Abstract
Planar cell polarity (PCP) refers to the polarization of cells within the plane of a cell sheet. A distinctive epithelial PCP in vertebrates is the uniform orientation of stereociliary bundles of the sensory hair cells in the mammalian cochlea. In addition to establishing epithelial PCP, planar polarization is also required for convergent extension (CE), a polarized cellular movement that occurs during neural tube closure and cochlear extension. Studies in Drosophila and vertebrates have revealed a conserved PCP pathway, including Frizzled (Fz) receptors. Here we use the cochlea as a model system to explore the involvement of known ligands of Fz, Wnt morphogens, in PCP regulation. We show that Wnt5a forms a reciprocal expression pattern with a Wnt antagonist, the secreted frizzled-related protein 3 (Sfrp3 or Frzb), along the axis of planar polarization in the cochlear epithelium. We further demonstrate that Wnt5a antagonizes Frzb in regulating cochlear extension and stereociliary bundle orientation in vitro, and that Wnt5a−/− animals have a shortened and widened cochlea. Finally, we show that Wnt5a is required for proper subcellular distribution of a PCP protein, Ltap/Vangl2, and that Wnt5a interacts genetically with Ltap/Vangl2 for uniform orientation of stereocilia, cochlear extension, and neural tube closure. Together, these findings demonstrate that Wnt5a functions in PCP regulation in mice.
INTRODUCTION
The mammalian auditory sensory organ, the organ of Corti, consists of four rows of sensory hair cells interdigitated with supporting cells along the length of the spiral cochlear duct. The innermost row of cells toward the center (medial aspect) are the inner hair cells (IHCs); the three rows toward the periphery (lateral aspect) of the cochlea are the outer hair cells (OHCs) (Fig. 1A, B). On the apical surface of each hair cell, bundles of finger-like extensions (stereocilia) are arranged in a “V”-shaped staircase. Invariably, the vertices of the “V”-shaped stereocilia of all the hair cells point to the periphery of the cochlea, displaying a distinctive planar cell polarity (PCP) (Gubb and Garcia-Bellido, 1982) along the mediolateral axis of the cochlea (Fig. 1B). The PCP of the cochlea is essential for auditory transduction. Deflection of the stereocilia bundle towards the tallest row of stereocilia during sound stimulation mechanically opens transduction channels near the tips of stereocilia, allowing influx of cations and depolarization of the hair cell. Thus, the coordinated orientation of stereocilia bundles is critical for effective function of the cochlea as it permits synchronous activation of groups of hair cells and their associated afferent neurons. During mouse development, the organ of Corti differentiates from a shorter and thicker primordium through cellular rearrangements characteristic of convergent extension (CE) (Keller, 2002) concurrently with the establishment of PCP from embryonic day 14.5 (E14.5) to E18.5 (Chen et al., 2002; McKenzie et al., 2004; Wang et al., 2005).
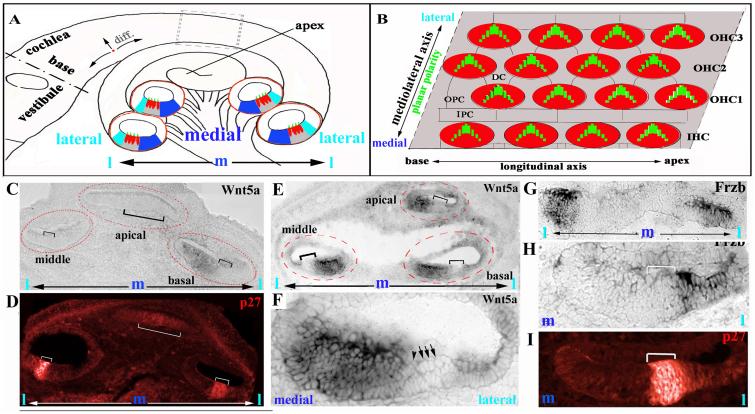
Fig. 1. Reciprocal expression of Wnt5a and Frzb along the mediolateral axis of the cochlea.
(A): Diagram of a cross section of the cochlear duct at different regions along the longitudinal axis (outlined by red circles). The IHCs are localized toward the center, or medial; the three rows of OHCs are at the periphery, or lateral region. The differentiation gradient of the organ of Corti is designated by arrows near base of the cochlea (A). The dark and light blue regions correspond, respectively, to the Wnt5a- and Frzb-expressing domains.
(B): Stereocilia are uniformly oriented along the mediolateral axis illustrated in this diagram of a whole mount of the organ of Corti.
(C,D): At E14.5, Wnt5a mRNA was detected in the region medial (C) to the developing organ of Corti (indicated by brackets) at the basal region of the cochlea. The expression of p27/Kip1 (D, red) marked distinctively the developing organ of Corti at this stage. In the middle and apical regions of the cochlea, Wnt5a expression was not detected.
(E,F): At E16.5, Wnt5a is expressed in the region medial to the organ of Corti (indicated with brackets) along the length of the cochlear duct. (F) is a higher magnification view of the middle cochlear cross section of (E). The IHC and OHC are indicated by an arrowhead and arrows, respectively.
(G-I): At E14.5, Frzb is expressed in the regions lateral (H, I) to the developing organ of Corti (indicated by brackets) marked by p27/Kip1 expression (J). (I): a higher magnification view of (H). m: medial. l: lateral.
Genetic studies in Drosophila have identified a set of so-called core PCP genes that are required for diverse forms of planar polarity (Klein and Mlodzik, 2005; Strutt and Strutt, 2005; Tree et al., 2002). In vertebrates, a similar cassette of genes, including Frizzled (Fz), Dishevelled, Ltap/Vangl2, and Celsr1, regulate CE and the establishment of PCP (Keller, 2002; Mlodzik, 2002; Wallingford et al., 2000; Wang et al., 2006a). Mutations in mouse PCP genes cause defective planar polarization of the cochlear epithelium and consequently stereociliary bundle misorientation (Curtin et al., 2003; Lu et al., 2004; Montcouquiol et al., 2003; Wang et al., 2005; Wang et al., 2006b). These mutations are also associated with a shortened and widened cochlear duct and a completely open neural tube, presumably resulting from defective CE during cochlear extension and neurulation, respectively (Montcouquiol et al., 2003; Wang et al., 2006a; Wang et al., 2005). During planar polarization, PCP components such as Fz3 and Fz6 (Montcouquiol et al., 2006; Wang et al., 2006b), dishevelled2 (Wang et al., 2005) , and Ltap/Vangl2 (Montcouquiol et al., 2006) are sorted asymmetrically along the mediolateral axis and display polarized subcellular localizations across the organ of Corti. Polarized membrane localization of Celsr1 was observed in the chicken auditory sensory organ (Davies et al., 2005). During CE in vertebrates, PCP proteins and other polarization complexes also display polarized subcellular distribution in polarized lamellipodial protrusions which are presumed to exert traction, pulling the cells toward one another mediolaterally. This causes the tissue to narrow along the mediolateral axis and, concomitantly, extend along a perpendicular axis (Ciruna et al., 2006; Hyodo-Miura et al., 2006; Jiang et al., 2005; Mlodzik, 2006) .
