Abstract
Rhesus rotavirus (RRV)-inoculated neonatal BALB/c mice develop an immune mediated inflammation of both extra- and intrahepatatic bile ducts which progresses to biliary obstruction and death by 3 weeks of age. The livers of diseased animals demonstrate increased numbers of T-lymphocytes along with elevated expression of Th1 type cytokines at 1 week, which transitions to increased numbers of macrophages and high expression of the pro-inflammatory cytokine TNF-α by 2 weeks. We sought to determine whether this correlation with high TNF-α expression played a causal role in disease pathology by employing both pharmacologic and genetic approaches for attenuation of TNF-α-induced injury. First, RRV-BALB/c mice were subjected to multiple treatments of either the TNF-RI-Fc fusion protein etanercept or rat neutralizing antibodies to mouse TNF-α (cV1q) beginning on day 8-10 post RRV infection. Also, TNF-RI−/− mice were also injected with RRV in the same manner as wild-type mice. In all cases TNF-inhibition did not reduce severity or incidence of disease. Survival curves of RRV-infected mice given blocking agents were similar to control RRV mice, and survival of challenged TNF-RI−/− mice was actually worse than wild-type, most likely due to the prolonged presence of infectious RRV within the liver. In all experimental groups, serum bilirubin, liver cellular infiltrate, and extra- and intrahepatic bile duct inflammation were no different than control mice. In summary, while RRV-BALB/c mice have highly elevated expression of TNF-α, this cytokine does not play an obligate role in disesae progression.
Keywords: rodent, cytokine, viral infection, inflammation
INTRODUCTION
Biliary atresia (BA) is a progressive, inflammatory cholangiopathy of infancy that leads to fibrosis and obliteration of both the extrahepatic and intrahepatic bile ducts (1). At the time of diagnosis, the extrahepatic bile ducts are partially or entirely obliterated with residual inflammatory cells present within duct remnants (2). The intrahepatic ducts at this time have an ongoing inflammatory response with lymphocytes surrounding and invading the ducts (3). Without intervention, children with BA die from biliary cirrhosis in the first 2 years of life and this disease is the indication for greater than 50% of pediatric liver transplants (4).
The etiology of BA is unknown and theories of pathogenesis involve viral infection, immune-mediated ductal destruction, and abnormalities in ductal development (4-8). The pathogenesis of BA may involve both an initial viral infection and a subsequent autoreactive immune response.
Numerous studies have investigated the role of the immune system in the pathogenesis of BA (9-13). Our initial studies characterizing this immune response have confirmed previous reports and have indicated that there is an increase in both CD4+ and CD8+ T cells and macrophages infiltrating the portal tracts. The portal tract infiltrating cells secrete the Th1 inflammatory cytokines TNF-α, IFN-γ, IL-2 and IL-12, which further induce Th1-cell mediated ductal destruction(12).
In order to explore the potential sequence of pathologic events leading to biliary obstruction in BA, we are utilizing the rotavirus-induced murine model of biliary atresia. The Rhesus group A rotavirus (RRV) neonatal murine model of biliary obstruction entails a virus-induced, progressive inflammatory destruction of extrahepatic and intrahepatic bile ducts leading to extrahepatic ductal fibrosis and obliteration (14-17). By two weeks of age, the biliary obstruction is associated with acholic stools, direct hyperbilirubinemia and intense portal tract and extrahepatic ductal inflammation (12). Previous reports demonstrate that although the virus is no longer detected in the liver by this time point, the inflammation persists and the mice die from liver failure by 1 month of life (14).
TNF-α is a pro-inflammatory cytokine which has many effector functions, including directly stimulating of production of nitric oxide by macrophages, and induction of apoptosis through direct interactions with TNF-Receptor p55 or, indirectly, by enhancing Fas expression in the Fas/Fas ligand apoptotic pathway (18, 19). We have shown increased expression of TNF-α at the time of diagnosis in human BA and increased expression of hepatic TNF-α and inducible nitric oxide synthase (iNOS) at day 14 post RRV-infection in the murine model (12, 20).
The objective of this study was to determine if TNF-α played an obligate role in disease progression. More specifically, we sought to determine the outcome of treating RRV-BALB/c mice with [1] TNF-α -blocking reagents (the TNF-RI-Fc fusion protein, etanercept; or a neutralizing monoclonal antibody to mouse TNF-α (cV1q) or by [2] ablation of the TNF signaling pathway using TNF-RI−/− mice on the BALB/c background injected with RRV in the same manner as wild-type mice.
