Abstract
The incidence of cancer has increased over the last decade, mainly due to an increase in the elderly population. Vaccine therapy for cancer is less toxic than chemotherapy or radiation and could be, therefore, especially effective in older, more frail cancer patients. However, it has been shown that older individuals do not respond to vaccine therapy as well as younger adults. This has been attributed to T cell unresponsiveness, a phenomenon also observed in cancer patients per se. This review summarizes the current knowledge of T cell unresponsiveness in cancer patients and elderly, the results of cancer vaccination in preclinical models and in clinical trials, and recent data of cancer vaccination at young and old age in preclinical models. Finally, experimental approaches will be proposed how to make cancer vaccines more effective at older age.
Keywords: Cancer vaccines, Aging, Immune system, Breast cancer, Preclinical models
T cell unresponsiveness in cancer patients
Cytotoxic T lymphocytes (CTL) are considered to be the most important players in anti-tumor reactions. The T cell receptor (TCR) of CTL recognizes tumor-associated antigens (TAA) in association with major histocompatibility complex (MHC) molecules on the tumor cells. As has become evident from in vitro studies, these CTL are activated when exposed simultaneously to both TAA/self-MHC complexes and co-stimulatory molecules, resulting in CTL-mediated tumor cell cytolysis. In cancer patients, CTL are often found at the site of the tumor, but have evidently been unable to destroy the tumor cells (Gravekamp et al., 1990). Multiple possible causes have been described for this unresponsiveness of the CTL in cancer patients (for a review, see Schreiber, 1998; Gravekamp 2001), some of which are discussed below. For example, low expression of the co-stimulatory molecule B7.1 on human metastatic carcinoma cells in gastrointestinal tumors (Koyama et al., 1998) has been suggested as a possible cause of T cell unresponsiveness because the interaction between co-stimulatory molecules and its ligand is an absolute need for T cell activation. Other described co-stimulatory or adhesion molecules that may play a role in T cell unresponsiveness are ICAM-1, VCAM-1 or ELAM-1 (Maurer et al., 1998). In mice, the importance of co-stimulatory molecules such as 4-1BBL or B7.1, for CTL activation, has been reported as well, (Loo et al., 1997; Melero et al., 1997). T cell unresponsiveness could also be due to low expression of self-MHC on tumor cells, which is required for recognition of tumor-specific antigens by the TCR. Low expression of MHC molecules has been commonly found in human metastatic tumor cells, such as metastatic melanoma, breast cancer or colon cancer (Garrido et al., 1997). Similarly, low expression of MHC molecules has been observed in mouse tumors, such as primary brain tumors (Akbasak et al., 1991), fibrosarcoma (Pedrinaci et al., 1999), melanoma (Weber and Rosenburg, 1990), or lung carcinoma (Blieden et al., 1998). TAA-based vaccination circumvents the poor antigen-presenting function of tumor cells, since the TAA are delivered to professional antigen-presenting cells (APC), such as dendritic cells (DC) and macrophages, which highly express MHC, TAA (derived from the vaccine), and co-stimulatory molecules, and subsequently activates TAA-specific CTL.
