olfactory bulbectomised rats. Med Sci Res. 1996;24:335–337. [Google Scholar]
- 17.Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, Eaves LJ, Walters EE, Neale MC, Heath AC, Kessler RC. The identification and validation of distinct depressive syndromes in a population-based sample of female twins. Arch.Gen.Psychiatry. 1996;53:391–399. doi: 10.1001/archpsyc.1996.01830050025004. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Whang WW, Kim HT, Pyun KH, Cho SY, Hahn DH, Lee HJ, Shim I. Expression of neuropeptide Y and cholecystokinin in the rat brain by chronic mild stress. Brain Res. 2003;983:201–208. doi: 10.1016/s0006-8993(03)03087-7. [DOI] [PubMed] [Google Scholar]
- 20.Kimbrough TD, Weekley LB. The effect of a high-fat diet on brainstem and duodenal serotonin (5-HT) metabolism in Sprague-Dawley and Osborne-Mendel rats. Int J Obes. 1984;8:305–310. [PubMed] [Google Scholar]
- 21.King BM, Cook JT, Rossiter KN, Rollings BL. Obesity-inducing amygdala lesions: examination of anterograde degeneration and retrograde transport. Am J Physiol Regul Integ Comp Physiol. 2003;284:R965–R982. doi: 10.1152/ajpregu.00249.2002. [DOI] [PubMed] [Google Scholar]
- 22.King BM, Rossiter KN, Stines SG, Zaharan GM, Cook JT, Humphries MD, York DA. Amygdaloid-lesion hyperphagia: impaired response to caloric challenges and altered macronutrient selection. Am J Physiol Regul Integ Comp Physiol. 1998;275:R485–R493. doi: 10.1152/ajpregu.1998.275.2.R485. [DOI] [PubMed] [Google Scholar]
- 23.Kishi T, Elmquist JK. Body weight is regulated by the brain: a link between feeding and emotion. Mol Psychiatry. 2005;10:132–146. doi: 10.1038/sj.mp.4001638. [DOI] [PubMed] [Google Scholar]
- 24.Kopf D, Westphal S, Luley CW, Ritter S, Gilles M, Weber-Hamann B, Lederbogen F, Lehnert H, Henn FA, Heuser I, Deuschle M. Lipid metabolism and insulin resistance in depressed patients: significance of weight, hypercortisolism, and antidepressant treatment. J Clin Psychopharmacol. 2004;24:527–531. doi: 10.1097/01.jcp.0000138762.23482.63. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, York DA, Bray GA. Comparison of Osborne-Mendel and S5B/PL strains of rat: central effects of galanin, NPY, beta-casomorphin and CRH on intake of high-fat and low-fat diets. Obes Res. 1996;4:117–124. doi: 10.1002/j.1550-8528.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma S, Morilak DA. Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neurosci. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Marcilhac A, Maurel D, Anglade G, Ixart G, Mekaouche M, Hery F, Siaud P. Effects of bilateral olfactory bulbectomy on circadian rhythms of ACTH, corticosterone, motor activity and body temperature in male rats. Horm Metab Res. 1999;105:552–559. doi: 10.1076/apab.105.6.552.3273. [DOI] [PubMed] [Google Scholar]
- 28.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65:634–51. doi: 10.4088/jcp.v65n0507. [DOI] [PubMed] [Google Scholar]
- 29.McNish KA, Davis M. Olfactory bulbectomy enhances sensitization of the acoustic startle reflex produced by acute or repeated stress. Behav Neurosci. 1997;111:80–91. doi: 10.1037//0735-7044.111.1.80. [DOI] [PubMed] [Google Scholar]
- 30.Meguid MM, Gleason JR, Yang ZJ. Olfactory bulbectomy in rats modulates feeding pattern but not total food intake. Physiol Behav. 1993;54:471–475. doi: 10.1016/0031-9384(93)90238-b. [DOI] [PubMed] [Google Scholar]
- 31.Meguid MM, Koseki M, Yang ZJ, Gleason JR, Laviano A. Acute adaptive changes in food intake pattern following olfactory ablation in rats. Neuroreport. 1997;8:1439–1444. doi: 10.1097/00001756-199704140-00023. [DOI] [PubMed] [Google Scholar]
- 32.Morley JE, Levine AS, Gosnell BA, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol. 1987;252:R599–R609. doi: 10.1152/ajpregu.1987.252.3.R599. [DOI] [PubMed] [Google Scholar]
- 33.Nikisch G, Agren H, Eap CB, Czernik A, Baumann P, Mathe AA. Neuropeptide Y and corticotrophin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int J Neuropsychopharmacol. 2005;8:403–410. doi: 10.1017/S1461145705005158. [DOI] [PubMed] [Google Scholar]
- 34.Okada S, York DA, Bray GA, Mei J, Erlanson-Albertsson C. Differential inhibition of fat intake in two strains of rat by the peptide enterostatin. Am J Physiol. 1992;262:R1111–R1116. doi: 10.1152/ajpregu.1992.262.6.R1111. [DOI] [PubMed] [Google Scholar]
- 35.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–1147. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 36.Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav. 2005;81:901–906. doi: 10.1016/j.pbb.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Papakostas GI, Petersen T, Iosifescu DV, Burns AM, Nierenberg AA, Alpert JE, Rosenbaum JF, Fava M. Obesity among outpatients with major depressive disorder. Int.J Neuropsychopharmacol. 2005;8:59–63. doi: 10.1017/S1461145704004602. [DOI] [PubMed] [Google Scholar]
