Summary
Barrier to autointegration factor (BAF) is an essential DNA binding protein that is found in both the nucleus and cytoplasm. BAF is known to be involved in the establishment of nuclear architecture during mitosis, and since phosphorylation of BAF increases during mitosis and can sharply inhibit its DNA binding ability, post translational modification of BAF is likely to have biological importance. Herein, we demonstrate a role for cytoplasmic BAF during poxviral infections. BAF acts as a potent inhibitor of poxvirus replication unless its DNA-binding activity is blocked by phosphorylation mediated by the virally encoded B1 protein kinase. These data position BAF as the effector of a novel innate immune response that prevents replication of exogenous viral DNA in the cytoplasm. By usurping a signaling pathway employed by the cell, the poxviral B1 kinase has evolved to enable the virus to evade this defense.
Introduction
The barrier to autointegration factor (BAF) is an 89 amino acid dimeric DNA binding protein found in both the nucleus and cytoplasm of many cell types. BAF has been shown to participate in nuclear reorganization during mitosis and is thought to facilitate nuclear reassembly by interacting with both DNA and inner nuclear membrane proteins, thus acting as a bridge to recruit chromatin to the nuclear periphery (Furukawa et al., 2003; Haraguchi et al., 2001; Margalit et al., 2005; Segura-Totten et al., 2002; Segura-Totten and Wilson, 2004; Shumaker et al., 2001). The contribution of cytoplasmic BAF to cell function has remained obscure, although it has been found to act as a retroviral cofactor. Specifically, cytoplasmic BAF has been found within retroviral preintegration complexes (PICs), where it prevents autointegration of proviral DNA in vitro; a role for BAF during the integration of the HIV and MLV proviral DNA into the cellular genome may also occur, but is currently somewhat controversial (Chen and Engelman, 1998; Harris and Engelman, 2000; Jacque and Stevenson, 2006; Lee and Craigie, 1998; Lin and Engelman, 2003; Shun et al., 2006). The roles of BAF both during normal cell division and retroviral infection rely heavily on its ability to bind double stranded DNA in a sequence-independent manner. This binding involves autonomous domains on each BAF monomer (Bradley et al., 2005; Harris and Engelman, 2000; Segura-Totten et al., 2002; Zheng et al., 2000).
Given the fact that BAF participates in the process of nuclear envelope assembly/disassembly during mitosis, its temporal and/or spatial regulation by post-translational modifications has seemed likely. Indeed, we have recently reported that the binding of BAF to DNA in vitro is strongly inhibited upon the phosphorylation of its N-terminus by the cellular Vaccinia-Related Kinases VRK-1 and VRK-2 (Nichols et al., 2006). The sites on which BAF is phosphorylated by VRKs are highly conserved in diverse eukaryotes and can be similarly phosphorylated in vitro by the closely related B1 protein kinase encoded by vaccinia virus (Nichols et al., 2006). (The nomenclature B1, which has been used in the literature, refers to the fact that this viral protein is encoded by the 1st ORF in the HIndIII B fragment of the viral genome; since the complete sequence of the WR strain has been completed, the nomenclature WR183 has also been introduced for this gene). However, a role for BAF phosphorylation during poxviral infection has not been elucidated.
Vaccinia is the prototypic member of the poxvirus family, which comprises complex DNA viruses whose more notorious members include the etiological agents of smallpox and monkeypox. These DNA viruses are unique in that they replicate exclusively in the cytoplasm of infected cells. The vaccinia B1 kinase is an essential protein that is expressed at early times after infection and encapsidated at low levels in nascent virions (Banham and Smith, 1992; Lin et al., 1992; Rempel and Traktman, 1992). Importantly, mutations which disrupt B1 compromise viral DNA replication, and therefore the production of infectious virus (Boyle and Traktman, 2004; Rempel et al., 1990; Rempel and Traktman, 1992). The mechanism through which B1 contributes to DNA replication remains elusive, as it does not appear to phosphorylate any of the known components of the viral replication machinery (Boyle and Traktman, 2004 and P.T. unpublished observations). However, since a B1 defect can be complemented by expression of hVRK1 from the viral genome (Boyle and Traktman, 2004), and since both hVRK1 and B1 can efficiently phosphorylate BAF in vitro (Nichols et al., 2006), it seemed plausible to propose an important role for B1-mediated phosphorylation of BAF during vaccinia infection.
Results
BAF Phosphorylation Increases During Vaccinia Infection
To first investigate whether BAF is phosphorylated during poxviral infections, we expressed BAF as a 3X-FLAG fusion protein, either transiently in U2OS or BSC40 cells, or stably by engineering a novel derivative of tetracycline-inducible 293 cells (293:BAF). In uninfected cells, BAF appears as two closely migrating forms in immunoblot analysis (Fig. 1A). Only the more slowly migrating form is radiolabeled when transfected cells are incubated in the presence of 32PPi, indicating that the post-translational modification induces a shift in BAF’s electrophoretic mobility (Fig 1A, lane 2, U2OS lysates, and (Nichols et al., 2006)). When all three cell lines were infected with wild-type (wt) vaccinia virus, the proportion of BAF present as the phosphorylated form increased, as did the intensity of the 32P signal in the labeled U2OS cells (lanes 3). To examine whether this phosphorylation was indeed mediated by the virally encoded B1 kinase, we utilized the ts2 temperature sensitive mutant, which has a point mutation in the B1 locus that greatly diminishes both the catalytic activity and stability of the protein (Rempel and Traktman, 1992). Importantly, the phosphorylation of BAF failed to increase during nonpermissive infections with ts2 (Figure 1A, compare lanes 2 and 4), supporting the conclusion that B1 phosphorylates BAF in vivo. To confirm that the lack of BAF phosphorylation under these conditions was a direct consequence of B1’s inactivation, rather than an indirect effect of the subsequent arrest of the viral life cycle, we mimicked the latter by performing infections with wt virus in the presence of cytosine arabinoside (araC). Under these circumstances, in which B1 is active but post-replicative events are blocked (Oda and Joklik, 1967), the increased phosphorylation of cytoplasmic (soluble) BAF was still observed (Fig. 1B, compare lanes 1,3 with 5,7). Finally, we monitored the phosphorylation status of BAF following infection with ts2/hVRK1, a derivative of ts2 that has been complemented by the insertion of the human VRK1 cDNA (Boyle and Traktman, 2004). Following infection with ts2/hVRK1, the proportion of BAF in the phosphorylated form also increased (Fig 1A, compare lanes 5 and 3).
Fig. 1.