T cell tolerance may be the most important obstacle for successful immunotherapy against cancer (for a review, see Zou, 2006). Since most TAA are weakly expressed on normal cells, the immune system will recognize them as self. Earlier in life T cells are taught in the primary lymphoid organs not to respond to self-antigens. Therefore, it is difficult to induce a strong immune response against most TAA. Evidence exists in humans and mice that regulatory T cells (Tregs), expressing CD4CD25, are crucial for maintaining T cell tolerance to self-antigens. More recent studies have shown that transcription factor forkhead box P3 (FOXP3) is crucial for the development of functional Tregs (CD4CD25FOXP3). Many strategies have been discussed and tested in animal models that target the inactivation or elimination of Tregs, in order to enhance vaccine efficacy. For instance, treatment with a tumor cell-based vaccine secreting GM-CSF, combined with anti-CTLA-4 antibodies (CTLA-4 is constitutively expressed in Tregs) in the B16/B6 melanoma mouse tumor model resulted in eradication of primary tumors and prevention of lung metastases, This was accompanied with improved antitumor responses (van Elsas et al., 1999). However, in humans with stage IV melanoma or renal cancer administration of anti-CTLA-4-specific antibodies did not inhibit suppressive activity of Tregs in vitro or in vivo (Maker et al., 2005). Questions arise whether the age of cancer patients may have played a role here. While the C57BL/6 mice injected with the B16-BL6 tumor cells were relatively young at the time of vaccination (8–12 weeks of age), most cancer patients, including patients with stage IV melanoma or renal carcinoma, are relatively old (> 50 years of age). Genetic instability often occurs in tumors of humans and mice resulting in loss of antigen expression (Corver et al., 2000; Sypniewska et al., 2005). Tumor cells that have lost TAA expression are able to escape vaccine-induced immune responses and generate new tumors. This underlines the need of multi-antigen vaccines. In humans and mice, many tumors secrete lymphokines or factors that inhibit vaccine-induced immune responses. Examples are TGFβ, IL-6, cyclooxygenase-2 (COX-2) and its products prostaglandine E, PD1-ligand, or indolamine 2,3-dioxygenase (IDO) (Gajewski et al., 2006; Park et al., 2004). Reduction of these lymphokines or factors may enhance vaccine efficacy.
In order to develop effective cancer vaccines, many obstacles have to be overcome. However, an additional problem almost totally ignored in the development of cancer vaccines, is ageing of the immune system.
T cell unresponsiveness in elderly
T cell unresponsiveness has also been reported for the elderly in general, including non-cancer patients (Miller et al., 1996). Below, alteration in several immunological parameters that may contribute to the age-related decline in T cell responses is discussed. In this discussion, human and mouse are compared. For instance, a decrease in the number of naive T cells (capable of reacting to new antigens) and an increase in the number of memory T cells (capable of reacting to previously exposed antigens) in elderly humans as compared to young adults have been reported (Utsuyama et al., 1992). It has been suggested that continual activation of the immune system by new antigens during the life span would lead to a depletion of naive T cells from the thymus, and a clonal expansion of memory T cells. With the involution of the thymus almost complete at the age of 60 years, new naive T cells at old age can no longer be generated (Grubeck-Loebenstein, 1997). The host is then dependent on the pool of naïve T cells generated earlier in life. Analogous to the situation in humans, a decrease of naïve T cells and an increase of memory T cells have also been described for aging mice (for a review see George and Ritte, 1996). Other possible causes for diminished T cell responses in aged humans and mice have been described, such as defects in TCR/CD3-mediated phosphorylation events or aberrant regulation of tyrosine kinases associated with the TCR (Tamir et al., 2000). An age-related decrease in the αβ repertoire of the human TCR has been described that may lead to diminished T cell responses (Wack et al., 1998). Another molecule important for T cell activation is CD28. CD28 is expressed at the cell membrane of T cells, and is the ligand for co-stimulatory molecule B7, expressed on APC. Clinical studies have documented that high proportions of CD8 T cells that lack CD28 are correlated with reduced antibody response to influenza vaccination (for a review, see Effros, 2006). Also in mice, CD8 T cells lacking CD28 expression have been reported (for a review, see Effros, 2004). Moreover, it has been shown that CD28-lacking CD8 T cells can suppress antigen-specific CTL responses (Filaci et al., 2004).
It is only during the last few years that vaccine studies are focused on the elimination of regulatory T cells. Regulatory T cells (CD4+CD25+) are naturally occurring regulatory/suppressive CD4 T cells, present at young and old age. They are able to inhibit TAA-induced immune responses (For a review see Zou, 2006). Recently, it has been shown that the age-related decline in T-cell-mediated immune responses can be ascribed to changes in the CD4+CD25−FOX3+ T cell population and not to a functional augmentation of suppressive CD4+CD25+FOX3+ T cells (Nishioka et al., 2006).
