Abstract
Background
Abnormalities in language processing and the related brain structures have been reported in people with schizophrenia. It has been proposed that the brain pathways for language processing are anomalous in these individuals and form the underlying basis for the positive symptoms of the illness. If language pathway abnormalities can be detected early in people at high-risk for schizophrenia prior to the onset of symptoms, early treatment can ensue.
Methods
Fifteen young adults at high genetic risk for developing schizophrenia were compared with 15 of their siblings with schizophrenia or schizoaffective disorder and 15 age and sex matched individuals at low risk for schizophrenia using a visual lexical decision task during fMRI. The data were analyzed by contrasting activation obtained during a real word–pseudoword discrimination task to activation obtained during a nonlinguistic discrimination task, and the differential activations were examined.
Results
Patterns of brain activation while reading and discriminating between real and pseudowords differed across groups, with more bilateral activation in schizophrenia patients and their high-risk siblings than controls. In control subjects discrimination of words from psuedowords significantly activated Brodmann’s area 44 more strongly than when non-linguistic symbols were discriminated. However, high-risk subjects and their siblings with schizophrenia activated this region similarly for both language and non-language tasks.
Conclusions
Normal individuals can be distinguished from subjects at high genetic risk for schizophrenia and patients with schizophrenia by their more lateralized and stronger activation of Brodmann’s area 44 to word compared with symbol discrimination tasks. Thus, evaluation of language processing by fMRI may be a valuable tool for use in the prediction of individual risk for developing schizophrenia.
Keywords: fMRI, Language, Early detection, Prodrome, High-risk, Endophenotype
Introduction
Anomalies in language processing have been hypothesized to underlie the characteristic symptoms of schizophrenia (Crow, 1998; DeLisi, 2001). The brain anatomical pathways associating cortical regions for both perceptive and productive speech may be disrupted such that inner thoughts are perceived as auditory experiences and processed heard speech is distorted as the basis for delusional perceptions. Speech is sometimes produced in a disorganized set of sentences in severe cases such that meaningful content is lacking.
An extensive previous literature documents various abnormalities of linguistic function in schizophrenia (Chaika, 1990; reviewed in DeLisi, 2001). Some past epidemiological and clinical reports indicate that this may have resulted from an early developmental problem as evidenced from noted delays in language acquisition and reading abilities (e.g. Crow et al., 1995; DeLisi et al., 1991). Recent fMRI studies provoking activation with a language production paradigm in patients who already have the diagnosis of acute or chronic schizophrenia (Boksman et al., 2005; Kircher et al., 2001, 2005; Koeda et al., 2006; Kubicki et al., 2003; Sommer et al., 2001, 2003; Weiss et al., 2006) and in those during the prodromal stage prior to illness onset and/or at high-genetic risk for illness (Whalley et al., 2004, 2005, 2006) have shown disruption in the normal lateralized activation in the frontal and temporal cortical circuits for language processing and further evidence that this pattern is heritable (Sommer et al., 2004). Other studies, mostly focusing on activation during tasks engaging working memory (Callicott et al., 2003; Keshavan et al., 2002; Seidman et al., 2006; Thermenos et al., 2004), attentional processes (Morey et al., 2005) in the prefrontal cortex, or facial expression and amygdala response (Habel et al., 2004), have suggested that these functional changes also occur early on and could be vulnerability markers for the illness.
The current study is a further focus on language activation in subjects who are at high-risk for schizophrenia. We used a word/pseudoword discrimination task based on the tasks used in previously published studies that required either reading words silently or aloud (Binder et al., 2005; Mechelli et al., 2005; Paulesu et al., 2000; Xiao et al., 2005). This and similar tasks used in normal control individuals have been shown to consistently activate Brodmann’s area 44 and 45. Heim et al. (2005) recently demonstrated that the lexical decision task more strongly activated Brodmann’s area 44 and 45 than did a phonological decision task, and therefore we adopted a reading-only version of the lexical word/psuedoword discrimination task. Either the sentence completion task involving retrieval of appropriate words, as employed in the Whalley et al. studies noted above or a task focused on recognition of words as employed in this study could provide fMRI measures that may have future utility for early detection of schizophrenia and provide an understanding of the biological basis for why individuals are at high-risk for developing schizophrenia. To our knowledge, the study presented here is the first study to use a word discrimination task to examine individuals at high-risk for developing schizophrenia by fMRI.