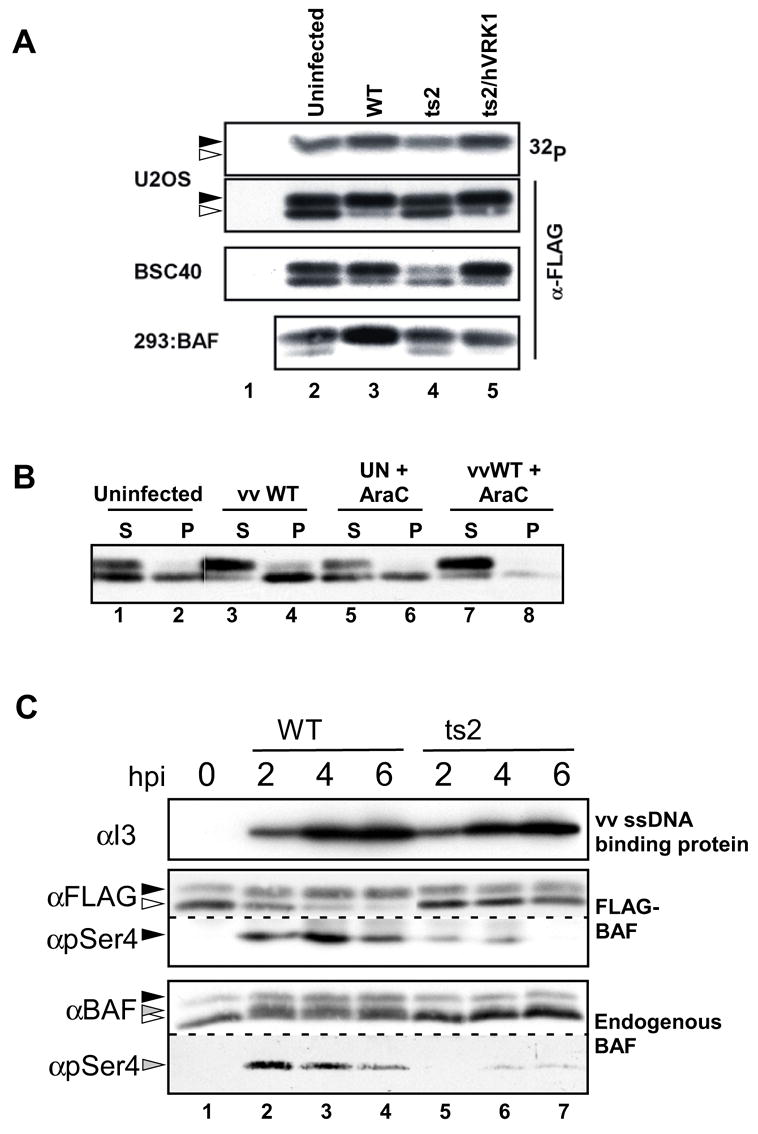
BAF is phosphorylated during vaccinia infection in a B1-dependent manner. BSC40, U2OS, and 293:BAF cells were engineered to express FLAG-BAF either through transient transfection (BSC40 and U2OS) or TET induction (293:BAF cells). Cells were left uninfected or infected for 5h with wild-type vaccinia virus, ts2, or ts2/hVRK1 at 39.7 C, as shown (MOI = 10). A) Lysates were prepared from all cells using 0.5% Triton X-100. The U2OS cells were metabolically labeled with 32PPi for 5 h prior to harvest, and the BAF was retrieved by immunoprecipitation with rabbit α-FLAG antibody. These immunoprecipitates, and the soluble lysates prepared from the other cell lines, were resolved by SDS-PAGE and either subjected to immunoblot analysis with the M2 α-FLAG antibody or visualized by autoradiography (top panel only). Open and filled triangles indicate unphosphorylated and phosphorylated BAF, respectively. B) BSC40 cells expressing FLAG-BAF were infected +/− 20μM AraC. Lysates were prepared in the presence of 0.5% Triton X-100 as in (A), and the BAF protein in both the soluble (S) and insoluble (P) fraction was visualized by immunoblot analysis. C) 293:BAF whole cell lysates were harvested at the indicated time post infection. Endogenous BAF and FLAG-BAF were detected either with an antibody specific to the N-terminus of BAF or M2 antibody, respectively. Phosphorylated endogenous BAF and phosphorylated FLAG-BAF were detected using an antibody raised against a BAF peptide phosphorylated at Ser-4. Vaccinia I3 was detected using α-I3 serum.
To verify that our observations using FLAG-tagged BAF were also observed with endogenous BAF, we examined the phosphorylation of both the endogenous and exogenous BAF proteins during vaccinia infections. This was accomplished using an antibody that we generated which specifically recognizes BAF molecules containing a phosphate moiety on Ser4, the site preferentially modified by B1 and VRK1 in vitro and in vivo (Nichols et al., 2006) (Supp Fig. 1). Indeed both FLAG-BAF and endogenous BAF showed a robust shift to the phosphorylated form soon after infection with wt virus (compare Fig. 1C, lanes 1 and 2), but not after ts2 infection (compare lanes 2 and 5). More specifically, quantitation of endogenous BAF modification at 2 hpi revealed a 10-fold greater phosphorylation during wt vs. ts2 infection. These data confirm that, in the context of viral infection, expression of B1 (or VRK1) from the cytoplasmic viral genome leads to an increase in the fraction of BAF that is phosphorylated.
BAF Associates with Viral DNA Replication Sites in Absence of the B1 Kinase
We next sought to understand how phosphorylation of BAF might be important during vaccinia infection. In light of the fact that (1) the ability of BAF to bind DNA is strongly inhibited by phosphorylation and (2) a catalytically active B1 kinase is needed for ongoing viral DNA replication, we hypothesized that unphosphorylated, cytoplasmic BAF would bind to vaccinia DNA upon its release from the viral core into the cytoplasm. This uncoating event occurs after the phase of early viral gene expression and enables DNA replication to commence. Once BAF would have access to the released genome, its ability to crossbridge DNA (Bradley et al., 2005; Suzuki and Craigie, 2002) might, in essence, lead to a sequestering of the genome that would block replication. This model would be consistent with the observation that catalytically active hVRK1, but not an inactive variant, rescues the replication defect of ts2 (Boyle and Traktman, 2004). Based on this hypothesis, we first predicted that BAF would be recruited to viral DNA replication sites during a nonpermissive ts2 infection (39.7ºC), but not during an infection with wt virus or with ts2 at the permissive temperature (31.5ºC). 293 cells stably expressing FLAG-BAF were utilized to study BAF localization, because the anti-BAF antibodies that are available cannot be used to detect endogenous BAF by immunofluorescence. FLAG-BAF was found in both the nucleus and the cytoplasm (Fig. 2, panel A), as has been previously observed for the endogenous protein (Haraguchi et al., 2001; Segura-Totten et al., 2002). These cells were then infected with wt vaccinia virus and the intracellular localization of FLAG-BAF was assessed. At 9 hpi the protein was present throughout the cell (Fig. 2, Panel C), exhibiting no colocalization with, or concentration at, the cytoplasmic sites of viral replication, which were visualized both by DAPI staining (Panel E) and with an antibody specific for the viral single strand DNA binding protein (I3) (arrowheads, Panel D) (Rochester and Traktman, 1998). We next monitored the localization of BAF following infection with ts2 for 9 hours at the permissive temperature; under these conditions, BAF retains a diffuse localization in the great majority of cells (Panel F). However, in keeping with the fact that the phenotype of ts2 is not fully wild-type even at the permissive temperature (Rempel et al., 1990), we did observe co-localization of BAF with 10–15% of the replication sites (arrows, Panels F-H). To examine whether BAF would consistently localize with foci of viral DNA during nonpermissive ts2 infections, we performed the initial 7h of infection at the permissive temperature, so that replication would begin and macromolecular sites of DNA accumulation would form, and then shifted the cultures to the nonpermissive temperature for an addition 2h. Importantly, this “shift-up” protocol leads to the cessation of viral DNA synthesis within 30 minutes (Rempel and Traktman, 1992). When these cultures were examined, we saw a striking colocalization of BAF with ~90% of the viral DNA replication sites (arrows in the three examples shown in panels I-Q). Together, these data are consistent with a model in which BAF remains diffuse during wt infection, when it is fully phosphorylated by B1 and hence does not bind to viral DNA. In contrast, under conditions where active B1 is lacking, the vast majority of BAF remains unphosphorylated and therefore binds to viral DNA replication complexes.
Fig 2.
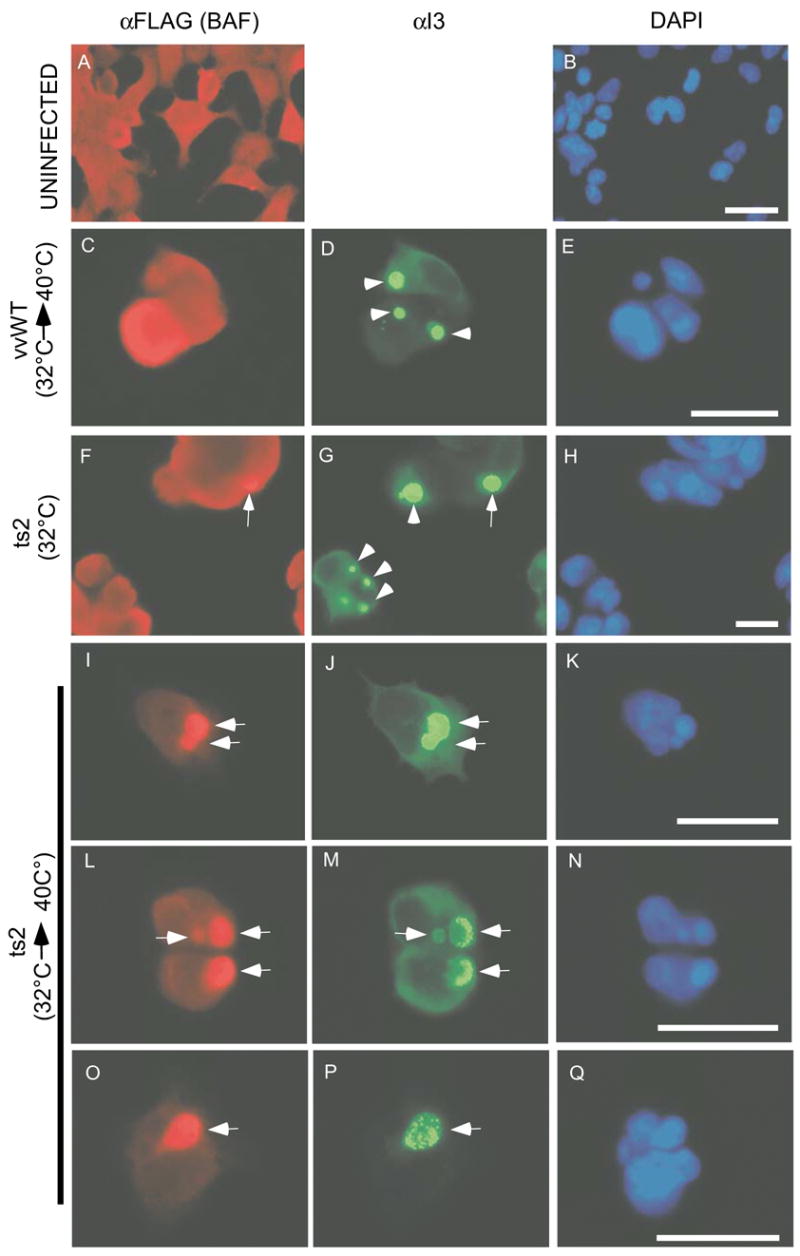
BAF co-localizes with vaccinia DNA replication sites in the absence of functional B1, but not during infections with wt virus. 293:BAF cells were induced to express FLAG-BAF and left uninfected (panels A and B) or then infected (MOI=5) with wt virus at 31.5 C for 7 h followed by a shift to 39.7 C for an addition 2 h (panel C-E), ts2 at 31.5 C (panels F-H), or ts2 at 31.5 C for 7 h followed by a shift to 39.7 C for an addition 2 h (3 examples in panels I-K, L-N and O-Q). At 9 hpi, cells were fixed and proteins visualized with M2 α-FLAG and α-I3 primary antibodies followed by fluorescent secondary antibodies. Arrowheads indicate viral DNA replication sites that exhibit no BAF colocalization, whereas arrows indicate replication sites that do show BAF colocalization. DNA was visualized using DAPI. Scale bars represent 50 μm in panels A and B and 25 μm in C-Q.