In addition to the problems at the level of T cells and/or tumor cells, defects in cytokine production have been observed in aged humans. An example is a human vaccine study in which significantly lower IL-2 was produced by T cells of older individuals stimulated with an influenza vaccine in vitro compared to those of young individuals (McElhaney et al., 1994). Similarly, significantly lower IFNγ was produced by peripheral blood nuclear cells (PBNC) from elderly individuals immunized with an influenza vaccine compared to young individuals (Quyang et al., 2000). IL-2 promotes T cell activation and proliferation, as well as release of IFNγ by T cells. The lower IL-2 production following in vitro stimulation with the influenza vaccine may explain the lower IFNγ production. IFNγ is involved in activation of APC such as macrophages. These macrophages are important for CTL priming.
Defects in other cell types than CTL and/or tumor cells may also explain T cell unresponsiveness in cancer. Antigen presenting cells (APC), such as dendritic cells (DC) in blood or Langerhans cells in skin, play a central role in T cell activation. These cells are often called “professional“ APC because they enable efficient processing of foreign proteins into peptides, and because they express MHC and co-stimulatory molecules at the high levels required for optimal presentation of the peptides to the immune system and subsequent stimulation of T cells. Tumor cells are poor APC, because co-stimulatory molecules and self-MHC, as well as TAA required for CTL stimulation are often weakly expressed. Therefore, DC loaded with peptides or fused with autologous tumor cells are frequently used in clinical trials as an anticancer immunotherapy to stimulate T cells in cancer patients. Since APC play such an important role in T cell activation, one might question whether this cell type could be involved in the age-related decline of T cell responsiveness. Although it has been shown in mice and humans that the number of Langerhans cells decreases with age, resulting in impaired immune function of the skin (Sprecher et al., 1990; Sunderkotter et al., 1997), the function of blood DC appeared to be unimpaired. For instance, it has been demonstrated that blood DC from old individuals can still function as powerful antigen-presenting cells when exposed to purified protein derivate (PPD) of Mycobacterium tuberculosis (Lung et al., 2000). Similarly, the responsiveness of blood DC to stimulation with influenza vaccine was unimpaired at old age (Sauerwein-Teissl et al., 1998). In both cases, expression patterns of MHC molecules and co-stimulatory molecules were found to be similar on blood DC of young and old individuals. These data suggest that DC in blood are not the primary target cell for the age-related decline of T cell responsiveness. However, DC have never been studied in older cancer patients. Therefore, especially those DC in the lymph nodes that present TAA to naïve T cells might be an interesting target in the study of age–related declines in T cell responsiveness in relation to cancer. Other APC, such as macrophages, appeared to secrete significantly lower levels of IL-6 and TNFα when stimulated with known ligands for the Toll-like receptors TLR1/2, TLR2/6, TLR3, TLR4, TLR5, and TLR9 in old than young mice (Renshaw et al., 2002). TLR are pattern recognition receptors that recognize conserved molecular patterns on microbes and activate the innate and adaptive immune system. Table 1 schematically lists the possible changes in antigens and receptors on tumor-specific CTL, tumor cells or APC, or factors secreted by tumor cells that could potentially be involved in diminished T cell responsiveness to cancer at old age.
Table 1. Tumor-immunological parameters potentially involved in T cell unresponsiveness.
| *Molecules on the membrane of Tumor cells and/or APC | CTL | **Lymphokine/receptors | ***Immune-inhibiting factors or cells |
|---|---|---|---|
| MHC/TAA | TCR | IL-2/IL-2 receptor | TGFβ |
| B7.1 | CD28 | IFNγ/IFNγ receptor | IL-6 |
| B7.2 | CTLA-4 | IDO | |
| ICAM-1/2 | CD49d | COX-2 | |
| VCAM-1 | LFA-1 | Prostaglandine E | |
| ELAM-1 | CLA | CD4+CD25−FOXP3+ T cells | |
| ECM | 4-1BB | CD8+CD28− T cells | |
| TLR1/2 | |||
| TLR2/6 | |||
| TLR3 | |||
| TLR4 | |||
| TLR5 | |||
| TLR9 | |||
| PD-L1-ligand |
Antigens, receptors, co-stimulatory molecules, or ligands expressed on the membrane of tumor cells, APC, or CTL. These molecules are involved in T cell activation. However, these molecules are often weakly expressed or altered or even absent in elderly individuals or mice or in cancer patients, which may lead to T cell unresponsiveness, compared to normal expression levels in young healthy individuals or mice.