2. Methods
2.1. Subjects
Subjects who were at high genetic risk for schizophrenia and age and sex matched subjects who were at low risk were recruited for clinical and MRI evaluations. Individuals were considered at high genetic risk if they originated from families in which at least one individual had a diagnosis of schizophrenia or schizoaffective disorder by DSM-IV criteria and they were still within the peak age of risk for schizophrenia (defined as ages 12–30; see review of age of onset by DeLisi, 1992).
Individuals were considered at low risk for schizophrenia and were eligible for participation if they had no family history of any psychotic disorder, psychiatric hospitalization or suicide in a first or second-degree relative. The low-risk controls were not included if on a structured interview evaluation they were found to have evidence of a psychotic illness (schizophrenia, bipolar disorder or psychosis not otherwise specified). Siblings of the individuals at high-risk for schizophrenia who had a diagnosis of schizophrenia or schizoaffective disorder were also recruited for comparison evaluations.
Recruitment of the high-risk cohort was possible by placing advertisements in newspapers and newsletters distributed by multiple chapters of The National Alliance for The Mentally Ill (NAMI). In addition, families who previously participated in other genetic studies on schizophrenia conducted by Dr. DeLisi were contacted for eligibility for the current study (DeLisi et al., 2002). Those individuals with schizophrenia thus came from these families or those newly recruited with a high-risk proband. Controls were solicited from the community by public advertisement.
A total of 45 subjects were included in this study and categorized into 3 diagnostic groups with 15 subjects in each group. Low-risk controls were age and sex matched as close as possible to the individuals at high-risk for illness (Table 1). All subjects were interviewed using the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994), information about them was obtained from a family member, and as appropriate, when available, medical records were obtained. A diagnosis was made using DSM-IV criteria. In addition, all subjects had verbal cognition tested during the preliminary evaluation and anyone with an IQ less than 85 was not included in the study. Two measures of verbal cognition were included in the current study (see Table 1), the Verbal Comprehension Index (VCI) from the Wechsler Intelligence Scales and the Wide Range Achievement Test-Reading (WRAT-Reading). Since only selected subtests from the full age appropriate Wechsler Scales (WAIS-III or WISC-IV; Wechsler, 1997, 2004) were administered, but not enough to calculate a Verbal IQ (FSIQ), the VCI was used. The VCI is a pure measure of Verbal IQ and includes the Information, Vocabulary, and Similarities subtests of the WAIS. The WRAT-Reading was used as a standardized measure that was close to the task used in the fMRI procedure (see below) and assesses differences in ability to decode a list of progressively harder words. One of the controls was unable to complete any of the expressive language tests due to a severe stutter that interfered with his speech and invalidated these data.
Table 1.
Subject characteristics analyzed by one-way ANOVA
| Low-risk controls (n=15) | High-risk subjects (n=15) | Siblings with schizophrenia (n=15) | F | df | P< | |
|---|---|---|---|---|---|---|
| Age | 23.9±5.2 | 21.7±6.1 | 35.3±8.8 | 16.141 | 2,42 | 0.000 |
| (range) | (16–35) | (13–30) | (20–55) | |||
| Male/female | 7/8 | 7/8 | 11/4 | 2.9 | 2,42 | 1.0 |
| Left/right handed | 1/14 | 1/14 | 1/14 | – | – | – |
| Education (years) | 14.2±2.21 | 12.6±2.75 | 14.5±2.20 | 0.815 | 2,42 | 0.606 |
| VCI** | 113.14±20.9 | 108.07±14.6 | 105.53±14.3 | 0.57 | 2,42 | 0.57 |
| WRAT reading | 106.71±12.5 | 106.07±9.1 | 102.33±10.6 | 0.13 | 2,42 | 0.88 |
| Racial composition | 10 Caucasian, 5 African–American | 13 Caucasian, 2 mixed race | 13 Caucasian, 2 mixed race | Chi-sq.=2.5 | 2,42 | 0.1 |
= Verbal Comprehension Index. Selected subtests from the full Wechsler Intelligence Scales (the WAIS-III or the WISC-IV) were administered, but not enough to calculate a Full Scale IQ (FSIQ). Therefore the Verbal Comprehension Index (VCI) was used. This is a pure measure of Verbal IQ and includes the Information, Vocabulary, and Similarities subtests of the WIS. A MANCOVA with VCI and WRAT Reading as the dependent variables and age and gender as covariates were used here to test for group difference.