We next examined the rate of DNA accumulation in these same 293:BAF cells using the identical “shift up” protocol. This experiment has previously been performed in L929 cells, in which a shift to the nonpermissive temperature during ts2 infection results in a precipitous drop in the rate of DNA replication while no change is observed during wt vaccinia infection (Rempel and Traktman, 1992). To determine whether this also occurs in 293:BAF cells and would coincide with the relocalization of BAF to sites of viral DNA replication, we infected 293:BAF cells at 31.5ºC with wt virus and ts2. The amount of viral DNA was assessed at 3, 7, 9, and 12 hpi by dot blot hybridization; we examined infections which were either performed entirely at 31.5ºC or shifted to 39.7° C at 7 hpi, a protocol which mirrors the procedure used in the immunofluorescence studies (Fig. 2). We observed that during wt infection, there was no difference in the rate of DNA synthesis in infections performed at 31.5ºC vs. those shifted to 39.7ºC (Fig. 3A, solid black vs dashed black lines). In contrast, as has been seen in other cell lines, the rate of DNA synthesis during ts2 infections was reduced overall compared to that seen with wt virus (compare solid gray with solid black lines). Moreover, the rate of DNA synthesis slowed significantly after the cultures were shifted to 39.7ºC (compare solid gray vs dashed gray lines). These data demonstrate that the relocalization of BAF to the sites of viral DNA accumulation which is seen when ts2 infections are shifted to 39.7° C (Fig 2) is correlated with an inhibition of further DNA replication.
Fig 3.
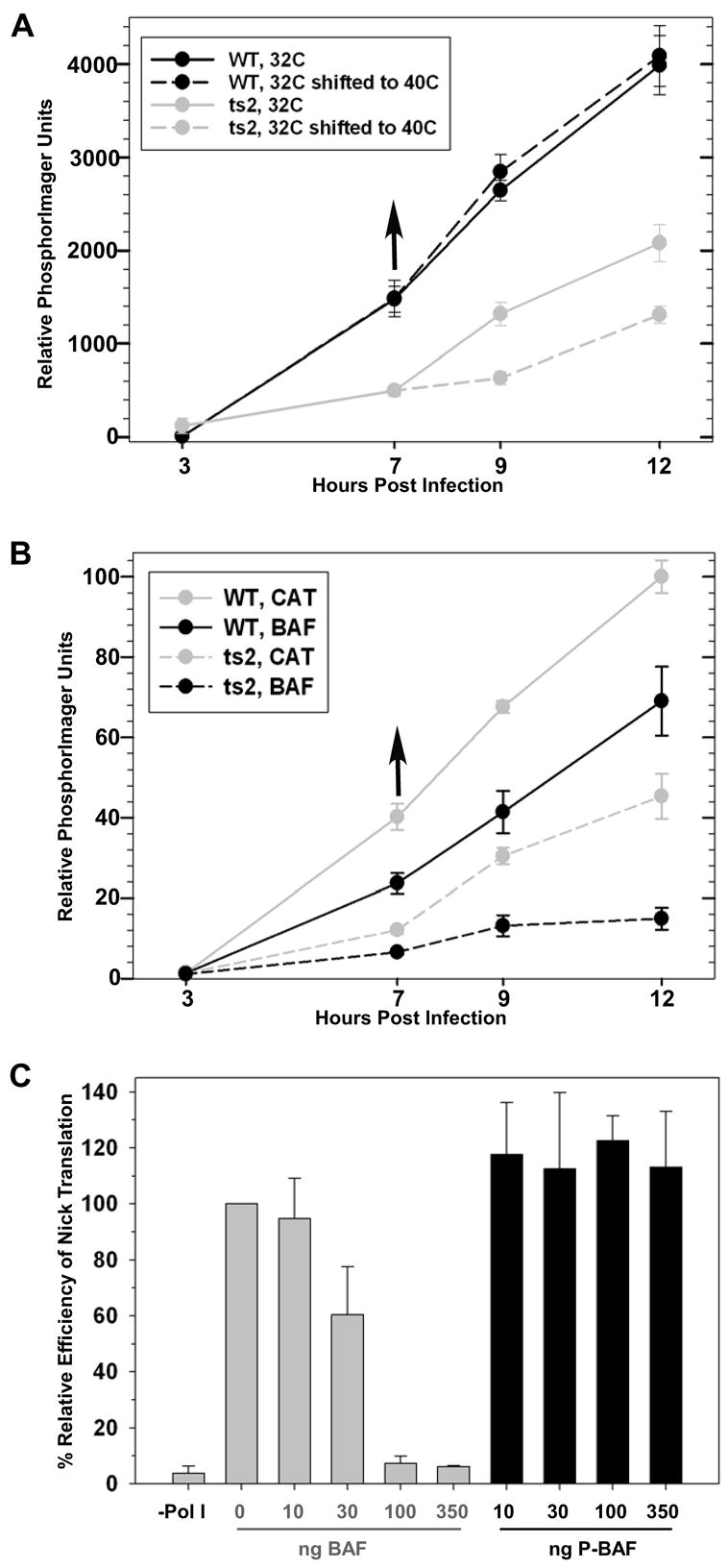
BAF Can Inhibit DNA Replication In Vivo and In Vitro. A-B) Relative rates of viral DNA accumulation were assessed by dot blot hybridization of cell lysates following wt and ts2 infection. In panel A, infections were initated in 293:BAF cells at 31.5ºC; infected cells were either left at that temperature throughout the experiment or shifted to 39.7ºC at 7 hpi. In panel B, both 293:CAT and 293:BAF cells were used and all infections were shifted to 39.7ºC at 7 hpi. Duplicate experiments were performed and analyzed in triplicate; the average values are shown, with standard errors indicated. C) Nick translation reactions were preincubated with unphosphorylated or phosphorylated BAF prior to the addition of E. coli Pol I for 1 hour, and the incorporation of radiolabeled dNTPs into DNA was measured. The average of two experiments is shown as percent efficiency compared to reactions with no BAF, with standard errors shown.
Increased BAF Levels Correlate with Decreased DNA Synthesis In Vivo and In Vitro
If BAF is capable of inhibiting viral DNA synthesis, an inverse correlation should exist between the abundance of BAF in the cytoplasm and the levels of viral DNA that are produced. To test this prediction, we compared DNA accumulation during infections performed with wt virus or ts2 in the control 293:CAT cell line and the 293:BAF cell line, each of which had been grown in 1 μg/ml tetracycline. In all cases, infections were performed at 31.5ºC for 7h, and then shifted to 39.7ºC. For both viruses, BAF overexpression correlated with a reduction of the amount of viral DNA that had accumulated at each time point assayed (Fig. 3B). At 12 hpi, the amount of DNA accumulated in 293:BAF cells infected with wt virus (black solid line) was 70% of that seen in 293:CAT cells (gray solid line). The impact of BAF overexpression on ts2 was more dramatic: the amount of DNA seen in 293:BAF cells at 12 hpi was only 33% of that found in the 293:CAT cells (compare the black and gray dashed lines).