Lower expression levels of lymphokines and their receptors have been found in T cells of elderly individuals or mice, or in cancer patients, that may lead to T cell unresponsiveness, compared to young individuals or mice.
Factors produced by tumor cells, or subpopulations of Tregs have been identified that may inhibit vaccine- and/or tumor-induced immune responses in cancer patients. APC= antigen-presenting cells, CD=cluster differentiation, CLA=cutaneous lymphocyte-associated antigen, COX=cyclooxygenase, CTL=cytotoxic T lymphocytes, CTLA=cytotoxic T cell antigen, ELAM=epidermal lymphocyte adhesion molecule, FOXP3= forkhead box P3, ICAM=inter cellular adhesion molecule, IDO=indoleamine 2,3-dioxygenase, IFN=interferon, IL=interleukin, LFA=leukocyte function-associated antigen, MHC=major histocompatibility complex, PD=programmed death, TAA=tumor-associated antigens, TGF=transforming growth factor, TLR=toll-like receptor, TCR=T cell receptor, Tregs= regulatory T cells, VCAM=vascular cellular adhesion molecules. CD28 or CTLA-4 binds B7.1 and B7.2. CD49d binds VCAM-1. LFA-1 binds ICAM-1/2. 4-1BB binds ECM. CLA binds ELAM-1.
Cancer vaccination: results in preclinical animal models
Suitable preclinical models, that adequately reflect human cancer, may teach us how to overcome the problems that occur in cancer patients in order to develop effective cancer vaccines. Criteria for suitable models are immune competence, developing human-like tumors, and expressing self-TAA. Many studies have become available in the last few years and results encourage vaccination as a non-toxic effective therapy against cancer. Table 2 summarizes the results of recent preclinical studies using mouse models with various types of tumors. Most studies report about the use of syngeneic models. In this type of models, a syngeneic tumor cell line (same genetic background as the receiving mouse) is injected into the mouse and tumor formation is within 1–4 weeks. Advantages of these models are the fast results obtained and the possibility to test vaccines at young and old age. However, transgenic models reflect human cancer more adequately, since the development of cancer in these models is more natural, i.e., they undergo normal-preneoplastic-neoplastic stages. These models are more useful to develop preventive vaccines. Disadvantages are the time frame in which the vaccines can be tested, since it takes 3–7 months before tumors appear in transgenic mice, and vaccines can only be tested at a relatively young age. Therefore, transgenic mice are not useful for developing vaccines at older age. Conditional mouse tumor models, in which tumor development can be induced at young and old age by the administration of antibiotics or drugs, are most suitable. However, vaccine studies in such mouse tumor models have not been reported so far. With respect to tumor protection, DNA-based Her2/neu vaccines were most promising in transgenic breast tumor model neuT (Quaglino et al., 2004, Spadaro et al., 2005). Complete protection was obtained up to 1 year. However, to decrease morbidity and mortality prevention or elimination of metastases is crucial. In this respect, Mage-b DNA, GRP94/T41, and mOX40L/GM-CSF, proved most promising in metastatic breast tumor models 4TO7cg and 4T1 (Sypniewska et al., 2005; Liu et al., 2005; Ali et al., 2004).