One of the controls was unable to complete any of the expressive language tests due to a severe stutter that interfered with his speech and invalidated this data. His receptive language and other scores were within the normal range, so there is no reason to doubt his performance on the fMRI task.
This study received Institutional Review Board approval for human subjects’ research at the Nathan S. Kline Institute for Psychiatric Research, a New York State Institution, and at New York University School of Medicine. All subjects gave written informed consent for their participation after carefully being explained the nature of the study and its procedures.
2.2. Visual word discrimination task
Subjects were asked to perform a Visual Word Discrimination task using the block design illustrated in Fig. 1. Following a training and practice period outside the MRI unit, the task was repeated four times during the scan and fMRI data acquired as described below.
Fig. 1.

The block design within one sequence. O — Initial Fixation (8 s). A — Lexical Decision Task Block. B — Non-linguistic Control Task Block. C — Rest block.
“A” blocks were Lexical Decision Blocks. In each “A” block, English language words (all concrete nouns) were presented on a screen in front of the subject in a random fashion, interspersed with pseudowords matched to the real words for number of letters. “B” blocks were Non-Linguistic Control Blocks consisting of non-linguistic symbols that were not letters (Fig. 2), and “C” rest blocks contained a blank screen. Twenty-five different real English words and 25 different pseudowords were used, with 10 per Lexical Decision Block (5 real and 5 pseudowords) and the same number of each was presented in each scanning sequence. The real words were chosen from a public access database (http://www.psych.rl.ac.uk/) based on a rating scale given for number of letters and word imagibility (range 550–700). Pseudowords were chosen from Pexman et al. (2002). Concrete nouns were selected for the word stimuli. The range of “550–700” denotes the imagibility rating invented by linguists to assess whether one word is easier to image internally than another. The ratings that we used in the current work refer to words that are highly imagible. These types of words were used in previous similar studies. Details of the imagibility rating are in Gilhooly and Logie (1980). The number of letters in the pseudowords was matched to the number of letters in the words. However, pseudowords are not real words so they cannot be matched to words with respect to word type. Pseudowords are combination of letters that can be pronounced. They may sound like a word, but they do not have meaning. Subjects were instructed to use their right hand to press the left button when a word appeared and to press the right button when the letters displayed were not considered to be a real word.
Fig. 2.

Visual stimuli in the non-linguistic motor control task.
During the Non-Linguistic Control Block, participants were presented with two different kinds of nonsense strings as shown in Fig. 2. The subjects were instructed to press the left button in response to the symbols in Fig. 2(a) and the right button in the response to the symbols in Fig. 2(b). In one block, 5 left and 5 right symbols were randomly displayed on the screen in front of the subject. This task was included to control for motor response and activity in the visual cortex.
During the rest block a blank screen was presented and subjects were instructed to keep their eyes open, remain relaxed and motionless.
The visual word discrimination task was repeated four times. Each block was comprised of 10 stimuli, each presented for 1000 ms with an interstimulus interval of 3000 ms. Each sequence consisted of 8 s of initial fixation, followed by 11 stimulation blocks of 30 s each. The stimuli were presented in random order within blocks. The order of blocks within sequences and the order of trials within blocks differed across the three tasks, but were identical for all subjects.
Only the first three tasks for each subject were used in the final analysis. The fourth fMRI experiment exhibited substantial head motion and little activation in all groups, and was therefore excluded in subsequent analysis. No other data sets were excluded.