We hypothesized that the recruitment of BAF to the sites of DNA synthesis and the subsequent inhibition of DNA replication was mediated by direct binding of nonphosphorylated BAF to viral DNA. Although several studies characterizing the BAF-DNA interaction have been published, the implications of this interaction on transcription or replication of the coated DNA have not been addressed. To assess whether BAF-bound DNA could still serve as a replication template, E. coli DNA polymerase I and plasmid DNA were incubated in standard nick translation reactions in the presence of increasing concentrations of either unphosphorylated or phosphorylated BAF. When increasing amounts of unphosphorylated BAF were added to the DNA template for 10 minutes prior to the addition of DNA Polymerase I, there was a sharp, dose-dependent decrease in the amount of nascent DNA synthesis (Fig. 3C). However, when the same experiment was performed using a BAF preparation that had been stoichiometrically phosphorylated by the B1 kinase, DNA synthesis proceeded unabated. These data clearly demonstrate that, when it is not phosphorylated, the direct interaction of BAF with DNA can act as a potent inhibitor of DNA synthesis.
Overexpression of BAF reduces viral yield; shRNA-mediated depletion of BAF rescues the temperature-sensitive phenotype of ts2
Since overexpression of BAF reduces viral DNA replication, it seemed reasonable to predict that it might also affect virus production, and so exacerbate the temperature-sensitivity of ts2. 293 cells induced to express FLAG-BAF or the chloramphenicol acetyltransferase (CAT) protein (control) were infected with wt virus or ts2 at both 31.5°C and 39.7°C, and the yield of infectious virus was determined at 24 hpi (Fig. 4A). Under the conditions used, this induction resulted in a 4 to 5 fold overexpression of FLAG-BAF vs endogenous BAF, as determined by quantitative immunoblot analysis (representative experiment shown in Fig. 4B). While wt vaccinia virus produced comparable yields in 293:CAT cells at both temperatures, ~3-fold less progeny were produced in 293:BAF at 39.7° C than at 31.5° C, indicating that overexpression of BAF might be somewhat inhibitory even in the presence of an active B1 kinase. This conclusion is supported by our finding (see Fig 3) that the levels of DNA synthesized by wt virus were reduced when BAF was overexpressed. The expected temperature-sensitivity of ts2 was evident in the control 293:CAT cell line, in which the yield of virus produced at 39.7° C was only 6.5% of that produced at 31.5° C; this phenotype is comparable to what has been previously observed in BSC40 cells (Boyle and Traktman, 2004). Even at the permissive temperature, the viral yield in the cells overexpressing BAF was 3-fold less than what was seen in the control cells, The temperature-sensitivity of the virus was also exacerbated: in the 293:BAF cells, the yield of virus produced at 39.7° C was only 1.6% of that produced at 31.5°C. Furthermore, when DNA replication was monitored in 293:BAF cells infected at 39.7° C, the levels of DNA that accumulated in ts2-infected cells was only 7% of that seen in wt-infected cells. (Supp Fig 2). These data indicate that when the B1 kinase is impaired, and the levels of BAF are elevated, completion of a productive infectious cycle is impaired.
Fig 4.
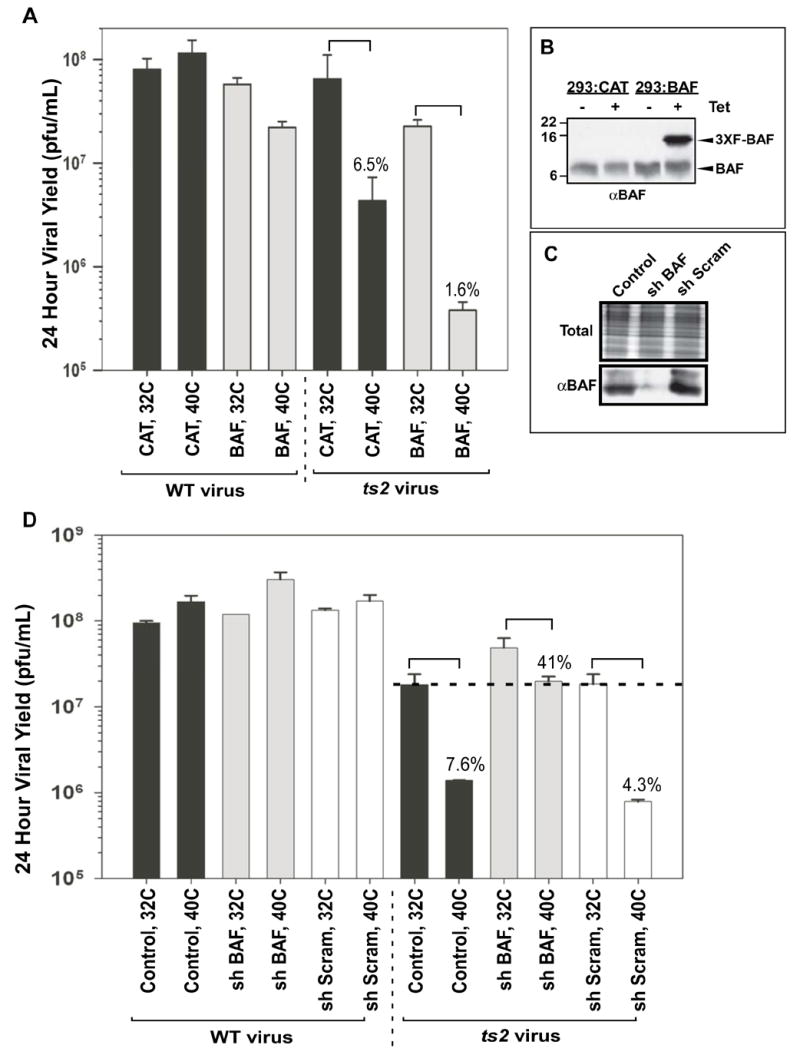
Overexpression of wild type BAF from the cellular genome exacerbates the ts2 phenotype, while shRNA-directed depletion of BAF rescues the ts2 phenotype. A-B) 293:CAT or 293:BAF cells were grown in media containing tetracycline for 6 days and either harvested for immunoblot analysis using a rabbit αBAF antibody (panel B) or plated in fresh media containing TET and then infected with wt virus or ts2 (MOI=5) at 31.5 or 39.7 C (noted as 32 and 40° C for simplicity) (panel A). All infections were harvested at 24 hpi and viral yield was determined. For each cell line, the yield of ts2 obtained at 39.7° C is expressed as a percent of that obtained at 31.5° C. C-D) 293 cells stably expressing shRNAs for 96 hours were either (C) harvested for immunoblot analysis using a rabbit αBAF antibody (total protein visualized using Coomassie) or (D) infected with wt virus or ts2 for 24h as described above. All data represent viral yields from infections titrated in duplicate or triplicate, with standard errors shown.
If unphosphorylated BAF represents a barrier to infection, then its absence should rescue the temperature-sensitivity of ts2 infections. We therefore generated replication-incompetent lentiviruses that would allow shRNA-mediated depletion of endogenous BAF. Cells were treated with a control lentivirus, a lentivirus encoding a scrambled shRNA with no target specificity, or a lentivirus encoding an shRNA whose sequence is known to target BAF efficiently (Jacque and Stevenson, 2006). Using the latter lentivirus, we consistently observed an ~85% decrease in the levels of endogenous BAF at 96 hpi (Fig 4C). After this pretreatment, each of the cultures was infected with wt vaccinia virus or ts2 at both 31.5°C and 39.7°C, and the yield of viral progeny was determined after 24h (Fig. 4D). wt virus produced comparable viral yields under all conditions. The temperature sensitivity of ts2 was clearly observed in both of the control cell lines, with the yield of virus obtained at 39.7° C being <8% of that produced at the permissive temperature. However, in the shBAF-treated cells, the yield of virus produced by ts2 at the non-permissive temperature was dramatically increased to 41% of that produced at the permissive temperature. Stated another way, in the context of a defective B1 kinase, a 6.7-fold reduction in BAF levels led to a 14-fold increased in virus production at 39.7° C. Clearly, depletion of BAF leads to a near reversal in the temperature-sensitive defect of ts2.