MATERIALS AND METHODS
Mice
Timed-pregnant female BALB/c mice (11-14 days post conception) were purchased from rotavirus-free colonies of The Jackson Laboratory (Bar Harbor, ME). TNF-RI−/− mice on the BALB/c background were supplied by N. Mukalda (Kanazawa University, Japan) and have been described elsewhere (21, 22). Average litter size was 3-7 pups. Mice were injected i.p. at 12-18 hours of life with either 4.0 × 104 pfu of virus in HBSS or HBSS alone. Mouse liver specimens were pooled for all experiments (average N=10). All animals were housed and handled in accordance with the UCDHSC Office of Laboratory Animal Medicine
Virus culture/titering
Rhesus rotavirus (RRV) strain MMU 18006 was grown in MA-104 African green monkey kidney cells (ATCC) and assayed for concentration by infectious plaque assay as previously described (20). Determination of presence of infectious virus in liver tissue was performed in the same manner following addition of 100% w/v HBSS and homogenization.
Treatment of mice with TNF-α blocking agents
Rat anti-murine TNF-α neutralizing antibody (clone cV1q) and isotype control antibody (clone CNTO1322) were provided by Centocor, Inc. (Horsham, PA) at a concentration of 10mg/mL. Jaundiced animals were treated with 40μL antibody administered i.p. on days 8, 11, 14, and 17 post-RRV inoculation. The TNF-RI-Fc fusion protein etanercept (Enbrel, Amgen, Inc., Thousand Oaks, CA) was purchased from a pharmacy and resuspended at 2mg/mL in normal saline (0.9% NaCl). Jaundiced animals were treated with 50μL etanercept i.p. on days 10, 13, and 16 post-RRV inoculation.
TNF-α Bioassay
The TNF-α sensitive murine fibrosarcoma cell line WEHI-164 was cultured in DMEM, 10% FCS, and 10ng/mL TNF-α (BD PharMingen) for 12 hours in order to induce apoptosis. Cells were also cultured with TNF-α with increasing concentrations of either cV1q anti-TNF-α antibody or etanercept to measure the ability of each reagent to block activity. Apoptosis was measured first by assaying induction of caspase expression by staining with the caspase 3/7-specific reagent FAM-DEVD-FMK (Carboxyfluorescein FLICA Apoptosis Detection Kit, Immunochemisty Technologies, Bloomington, MN). Induction of cell death was also measured by YO-PRO-1 staining according to manufacturer's instructions (Vybrant Apoptosis Assay Kit #4, Molecular Probes, Eugene, OR).
Clinical assessment
Daily assessment post infection was performed looking for the appearance of jaundice in non-fur-covered areas and acholic stools. Mortality was determined at day 7, 14 and 21 of life. Gross examination of the liver and biliary system of inoculated animals was carried out with a dissecting microscope.
Serum Bilirubin Assay
Blood was collected from the renal artery of RRV- and HBSS-treated mice at time of sacrifice, allowed to clot and centrifuged to purify serum. Direct bilirubin was determined using the Direct Bilirubin Assay from Diagnostic Chemicals Limited (Oxford, CT).
Histopathology
Consecutive sections of liver and extrahepatic bile ducts were fixed in formalin, embedded in paraffin, sectioned with a cryotome at various levels and 6 μm sections were stained with hematoxylin and eosin.
Flow cytometry
Fluorochrome-conjugated antibodies to murine CD3, CD4, CD8a, CD11b, CD45, and isotype matched controls were purchased from eBiosciences. Liver tissue was homogenized over wire mesh, hepatocytes pelleted and remaining cells subjected to RBC lysis. Cells were blocked with anti-murine CD16/32 (eBiosciences) and stained with PE-conjugated anti-CD45 and other cell-surface antibodies. Expression of cell surface markers was determined on a FACS Caliber flow cytometer (Becton-Dickinson, Mountain View, CA) using CELL Quest software for analysis. Leukocyte populations were gated for study based on their CD45-staining.
Statistical analysis
All results were based on at least 3 separate experiments and are shown as mean ± SEM. Where appropriate, differences between the relevant groups were analyzed statistically with the Student's t-test for unpaired samples (InStat program, SanDiego, CA). Differences in means were considered significant for P- values < 0.05.