2.3. Magnetic resonance imaging protocol
Functional Imaging (fMRI) was performed on a 1.5T Siemens Vision system (Erlangen Germany). During each scan 169 functional volumes sensitive to blood oxygen level dependent (BOLD) contrast were acquired with a T2-weighted sequence (TR=2 ms, TE=50 ms, flip angle=85°, Matrix=64×64, FOV=224, pixel size= 3.5×3.5, time=5 min 38 s). Each volume comprised 22 axial slices with 5 mm slice thickness and no gap. The first four volumes acquired during the initial fixation were discarded. Following the fourth fMRI acquisition series, a T2-weighted fast spin echo data set was acquired using the same slice orientation parameters as in the fMRI acquisition sequence but higher in-plane resolution. Finally, a high resolution 3-D magnetization prepared rapid gradient echo data set was acquired for spatial mapping of fMRI data.
2.4. fMRI analyses
Data were analyzed using the FEAT fMRI Analysis tool in FSL3.3 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). In the first-level data analysis of each sequence, slice timing correction, intensity normalization and high pass temporal filtering were applied, non-brain structures were removed using the BET Brain Extraction tool (Smith, 2002). To correct for head motion, MCFLIRT was used, which is a linear registration tool applying rigid-body transformations (Jenkinson et al., 2002). Each image was smoothed with an 8-mm FWHM Gaussian spatial filter. Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al., 2001). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of P<0.05 (Worsley et al., 1992). Scans were registered to an average T1-weighted brain template in the standard Talairach space with FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002). Each of the first three fMRI acquisition sequences conducted on each subject was analyzed and the average response was obtained. In-Group analysis was carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) (Beckmann et al., 2003; Woolrich et al., 2004). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of P<0.05 (Worsley et al., 1992).
The two blocks (A and B as described in Fig. 1) were analyzed for each individual, and a group mean obtained for each group. In a first analysis we used an “exclusive mask” (A–B) by setting the A event as 1 and B event as −1 in the contrasts and F-tests options. Meanwhile we masked this real contrast with positive A and positive B in the Contrast Masking options. This “exclusive mask analysis” differentiated the lexical from the non-linguistic control task and permitted extraction of those regions predominantly activating with the lexical task. In a second analysis, we used an “inclusive mask” (activated A regions masked by activated regions of B in the Contrast Masking options) that determined the regions of activation common to both tasks (“inclusive mask analysis”). Thus, for each group, we obtained Z-thresholded activation maps for the “A” task (word/pseudoword discrimination), the “B” task (non-linguistic discrimination), for “A–B”(exclusive mask) and for “A mask B” (inclusive mask).
For between-group analyses, the FSL FLAME package was utilized. The “A mask B” (inclusive mask) condition for the three groups were analyzed controlling for age and sex. Z (Gaussianized T/F) statistic images were thresholded at P =0.05 (cluster corrected). In this study, all first order analyses were restricted to regions of positive activation. In this report, regions which exhibited negative activation are not considered.
3. Results
The siblings with schizophrenia were significantly older than the high-risk and low-risk groups; however, the groups did not differ significantly on sex, handedness, racial distribution, or level of education (see Table 1). Age and sex were controlled for in analyses below.
In the within-group Exclusive Mask Analyses (language specific activity), the normal controls showed one cluster of significantly more activation in the left inferior frontal gyrus, i.e. Brodmann’s areas 44 (see Fig. 3). In the high-risk subjects and their siblings with schizophrenia, activation was similar for both the word discrimination and the non-linguistic tasks. Thus no significantly activated brain region was found during this subtraction paradigm.
Fig. 3.

Regions of significantly more brain activation for the word discrimination task compared with the non-linguistic task in the control group. Note the activation of Brodmann’s area 44. There were no regions of significantly more activation for the word discrimination task in the subjects at high-risk or their siblings with schizophrenia.