Unphosphorylatable BAF Cannot be Expressed from the Viral Genome
Our final prediction was that, if depletion or phosphorylation of BAF is a prerequisite for efficient viral DNA replication, then the cytoplasmic expression of an nonphosphorylatable variant of BAF should inhibit viral replication. We previously identified such a variant during our characterization of B1- and VRK1-mediated phosphorylation of BAF (Nichols et al., 2006). The BAF-MAAAQ protein, in which the B1 target sites Thr-2, Thr-3, and Ser-4 have been replaced with alanine, is no longer modified by B1 (or hVRK1) and retains DNA binding activity in the presence of the kinase. These N’ terminal amino acid substitutions prevent the phosphorylation of BAF without affecting its intrinsic DNA binding activity or its stability in vivo. To compare the impact of wt BAF and BAF-MAAAQ on the vaccinia life cycle, we attempted to generate viral recombinants expressing these proteins. The pJS4-RFP plasmid (shown in Fig. 5A), contains 2 divergent vaccinia promoters, one of which was used to drive the expression of RFP (red fluorescent protein), and the second to express the relevant BAF protein. Sequences derived from each half of the vaccinia TK locus flank this cassette and enable its stable insertion into the viral genome via homologous recombination. This recombinational insertion leads to the inactivation of the TK locus, which in turn renders the virus resistant to bromodeoxyuridine (BrdU).
Fig 5.
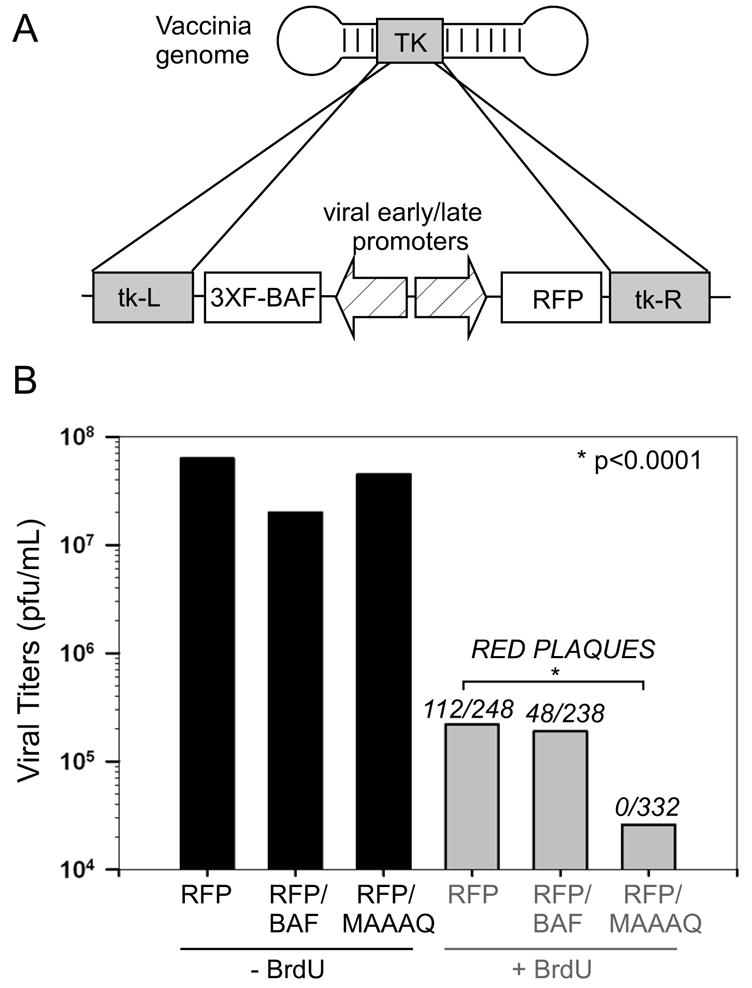
A non-phosphorylatable derivative of BAF is lethal for viral infection when expressed from the viral genome. Expression of BAF from the viral genome: Linearized pJS4-RFP, RFP+BAF, and RFP+MAAAQ plasmid DNA was introduced into BSC40 cells previously infected with wt vaccinia virus (MOI=0.03), and recombinational insertion of the transcriptional cassette into the viral TK locus was allowed to proceed during 2 days of incubation. One passage was then performed in human TK- cells +/− BrdU to select for TK-deficient virus. The viral yield was then determined by titration on BSC40 cells, and red fluorescent plaques were counted to determine the number of TK- viruses expressing RFP, and by inference, BAF or BAF-MAAAQ. The frequency of red plaques obtained in each of the three experiments was compared in pairwise fashion, and the difference between each was found to be significant using a Fisher’s exact test (p<0.0001).
Cells were infected with wt virus and transfected with linearized plasmid, the expression of which was verified by RFP fluorescence in the initial transfection (data not shown). The viral yield from these infections/transfections was passaged in the absence or presence of BrdU (to amplify all the virus present or to select for TK- viruses, respectively). In the absence of BrdU selection, all three stocks contained a similar viral titer (Fig. 5B). The total viral yield decreased markedly following BrdU selection, but equivalent titers were obtained from infections/transfections involving plasmids expressing RFP alone or RFP and wt BAF. Moreover, a significant fraction of these TK-plaques were red, confirming that their BrdU-resistance was a result of integration of the RFP (or RFP BAF) expressing cassette, rather than the acquisition of random mutations that had inactivated the TK locus. Viruses expressing wt BAF were isolated at a lower frequency than those expressing RFP alone, suggesting that constitutive expression of BAF may render these viruses somewhat less fit, although this possibility has not been explored further. Nonetheless these viruses could be readily generated, and the expression of BAF from their genome was verified by immunoblot analysis (Supp. Fig 3). We also attempted to insert the RFP or RFP↔BAF cassette into the ts2 genome at 31.5° C. While ts2 viruses expressing RFP alone were readily isolated, red plaques that were presumed to contain the RFP↔BAF cassette were ~25% of the size of non-red plaques and could only be passaged for two infectious cycles (data not shown) before infectivity was lost. Presumably, even at the permissive temperature, the compromised B1 protein produced by ts2 was insufficient to overcome the repression mediated by overexpression of BAF from the viral genome.
Our attempts to isolate wt viruses containing the RFP↔BAF MAAAQ cassette proceeded far differently than had our successful efforts to generate viruses expressing RFP or RFP + wt BAF (see above). Only 10% as much virus was recovered after passage of the progeny obtained from the infection/transfection in the presence of BrdU, and none (0 of 332) of these TK- plaques were red (Fig 5B). The inability to obtain recombinants expressing BAF MAAAQ strongly supports the conclusion that the expression of a nonphosphorylatable derivative of BAF in the cytoplasm is lethal for the virus, presumably as a result of sequestration of the viral genome.
Discussion
The cellular BAF protein is known to play important roles in regulating nuclear architecture during the cell cycle via interactions with DNA and inner nuclear membrane proteins containing LEM domains. BAF’s closest relative, BAF-like protein (BAF-L) has 40% homolog to BAF, but appears unable to bind DNA or LEM domains, indicating that these are attributes unique to BAF (Tifft et al., 2006). In addition to its nuclear localization, BAF has been observed in the cytoplasm of interphase cells (Segura-Totten and Wilson, 2004), although it has been unclear how this pool of BAF contributes to cellular function. However, early in our studies we made the observation that transiently expressed GFP-BAF could be seen to colocalize with transfected DNA, which formed DAPI-positive speckles in the cytoplasm. These data suggested that cytoplasmic BAF can, and does, recognize and bind to DNA (MSW and PT, unpublished observations).
It has become clear that BAF’s functions within the cell are regulated by phosphorylation. We have shown that the nuclear VRK1 kinase phosphorylates BAF in vitro and in mammalian cell culture, regulating its DNA-binding activity, its interaction with LEM domains, and its intracellular localization (Bengtsson and Wilson, 2006; Nichols et al., 2006). The interplay of VRK and BAF has recently been found to be operative in C. elegans as well, where is appears to regulate the association of BAF with chromatin during nuclear reassembly at the end of mitosis (Gorjanacz et al., 2007). Interestingly, the VRK kinases are closely related to the B1 kinase encoded by the vast majority of poxviruses, which are complex DNA viruses that replicate exclusively in the cytoplasm of infected cells. The fact that VRK1 can complement the replication defect of a B1 mutant of vaccinia virus indicates that the viral and cellular kinases share functional as well as structural similarity, and indeed B1 retains the ability to repress BAF’s DNA binding capability in a manner that is nearly indistinguishable from VRK (Nichols et al., 2006).