Table 2. Cancer Vaccination in Preclinical Models.
| Cancer | Antigen | Vaccine | Results So Far | Animal model | References |
|---|---|---|---|---|---|
| Melanoma | Tumor lysate | Fls/DC | RTG | B16/syng/P | Yoshikawa et al., 2006 |
| Tumor lysate/CpG | DC/DNA | RTG/PS | B16/syng/P/T | Pilon-Thomas et a., 2006 | |
| Breast | Her2/neu/extrcell. | DNA/electroporation | CP up to 1 year p<0.05 | neuT/transg/P/T | Quaglino et al., 2004 |
| Her2/neuIL-12 | DNA electroporation | CP up to 1 year p<0.05 | neuT/transg/P. | Spadaro et al., 2005 | |
| Her2/neu | DNA | CP up to 60 days p<0.05 | Her2/neu/syng/P | Chan et al., 2006 | |
| Her2/neu | DC/neu | TD p<0.05 | Her2/neu/transg/P | Chan et al., 2006 | |
| Her2/neu | Listeria | TR/Her2/CTLR p<0.05 | Her2/neu/syng/T | Singh et al., 2005 | |
| Mage-b | DNA | PM p<0.05/RTG p<0.05 | 4TO7cg/syng/P | Sypniewska et al., 2005 | |
| BORIS/CD80 | DNA/Adjuvant | PS/RTG | 4T1/syng/P | Loukinov et al., 2006 | |
| GRP94/4T1 | DNA/Radiation | PM/TD p<0.05 | 4T1/syng/T | Liu et al., 2005 | |
| mOX40L/GM-CSF | Fusion protein | PM/RTG p<0.05 | 4T1/syng/P/T | Ali et al., 2004 | |
| Ovarian | IL-2/TGFβ-AS | Fibroblast/Tumor | TP p<0.01 | MOT/syng/P | Dorigo et al., 1998 |
| Colon | mOX40L/GM-CSF | Fusion protein | RTG | CT26/syng/P/T | Ali et al., 2004 |
| SVF10-E-IL18 | RNAsuicide vector | TR | CT26/syng/T | Chikkanna et al., 2006 | |
| AFP | S.typhimurium | ILS p<0.05 | CT26/syng/P | Chou et al., 2006 | |
| Prostate | CDL40 | Adenovirus | RTG p<0.001 | TRAMP-C2/syng/P/T | Dzojic et al., 2006 |
AFP=alpha feto protein, CP=complete protection, CTLR=cytotoxic T lymphocyte responses, DC= dendritic cells, FLs=fusogenic liposomes, GM-CSF=granulocyte macrophage-colony stimulating factor, GRP=glucose-regulated protein, HCC= hepato cellular carcinoma, IL =interleukin, ILS=increased life span, Mage=melanoma-associated antigen, MOT= murine ovarian teratoma, P=preventive vaccination, PM=prevention metastases, PS=prolonged survival, RTG=reduced tumor growth, SFV-10E= enhanced Semliki Forest virus, T=therapeutic vaccination, TD=tumor delay, TGF=transforming growth factor, TP=tumor prevention, TR=tumor regression.
Cancer vaccination: the results of human clinical trials
Table 3 summarizes the results of most recent and promising human clinical trials of various types of cancer treated with vaccines. The most promising vaccine is human papilloma virus (HPV), capable of complete prevention of cervix carcinoma, up to 3.5 years (average) (Mao et al., 2006). A main reason for this success might be that the HPV-16 vaccine contains a viral antigen and will be recognized as foreign. Important is that HPV-16 vaccination is preventively effective. The potential negative effects of tumors on vaccine-induced immune responses that may occur in therapeutic vaccinations can be circumvented in preventive vaccinations. Like in the preclinical models, vaccination with MAGE (against metastatic melanoma) again showed regression of metastases (Salcedo et al., 2005). Also vaccination with TAA survivin showed regression of metastases (Wobser et al., 2006). Although HER2/neu vaccination was promising, the efficacy was lower than in the preclinical models, i.e., some reduction in recurrence and prolonged stable disease (Dees et al., 2004; Peoples et al., 2005). Overall, the results from preclinical models were more successful than results from human clinical trials. One reason might be that most vaccines in the preclinical models have been tested preventively, and therapeutic vaccinations in the preclinical models may have started relatively earlier when tumors were very small or not palpable yet, compared to vaccinations in human clinical trials. However, another or additional important reason might be the age of cancer patients at the time of vaccination, who were generally over 50 years of age. As shown below, the age is an important factor for efficacy of cancer vaccines.