Wnts are known ligands for Fz receptors (Bhanot et al., 1996; Yang-Snyder et al., 1996), which are essential components of PCP signaling (Vinson et al., 1989; Wang et al., 2006b). However, whether Wnts are involved in PCP regulation remains controversial. Studies in Drosophila wing have pointed to a Wnt-independent mechanism (Amonlirdviman et al., 2005; Klein and Mlodzik, 2005; Strutt and Strutt, 2005; Tree et al., 2002) . Two recent studies show that Wnt is involved in orientating the denticles (actin-based cell projections) on segmentally repeated subsets of ventral epidermal cells in the Drosophila embryos (Colosimo and Tolwinski, 2006; Price et al., 2006), and that Wnt may function together with hedgehog (Hh) as instructive polarizing cues that help establishing directionality within the epidermis (Colosimo and Tolwinski, 2006). In Xenopus and zebrafish, Wnt5 and Wnt11 are required for CE, although their role may be permissive instead of instructive (Smith et al., 2000; Tada and Smith, 2000; Topczewski et al., 2001; Ulrich et al., 2003).
So far, Wnts have not been demonstrated for an in vivo role in PCP signaling in mammals. In the mouse cochlea, Wnt7a is expressed in a type of supporting cells, the pillar cells, within the organ of Corti (Dabdoub et al., 2003). Addition of Wnt7a-conditioned medium (CM) or the Wnt antagonists Sfrp1 and Wif1 leads to misorientation of stereocilia in culture (Dabdoub et al., 2003). However, no PCP defect is observed in Wnt7a knockout mice (Dabdoub et al., 2003). In this study, we used the cochlea as a model system and explored the involvement of Wnt morphogens in PCP regulation. We provide in vitro and in vivo evidence supporting a role for Wnt5a in PCP regulation in mice.
MATERIALS AND METHODS
Mouse strains and animal care
The animals used are Wnt5a+/− (Yamaguchi et al., 1999), Looptail LtapLp/+ (Kibar et al., 2001), Cre/Esr1 (Nat) (Badea et al., 2003), Gt(Rosa)26Sor animal (Jackson Laboratory), Math1/GFP (Chen et al., 2002; Lumpkin et al., 2003) and BAT-gal (Maretto et al., 2003) animals. The LtapLp/+ colony was in a mixed C57BL/6 × 129 background. Wnt5a+/− animals (129S7/SvEvBrd-Hprt) were bred with C57/B6Crl animals (Charles River Laboratory). To generate transgenic mice expressing Ltap-GFP fusion protein, we inserted the coding sequence for GFP before the stop codon of the Ltap gene in a bacterial artificial chromosome (BAC) (BACPAC, RP23-244J9) using a reported method (Yang et al., 1997). Animal care and use was in accordance with NIH guidelines and was approved by the Animal Care and Use Committee of Emory University and the University of California, Irvine. The morning after the mating of the animals is designated as E0.5. BrdU cell proliferation analyses were performed as described (Chen et al., 2002). The number of animals used for each genotype at each stage is least three and indicated where data are presented.
In-situ hybridization and histology analyses of inner ear tissues
Inner ear dissection, sectioning, immunostaining, and in-situ hybridization were performed as described (Radde-Gallwitz et al., 2004). Primary antibodies and dyes used were: acetylated α-tubulin (mouse monoclonal, 1:50-100, Sigma); FITC- and rhodamineconjugated phalloidin (5 pM, from Molecular Probes); p27/Kip1 (mouse monoclonal, 1:100, BD Transduction Laboratories); BrdU (mouse monoclonal, 1:100, Roche). Secondary antibodies were purchased from Jackson ImmunoResearch. For p27/Kip1 and BrdU staining, the sections were subjected to citric acid steam treatment prior to immunostaining.
For analysis of PCP, we stained the whole mount preparations of the organs of Corti with an antibody against acetylated α-tubulin and rhodamine- or FITC-conjugated phalloidin that label the kinocilium and the stereocilia, respectively (Wang et al., 2005). The samples were analyzed and images were acquired with Zeiss LSM510 for less than 1 μm optical sections.
cDNAs for Wnt5a and Frzb were cloned from theE14.5 inner ear epithelium by RT-PCR with oligonucleotides listed in supplemental Table I.
Scanning electronic microscope (SEM)
SEM imaging was performed as described (Wang et al., 2005). Briefly, inner ears were fixed in 2.5% glutaraldehyde in 0.7M Cacodylate buffer (pH 7.3) for 4 hours at room temperature, and transferred to PBS, pH7.4. The specimens were further treated with 1% tannic acid, followed by osmium tetroxide treatment. The specimens were dehydrated through series ethanol, and critically dried. The dried samples were mounted on metal stubs, and coated with gold particles, and ready for examination.
Organ Culture
We dissected cochleae from E14.5 embryos and cultured either the intact cochlear duct or bisected cochlear ducts on polyD-lysine- (Sigma) and fibronectin-coated glass-bottomed dishes in control, Frzb-containing, or Wnt5a- and Frzb-treated culture media for 4-6 days in vitro (DIV) as described (Wang et al., 2005). For suppression of Frzb by Wnt5a, we pre-incubated molar ratios of Frzb and Wnt5a for 20 minutes at 37°C before addition to the culture media. For cochlear grafting cultures, two cochleae were placed close or distant to each other. The mediolateral axes of the two cochleae cultured were parallel or anti-parallel to determine whether there is an intrinsic difference between the cochlear tissues in the medial and the lateral regions for the orientation of stereociliary bundles.
Preparation of Wnt5a-conditioned media and Western blot analysis
Both the control L cells (ATCC, Cat# CRL-2648) and Wnt5a producing L cells (ATCC, Cat# CRL-2814) were cultured according to ATCC instructions. The media collected from both control cells and the Wnt5a-producing cells were concentrated with Centriplus YM-10 (Millipore, Cat# 4411). The concentration of Wnt5a in the conditioned media was determined by Western blot analysis using the recombinant Wnt5a expressed in CHO cells (R&D, Cat# 645WN) as the standard and an antibody against Wnt5a (Neuromics, Cat# GT15034, 1:2500). Western blot analyses were carried out using standard protocol. Cochlear and brain protein extracts were isolated from E15.5-E16.5 embryos and subjected to Western blot analysis.
Quantification of stereociliary bundle orientation and cochlear extension
We measured the length of cochlear ducts at the beginning and end of culture using NIH ImageJ software. The lengthening in the culture was scored as the ratio of the length of the culture at the beginning to the length of the culture at the end. To determine stereociliary bundle orientation, we drew a line from the position of the kinocilium through the middle of the “V”-shaped stereocilia (bisecting line). The angle formed between this line and the line parallel to the mediolateral axis was used for quantifications. In wild type animals, this angle is closed to 0. For histogram plots of distribution of the angles, the angles forms at the right side or the left side of the line representing mediolateral axis are designated to be positive (+) or negative (−), respectively. For quantification of the average deviation from the normal orientation (angle=0), only the angles were scored without positive (+) or negative (−) designations. At least 50 hair cells in each row at the basal, middle, and apical regions were quantified for each sample. The same quantification method was used for quantification of stereociliary bundle orientation for whole mount preparations of the organs of Corti and the organs of Corti in vitro. SPSS (11.0) was used for statistic analyses.
Canonical Wnt activity assay
Embryos were collected from timed-pregnant BAT-gal (Maretto et al., 2003) transgenic females at E10.5, E12.5, E14.5, E16.5 and E18.5. Embryonic heads were partially dissected in ice-cold PBS (pH 7.4) and briefly fixed in 2% paraformaldehyde (PFA) in PBS on ice for thirty minutes. The ears were completely dissected and washed three times in fresh rinse buffer (100mM sodium phosphate pH7.3, 2mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40) and incubated for LacZ activity in staining solution (rinse buffer plus 5mM potassium ferricyanide, 5mM potassium ferrocyanide, and 1mg/ml X-Gal) in the dark overnight (16-20 hours) at room temperature. The samples were washed and post-fixed in 4% PFA , and further treated for whole mount or cryosection according to our standard protocol. To visualize the tissue structure, tissue sections were sometimes counterstained with eosin and mounted.