Based on these observations, we postulated that the DNA-binding activity of cytoplasmic BAF might represent a novel, intrinsic host defense against the expression of foreign DNA. Because VRK1 shows a nuclear localization, and is not available to phosphorylate cytoplasmic BAF (Lopez-Borges and Lazo, 2000; Nichols and Traktman, 2004), we reasoned that the viral B1 kinase had evolved to target the same residues on BAF which would be targeted VRK1. B1 is both encapsidated in vaccinia virions and expressed immediately upon infection (Banham and Smith, 1992; Lin et al., 1992; Rempel and Traktman, 1992), and is therefore poised to phosphorylate BAF and block its ability to bind to the viral genome as it is released from the core, allowing viral DNA replication to proceed efficiently.
In this report, we have demonstrated that endogenous BAF is indeed phosphorylated in a B1-dependent manner during vaccinia virus infections, as is a stably expressed epitope-tagged BAF. When active B1 is present, this epitope-tagged BAF is found throughout the cell; however, when infection proceeds in the presence of an impaired B1 kinase, BAF relocalizes rapidly and extensively to the sites at which viral DNA has accumulated. This localization correlates with a diminution of further viral DNA replication. Overexpression of BAF exacerbates the temperature-sensitive phenotype of the ts2 mutant, greatly reducing DNA synthesis and further diminishing virus production at the non-permissive temperature. Indeed, the overexpression of BAF has a dampening effect on viral DNA replication and the production of infectious progeny even in the presence of a wild-type B1 kinase. Our hypothesis that the association of BAF with viral DNA in vivo would impair replication, and perhaps transcription, is supported by our demonstration that BAF inhibits DNA synthesis in vitro in a dose dependent manner. Phosphorylated BAF, which lacks DNA-binding activity, has no such effect. Finally, while it remains possible that BAF also inhibits the activity of vaccinia proteins in vivo through direct protein:protein interactions, our in vitro studies utilized no viral proteins, demonstrating that BAF’s repressive ability relies only on its interaction with DNA.
To complement our analysis of the impact of BAF overexpression, we utilized shRNA technology to assess the impact of BAF depletion. Depletion of BAF had a striking effect, leading to a near reversal of the temperature-sensitive phenotype of the ts2 mutant. These data imply that the primary and most vital role played by the B1 kinase during the vaccinia virus life cycle is to overcome the repressive effect of BAF.
As another test of whether phosphorylation of BAF was as vital as it appeared to be for efficient viral replication, we attempted to generate viral recombinants expressing wt BAF or a nonphosphorylatable derivative of BAF. While viruses expressing BAF were readily isolated, we were unable to isolate any recombinants expressing the BAF-MAAAQ derivative. The expression of nonphosphorylatable BAF from the viral genome, where it is translated in close proximity to the viral genome, is in fact lethal to the virus.
Cells have numerous mechanisms at their disposal to detect invaders, but viruses have evolved equally numerous countermeasures that enable them to direct successful infections. It is the efficiency with which these countermeasures work that often define the host range of a particular virus. Interestingly, molluscum contagiosum virus (MCV) is the only poxvirus that lacks a B1 homolog, and this virus exhibits a very narrow host range. It is only able to replicate in the stratified epithelium of human skin, and has not been successfully passaged in any cell culture system (Birthistle and Carrington, 1997). We speculate that the keratinocyte layer that is permissive for MCV infection may either have very low levels of cytoplasmic BAF or contain a cytoplasmic pool of a kinase (like VRK) that can phosphorylate and neutralize BAF. Alternatively, MCV may encode an as yet unknown protein that overcomes BAF via a distinct mechanism.
This duel between BAF and B1 is a newly identified example of the intricate interplay between pathogens and their hosts. Among the most well-characterized mediators of this innate surveillance against infection are the toll-like receptors, which are capable of responding to a variety of foreign molecules including DNA and RNA (Vasselon and Detmers, 2002). However, the absence of TLRs from most non-immune cells has stimulated speculation that other sensors of foreign nucleic acid must exist (Ishii et al., 2006; Stetson and Medzhitov, 2006; Wagner and Bauer, 2006). Indeed, the elucidation that the RNA helicase RIG-I can act as a cytoplasmic RNA sensor, and indeed can trigger the downstream activation of host response genes indicates that this is true (Meylan and Tschopp, 2006). Because BAF is a highly conserved protein (Supp Fig 4) which has been found in all eukaryotic dividing cells tested (Mansharamani et al., 2003), and which binds DNA avidly and without regard to sequence specificity, our findings raise the possibility that BAF might be a central component of a widespread host defense which recognizes and silences foreign DNA. It will be of significant interest to determine whether, subsequent to its binding to foreign DNA, BAF may function like RIG-I and contribute to a signaling cascade that alerts cells of a possible infection.
Experimental Procedures
Cloning and Production of Stable Cell Lines
pcDNA5/FRT/TO/3XFLAG-BAF was constructed by amplifying the BAF alleles from p3XFLAG-BAF (Nichols et al., 2006) with the primers FLAGBAF-UPBam (5’-GAGGGTACCGGATCCGCACCATGGACTACAAA -3’) and BAF-DN. This places a BamHI sequence (underlined) both upstream and downstream of the ORF, which were used to clone into the BamHI site present in the pcDNA5/FRT/TO.
Stable integration of CAT and 3XFLAG-BAF in 293 cells was accomplished using the Flp-In System (Invitrogen, Carlsbad, CA), which employs 293 Trex cells (Invitogen) containing two stably integrated plasmids. The first plasmid pcDNA6/TR constituitively expresses TetR; a single integrated copy of the second plasmid pFRT/lacZeo contains the yeast FRT recombination site. These cells were co-transfected with pcDNA5/FRT/TO-CAT or -3XFLAG-BAF and pOG44, a plasmid expressing the S. cerevisiae Flp recombinase. Expression of Flp mediates a single recombination event between the FRT site in pcDNA5/FRT/TO plasmids and the FRT site integrated into the 293 genome, thus ensuring that the resulting 293:CAT and 293:BAF cells express each of their exogenous genes from a single copy at the same genetic locus. Properly targeted cells are selected by growth in 100 μg/ml hygromycin and 15 μg/ml blasticidin; expression is uniform because all cells express the protein from a single copy of a cDNA inserted at the same genomic locus under the regulation of the same promoter.
Cloning and production of recombinant viruses
pJS4-RFP-BAF and –MAAAQ were constructed by amplifying the BAF alleles from p3XFLAG-BAF and p3XFLAG-BAF-MAAAQ with the primers FLAG-BAF-UPXho (5’-CAGCTCGAGGCCACCATGGACTACAAAGACC -3’) and BAF-DN (5’-GCTGAATTCGGATCCTCACAAGAAGGCG -3’). This places a XhoI site (bold) upstream and a BamHI site (underlined) downstream of the ORF, which were used to clone into the corresponding sites present in pJS4-RFP (Chakrabarti et al., 1997).
Virus and Infections
Virus preparation- Wild-type vaccinia virus (WR strain), ts2 and ts2:hVRK1 (Boyle and Traktman, 2004) were grown in BSC40 cells at 37°C or 31.5°C, respectively. Viral stocks were prepared from cytoplasmic lysates of infected cells by ultracentrifugation through 36% sucrose and then titrated on BSC40 cells. During infections, 31.5 and 39.7°C were used as the permissive and nonpermissive temperatures, respectively, and are referred to in Figures as 32 and 40°C.
24 hour yield- Confluent 35-mm-diameter dishes of 293:CAT or 293:BAF cells were infected at an MOI of 5 and maintained at 31.5 or 39.7°C. Cells were harvested at 24 hpi in 1 mM Tris (pH 9.0). Virus yields were assessed by plaque assays performed on BSC40 cells at 31.5°C. Three separate experiments were performed, and each was titrated in triplicate; the average value is shown with standard errors indicated.
Immunofluorescence
293:BAF cells were grown in 1 μg/ml tetracycline for 3 days prior to plating on chamber slides (Lab-Tek, Naperville, IL) 48 hours before infection and/or fixation. Infections were performed at an MOI=5. At 9 hpi cells were fixed in 4% PFA, 4% sucrose in phosphate-buffered saline (PBS; 140 mM NaCl, 2 mM KCL, 10 mM Na2HPO4, 1 mM KH2PO4, [pH 7.4]) at room temperature for 15 min, permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature and then blocked in PBS/5% BSA for 30 min. Cells were then incubated with mouse α-FLAG M2 antibody (Sigma) and rabbit α-I3, both at a dilution of 1:400 in PBS/1% BSA. This was followed by Alexa-fluor 594-conjugated goat α-mouse and Alexfluor 488-conjugated goat α-rabbit-488 (Molecular Probes) at 1:500 in PBS/1% BSA. DNA was stained with DAPI. Proteins were observed by indirect fluorescence on a Nikon Eclipse TE2000-U microscope (Melville, NY). Images were pseudocolored using MetaMorph software (Sunnyvale, CA) and labeled using Canvas 8 (Deneba Systems, Miami, FL).