Table 3. Cancer Vaccination in Clinical Trials.
| Cancer | Antigen | Vaccine | Results So Far | References |
|---|---|---|---|---|
| Melanoma | MAGE-A1/3 | Tumor DC/Tumor lysate | TR | Salcedo et al., 2005 |
| MART-1/gp100/tyros | Peptide/low dose GM-CSF | ICO | Markovic et al., 2006 | |
| VACCIMEL | Allogeneic melanoma/BCG | HR p<0.05 | Barrio et al., 2006 | |
| Breast | 1E10 (GM3 ganglioside) | anti-Id Ab | HR/CR p<0.05 | Guthmann et al., 2006 |
| Membrane Ags | DC/Tumor | SD/DR/TCR | Avigan et al., 2004 | |
| HER2/neu | HER2/neupeptide/DC | SD/TCR | Dees te al., 2004 | |
| HER2/neuE75 | Peptide/GM-CSF | RR p<0.19/PSIR | Peoples et al., 2005 | |
| P53 | Peptide/DC | SD/TCR | Svane et al., 2004 | |
| Cervix Carcinoma | HPV-16L1 | polypeptide/VLP | CP up to 48 months | Mao et al., 2006 |
| Pancreas | Survivin | Peptide/Adjuvant | RM/IR (case report) | Wobser et al., 2006 |
| Renal Carcinoma | RNA Tumor | RNA transf DC/depl Tregs | TSTCR p<0.05 | Dannull et al., 2005 |
| Prostate Cancer | PSA | Peptide/GM-CSF | PSA-spec TCR | Perambakam et al., 2006 |
| Follicular Lymphoma | Id epitope | Protein | DFS/HR/CR | Inoges et al., 2006 |
Ab= antibody, CP=complete prevention, BCG=Bacillus Calmette Guerin, CR=cellular responses, DC=dendritic cells, DFS=disease free survival, DR=disease regression, GM-CSF=granulocyte macrophage-colony stimulating factor, PSA=prostate specific antigen, HR=humoral responses, ICO=improved clinical outcome, Id=idiotypic, IR=immune responses, PSA=prostate specific antigen, PSIR=peptide specific immune responses, RM=regression metastases, RR= recurrence reduced, SD= stable disease, TCR=T cell responses, TR=tumor regression, Tregs=regulatory T cells, TSTCR=tumor-specific T cell responses, VLP= virus-like particle.
Comparison of cancer vaccination at young and old age in preclinical models
More than 50% of all cancer patients are 65 years or older (Muss, 2001). However, as discussed earlier, the immune system at older age is impaired, due to T cell unresponsiveness. In almost all preclinical studies, cancer vaccines have been tested at young age (Table 2). Very recently, results of a few vaccine studies in preclinical models at old age became available, including studies with Mage-b vaccination performed in our laboratory. These are the first studies that show that cancer vaccines are less effective at old than at young age. A first vaccine study in young and old mice reported is from Provinciali et al. in 2000. A syngeneic mammary adenocarcinoma cell line TS/A was genetically engineered to release IL-2 (TS/A-IL-2). Young and old mice were immunized with TS/A-IL-2 cells and subsequently challenged with the parental TS/A cell line. While TS/A-IL-2 protected 90% of the young mice, only 10% was protected of the old mice.
Many vaccine studies have been performed with the Her2/neu DNA vaccine in young mice. Provinciali developed a pCMVneuNT DNA vaccine and tested its efficacy in young and old mice that were subsequently challenged with syngeneic TUBO cells, overexpressing HER2/neu (Proviciali et al., 2003). Young mice were completely protected while less than 60% of the old mice were protected against TUBO challenge. Anti-neu antibodies, induced by the vaccine, and proliferation after restimulation in vitro, was higher at young than at old age.