RESULTS
Wnt5a is expressed in a reciprocal pattern with a Wnt antagonist in the cochlea along the planar polarization axis
During mouse development, precursor cells giving rise to the organ of Corti withdraw from the cell cycle around E13.5-E14.5 (Chen and Segil, 1999; Ruben, 1967). Subsequently, hair cells start to differentiate in a gradient starting near the base of the cochlear duct at the IHC location and extending along the mediolateral and longitudinal axes (Fig. 1A). The development of stereocilia follows the differentiation gradient of hair cells. By E18.5, the organ of Corti is patterned along the entire length of the cochlea into four rows of hair cells. The polarization of stereocilia along the mediolateral axis is readily recognizable in the base to the middle region of the cochlear duct by E18.5.
Since Wnts are implicated in the orientation of stereocilia in vitro but Wnt7a does not appear to be essential for PCP regulation in vivo (Dabdoub et al., 2003), we sought to determine whether other Wnts are involved in planar polarization of the cochlea. We found transcripts for several Wnt molecules in the cochlear epithelium from E14.5 to E18.5 (data not shown). In particular, we detected the expression of Wnt5a at E14.5 near the base in the region medial to the p27/Kip1-expressing domain that demarcates the developing organ of Corti in the cochlear epithelium (Fig. 1C,D) (Chen and Segil, 1999). By E16.5, Wnt5a is expressed in the region medial to the organ of Corti from the base to the apex of the cochlea (Fig.1E,F).
A family of secreted Fz-related proteins, or Sfrps, shares the extracellular Wnt-binding domain of Fz. Sfrps can bind Wnts and sequester Wnts from Fz receptors, thus acting as Wnt antagonists (Rattner et al., 1997; Wang et al., 1997). In the cochlear epithelium, Sfrp3, also known as Frzb, is expressed in the region lateral to the developing organ of Corti when Wnt5a is expressed in the region medial to the organ of Corti (Fig. 1G-I). Therefore, a Wnt antagonist is expressed in a reciprocal pattern with Wnt5a along the mediolateral axis. In addition, the onset of Wnt5a expression also displays a basal-to-apical polarity along the longitudinal axis of the cochlear duct (Fig. 1).
Wnt5a and Frzb interact in vitro for stereociliary bundle orientation and cochlear extension
Wnt molecules upon binding to Fz can activate the so-called canonical signaling pathway, which culminates in the formation of nuclear β-catenin/LEF/TCF transcriptional complexes that activate expression of target genes (Logan and Nusse, 2004). We therefore examined the status of canonical Wnt signaling in the cochlea at the time of Wnt5a expression using BAT-gal reporter mice, where LacZ expression is under the control of LEF/TCF binding sites (Maretto et al., 2003). No canonical Wnt activity was detected in the cochlea at the same stage that Wnt5a is expressed in wild-type cochleae (supplement Fig.1), suggesting that Wnt5a expressed in the cochlea might function in a fashion that is independent of β-catenin-mediated transcriptional activation.
We next used organ culture to test whether Wnt5a and Frzb have any effect on the orientation of stereocilia or cochlear extension (Figs. 2,3). We isolated cochlear ducts at E14.5 and cultured the intact cochlear ducts or bisected cochlear ducts on polyD-lysine-and fibronectin-coated cover glass for 4-6 days in vitro (DIV). Under this condition, the organ of Corti will differentiate and achieve nearly normal PCP (Fig. 2A)(Wang et al., 2005). The addition of Frzb affected the polarization of stereocilia in a dose-dependent manner (Fig. 2A-D). This effect was suppressed by pre-incubation of Frzb with molar ratios of Wnt5a in Wnt5a-conditioned medium for 20 minutes before addition to the cultures (CM) (Fig. 2C,D).
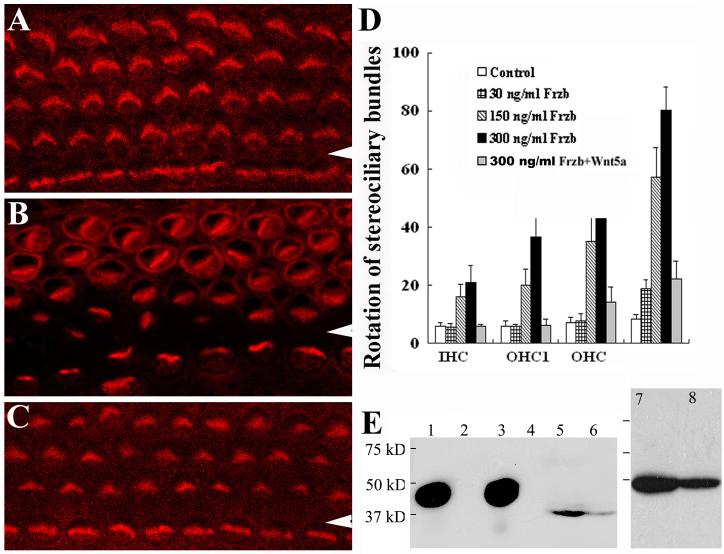
Fig. 2. Wnt5a and Frzb interact in vitro for stereociliary bundle orientation.
(A-D): The cochleae from wild-type E14.5 embryos undergo differentiation in culture. Stereocilia were normally oriented in the control medium as shown by phalloidin staining (A). The addition of Frzb to the medium affected stereociliary bundle orientation (B), but this effect was suppressed by pre-incubation with Wnt5a (C). Arrowheads indicate the pillar cell region that separates the IHCs from OHCs. Stereociliary bundle misorientation in the culture was quantified (D). The OHC3 and additional rows of OHCs in culture are quantified as a single group, OHC3+. The effect of Frzb on stereociliary bundle orientation ([Frzb]> 150 ng/ml, p<0.001) and the rescue by Wnt5a pre-incubation (p<0.000) were statistically significant. (E): Western blots with an antibody against Wnt5a. Lane 1: purified recombinant Wnt5a (R&D); lanes 2 and 4: media from the control cell line; lane 3: media from Wnt5a-expressing cells (Wnt5a-CM); lanes 5, 6, 8: cochlear extracts; lane 7: brain extracts.
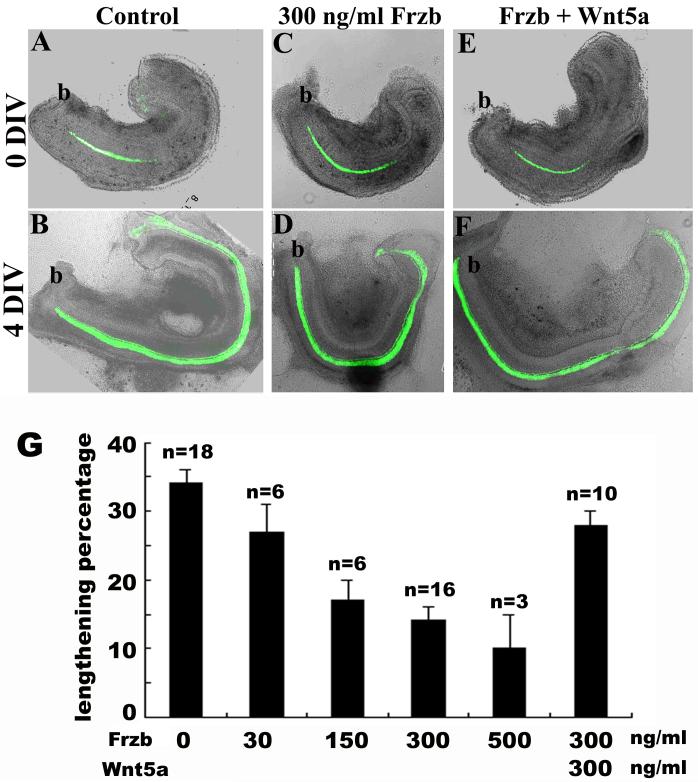
Fig. 3. Wnt5a suppressed the effect of Frzb on cochlear extension in vitro.