Cell Culture and Reagents
Human thymidine kinase-negative 143B osteosarcoma cells (TK-) and African green monkey BSC40 kidney epithelial cells were maintained as monolayers in DMEM, supplemented with 5% fetal bovine serum (FBS; GIBCO/Invitrogen, Carlsbad, CA) in the presence of 5% CO2. Human osteosarcoma U2OS cells were maintained in DMEM with 10% FBS. Flp-In Trex 293 HEK cells were obtained from Invitrogen and grown in DMEM with 10% FBS, 50μg/ml hygromycin, and 7.5μg/ml blasticidin after they had been stably transfected with pcDNA5/FRT/TO-CAT or -3XFLAG-BAF. The following plasmids were used in this study: p3XFLAG-BAF (Nichols et al., 2006), and pcDNA5/FRT/TO (Invitrogen). pJS4 was generously provided by B. Moss (NIAID, Bethesda, MD); the pJS4:RFP derivative was previously generated in our laboratory by Jason Mercer (unpublished).
Cell Lysis and Immunoblotting
Approximately 1 x 106 U2OS or BSC40 were transiently transfected with 1 μg of p3XFLAG-BAF or empty vector using Lipofectamine 2000. 1 x 106 293:BAF cells were induced to express the relevant protein with 1 μg/ml tetracycline. At 16 h posttransfection or post-induction the cells were infected with wild type virus, ts2, or ts2/hVRK1 and at 5 hpi were resuspended in ice-cold fractionation buffer (20 mM Tris pH 7.4, 5 mM MgCl2, 5 mM DTT, 150 mM KCl, and 0.5% Triton X-100) containing 1 mM NaF, 1 mM sodium orthovanadate, 40 mM β-glycerol phosphate, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 mM PMSF. After 10 min of incubation on ice, sucrose was added to 10% and the insoluble fraction was retrieved by centrifugation at 1000 x g for 10 min. The supernatant was resolved by SDS-PAGE and subjected to immunoblot analysis with α-FLAG M2 antibody. When indicated, U2OS cells were radiolabeled by replacing the media with phosphate-free DMEM (MP Biomedical, Irvine, CA) supplemented with 100 μCi/ml 32PPi from 20 to 24 h posttransfection, and FLAG-BAF was then retrieved from the soluble fraction of radio-labeled extracts using rabbit α-FLAG sepharose, washed, and eluted in SDS sample buffer before analysis by immunoblot and autoradiography.
Antibodies
Antiserum specific for vaccinia single stranded binding protein I3 has been described (Rochester and Traktman, 1998). Rabbit α-BAF antibody recognizing total BAF was generously provided by Dr. Katherine Wilson (Johns Hopkins Univ., Baltimore, MD). An antibody specific for BAF modified by phosphorylated on Ser-4 was generated in rabbits by Bethyl Laboratories, Inc. (Montgomery, TX). Antibody was raised against the KLH-conjugated peptide MTT(pS)QKHRDFC, and phosphospecific antibody was isolated from serum by sequential negative and positive affinity purification.
Production and Use of shRNA Lentiviruses
The pLL3.7-shBAF construct was produced by annealing the primers shBAF-UP (5’-TGGCCTATGTTGTCCTTGGCTTCAAGAGAGCCAAGGACAACATAGGCCTTTTT GGAAAC-3’) and shBAF-DN (5’-TCGAGTTTCCAAAAAGGCCTATGTTGT CCTTGGCTCTCTTGAAGCCAAGGACAACATAGGCCA-3’) and cloning them into the HpaI and XhoI sites of pLL3.7/Puro ((Rubinson et al., 2003)#). The pLL3.7-Scram construct was produced by annealing shScram-UP (5’-TCAGTCGCGTTTGCGACTGGTTCAAGAGACCAGT CGCAAACGCGACTGTTTTTGGAAAC-3’) and shScram-DN (5’-TCGAGTTTCCAAAAACAGTCGCGTTTGCGACTGGTCTCTTGAACCAGTCGCAAACG CGACTGA-3’) and cloning the product into the HpaI and XhoI sites of pLL3.7/Puro. Lentiviruses expressing shRNAs were produced in 293T cells following transfection with either pLL3.7, pLL3.7-shBAF, or pLL3.7-shScram in combination with pMD2.G, pRSV REV, and pMDLg/pRRE encoding the VSV-G, Rev, and Gag/Pol genes, respectively. At 16 hours post transfection, fresh media was added to cells. At 48 hours post transfection, the media containing recombinant virus was harvested, polybrene was added to 8 μg/mL, and stocks were aliquoted for storage at -80ºC.
To inhibit BAF expression, 293:CAT cells were infected with lentivirus containing pLL3.7, -shBAF, or –shScram for 24 hours, followed by selection of stable integrants in the presence of 5 μg/ml puromycin. At 96 hpi with lentivirus, cells were infected with either wt or ts2 vaccinia virus (MOI=5) and placed at 31.5ºC or 39.7ºC for 24 hours. Cells were then harvested and the yield of vaccinia virus was determined. Alternatively, at 96 hpi with lentivirus, cells were harvested and the rabbit α-BAF antibody was used in immunoblot analysis to assess the extent of BAF depletion.
Dot blot hybridization to assess viral DNA accumulation
Confluent 35-mm-diameter dishes of 293:CAT or 293:BAF cells were infected with WT virus or ts2 at an MOI of 5 and incubated at 31.5 or 39.7°C. Cells were harvested at the indicated time points (3, 7. 9, and 12 hpi). Subsequent lysis of the cells and dot blot hybridization was performed as previously described (Boyle and Traktman, 2004).
Production of recombinant BAF and Use in Nick Translation Studies
Dimeric BAF was expressed and purified from BL21(DE3)pLysS cells as described (Nichols et al., 2006). BAF was phosphorylated in vitro using purified, recombinant B1 kinase (or included in mock reactions containing no kinase) under conditions known to phosphorylate BAF to completion (Nichols et al., 2006). These reactions were used as the source of BAF in the nick translation reactions described below.
Nick translation reactions (11 μl) were assembled by first combining the pBR322:vvHindIII E plasmid, unlabeled GTP and CTP, radiolabeled ATP and TTP, and DNase I (200ng/mL final concentration) on ice. Various amounts of phosphorylated or unphosphorylated recombinant BAF (0, 10, 30, 100, 350 ng) were then added to the reactions, which were moved to room temperature for 10 minutes. DNA Polymerase I (New England Biolabs, Ipswitch, MA) was then added to the reactions, which were then placed at 16ºC for 1 hour. Reactions were stopped by the addition of 40 μL of TE buffer and placed on ice; unincorporated nucleotides were removed by size exclusion spin chromatography. DNA synthesis, as reflected by the incorporation of radiolabeled dNTPs into DNA was quantified by Çerenkov counting.
Supplementary Material
Supp Fig 1: Rabbit αpSer4-BAF is specific for phosphorylated BAF. The indicated amounts of recombinant BAF, either unphosphorylated or phosphorylated by VRK1, were subjected to immunoblot analysis with the αpSer4-BAF antibody or with an αBAF serum that recognizes both modified and unmodified BAF. The unphosphorylated BAF was not recognized by the αpSer4-BAF antibody at any of the amounts tested.
Supp Fig 2: Minimal DNA replication occurs during non-permissive infections of 293:BAF cells by ts2. 293:BAF cells were infected with either wt virus or ts2; infections were initated and maintained at 39.7° C. Parallel cultures were harvested at 7, 12, and 24 hpi, and the temporal profile of viral DNA replication was assessed by dot blot hybridization. Duplicate experiments were performed and analyzed in duplicate; the average values are shown, with standard errors indicated.
Supp Fig 3: Recombinant virus containing pJS4-RFP-BAF expresses FLAG-BAF. Red plaques identified in studies shown in Figure 5 were isolated and the viruses expanded on BSC40 cells. Infected cell lysates were subjected to immunoblot analysis using αFLAG or αI3 antibody.
Supp Fig 4: BAF is highly conserved in diverse eukaryotes. Conservation of N-terminal serine and threonine residues known to be targeted by B1 are boxed in red. Residues known to contribute to DNA binding and α-helices have been previously defined (Bradley et al., 2005; Harris and Engelman, 2000; Segura-Totten et al., 2002; Zheng et al., 2000).