In the within-group Inclusive Mask Analyses, regions of activation common to both the non-linguistic control and linguistic blocks were examined (Fig. 4a,b,c). Control subjects had significant activation on the left in Brodmann’s areas 44 and 45. However, both high-risk and subjects with schizophrenia had significant activation during both linguistic and non-linguistic control blocks in Brodmann’s areas 44 and 45 bilaterally, where activation on the right side (region of the right inferior frontal gyrus) was not present in the low-risk subjects. Thus, when contrasted with the exclusive mask analysis, the high-risk subjects and patients experienced less ‘task-specialization’ of Brodmann’s areas 44 and 45.
Fig. 4.
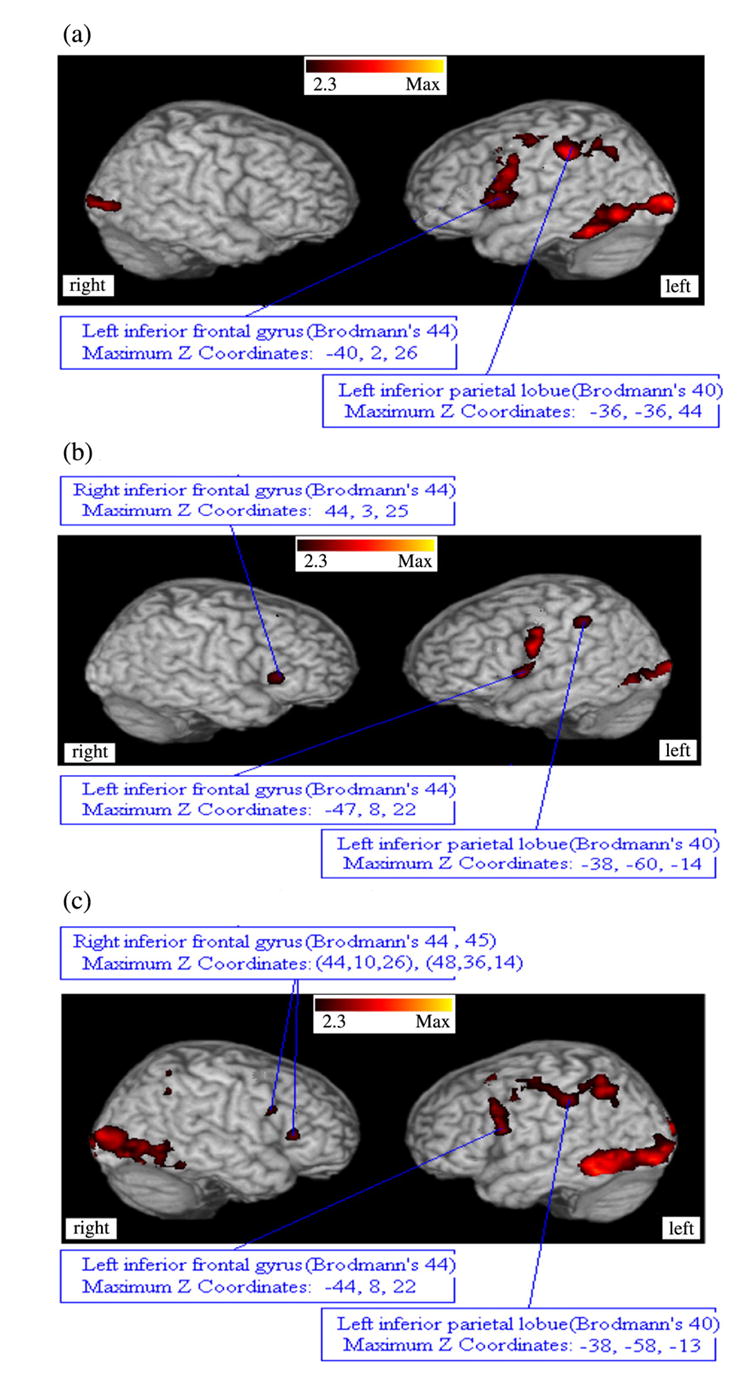
Significant regions of brain activation in the three groups when the Visual Word Decision Task and the Non-word Control Task were analyzed using an inclusive (logical ‘and’ mask). Note the laterality present in Brodmann’s area in the normal control subjects compared with the subjects at high-risk for schizophrenia or subjects with schizophrenia. Conversely, visual activation associated with the fMRI tasks resulted in bilateral activation in control subjects and schizophrenic patients, but not in subjects at high-risk for schizophrenia. (a) low-risk controls. (b) high-risk subjects. (c) subjects with schizophrenia.