RESULTS
TNF-α-blocking agents are effective at reducing TNF-α activity
The ability of the anti-murine TNF-α antibody cV1q and the TNF-RI-Fc receptor fusion protein etanercept to block the apoptosis-inducing activity of TNF-α was tested using the WEHI 164 cell line bioassay. In this assay, the WEHI-164 murine fibrosarcoma cell line was cultured in the presence of increasing concentrations of TNF-α to determine the optimal amount for inducing apoptosis. Apoptosis was measured by two different assays: (1) percent of cells that stain positive for FLICA FAM-DEVD-FMK, a fluorochrome inhibitor of caspase which binds specifically to activated caspase-3 and -7, known markers of apoptosis; and (2) percent of cells that stain positive for YO-PRO-1, a green fluorescent dye which can enter apoptotic cells. It was determined that 10ng/μL recombinant TNF-α yielded approximately 50% apoptotic cells by both assays (data not shown) after 16 hours of culture. Next, WEHI-164 cells were cultured with TNF-α with and without either cV1q antibody or etanercept ranging in concentrations from 1 to 106 ng/μL. Both reagents inhibited the TNF-α induced apoptotic activity, in a dose-response fashion, as measured by both FLICA (Figure 1A) and YO-PRO-1 (Figure 1B). Etanercept was completely effective in blocking all TNF-α activity at 103 ng/μl, whereas cV1q reduced activity to levels approaching background at 106 ng/μL.
Figure 1. Etanercept and cV1q antibody are effective at blocking TNF-α induced cell death.
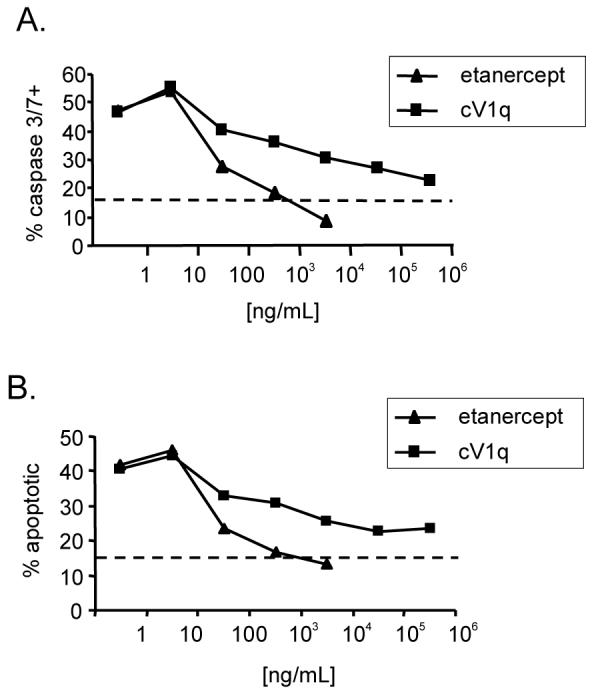
WEHI 164 cells were cultured for 12 hours with 10ng/mL TNF-α with increasing concentrations of either cV1q anti-TNF-α antibody or etanercept. A. Percent of cells positive for caspase 3/7. B. Percent of cells positive for the apototic cell-specific stain YO-PRO-1. Dashed line represent the background staining for each marker on non-TNF-α treated WEHI 164 cells.
Disease manifestation in mice with non-functional TNF-α-pathways
Survival
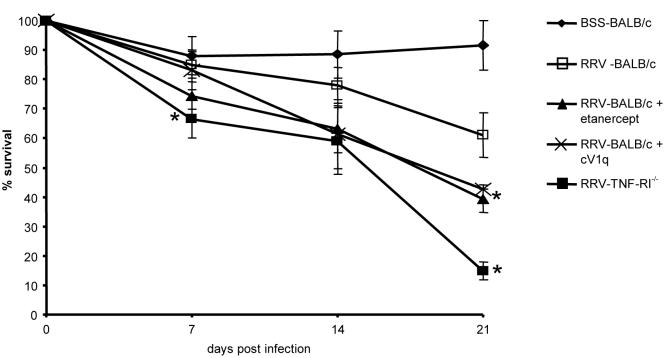
We next used ph both pharmacologic and genetic approaches to inhibit TNF-α mediated injury in vivo following RRV-inoculation. Figure 2 shows the survival curves of experimental mice as compared to intact, untreated RRV mice or control mice given BSS. Contrary to our initial hypothesis, neither etanercept or cV1q treatment nor ablation of TNF-RI alleviated biliary disease in these animals. In fact, both types of mice had elevated levels of mortality as compared to both intact RRV-BALB/c or BSSBALB/c mice. This increased disease is most evident by three weeks when approximately 40% (± 4.7%) of RRV-mice given TNF-blocking agents were alive whereas 61% (± 7.6%) of untreated RRV-mice survived to 3 weeks (p=0.04). Strikingly, TNF-RI−/− mice inoculated with RRV had the most severe course of disease. By 1 week, survival of these mice was significantly worse than wild-type BALB/cs (66.3% ± 6.4% survival vs. 85.1%± 6.3%, p=0.048) and these differences only increased with time. By 3 weeks, only 14% ± 3.1% (p=0.0045) of TNF-RI−/− mice were still alive. The gross appearance of these mice was also different. Wild type animals inoculated with RRV appear as highly jaundiced on all non-fur bearing areas and the peritoneal contents had a yellow discoloration. However, TNF-RI−/− mice were less jaundiced and manifested an oily fur appearance.