Lusgarten et al., (2004) immunized young and old mice with a syngeneic pre-B cell lymphoma cell line (BM-185), expressing enhanced Green Fluorescent Protein (EGFP) and a co-stimulatory molecule CD80 (B7.1). While the young mice developed a long-lasting memory response capable of rejecting BM-185 wild type tumors, the old mice did not develop long-lasting memory responses. However, when the BM-185-EGFP-CD80 plus agonist anti-OX40 mAb were injected in old mice, long-lasting memory responses were capable of rejecting BM-185 wild type tumor cells with the same vigor as in young mice. In another study by the group of Lusgarten, DC vaccination plus rIL-2 protected 60% of the young mice from challenge with syngeneic TRAMP-C2 tumor cells (adenocarcinoma of the prostate), while only a minimal effect was observed in the old mice (Sharma et al., 2006). However, when co-administered with anti-OX-40 or anti-4-1BB mAbs (leukocyte differentiation antigen on T and NK cells, and DC) a vigorous antitumor reponse in both young (85–90%) and in old (70–75%) mice was observed.
In our laboratory, we developed a DNA vaccine of Mage-b and tested this vaccine at young and old age in a syngeneic mouse tumor model, 4TO7cg. This mouse tumor model is moderately metastatic (range: 2–20 metastases per mouse) and overexpresses Mage-b in primary tumor and metastases (Sypniewska et al., 2005). Preventive vaccination of young and old mice with pcDNA3.1-Mage-b protected 90% of the young mice from metastases, while only 60% of the old mice remained free of metastases (Figure 1). Analysis of spleen cells of tumor-bearing mice after in vitro restimulation, showed high levels of IL-2 and IFNγ at young age but undetectable levels at old age (Figure 2). We repeated this vaccine study in a much more aggressive metastatic model 4T1 (range: 5–300 metastases per mouse), also overexpressing Mage-b, but this time we mixed the pcDNA3.1-Mage-b DNA vaccine with plasmid DNA secreting GM-CSF. To recruit APC more effectively to the peritoneal cavity (pc), thioglycollate was injected into the pc, prior to each vaccination. Thioglycollate-stimulated macrophages are not fully differentiated, highly express MHC class II, and are highly phagocytic (Cook et al., 003). Evidence exists that thioglycollate-stimulated macrophages can function as APC (Rock et al., 1993). Although the effect in the young mice was stronger than in the old, a significant reduction in the frequency of metastases was observed in both young and old mice (Figure 3). However, when analyzing the draining lymph nodes of tumor-bearing mice, moderate levels of IL-2 and IFNγ were detected after restimulation at young age but not at old age (Figure 4). FACS analysis of the draining lymph nodes of Mage-b vaccinated tumor-bearing mice at young and old age after restimulation, showed CD4 and CD8 responses (intracellular IL-2 and/or IFNγ production) at young age but not at old age (data not shown). At old age macrophages and NK cells were more active (intracellular production of IFNγ and IL-2 receptor expression), suggesting that the innate immune response may have contributed to the antitumor response in the mice.
Figure 1.
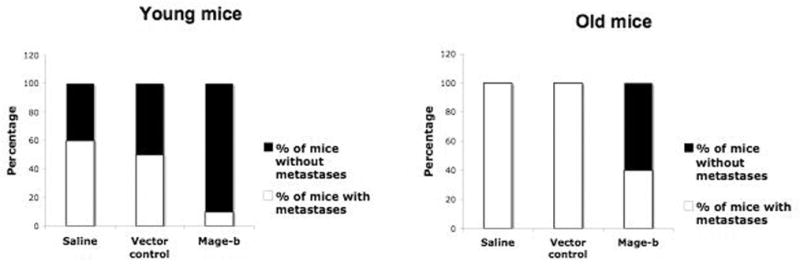
Preventive effect of Mage-b vaccination on metastases in a 4TO7cg model at young and old age. Young (3 months) and old mice (20 months) were preventively immunized with Mage-b plasmid DNA and challenged with 4TO7cg tumor cells. At young age, the percentage of mice without metastases was significantly higher in the Mage-b-vaccinated group than in the control groups (Fisher’s exact test p<0.0001). At old age, the percentage of mice without metastases was also significantly higher in the Mage-b-vaccinated group than in the control groups (Fisher’s exact test p<0,0001), but the effect at old age was less vigorous than at young age. (Submitted for publication, 2006)
Figure 2.