Three between-groups comparisons for the inclusive mask analysis were performed. First, high-risk subjects were compared with controls. Fig. 5 shows regions of significantly increased and decreased activation in high-risk subjects relative to controls when the activation common to both the linguistic and non-linguistic tasks were examined. Significantly increased activation occurred in multiple regions in the right hemisphere (e.g. inferior frontal gyrus: Talairach coordinates 52, 15,10; middle and superior temporal gyri: Talairach coordinates 63, −12, 7; and inferior parietal lobule: Talairach coordinates 44, −50, 48); whereas, regions in the right fusiform gyrus (Talairach coordinates 40, −48, −18), and the right middle temporal gyrus (Talairach coordinates (64, −20, −11) had significantly decreased activation in the high-risk subjects compared with controls.
Fig. 5.

Differences between subjects at high genetic risk for schizophrenia and low-risk controls (P<0.01) for the ‘inclusive mask analysis’. (a) High-risk>Controls. (b) High-risk<Controls.
Secondly, the patients with schizophrenia were compared with controls. Fig. 6 shows significantly increased and decreased regions of activation for the patients with schizophrenia compared with normal controls when the activation common to both the linguistic and non-linguistic tasks were analyzed. A number of regions showed significantly increased activation on the right side, including left superior temporal gyrus (Talairach coordinates −66, −7, 5), right and left side inferior parietal lobule (Talairach coordinates ±43, −45, 54) and right side inferior frontal gyrus (Talairach coordinates 50, 11,10) in the patients. The regions with significantly decreased activation in the patients include left fusiform gyrus (−40, −42, −21) and right parahippocampal gyrus (26, −40, −2).
Fig. 6.

Differences between subjects with schizophrenia and low-risk controls (P<0.01) for the ‘inclusive mask analysis’ (P<0.01). (a) Patients>Controls. (b) Patients<Controls.
Thirdly, the high-risk subjects were compared with their siblings who had schizophrenia. The cluster corrected results reported neither significantly increased nor significantly decreased regions of activation, related to language processing, for the high-risk subjects compared with the patients.
4. Discussion
The current fMRI study used a visual word decision task to define regions of brain activation that are significantly different between individuals who have schizophrenia compared with controls, and to determine whether these differences can also be seen in their relatives who are still within the age of risk for schizophrenia and thus at a higher-risk than the general population. The data suggest that overall, our subjects with schizophrenia exhibited more right sided (bilateral) activation during the language task than did controls, as did their family members at high-risk for the disorder. In addition, the same brain regions on the right were also activated by a non-linguistic visual task. In contrast, some regions, such as the left inferior frontal gyrus, were activated in the controls significantly more during the linguistic than the control task, while they were activated by both tasks equally in the high-risk and schizophrenia subjects. Thus, the present data appear to show clear activation differences in controls when processing a linguistic compared with a non-linguistic task, but no regional activation differences specific to language in people at genetic high-risk for schizophrenia nor those with schizophrenia. It can be concluded that a more diffuse and bilateral set of regions are activated in both the high-risk and schizophrenia subjects than normal controls possibly signifying anomalous circuitry and less efficiency to the processing of language.
In addition, the current study shows some evidence of a difference in visual activation between controls and the subjects with schizophrenia, but not those at high-risk. Visual processing deficits have been found previously in studies of patients with schizophrenia (e.g. Kim et al., 2006) and in another study related to the genetic vulnerability for developing illness (Green et al., 2006). While our data do not support the latter, it is possible that visual activation differences could implicate visual processing pathways that are relevant to the recognition of language and thus contribute to some of the changes then seen in the processing of words in frontal and temporal lobes.