Figure 2. Mice with non-functional TNF pathways are more sensitive to RRV-induced mortality.
BALB/c mice were analyzed for survival for 3 weeks post RRV-infection (or BSS injection for controls). Each group was assessed 3 or more times with n ≥ 15 for each set. Average survival and standard error of the mean is shown for each timepoint. Points with a P-value <0.05 are indicated with asterisks.
Serum bilirubin
Direct bilirubin was measured in the serum of 2 week old animals. BSS control mice consistently had undetectable levels of direct bilirubin whereas RRV mice measured greater than 10mg/dL (Figure 3). The levels of serum direct bilirubin in RRV mice treated with etanercept or cV1q were not significantly different than that of the untreated RRV mice (7.5mg/dL for etanercept and 9.5mg/dL for cV1q). The direct bilirubin levels of RRV- TNF-RI−/− mice (5.5 ± 2.1 mg/dL) was significantly lower than RRV-BALB/c mice (p=0.043), consistent with the reduced jaundice in these animals.
Figure 3. TNF-RI ablation, but not TNF-α blockade, alters serum bilirubin in RRV-BALB/c mice.
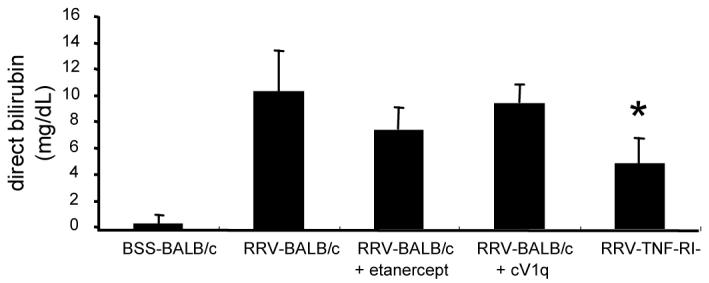
Blood was collected from mice at time of necropsy at 2 weeks post-RRV-infection. Clarified serum was tested for direct bilirubin in three independent pools from each group. Average direct bilirubin concentration and standard error of the mean are shown. Points with a P-value <0.05 are indicated with asterisks.
Cellular infiltrate
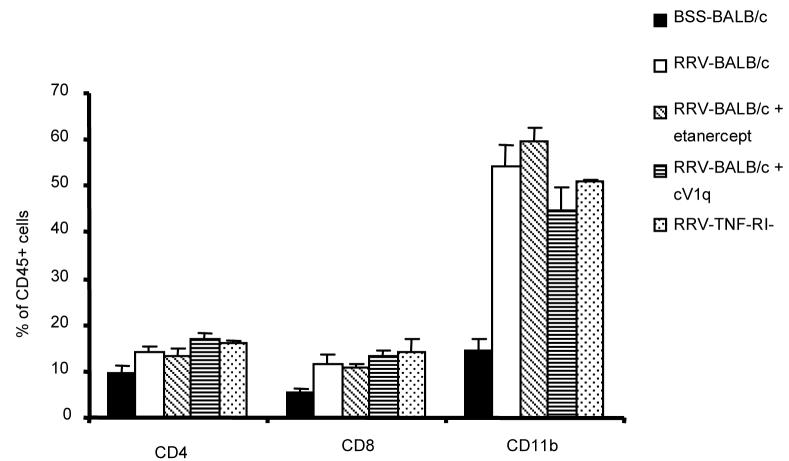
Previous studies in this laboratory have demonstrated an influx of portal tract CD4+ and CD8+ T-cells and a massive increase of CD11b+ macrophages in RRV-BALB/c mice at 2 weeks of age. Livers from RRV-BALB/c mice given TNF-blocking agents or in TNF-RI−/− mice were analyzed for the presence of cells that either make or are responsive to TNF-α. In all cases, cell numbers of treated or TNF-RI−/− mice were indistinguishable from those present in RRV-BALB/c mice (Figure 4). All mice had statistically significant increases in CD4+, CD8+, and CD11b+ cells compared to BSS-BALB/c mice at 2 weeks.