Acknowledgments
We thank Drs. Kathy Boyle, Stephen Duncan, Amy Hudson, and R. Jeremy Nichols for critical analysis and comments during the preparation of this manuscript. This work was supported by a post-doctoral grant to MSW from the Great Lakes Regional Center for Excellence in Biodefense and Emerging Infectious Diseases (U54 AI-057153) and by grants to PT from the NIH (2 R01 AI 21758) and the MCW Advancing a Healthier Wisconsin Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Banham AH, Smith GL. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992;191:803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- Bengtsson L, Wilson KL. Barrier-to-autointegration factor phosphorylation on Ser-4 regulates emerin binding to lamin A in vitro and emerin localization in vivo. Mol Biol Cell. 2006;17:1154–1163. doi: 10.1091/mbc.E05-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birthistle K, Carrington D. Molluscum contagiosum virus. J Infect. 1997;34:21–28. doi: 10.1016/s0163-4453(97)80005-9. [DOI] [PubMed] [Google Scholar]
- Boyle K, Traktman P. Members of a novel family of mammalian protein kinases complement the DNA− phenotype of a vaccinia virus ts mutant defective in the B1 kinase. J Virol. 2004;78 doi: 10.1128/JVI.78.4.1992-2005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CM, Ronning DR, Ghirlando R, Craigie R, Dyda F. Structural basis for DNA bridging by barrier-to-autointegration factor. Nat Struct Mol Biol. 2005;12:935–936. doi: 10.1038/nsmb989. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- Chen H, Engelman A. The barrier-to-autointegration protein is a host factor for HIV type 1 integration. Proc Natl Acad Sci U S A. 1998;95:15270–15274. doi: 10.1073/pnas.95.26.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Sugiyama S, Osouda S, Goto H, Inagaki M, Horigome T, Omata S, McConnell M, Fisher PA, Nishida Y. Barrier-to-autointegration factor plays crucial roles in cell cycle progression and nuclear organization in Drosophila. J Cell Sci. 2003;116:3811–3823. doi: 10.1242/jcs.00682. [DOI] [PubMed] [Google Scholar]
- Gorjanacz M, Klerkx EP, Galy V, Santarella R, Lopez-Iglesias C, Askjaer P, Mattaj IW. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. EMBO J. 2007;26:132–143. doi: 10.1038/sj.emboj.7601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi T, Koujin T, Segura-Totten M, Lee KK, Matsuoka Y, Yoneda Y, Wilson KL, Hiraoka Y. BAF is required for emerin assembly into the reforming nuclear envelope. J Cell Sci. 2001;114:4575–4585. doi: 10.1242/jcs.114.24.4575. [DOI] [PubMed] [Google Scholar]
- Harris D, Engelman A. Both the structure and DNA binding function of the barrier-to-autointegration factor contribute to reconstitution of HIV type 1 integration in vitro. J Biol Chem. 2000;275:39671–39677. doi: 10.1074/jbc.M002626200. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- Jacque JM, Stevenson M. The inner-nuclear-envelope protein emerin regulates HIV-1 infectivity. Nature. 2006;441:641–645. doi: 10.1038/nature04682. [DOI] [PubMed] [Google Scholar]
- Lee MS, Craigie R. A previously unidentified host protein protects retroviral DNA from autointegration. Proc Natl Acad Sci U S A. 1998;95:1528–1533. doi: 10.1073/pnas.95.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Engelman A. The barrier-to-autointegration factor is a component of functional human immunodeficiency virus type 1 preintegration complexes. J Virol. 2003;77:5030–5036. doi: 10.1128/JVI.77.8.5030-5036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Chen W, Broyles SS. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J Virol. 1992;66:2717–2723. doi: 10.1128/jvi.66.5.2717-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Borges S, Lazo PA. The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene. 2000;19:3656–3664. doi: 10.1038/sj.onc.1203709. [DOI] [PubMed] [Google Scholar]
- Mansharamani M, Graham DR, Monie D, Lee KK, Hildreth JE, Siliciano RF, Wilson KL. Barrier-to-autointegration factor BAF binds p55 Gag and matrix and is a host component of human immunodeficiency virus type 1 virions. J Virol. 2003;77:13084–13092. doi: 10.1128/JVI.77.24.13084-13092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit A, Segura-Totten M, Gruenbaum Y, Wilson KL. Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc Natl Acad Sci U S A. 2005;102:3290–3295. doi: 10.1073/pnas.0408364102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Nichols RJ, Traktman P. Characterization of three paralogous members of the Mammalian vaccinia related kinase family. J Biol Chem. 2004;279:7934–7946. doi: 10.1074/jbc.M310813200. [DOI] [PubMed] [Google Scholar]
- Nichols RJ, Wiebe MS, Traktman P. The vaccinia-related kinases phosphorylate the N' terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol Biol Cell. 2006;17:2451–2464. doi: 10.1091/mbc.E05-12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K, Joklik WK. Hybridization and sedimentation studies on early and late vaccinia messenger RNA. J Mol Biol. 1967;27:395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Rempel RE, Anderson MK, Evans E, Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990;64:574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel RE, Traktman P. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J Virol. 1992;66:4413–4426. doi: 10.1128/jvi.66.7.4413-4426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester SC, Traktman P. Characterization of the single-stranded DNA binding protein encoded by the vaccinia virus I3 gene. J Virol. 1998;72:2917–2926. doi: 10.1128/jvi.72.4.2917-2926.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Segura-Totten M, Kowalski AK, Craigie R, Wilson KL. Barrier-to-autointegration factor: major roles in chromatin decondensation and nuclear assembly. J Cell Biol. 2002;158:475–485. doi: 10.1083/jcb.200202019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Totten M, Wilson KL. BAF: roles in chromatin, nuclear structure and retrovirus integration. Trends Cell Biol. 2004;14:261–266. doi: 10.1016/j.tcb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shumaker DK, Lee KK, Tanhehco YC, Craigie R, Wilson KL. LAP2 binds to BAF. DNA complexes: requirement for the LEM domain and modulation by variable regions. EMBO J. 2001;20:1754–1764. doi: 10.1093/emboj/20.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun MC, Daigle JE, Vandegraaff N, Engelman A. Wild-type Levels of Human Immunodeficiency Virus Type 1 Infectivity in the Absence of Cellular Emerin Protein. J Virol. 2006 doi: 10.1128/JVI.01953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Craigie R. Regulatory mechanisms by which barrier-to-autointegration factor blocks autointegration and stimulates intermolecular integration of Moloney murine leukemia virus preintegration complexes. J Virol. 2002;76:12376–12380. doi: 10.1128/JVI.76.23.12376-12380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tifft KE, Segura-Totten M, Lee KK, Wilson KL. Barrier-to-autointegration factor-like (BAF-L): a proposed regulator of BAF. Exp Cell Res. 2006;312:478–487. doi: 10.1016/j.yexcr.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Vasselon T, Detmers PA. Toll receptors: a central element in innate immune responses. Infect Immun. 2002;70:1033–1041. doi: 10.1128/IAI.70.3.1033-1041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Bauer S. All is not Toll: new pathways in DNA recognition. J Exp Med. 2006;203:265–268. doi: 10.1084/jem.20052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Ghirlando R, Lee MS, Mizuuchi K, Krause M, Craigie R. Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc Natl Acad Sci U S A. 2000;97:8997–9002. doi: 10.1073/pnas.150240197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Fig 1: Rabbit αpSer4-BAF is specific for phosphorylated BAF. The indicated amounts of recombinant BAF, either unphosphorylated or phosphorylated by VRK1, were subjected to immunoblot analysis with the αpSer4-BAF antibody or with an αBAF serum that recognizes both modified and unmodified BAF. The unphosphorylated BAF was not recognized by the αpSer4-BAF antibody at any of the amounts tested.
Supp Fig 2: Minimal DNA replication occurs during non-permissive infections of 293:BAF cells by ts2. 293:BAF cells were infected with either wt virus or ts2; infections were initated and maintained at 39.7° C. Parallel cultures were harvested at 7, 12, and 24 hpi, and the temporal profile of viral DNA replication was assessed by dot blot hybridization. Duplicate experiments were performed and analyzed in duplicate; the average values are shown, with standard errors indicated.
Supp Fig 3: Recombinant virus containing pJS4-RFP-BAF expresses FLAG-BAF. Red plaques identified in studies shown in Figure 5 were isolated and the viruses expanded on BSC40 cells. Infected cell lysates were subjected to immunoblot analysis using αFLAG or αI3 antibody.
Supp Fig 4: BAF is highly conserved in diverse eukaryotes. Conservation of N-terminal serine and threonine residues known to be targeted by B1 are boxed in red. Residues known to contribute to DNA binding and α-helices have been previously defined (Bradley et al., 2005; Harris and Engelman, 2000; Segura-Totten et al., 2002; Zheng et al., 2000).