The results of this study add to the increasing literature showing reduced language lateralization in schizophrenia (e.g. Sommer et al., 2003; 2001) and previous publications (Whalley et al., 2005, 2006) showing that a more bilateral pattern of activation can also be detected in people at high-risk for the disorder. These results also suggest that the underlying pathological basis for schizophrenia may be related to anomalies in the pathways for language processing or other functions as previously suggested (Crow, 1998; DeLisi, 2001; Morey et al., 2005).
Biological findings that distinguish individuals at high-risk for schizophrenia from controls are particularly of interest. If in further studies they are shown to be both highly sensitive to the prediction of who develops schizophrenia and highly specific to this disorder, then future use of any of these findings could be possible as a screening devise that may aid clinicians to make decisions about treatment for people who show non-specific signs of illness and be of prognostic value.
However, the current study was only an initial attempt at determining whether focusing on a language paradigm in fMRI may be a useful candidate method to develop further as a possible screening measure. Unfortunately in this first fMRI project aimed at distinguishing high-risk subjects from controls, we failed to save information on language task performance while subjects were performing the fMRI scan. However, in a prior training session, every subject could perform the task with ease and we were also unable to find any significant Verbal IQ or reading performance differences between groups when tested outside the scanner. Nevertheless, we do not know whether actual performance ability could have contributed to the difference in activation patterns. Our current study was also not as well matched for racial/ethnic and age effects as they could be, since it was difficult to find these types of subjects for study. While we did not find effects of these variables on the pattern of activation (and controlled for age and sex in all analyses), they need to be more carefully matched in further studies.
Future work will need a much larger cohort of high-risk individuals, followed longitudinally, to determine who eventually develops schizophrenia and whether the prior fMRi language study would have been predictive of illness. In addition, a focus on individual variation and analysis of the amount of activation in candidate brain regions detected by the current study will be an important further analysis.
Acknowledgments
This project was partially supported by a grant from NIMH, R21 MH071720.
References
- Beckmann C, Jenkinson M, Smith SM. General multi-level linear modelling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. NeuroImage. 2005;27(3):677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Boksman K, Theberge J, Williamson P, Drost DJ, Malla A, Densmore M, Takhar J, Pavlosky W, Menon RS, Neufeld RW. Schizophrenia Research. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Vershinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. American Journal of Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Chaika EO. Charles C. Thomas Publisher; Springfield, Illinois: 1990. Understanding Psychotic Speech: Beyond Freud and Chomsky. [Google Scholar]
- Crow TJ. Nuclear schizophrenic symptoms as a window on the relationship between thought and speech. British Journal of Psychiatry. 1998;173:303–309. doi: 10.1192/bjp.173.4.303. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Done DJ, Sacker A. Childhood precursors of psychosis as clues to its evolutionary origins. European Archives of Psychiatry and Clinical Neuroscience. 1995;245(2):61–69. doi: 10.1007/BF02190732. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Speech disorder in schizophrenia: review of the literature and new study of the relation to uniquely human capacity for language. Schizophrenia Bulletin. 2001;27:481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. The significance of the age of onset for schizophrenia. Schizophrenia Bulletin. 1992;18:209–215. doi: 10.1093/schbul/18.2.209. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Boccio AM, Riordan H, Hoff AL, Dorfman A, McClelland J, Kushner M, Van Eyl O, Oden N. Familial thyroid disease and delayed language development in first admission patients with schizophrenia. Psychiatry Research. 1991;38:39–50. doi: 10.1016/0165-1781(91)90051-p. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sherrington R, Shaw S, Nanthakumar B, Shields G, Smith AB, Wellman N, Larach NW, Loftus J, Razi K, Stewart J, Vita A, De Hurt M, Crow TJ, Sherrington R. A genome-wide scan of 382 affected sibling-pairs with schizophrenia suggests linkage to chromosomes 2cen and 10p. American Journal of Psychiatry. 2002;159:803–812. doi: 10.1176/appi.ajp.159.5.803. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Logie RH. Age of acquisition, imagery, concreteness, familiarity and ambiguity measures for 1944 words. Behaviour Research Methods and Instrumentation. 1980;12:395–427. [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biological Psychiatry. 2006;59(5):446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Habel U, Klein M, Shah NJ, Toni I, Zilles K, Falkai P, Schneider F. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. American Journal of Psychiatry. 2004;161(10):1806–1813. doi: 10.1176/ajp.161.10.1806. [DOI] [PubMed] [Google Scholar]