Figure 4. RRV-Mice with non-functional TNF pathways show no change in composition of inflammatory cellular infiltrate in the liver.
Fluorochrome-stained hepatocyte-depleted liver homogenate was gated for leukocytes based on scatter profile and CD45+ staining. Cells were then analyzed for lymphocyte (CD4 and CD8) and macrophage (CD11b) surface markers. Each bar represents a minimum of 3 experiments with standard error of the mean shown.
Histology
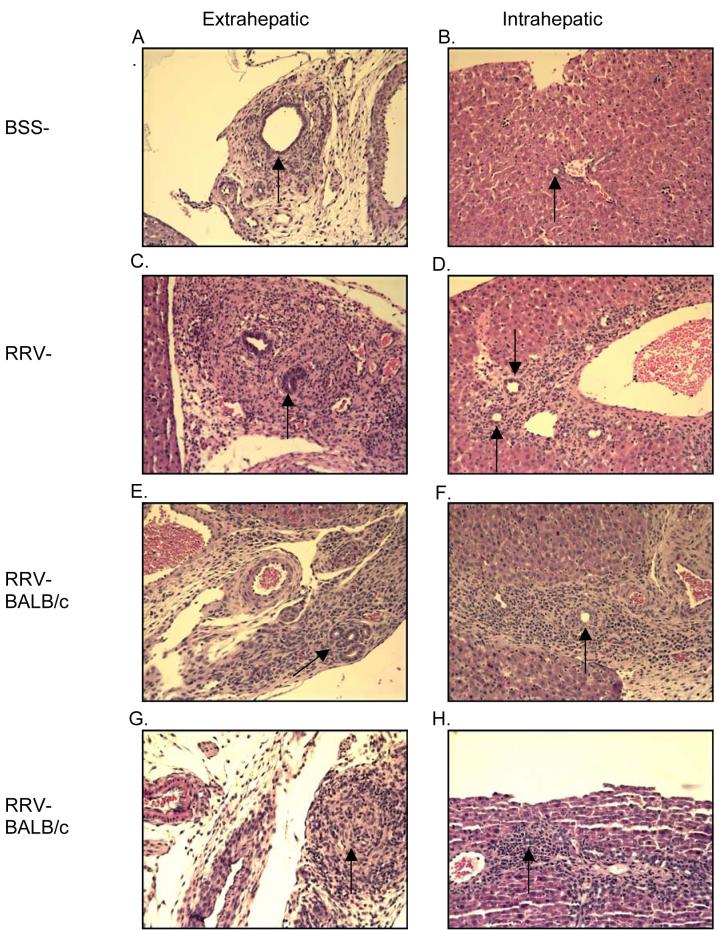
While mice with altered ability to respond to TNF-α appeared to have liver disease similar to untreated RRV-BALB/c mice, it was important to determine if bile duct narrowing or destruction secondary to inflammation was occuring to the same degree as control RRV-BALB/c mice. To this end, histology of all categories of mice was examined (Figures 5 and 6). In figures 5A and 5B, the extra- and intrahepatic bile ducts of BSS-BALB/c mice have low numbers of surrounding resident leukocytes. Figures 5C and 5D demonstrate the inflammatory infiltrate of a typical RRV-BALB/c mouse. The extrahepatic bile duct is completely inflamed with massive cellular infiltrate and severe narrowing of the ductal lumen. This infiltrate is also evident in the portal tract regions of the liver (Figure 5D). Based on the clinical outcomes it was not surprising that neither etanercept nor cV1q treatment were effective at influencing the pathology arising from RRV infection. In both the extrahepatic duct and the portal tracts of these mice, large numbers of invading leukocytes were present (Figures 5E-H) and the ductal lumen is compromised.
Figure 5. TNF-α blocking agents have no effect on bile duct histology in RRV-infected mice.
Representative histology of extra- and intrahepatic bile ducts of BSS-BALB/c mice demonstrates no infiltrate and patent bile ducts. RRV-BALB/c mice, both with and without TNF-α blocking agents, exhibit significant inflammation and narrowing of bile ducts. Magnification is 200x.