(A-F): The extension of the wild-type cochlea in culture was reduced in the presence of Frzb (A-D). This reduced extension was partially suppressed by pre-incubation with Wnt5a (E,F). Green signals are GFP expressed under the control of Math1 enhancers marking the hair cells. At the beginning of culture, only a single row of IHCs were differentiated near the base of the cochlea (A, C, E). b: base of the cochlea.
(G): Quantification of cochlear extension in vitro. The effect of Frzb on cochlear extension ([Frzb]>150 ng/ml, p<0.001) and the rescue by Wnt5a pre-incubation (p=0.001) were statistically significant.
Parallel to the association of cochlear extension and terminal differentiation of the organ of Corti in vivo, bisected wild-type cochleae isolated at E14.5 undergo extensive lengthening in the culture ( Fig. 3A,B) (Wang et al., 2005). The addition of Frzb affected cochlear extension (Fig. 3C,D), and pre-incubation with Wnt5a partially reversed this effect (Fig. 3E-G).
Together, these results suggest that excess Frzb affects stereociliary bundle orientation and cochlear extension. Since Wnt5a can suppress the effect of excess Frzb on stereociliary bundle orientation and cochlear extension, the endogenous pathway modulated by Frzb likely involves its Wnt-binding domain.
Interestingly, however, the effect of Wnt5a-CM alone appeared less specific. No effect was observed at similar concentrations to Frzb, while gross defects in the development of stereocilia were often seen at higher concentrations (>1.5 mg/ml) (data not shown), possibly due to non-optimized culture media containing Wnt5a-CM. This may suggest that Wnt5a, if involved, plays mainly a permissive function in stereociliary bundle orientation in vitro. Alternative, the Wnt5a protein present in the conditioned medium may be in a different biological form compared with the endogenous Wnt5a protein from cochlea (Fig. 2E) and brain (data not shown). The latter notion is supported by our observation of different mobility on SDS gel electrophoresis of the recombinant Wnt5a and the endogenous Wnt5a proteins from mouse brain and cochlea (Fig. 2E). The recombinant Wnt5a protein migrates as around 50 kD (Fig. 2E, lanes 1 and 3), as reported previously (Mikels and Nusse, 2006). However, the endogenous Wnt5a protein from mouse brain and cochlea migrates around 37 kD (Fig. 2E, lanes 5-8). Therefore, it is possible that the Wnt5a in the conditioned medium is capable of binding to Frzb while incapable of activating downstream pathways involved in regulating cochlear extension and stereociliary bundle orientation. An additional implication of this possibility is that the binding of Wnt5a in Wnt5a-CM to endogenous Frzb, or the loss-of-function of Frzb in vitro, does not affect the orientation of stereocilia.
Ablation of Wnt5a leads to characteristic CE defects in the cochlea
We next analyzed the function of Wnt5a in PCP regulation in vivo. PCP mutants exhibit a fully opened neural tube (craniorachischisis), misoriented stereocilia, and cochlear phenotypes characteristic of CE defects, including a shortened and widened cochlear duct (Curtin et al., 2003; Lu et al., 2004; Montcouquiol et al., 2003; Wang et al., 2005; Wang et al., 2006b). In particular, the apical region of the organ of Corti includes additional rows of sensory hair cells in these PCP mutants (Montcouquiol et al., 2003; Wang et al., 2005).
We examined cochleae isolated from Wnt5a−/−, Wnt5a+/−, and wild-type littermates (Yamaguchi et al., 1999). In gross appearance, the inner ears of Wnt5a−/− animals differed slightly from those of control littermates. The normal curvature at the base of the cochlea was not apparent in Wnt5a−/− animals and the hook region constituting the curvature at the base of the cochlear duct was shortened (Fig. 4A,B). The cochlear ducts from about 35% of Wnt5a−/− animals (n = 34) were shortened and the apical tip of the cochlear duct was bent (Fig. 4A,B). The organs of Corti in these 35% of Wnt5a−/− animals that had shortened cochlear ducts (n=34) had additional rows of hair cells along the entire length of the cochlear duct (Fig. 4C,D) in comparison to control samples (Fig. 4E,F). The widening of the organ of Corti was especially prominent in the apical half of the cochlear duct (Fig. 4C,D). One Wnt5a−/− animal also had craniorachischisis observed in all known mammalian PCP mutants (Curtin et al., 2003; Lu et al., 2004; Montcouquiol et al., 2003; Wang et al., 2005; Wang et al., 2006b), while three had the exencephaly phenotype (not shown). In the remaining 65% of Wnt5a−/− animals, the cochlear duct was slightly shortened and a discontinuous additional row of outer hair cells was observed (data not shown).
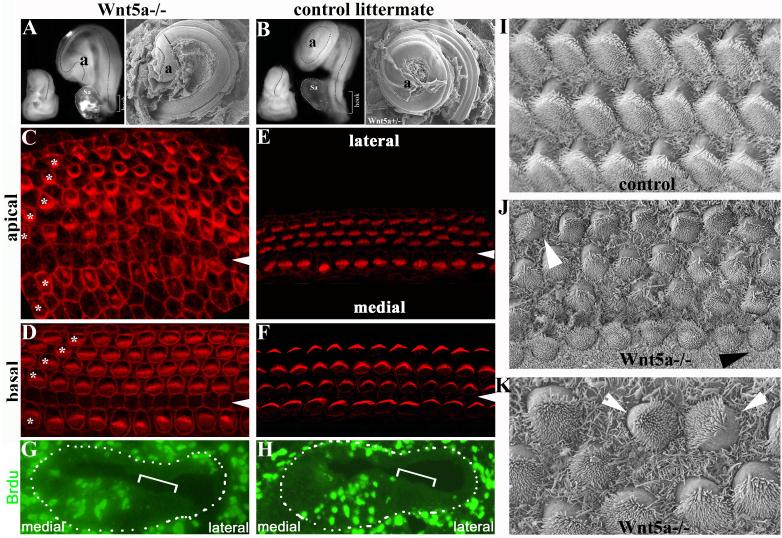
Fig. 4. Wnt5a null animals show characteristic CE defects in the cochlea.
(A-B): Cochleae from E18.5 Wnt5a−/− embryos (A) were shorter than those from control littermates (B) as shown in both dissection (left) and SEM (right) images. Dashes outline the curvatures of the cochlear ducts. Sa: vestibular saccule attached to the base of the cochlea.
(C-F) The organ of Corti from an E18.5 Wnt5a−/− embryo (C-D) stained with phalloidin showed additional rows of OHCs in both basal and apical regions compared with a control littermate (E,F). Asterisks mark each row of OHCs in the Wnt5a−/− sample. Arrowheads indicate the pillar cell region. The confocal plane for the Wnt5a−/− sample was close to the apical cortex of the cells in the organ of Corti, and therefore the phalloidin staining for cell-cell junctions in the Wnt5a−/− sample (C,D) appeared to be more intense than the staining in the control sample (E,F).