- Heim S, Alter K, Ischebeck AK, Amunts K, Eickhoff SB, Mohlberg H, Zilles K, von Cramon DY, Friederici AD. The role of the left Brodmann’s areas 44 and 45 in reading words and pseudowords. Cognitive Brain Research. 2005;25(3):982–993. doi: 10.1016/j.cogbrainres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2002;26(6):1143–1149. doi: 10.1016/s0278-5846(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82(1):1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TT, Bullmore ET, Brammer MJ, Williams SC, Broome MR, Murray RM, McGuire PK. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophrenia Research. 2001;50:27–40. doi: 10.1016/s0920-9964(00)00042-6. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Oh TM, Krammer MJ, McGuire PK. Neural correlates of syntax production in schizophrenia. British Journal of Psychiatry. 2005;186:209–214. doi: 10.1192/bjp.186.3.209. [DOI] [PubMed] [Google Scholar]
- Koeda M, Takahashi H, Yahata N, Matsuura M, Asai K, Okubo Y, Tanaka H. Language processing and human voice perception in schizophrenia: a functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:948–957. doi: 10.1016/j.biopsych.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG. An fMRI study of semantic processing in men with schizophrenia. NeuroImage. 2003;20:1923–1933. doi: 10.1016/s1053-8119(03)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience. 2005;17(11):1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Archives of General Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies: rationale, unique features and training. Archives of General Psychiatry. 1994;51:849–862. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, Pesenti S, Gallagher A, Perani D, Price C, Frith CD, Frith U. A cultural effect on brain function. Nature Neuroscience. 2000;3(1):91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Pexman P, Lupker S, Reggin L. Phonological effects in visual word recognition: investigating the impact of feedback activation. Journal of Experimental Psychology Learning, Memory, and Cognition. 2002;28:572–584. doi: 10.1037//0278-7393.28.3.572. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophrenia Research. 2006 doi: 10.1016/j.schres.2006.03.019.. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IEC, Ramsey NE, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophrenia Research. 2001;52:57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, Kahn RS. Language lateralization in female patients with schizophrenia: an fMRI study. Schizophrenia Research. 2003;60(23):183–190. doi: 10.1016/s0920-9964(02)00300-6. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, van Oel CJ, Kahn RS. Language activation in monozygotic twins discordant for schizophrenia. British Journal of Psychiatry. 2004;184:128–135. doi: 10.1192/bjp.184.2.128. [see comment] [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Seidman LJ, Breiter H, Goldstein JM, Goodman JM, Poldrack R, Faraone SV, Tsuang MT. Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: a pilot study. Biological Psychiatry. 2004;55(5):490–500. doi: 10.1016/j.biopsych.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition. Harcourt Assessment, Inc; San Antonio, TX: 2004. [Google Scholar]
- Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Felber S, Fleischhacker WW. Language lateralization in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Psychiatry Research Neuroimaging. 2006;146:185–190. doi: 10.1016/j.pscychresns.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain. 2004;127(Pt 3):478–490. doi: 10.1093/brain/awh070. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128:2097–2108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Morehead W, McIntosh A, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional imaging as a predictor of schizophrenia. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.11.013. PMID: 16460690[Pubmed] [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of FMRI data. NeuroImage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multi-level linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Zhang JX, Wang X, Wu R, Hu X, Weng X, Tan LH. Differential activity in left inferior frontal gyrus for pseudowords and real words: an event-related fMRI study on auditory lexical decision. Human Brain Mapping. 2005;25(2):212–221. doi: 10.1002/hbm.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]