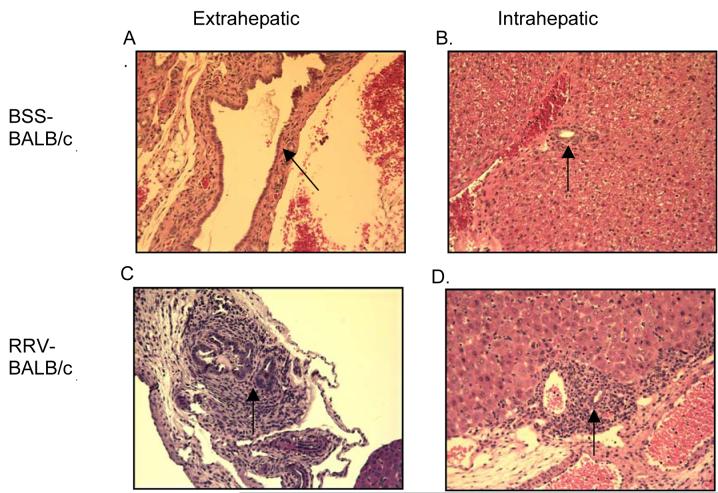
Figure 6. TNF-RI−/− mice infected with RRV show occluded extrahepatic bile ducts and cellular infiltrate similar to wild type mice.
Representative H&E staining of extra- and intrahepatic bile ducts of BSS- and RRV-TNF-RI−/− mice. BSS-control mice exhibit no infiltrate and patent ducts similar to BSS-BALB mice (Figure 5a, 5b). RRV- TNF-RI−/− mice show histology similar to RRVBALB/c (Figure 5c, 5d) (magnification 200x).
Histological analysis was also performed on the TNF-RI−/− mice. Mice given BSS appeared identical to intact BSS-BALB/c in both the extra- and intrahepatic ducts (Figure 6A and 6B). Administration of RRV to TNF-RI−/− mice resulted in the same degree of periductular cell infiltrate as wild-type RRV mice. Figure 6C shows the pertubation of the extrahepatic duct and 6D reveals extensive inflammation within a portal tract.
Ability to clear viral infection is impaired in TNF-RI−/− mice but not in mice given TNF-α blocking agents
As the survival curves of all experimental groups with attenuated TNF-α were significantly worsened relative to RRV-BALB/c mice, it was hypothesized that these mice might have differential ability to clear the RRV infection than intact, untreated mice. Infectious plaque assays were performed to determine the levels of residual RRV present in the livers of mice 2 weeks post-infection (Table I). RRV-BALB/c mice were capable of eliminating infectious RRV by 2 weeks and these results were unchanged in RRV-BALB/cs given TNF-α blocking agents. This is probably due to the fact that treatment with entanercept or cV1q commenced at days 8-10 post-infection and that the majority of viral clearance takes place in the first week. TNF-RI−/− mice, on the other hand, were unable to completely abolish infectious RRV by 2 weeks. These liver samples had 1.7 × 104 ± 0.5 × 104 pfu RRV/g of liver tissue as compared to undetectable levels in all other mice. The inability to clear infectious RRV may have contributed to the increased mortality of these mice.
Table I.
Plaque assay (2 week p.i. liver homogenate)
| pfu/mg | SE | |
|---|---|---|
| BSS-BALB/c | <10 | |
| RRV-BALB/c | <10 | |
| RRV-BALB/c + etanercept |
<10. | |
| RRV-BALB/c + cV1q |
<10 | |
| RRV-TNF-RI−/− | 1.7 × 104 | 0.5 × 104 |
| RRV-BALB/c (1 week p.i.) |
50 × 104 | 8.0 × 104 |
DISCUSSION
Biliary Atresia is a progressive inflammatory, sclerosing cholangiopathy of infancy which leads to biliary cirrhosis. However, the etiology of BA is not known and may invlove immune-mediated bile duct destruction. We have previously shown greatly increased expression of TNF-α at the time of diagnosis in human BA (12) and at day 14 post RRV-infection in the murine model (20). If this pronounced increase in TNF-α played a role in disease progression, we hypothesized that elimination of a functional TNF-α pathway in RRV-infected BALB/c mice would result in improved survival and enhanced recovery from the inflammatory process and biliary obstruction. In contrast to our hypothesis, in vivo data demonstrated that neither pharmacologic nor genetic methods reduced the manifestations of BA nor improved survival. Survival curves of RRV mice given blocking agents were not improved over untreated RRV mice. Moreover, the survival curve for the TNF-RI−/−-RRV mice was significantly worse in comparison to untreated RRV mice or RRV mice treated with TNF-α blocking agents. This is likely due to the continued presence of infectious RRV up to 2 weeks post-infection. The clearance of the virus may be necessary for improved survival, although clearance does not prevent the progressive inflammatory process.