(G-H): BrdU-injected E18.5 cochlear sections from Wnt5a−/− (G) and control (H, Wnt5a+/−) littermates stained with BrdU antibody (green) to reveal dividing cells. Brackets indicate the organ of Corti. Dashes dots outline the cochlear ducts. No statistic difference in the number of BrdU+ cells was detected in comparable cochlear sections from Wnt5a−/− and control embryos.
(I-K): In contrast to normal polarity of the stereocilia across the organ of Corti in the control littermate (I), the organ of Corti from Wnt5a−/− animals showed limited misorientation of stereocilia with varying degrees (J, K). The white arrowheads indicate a rotated outer hair cell (J) and a pair of outer hair cells facing each other (K). The black arrowhead indicates a rotated inner hair cell (J).
Wnt5a−/− animals show a general reduction in outgrowth of diverse embryonic structures whose development requires extension from the primary body axis (Yamaguchi et al., 1999). The embryos were truncated caudally including a loss of tail and significant shortening of the embryonic anterior-to-posterior axis, the snout, and manidible and tongue were truncated, reduced outgrowth of the external ear was apparent, both fore- and hindlimbs were shortened along the proximal-to-distal axis, and genital tubercle was absent (Yamaguchi et al., 1999). The outgrowth defects in Wnt5a−/− animals have been attributed partly to proliferation defects in these tissues (Yamaguchi et al., 1999). The expression of Wnt5a in the cochlea medial to the developing organ of Corti is detected at E14.5, when cells in the organ of Corti have withdrawn from the cell cycle, but the cells medial to the organ of Corti are still dividing at this stage and will undergo a few further rounds of cell proliferation (Fig. 4G, H) (Chen and Segil, 1999; Ruben, 1967). To determine whether loss of Wnt5a affects cell proliferation in regions that undergo active cell division after the expression of Wnt5a is detected at E14.5, we administered BrdU to pregnant females three times at E14.5, and then compared cell proliferation in Wnt5a−/− and wild-type embryos (Fig. 4G,H). We observed comparable numbers of BrdUpositive cells in each cochlear section from Wnt5a−/− animals and their control littermates. Collectively, the widening of the organ of Corti and the cochlear duct, the concomitant shortening of the cochlear duct itself, and the apparently normal cell proliferation in affected regions indicate a CE defect in Wnt5a−/− animals.
Wnt5a genetically interacts with Ltap/Vangl2 to regulate stereociliary bundle orientation, cochlear length, and neural tube closure
In contrast to 35% penetrance of shortened and widened cochleae in Wnt5a−/− animals, hair cell stereociliary bundles in Wnt5a−/− mice only showed occasional imperfect alignments (Fig. 4I-K). To explore a potential function of Wnt5a in stereociliary bundle orientation and to define its role in CE, we generated mice carrying mutant alleles for both Wnt5a and Ltap/Vangl2 (Kibar et al., 2001; Montcouquiol et al., 2003) (Figs. 5,6).
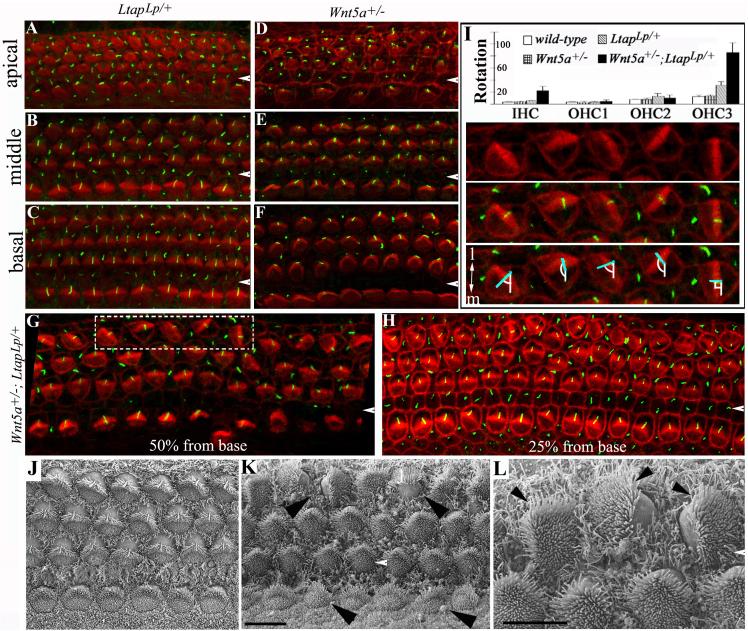
Fig. 5. Genetic interaction of Wnt5a and Ltap/Vangl2 in establishing uniform orientation of stereocilia.
(A-H): The cochlear ducts from E18.5 LtapLp/+ (A-C), Wnt5a+/− (D-F), and Wnt5a+/−; LtapLp/+ (G-H) littermates stained with phalloidin (red) and acetylated α-tubulin (green). The acetylated α-tubulin antibody labels the kinocilia located at the vertices of the stereocilia and phalloidin labels actin in stereocilia. In contrast to the heterozygous LtapLp/+ (A-C) and Wnt5a+/− (D-F) embryos, the Wnt5a+/−; LtapLp/+ double-heterozygous littermates (G-H) had misoriented stereocilia in the third row of OHCs. The white arrowheads indicate the pillar cell region separating the IHCs from OHCs.
(I): The orientation of stereocilia in Wnt5a+/−; LtapLp/+ (n= 16) mice was quantified and compared to that of LtapLp/+ (n = 4), Wnt5a+/− (n = 4) and wild-type (n = 4) animals. The quantification of stereociliary orientation (I) is illustrated by hair cells from the dashed box in (G), which is shown in the panels below the histogram in (I) with the top panel showing phalloidin labeling alone, the middle panel showing phalloidin plus acetylated atubulin, and bottom panel showing the measurement of the rotation. The angle formed between the mediolateral axis (I, bottom panel, white line) and the line bisecting the “V”-shaped stereocilia (I, bottom panel, blue line) was measured and plotted in the histogram.
(J-L): SEM images of the organ of Corti from E17.5 LtapLp/+ (J) and Wnt5a+/−; LtapLp/+ (K-L) embryos. Arrowheads in (K) mark two OHCs with an abnormal 90° or 180° rotation and two IHCs with minor degrees of rotation. The misalignment of stereocilia (L, arrowheads) is more clearly seen at high magnification (L, higher magnification of the top left corner of K). Scale bars: 10 μm (J-K) and 6 μm (L).
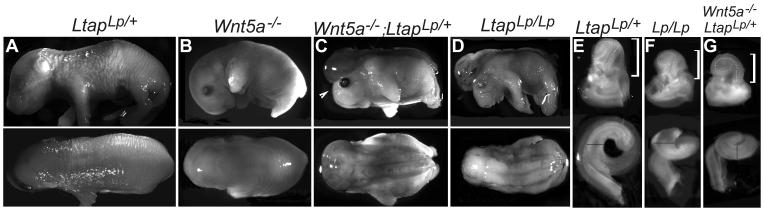
Fig. 6. Genetic interaction of Wnt5a and Ltap for neurulation and cochlear lengthening.