- 38.Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 39.Perret M, Aujard F, Seguy M, Schilling A. Olfactory bulbectomy modifies photic entrainment and circadian rhythms of body temperature and locomotor activity in a nocturnal primate. J Biol Rhythms. 2003;18:392–401. doi: 10.1177/0748730403254248. [DOI] [PubMed] [Google Scholar]
- 40.Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 41.Primeaux SD, Holmes PV. Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiol Behav. 1999;67:41–47. doi: 10.1016/s0031-9384(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 42.Primeaux SD, Holmes PV. Olfactory bulbectomy increases metenkephalin- and neuropeptide-Y-like immunoreactivity in rat limbic structures. Pharmacol Biochem Behav. 2000;67:331–337. doi: 10.1016/s0091-3057(00)00358-0. [DOI] [PubMed] [Google Scholar]
- 43.Primeaux SD, Wilson MA, Wilson SP, Guth AN, Lelutiu NB, Holmes PV. Herpes virus-mediated preproenkephalin gene transfer in the ventral striatum mimics behavioral changes produced by olfactory bulbectomy in rats. Brain Res. 2003;988:43–55. doi: 10.1016/s0006-8993(03)03337-7. [DOI] [PubMed] [Google Scholar]
- 44.Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Peptides. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 45.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. Int.J Obes Relat Metab Disord. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 46.Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? Am J Epidemiol. 2000;152:163–170. doi: 10.1093/aje/152.2.163. [DOI] [PubMed] [Google Scholar]
- 47.Roberts RE, Strawbridge WJ, Deleger S, Kaplan GA. Are the fat more jolly? Ann Behav Med. 2002;24:169–180. doi: 10.1207/S15324796ABM2403_02. [DOI] [PubMed] [Google Scholar]
- 48.Rutkoski NJ, Lerant AA, Nolte CM, Westberry J, Levenson CW. Regulation of neuropeptide Y in the rat amygdala following unilateral olfactory bulbectomy. Brain Res. 2002;951:69–76. doi: 10.1016/s0006-8993(02)03136-0. [DOI] [PubMed] [Google Scholar]
- 49.Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res. 2002;10:1188–1196. doi: 10.1038/oby.2002.161. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz MW, Morton GJ. Keep hunger at bay. Nature. 2002;418:595–597. doi: 10.1038/418595a. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 52.Sergeyev V, Fetissov S, Mathe AA, Jimenez PA, Bartfai T, Mortas P, Gaudet L, Moreau JL, Hokfelt T. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacol. 2005;178:115–124. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu H, Fisler JS, Bray GA. Extracellular hypothalamic monoamines measured by in vivo micro-dialysis in a rat model of dietary obesity. Obes Res. 1994;2:100–109. doi: 10.1002/j.1550-8528.1994.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 54.Sjoberg RL, Nilsson KW, Leppert J. Obesity, shame, and depression in school-aged children: a population-based study. Pediatrics. 2005;116:389–392. doi: 10.1542/peds.2005-0170. [DOI] [PubMed] [Google Scholar]
- 55.Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18:207–211. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- 56.Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 57.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6:1205–1211. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 58.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: A powerful stimulant of feeding behavior. Proc Natl Acad Sci U S A. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Tichomirowa MA, Keck ME, Schneider HJ, Paez-Pereda M, Renner U, Holsboer F, Stalla GK. Endocrine disturbances in depression. J Endocrinol Invest. 2005;28:89–99. doi: 10.1007/BF03345535. [DOI] [PubMed] [Google Scholar]
- 61.van der Stelt HM, Breuer ME, Olivier B, Westenberg HG. Permanent deficits in serotonergic functioning of olfactory bulbectomized rats: an in vivo microdialysis study. Biol Psychiatry. 2005;57:1061–1067. doi: 10.1016/j.biopsych.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 62.Welch CC, Grace MK, Billington CJ, Levine AS. Preference and diet type affect macronutrient selection after morphine, NPY, norepinephrine, and deprivation. Am J Physiol. 1994;266:R426–R433. doi: 10.1152/ajpregu.1994.266.2.R426. [DOI] [PubMed] [Google Scholar]
- 63.Werrij MQ, Mulkens S, Hospers HJ, Jansen A. Overweight and obesity: The significance of a depressed mood. Patient Educ Couns. 2006;62:126–131. doi: 10.1016/j.pec.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Widdowson PS, Upton R, Henderson L, Buckingham R, Wilson S, Williams G. Reciprocal regional changes in brain NPY receptor density during dietary restriction and dietary-induced obesity in the rat. Brain Res. 1997;774:1–10. doi: 10.1016/s0006-8993(97)81680-0. [DOI] [PubMed] [Google Scholar]
- 65.Wolak ML, De Joseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- 66.Wortwein G, Husum H, Anderson W, Bolwig TG, Mathe AA. Effects of maternal separation on neuropeptide Y and calcitonin gene-related peptide in “depressed” Flinders Sensitive Line rats: a study of gene-environment interactions. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:684–693. doi: 10.1016/j.pnpbp.2006.01.027. [DOI] [PubMed] [Google Scholar]