Many reports in the literature address the necessity of TNF-α for adequate viral clearance in murine models. It has been reported that apoptosis mediated by TNF-RI, in combination with Fas, was sufficient for rapid clearance of cytomegalovirus (23). Also, TNF-RI was found necessary for complete viral clearance in a murine chronic lymphocytic choriomeningitis virus infection model (24). Thirdly, some reports demonstrate that TNF-α can enhance the anti-viral response to respiratory syncytial virus in mice (25). Data reported here show that TNF-RI is essential for elimination of RRV in BALB/c neonates. Thus, it appears that TNF-α is critical for clearance of a number of viruses, including RRV.
In addition to survival, the serum bilirubin, liver cellular infiltrate and histologic changes are similar between the untreated RRV mice, and RRV mice treated with TNF-α blocking agents or TNF-RI−/−-RRV mice. It is interesting to note that although survival is significantly worse in the TNF-RI−/− mice, liver cellular infiltrates and histologic changes are similar to untreated RRV mice or RRV mice treated with TNF-α blocking agents. We have demonstrated persistent viral load in the TNF-RI−/− mice. Thus, this exaggeration of disease may not be due to a direct effect on the immune-mediated progressive cholangiopathy in BA but rather that initial exposure, not sustained infection, to RRV initiates the immune mediated cholangiopathy with its inherent progressive destruction of the bile ducts.
TNF-α is believed to play a critical role in other immunological diseases such as Rheumatoid Arthritis and Crohn's Disease. It has been shown that the use of TNF-α neutralizing agents has high efficacy in the treatment of these diseases(26, 27). Despite these clinical observations, the murine studies shown here do not demonstrate any improvement in the progression of BA with TNF-α blockade. Based on these studies, TNF-α neutralizing agents should not be utilized in human BA until more is known about this cytokine in human disease.
Certainly, the progressive nature of BA is multifactorial and many other cytokines may be contributing, independent of TNF-α . In the murine model of BA, it has been shown that IFN-γ plays a critical role in disease progression (28) and there is increased expression of iNOS (20). Despite TNF-α blockade, either of these two potent molecules may have an effect on downstream modulation of the TNF-α pathway. Future studies will investigate other cytokines and chemokines, both individually as well as combinatorily. By blocking more than one pathway, one might be able to prevent progressive biliary destruction. Moreover, temporal blockade of more than one cytokine/chemokine may provide additional insight into disease progression and survival. Taken together, results indicate that despite the strong correlation of TNF-α expression with disease progression, this pro-inflammatory cytokine is dispensable for target injury.
ACKNOWLEDGEMENTS
We thank Patsy Ruegg (IHCTech, Denver) for providing invaluable histological services and Dr. Ronald Sokol for critical reading of this manuscript.