(A-D): LtapLp/+ (n>100) (A) and Wnt5a−/− (B, 95%, n = 26) mice had normal neural tube closure. Wnt5a−/−;LtapLp/+ (C, 100%, n = 4) and LtapLp/Lp embryos (D, 100%) show craniorachischisis observed in all the known PCP mutants. The arrowhead in (C) indicates the open- eyelid phenotype observed in several PCP mutants. (E-G): Inner ears and dissected cochlear ducts (below) from LtapLp/+ (E), LtapLp/Lp (F) and Wnt5a−/−;LtapLp/+ embryos (G). The brackets indicate the cochlear portion. The lines were drawn from the medial to the lateral sides of the cochlear ducts in the apical region to mark the width of the cochlear ducts.
Mice that are homozygous for a loss-of-function point mutation in Ltap, the looptail (LtapLp) mutation, showed misoriented stereocilia and a shortened, widened cochlear duct, as well as craniorachischisis (Montcouquiol et al., 2003). Heterozygous LtapLp/+ mice had normal stereociliary bundle orientation (Fig. 5A-C,I,J). In double-heterozygous Wnt5a+/−; LtapLp/+ mice, the third row of OHCs displayed high frequency of stereociliary bundle misorientation in the basal region, and almost all bundles were misorientation in the middle to apical region (Fig. 5G-I, K,L). The IHCs in Wnt5a+/−; LtapLp/+ double-heterozygous animals also showed some degree of stereociliary bundle misorientation (Fig. 5I, K).
In addition to the inner ear stereociliary bundle orientation phenotype, Wnt5a+/−; LtapLp/+ heterozygous mice displayed drastically decreased weight gain after birth; most of them died within one week. One Wnt5a+/−; LtapLp/+ heterozygous male survived and produced 16 litters, or 131 live embryos with Wnt5a+/− females. Of these embryos, four were Wnt5a−/−;LtapLp/+. All four exhibited craniorachischisis (Fig. 6), presenting a drastic increase in penetrance as compared to the craniorachischisis phenotype displayed by Wnt5a−/− (1 in 34) or LtapLp/+ animals (0 in more than 100). The inner ears of these Wnt5a−/−;LtapLp/+ embryos were similar to those of LtapLp/Lp PCP mutants and had shortened, widened cochlear ducts (Fig. 6).
These observations indicate that Wnt5a genetically interacts with the PCP gene Ltap/Vangl2. The PCP-specific phenotypes in the cochlea of Wnt5a+/−; LtapLp/+ double-heterozygous animals and in the neural tube of Wnt5a−/−;LtapLp/+ animals further indicate that Wnt5a plays a role in PCP signaling in mice.
Ltap distribution in the cochlea is abnormal in Wnt5a−/− mice
The hallmark of planar polarization for CE and establishment of epithelial PCP is polarized localization of core PCP proteins. We generated BACLtap-GFP mice through BAC-mediated transgenesis to examine the subcellular localization of Ltap/Vangl2 (Yang et al., 1997). BACLtap-GFP mice carry a transgene in which the coding sequence for GFP was inserted before the stop codon of the Ltap gene.
The subcellular localization of Ltap-GFP in wild-type mice was consistent with immunostaining using an antibody against Ltap/Vangl2 (Montcouquiol et al., 2006). In the wild-type cochlea at E18.5, Ltap-GFP was localized to the junctions between hair cells and supporting cells, and apparently appears on the medial side of hair cells (Fig. 7A,B). In Wnt5a−/− littermates, cellular geometries and contacts were altered in the apical region of the cochlea (Fig. 7C,D) where strong CE defects were observed (Fig. 4). In the apical region, Ltap-GFP was localized to the junctions between supporting cells and the polarized subcellular localization to the medial sides of hair cells was absent (Fig. 7C,D). In the basal region of the cochlear duct, Ltap-GFP showed a more normal distribution, being largely localized to the medial side of hair cells in Wnt5a−/− mice, although some abnormal localization of Ltap-GFP to the lateral side of hair cells was also observed (Fig. 7E,F). Therefore, the subcellular distribution of Ltap in the cochlea was affected by the absence of Wnt5a, particularly in the apical region. The strong spatial correlation between the extent of abnormalities in Ltap localization and cellular geometry and the extent of CE defects suggests possible cellular mechanisms that underlie the CE defects in the cochlea of Wnt5a−/− mice (Blankenship et al., 2006; Classen et al., 2005; Hyodo-Miura et al., 2006).
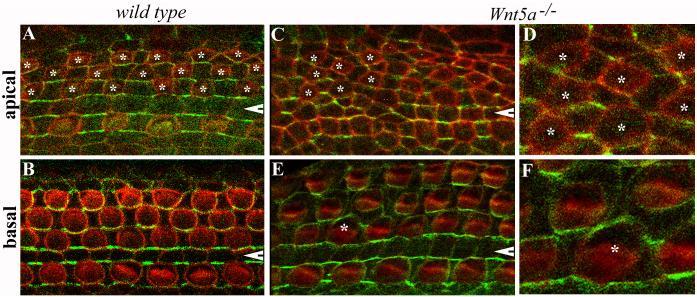
Fig. 7. Aberrant cellular geometries and distribution of Ltap in Wnt5a−/− mice.
(A-F): Whole mount of the organ of Corti from E18.5 wild-type (A-B) and Wnt5a−/− (C-F) mice carrying BACLtap-GFP. Hair cells were visualized by phalloidin staining (red). Ltap-GFP (green) was seen at the medial boundaries between hair cells and supporting cells in wild-type samples (A, B). In Wnt5a−/− mice, the localization of Ltap-GFP in the base region appeared normal on the medial side of hair cells (E), except around one hair cell (E, marked by an asterisk). In the apical region, cellular geometry was altered in Wnt5a−/− mice, and so was the localization of Ltap-GFP (C). OHCs in the apical region are marked with asterisks (A, C). White arrowheads indicate the pillar cell region. (D) and (F) are higher magnification images for the regions where hair cells are marked by asterisks in (C) and (E), respectively.
Mediolateral instructive cues for PCP in the cochlea
The expression and functional studies of Wnt5a (Figs. 1-7) strongly supports a role for Wnt5a in PCP regulation in mice. However, it is not clear whether there are instructive cues for PCP in the cochlea and, if so, whether Wnt5a contributes to such instructive cues. To address these issues, we grafted tissues in the organ culture to test whether instructive cues are present along the mediolateral axis of the cochlea for the orientation of stereociliary bundles (Fig. 8).
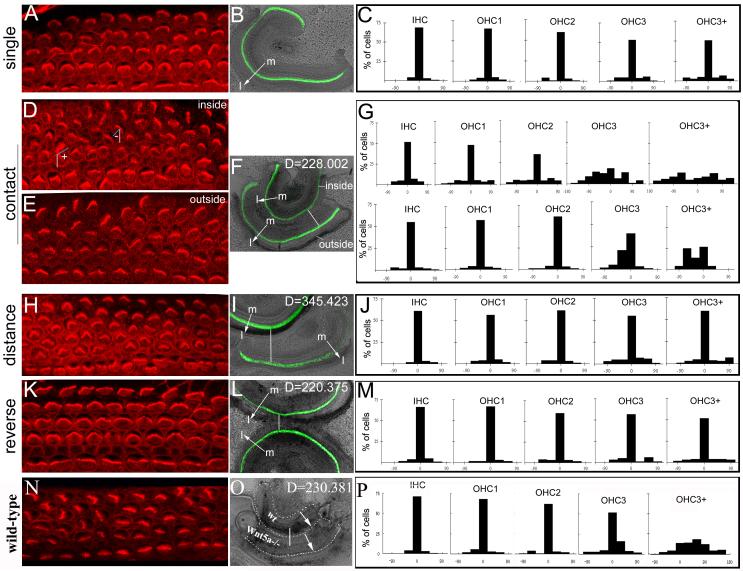
Fig. 8. Potential instructive cues along the mediolateral axis of the cochlea.