Footnotes
This work was supported by grants from the US National Institutes of Health (National Institute of Diabetes, Digestive, and Kidney Diseases) and the March of Dimes Institute
REFERENCES
- 1.Landing BH. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst--the concept of infantile obstructive cholangiopathy. Prog Pediatr Surg. 1974;6:113. [PubMed] [Google Scholar]
- 2.Gosseye S, Otte JB, De Meyer R, Maldague P. A histological study of extrahepatic biliary atresia. Acta Paediatr Belg. 1977;30:85. [PubMed] [Google Scholar]
- 3.Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J Pediatr Surg. 1995;30:515. doi: 10.1016/0022-3468(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 4.Sokol RJ, Mack C. Etiopathogenesis of biliary atresia. Semin Liver Dis. 2001;21:517. doi: 10.1055/s-2001-19032. [DOI] [PubMed] [Google Scholar]
- 5.Morecki R, Glaser JH, Cho S, Balistreri WF, Horwitz MS. Biliary atresia and reovirus type 3 infection. N Engl J Med. 1982;307:481. doi: 10.1056/NEJM198208193070806. [DOI] [PubMed] [Google Scholar]
- 6.Tyler KL, Sokol RJ, Oberhaus SM, Le M, Karrer FM, Narkewicz MR, Tyson RW, Murphy JR, Low R, Brown WR. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27:1475. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 7.Jevon GP, Dimmick JE. Biliary atresia and cytomegalovirus infection: a DNA study. Pediatr Dev Pathol. 1999;2:11. doi: 10.1007/s100249900083. [DOI] [PubMed] [Google Scholar]
- 8.Riepenhoff-Talty M, Gouvea V, Evans MJ, Svensson L, Hoffenberg E, Sokol RJ, Uhnoo I, Greenberg SJ, Schakel K, Zhaori G, Fitzgerald J, Chong S, el-Yousef M, Nemeth A, Brown M, Piccoli D, Hyams J, Ruffin D, Rossi T. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis. 1996;174:8. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Tracy TF, Jr., Dillon P, Fox ES, Minnick K, Vogler C. The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J Pediatr Surg. 1996;31:121. doi: 10.1016/s0022-3468(96)90333-4. [DOI] [PubMed] [Google Scholar]
- 10.Davenport M, Gonde C, Redkar R, Koukoulis G, Tredger M, MieliVergani G, Portmann B, Howard ER. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001;36:1017. doi: 10.1053/jpsu.2001.24730. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed AF, Ohtani H, Nio M, Funaki N, Shimaoka S, Nagura H, Ohi R. CD8+ T cells infiltrating into bile ducts in biliary atresia do not appear to function as cytotoxic T cells: a clinicopathological analysis. J Pathol. 2001;193:383. doi: 10.1002/1096-9896(2000)9999:9999<::aid-path793>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Mack CL, Tucker RM, Sokol RJ, Karrer FM, Kotzin BL, Whitington PF, Miller SD. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56:79. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 14.Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhnoo I, Rossi T, Fisher J, Ogra PL. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Petersen C, Biermanns D, Kuske M, Schakel K, Meyer-Junghanel L, Mildenberger H. New aspects in a murine model for extrahepatic biliary atresia. J Pediatr Surg. 1997;32:1190. doi: 10.1016/s0022-3468(97)90680-1. [DOI] [PubMed] [Google Scholar]
- 16.Petersen C, Grasshoff S, Luciano L. Diverse morphology of biliary atresia in an animal model. J Hepatol. 1998;28:603. doi: 10.1016/s0168-8278(98)80283-3. [DOI] [PubMed] [Google Scholar]
- 17.Czech-Schmidt G, Verhagen W, Szavay P, Leonhardt J, Petersen C. Immunological gap in the infectious animal model for biliary atresia. J Surg Res. 2001;101:62. doi: 10.1006/jsre.2001.6234. [DOI] [PubMed] [Google Scholar]
- 18.Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, Kim CH, Kim SJ, Kim JD, Choi IS, Choi IH. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999;162:1889. [PubMed] [Google Scholar]
- 19.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 20.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4(+) Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115:200. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 22.Ishida Y, Kondo T, Tsuneyama K, Lu P, Takayasu T, Mukaida N. The pathogenic roles of tumor necrosis factor receptor p55 in acetaminophen-induced liver injury in mice. J Leukoc Biol. 2004;75:59. doi: 10.1189/jlb.0403152. [DOI] [PubMed] [Google Scholar]
- 23.Fleck M, Kern ER, Zhou T, Podlech J, Wintersberger W, Edwards CK, 3rd, Mountz JD. Apoptosis mediated by Fas but not tumor necrosis factor receptor 1 prevents chronic disease in mice infected with murine cytomegalovirus. J Clin Invest. 1998;102:1431. doi: 10.1172/JCI3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suresh M, Gao X, Fischer C, Miller NE, Tewari K. Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection. J Virol. 2004;78:3906. doi: 10.1128/JVI.78.8.3906-3918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuzil KM, Tang YW, Graham BS. Protective Role of TNF-alpha in respiratory syncytial virus infection in vitro and in vivo. Am J Med Sci. 1996;311:201. doi: 10.1097/00000441-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Atzeni F, Turiel M, Capsoni F, Doria A, Meroni P, Sarzi-Puttini P. Autoimmunity and Anti-TNF-{alpha} Agents. Ann N Y Acad Sci. 2005;1051:559. doi: 10.1196/annals.1361.100. [DOI] [PubMed] [Google Scholar]
- 27.Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 2003;124:1774. doi: 10.1016/s0016-5085(03)00382-2. [DOI] [PubMed] [Google Scholar]
- 28.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, Bezerra JA. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]