(A-P): The orientation of stereociliary bundles was visualized with phalloidin staining (A,D,E,H,K,N) in wild-type cochleae cultured singly (A,B), in parallel closely (D-F), in parallel distantly (H,I), in anti-parallel (K,L), and between closely parallel Wnt5a−/− and wild-type cochleae (N,P) The distribution of stereociliary bundle orientation in each row of hair cells cochleae cultured singly (C, n=11), in closely parallel (G, n=4), in distantly parallel (J, n=4), in anti-parallel (M, n=4), and between Wnt5a−/− and wild-type cochleae (P, N=3) was quantified and plotted on the histograms. The angles formed between the mediolateral axis (D, white lines) and the lines bisecting the “V”-shaped stereocilia (D, blue lines) were used for quantifications as described in Methods. This angle is close to 0 in normally oriented stereocilia. As diagramed in (D), the angles formed at the right or the left sides of the mediolateral axis were designated to be positive or negative, respectively. The lines (F,I,L,O) and arrows (B,F,I,L,O) indicate the distance between the two cochleae and the orientation of the mediolateral axes of the cochleae, respectively. The green signal (B, F, I, L) is from GFP expressed under Math1 enhancers and marks the hair cells. In the grafts between Wnt5a−/− and wild-type cochleae (O), GFP was not present and the cochleae were outlined by dashed lines. D: units of the distance between the two cochleae cultured together using NIH ImageJ (F, I, L,O); m: the medial side of the cochlea; l: the lateral side of the cochlea.
As we showed previously (Fig. 2) (Wang et al., 2005), the cochleae cultured singly showed normal PCP (Fig. 8A-C). When two cochleae were cultured together in a parallel manner so that the medial side of one cochlea (outside) was grafted to the lateral side of the second cochlea (inside), the orientation of stereociliary bundles in the outer rows of OHCs in the inside cochlea that were the nearest to the medial region of the outside cochlea was significantly altered (Fig. 8D,F,G). The orientation of stereociliary bundles of the outer rows of OHCs in the outside cochlea showed a small alteration (Fig. 8E-G).
To further test whether the effect on the orientation of stereociliary bundles of the outer rows of OHCs is due to the intrinsic properties of the cochlear tissue grafted, we increased the distance between the two cochleae in parallel cultures (Fig. 8H-J), as well as reversed the relative orientation of the two cochleae so that the mediolateral axes of the two cochleae grafted together were anti-parallel (Fig. 8K-M). When the distance between the two cochleae in parallel cultures was increased, the orientation of stereociliary bundles in both the inside and outside cochleae was normal (Fig. 8H-J, and data not shown). In contrast to the parallel cultures (Fig. 8D-G), the orientation of stereociliary bundles was not altered even in regions where the distance between the two cochleae was close in the anti-parallel cultures where the mediolateral axes of the two cochleae were reversed and the lateral sides of the two cochleae were placed next to each other (Fig. 8K-M, and data not shown). These data together indicates that there is potentially an intrinsic difference between the medial and lateral regions of the cochlear epithelium for directing the orientation of stereociliary bundles and supports the presence of instructive cues along the mediolateral axis of the cochlear epithelium for establishing PCP in the outer rows of OHCs.
However, we could not determine the contribution of Wnt5a to the difference between the medial and lateral tissues of the cochlea for PCP in vitro. We grafted the medial region of cochleae from Wnt5a−/− animals to the lateral side of wild-type cochleae (Fig. 8N-P). No statistically significant difference was observed between cultures of Wnt5a−/− and wild-type grafts (Fig. 8N-P) and wild-type and wild-type grafts in the same experiments. This observation is consistent with the low penetrance of stereociliary misorientation in Wnt5a−/− mice and suggests the presence of redundant pathways for Wnt5a function in PCP signaling.
DISCUSSION
In this study, we demonstrate that Wnt5a plays a role in mice in two cellular processes regulated by the PCP pathway, establishment of epithelial PCP and CE.
We found a reciprocal expression pattern of Wnt5a and Frzb along the axis for planar polarization in the cochlea at the time that PCP is being established (Fig. 1). We showed that Wnt5a suppresses the effect of Frzb on stereociliary bundle orientation in vitro (Fig.2). We further observed imperfect stereociliary bundle alignments in Wnt5a−/− animals (Fig. 4) and a genetic interaction between Wnt5a and Ltap/Vangl2 in regulating stereociliary bundle orientation (Fig. 5). Together, these observations indicate that Wnt5a contribute to the establishment of uniform bundle orientation.
Although Frzb addition has a strong effect on PCP in vitro, only minor imperfection in the alignment of stereocilia in Wnt5a−/− mice was observed. This observation suggests that additional pathways parallel or redundant to Wnt5a are involved in PCP regulation in the cochlea. The presence of redundant pathways made it difficult to determine the molecular mechanism underlying the role of Wnt5a in PCP signaling in mice. It is not clear whether the reciprocal expression of Wnt5a and Frzb along the axis for planar polarization is involved in generating a graded Wnt signal to direct the establishment of PCP in the cochlea. The lack of demonstrated effectiveness of Wnt5a-CM on stereociliary orientation further prevented us from reversing the source of Wnt5a in vitro to test a possible instructive role for Wnt5a. However, the data from our grafted organ cultures (Fig. 8) indicates an intrinsic difference between the medial and lateral regions of the cochlear epithelium for PCP and supports the presence of instructive cues along the mediolateral axis of the cochlear epithelium.
The composition of potential mediolateral instructive cues, however, remains unknown. The putative compensatory pathways for Wnt5a may include additional Wnts (Colosimo and Tolwinski, 2006; Dabdoub et al., 2003; Price et al., 2006) and Hh (Colosimo and Tolwinski, 2006). BMPs might also contribute to PCP regulation in the cochlea, as BMP signaling can be modulated by a secreted frizzled molecule (Lee et al., 2006; Muraoka et al., 2006) and BMPs are expressed asymmetrically along the mediolateral axis of the cochlear epithelium (Morsli et al., 1998; Takemura et al., 1996). Future studies towards the understanding of additional pathways and the generation of genetic tools to interrupt or reverse the presence of Wnts and Wnt antagonists in vivo will be critical to determine the molecular role of Wnts in PCP regulation.
CE consists of cellular intercalations along the mediolateral axis and extension along the perpendicular longitudinal axis. In Xenopus and zebrafish, Wnt5 and Wnt11 are required for CE. However, their expression pattern is not consistent with an instructive but rather a permissive role (Heisenberg et al., 2000; Kilian et al., 2003; Smith et al., 2000; Tada and Smith, 2000; Ulrich et al., 2003). In the cochlea, the expression of Wnt5a is asymmetric along the mediolateral and the longitudinal axes (Fig. 1) and is appropriate to serve as a cue for CE along both axes. The effect of Frzb on cochlear extension in vitro and the suppression of this effect by Wnt5a provided the first hint that Wnts may be involved in CE in mammals (Fig. 3). Our observation of craniorachischisis and shortened and widened cochleae in some Wnt5a−/− animals (Fig. 4, and data not shown), and the genetic interaction between Wnt5a and Ltap/Vangl2 in enhancing the penetrance of cochlear CE and neural tube closure (Figs. 5,6) further supported a role for Wnt5a in the PCP pathway for CE regulation.
An intriguing question is how the mammalian PCP pathway concurrently regulates the establishment of PCP and CE in the cochlea during terminal differentiation. In Wnt5a mutants, phenotypes from the two processes show different penetrance. The cochlear CE defect has a higher penetrance than the stereociliary defect in Wnt5a−/− animals (Fig. 4), while Wnt5a+/−; LtapLp/+ double heterozygous animals have a 100% penetrant stereociliary orientation defect in the third row of OHCs but no apparent cochlear CE defect (Fig. 7). The dissociation of cochlear CE and stereocilia orientation defects in some Wnt5a mutants suggests that the molecular mechanisms underlying CE and stereociliary bundle orientation are not identical.
Wnts can trigger both canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) downstream pathways. No canonical Wnt activity was detected in the cochlea during terminal differentiation (supplemental Fig. 1), making it unlikely that Wnt5a acts via a β-catenin-dependent pathway. This said, it remains possible that canonical activity occurs at a level below the detection threshold with the BAT-gal reporter. However, our data is consistent with the current view on the modulation of the specificity of downstream Wnt pathways by the context of intracellular factors and receptor. Candidate vertebrate cytoplasmic PCP proteins have been shown to promote the PCP pathway while inhibiting the canonical Wnt pathway (Moeller et al., 2006; Schwarz-Romond et al., 2002; Simons et al., 2005). For example, a candidate PCP gene, Ankrd6/Diversin, inhibits canonical Wnt activity while promoting PCP signaling (Moeller et al., 2006; Schwarz-Romond et al., 2002). The presence of specific Fz receptors in the cochlea may further direct Wnts in the cochlea to act via a noncanonical pathway. Fz3 and Fz6 are expressed in the developing organ of Corti and are redundantly required for uniform stereociliary orientation (Wang et al., 2006b). However, Fz3 and Fz6 fail to mediate the activation of the canonical Wnt pathway in vitro when Wnt5a is juxtaposed with a domain enabling its binding to LRP5/6, the coreceptor required for the canonical Wnt pathway (Liu et al., 2005). Our study now provides the first complementary genetic evidence to demonstrate functional involvement of Wnt5a in the PCP signaling, a noncanonical Wnt pathway, in mammals. While additional studies are clearly needed to further dissect the exact molecular role and the signaling cascade downstream of Wnt5a in PCP regulation, it is tempting to speculate that an intricate modulation or suppression of canonical Wnt signaling by multiple components of the PCP pathway may have evolved in the vertebrates to divert Wnts to PCP signaling.
Supplementary Material
Supplemental Fig. 1. Lack of canonical Wnt activity in the cochlea upon expression of Wnt5a.
(A-E): LacZ activity in BAT-gal mice was limited to the vestibule and was not seen in the cochlea in the developing inner ear at E10.5 (A), E12.5 (B), E15.5 (C) and E18.5 (D-E).
(F): LacZ activity was readily detectable in the cochlea upon induced ubiquitous expression of β-galactosidase.
co: cochlea; CVG: cochleovestibular ganglion; cd: cochlear duct; sa: saccule of vestibule; ut: utricle of vestibule; cr: crista of vestibule. The blue dashes outline the otocyst (A) and the cochlear duct (B). The dashed lines separate the cochlea from the vestibule (B-C).
Brackets indicate the organ of Corti (D, F-I).
Acknowledgments
We thank Kristen Radde-Gallwitz and Shuanding (Amy) Li for assistance with in-situ hybridization, animal genotyping, and cryosection preparation; S. Piccolo at the University of Padua for BAT-gal animals; Jane E. Johnson at the University of Texas Southwestern for Math1GFP mice; Catherine Rhéaume at the University of California Irvine for preparation of BAT-gal samples; Andreas Fritz, Win Sale and Douglas Falls at Emory University for critical comments on the manuscript; and Ken Moberg at Emory University for helpful discussion on the interpretation of results. This work is supported by grants from the US National Institute of Health (to X.D. and P.C.), a training grant from the US National Institute of Health (to C. J.), the Woodruff Foundation (to P.C.), and the Wellcome Trust (K.P.S. and A.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–6. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–22. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–30. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–70. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–17. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Colosimo PF, Tolwinski NS. Wnt, Hedgehog and Junctional Armadillo/beta-Catenin Establish Planar Polarity in the Drosophila Embryo. PLoS ONE. 2006;1:e9. doi: 10.1371/journal.pone.0000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–84. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- Davies A, Formstone C, Mason I, Lewis J. Planar polarity of hair cells in the chick inner ear is correlated with polarized distribution of c-flamingo-1 protein. Dev Dyn. 2005;233:998–1005. doi: 10.1002/dvdy.20376. [DOI] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hyodo-Miura J, Yamamoto TS, Hyodo AC, Iemura S, Kusakabe M, Nishida E, Natsume T, Ueno N. XGAP, an ArfGAP, is required for polarized localization of PAR proteins and cell polarity in Xenopus gastrulation. Dev Cell. 2006;11:69–79. doi: 10.1016/j.devcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–76. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–76. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–59. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Bafico A, Aaronson SA. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Mol Cell Biol. 2005;25:3475–82. doi: 10.1128/MCB.25.9.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–95. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–12. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. A GAP in convergent extension scores PAR. Dev Cell. 2006;11:2–4. doi: 10.1016/j.devcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Moeller H, Jenny A, Schaeffer HJ, Schwarz-Romond T, Mlodzik M, Hammerschmidt M, Birchmeier W. Diversin regulates heart formation and gastrulation movements in development. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–35. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–38. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- Price MH, Roberts DM, McCartney BM, Jezuit E, Peifer M. Cytoskeletal dynamics and cell signaling during planar polarity establishment in the Drosophila embryonic denticle. J Cell Sci. 2006;119:403–15. doi: 10.1242/jcs.02761. [DOI] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477:412–21. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–63. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;(Suppl 220):1–44. [PubMed] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–84. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Conlon FL, Saka Y, Tada M. Xwnt11 and the regulation of gastrulation in Xenopus. Philos Trans R Soc Lond B Biol Sci. 2000;355:923–30. doi: 10.1098/rstb.2000.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–27. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Takemura T, Sakagami M, Takebayashi K, Umemoto M, Nakase T, Takaoka K, Kubo T, Kitamura Y, Nomura S. Localization of bone morphogenetic protein-4 messenger RNA in developing mouse cochlea. Hear Res. 1996;95:26–32. doi: 10.1016/0378-5955(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–64. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–24. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, Roehl H, Tada M, Wilson SW, Adams RJ, Soll DR, Heisenberg CP. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development. 2003;130:5375–84. doi: 10.1242/dev.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–4. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–5. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006a;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–66. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006b;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–23. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol. 1997;15:859–65. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–6. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Lack of canonical Wnt activity in the cochlea upon expression of Wnt5a.
(A-E): LacZ activity in BAT-gal mice was limited to the vestibule and was not seen in the cochlea in the developing inner ear at E10.5 (A), E12.5 (B), E15.5 (C) and E18.5 (D-E).
(F): LacZ activity was readily detectable in the cochlea upon induced ubiquitous expression of β-galactosidase.
co: cochlea; CVG: cochleovestibular ganglion; cd: cochlear duct; sa: saccule of vestibule; ut: utricle of vestibule; cr: crista of vestibule. The blue dashes outline the otocyst (A) and the cochlear duct (B). The dashed lines separate the cochlea from the vestibule (B-C).
Brackets indicate the organ of Corti (D, F-I).