IL-2 and IFNγ levels in spleen cultures of young and old mice with the 4TO7cg model. High levels of IL-2 and IFNγ after restimulation with Mage-b DNA were found in spleen cultures of young mice (3 months), but were almost undetectable in spleen cultures of old mice (20 months). At young age, IL-2 (ANOVA p<0.0001) and IFNγ (ANOVA p<0.001) levels were significantly higher in spleen cultures of Mage-b-vaccinated mice, compared to the spleen cultures of control mice. (Submitted for publication 2006)
Figure 3.

Preventive effect of Mage-b on metastases in the 4T1 model at young and old age. Young (3 months) and old (20 months) mice were preventively immunized with Mage-b plasmid DNA and challenged with 4T1 tumor cells. Prior to each vaccination, antigen-presenting cells (macrophages) were recruited to the peritoneal cavity with thioglycollate. At young age, the percentage of mice without metastases was significantly higher in the Mage-b-vaccinated group than in the control groups (Kruskal Wallis p=0.00102). At old age, the percentage of mice without metastases was also significantly higher in the Mage-b-vaccinated group than in the control groups (Kruskal Wallis p=0.0046), but the effect at old age was less vigorous than at young age. There was also an aging effect, i.e., old mice in general developed less metastases than young mice. (Submitted for publication, 2006)
Figure 4.

IL-2 and IFNγ levels in lymph node cultures of young and old mice with the 4T1 model. Moderate levels of IL-2 and IFNγ after restimulation with syngeneic 64pT breast tumor cells (expressing highly Mage-b) were found in cultures of lymph nodes of young mice (3 months), but were almost undetectable in cultures of lymph nodes of old mice (20 months). At young age, IL-2 (ANOVA p=0.0003) and IFNγ (ANOVA p=0.0001) levels were significantly higher in cultures of lymph nodes of Mage-b-vaccinated mice, compared to the cultures of lymph nodes of control mice. (Submitted for publication 2006).
Summary and future prospects
The main conclusion from studies in preclinical models at young and old age is that cancer vaccines are less effective at older age than in young adults. These results imply that vaccines may not be very effective in cancer patients, which are usually elderly, unless the vaccines are optimized for older age. The studies discussed in this review show the potential but also the need for tailoring cancer vaccination to old age. Below, a number of approaches are proposed that may contribute to further improvement of efficacy of cancer vaccination at older age.
DNA vaccines are of great value since any DNA sequence can be added to the vaccine vector that may improve T cell activation with minimal toxicity. Activation of T cells at older age could be achieved by expressing IL-2 and IFNγ from the vaccine vector, or GM-CSG or Flt3-ligand to the vaccine vector in order to activate macrophages and DC or improve processing and presentation of antigens by APC. Addition of a DNA sequence encoding IL-7 (Tan et al., 2001) may recruit only those naïve T cells that react with the vaccine antigen. Also, activation of co-stimulatory molecules by anti-OX-40 and anti-4-1BB Abs seems to be a promising approach as shown by Lusgarten et al. (2006). A first immunization at young age, when sufficient naïve T cells are still present, followed by boosting at old age may improve T cell responses at older age. This approach has shown to be effective for improving Ab responses at older age in mice (Stacy et al., 2003). Activation of the innate immune system by mixing a lipophylic glycopeptide L-TMP-PE (liposyl muramyl phosphatidylethanolamine) with the vaccine, which is capable of recruiting and activating killer macrophages (Nardin et al., 2006), is another approach if T cell activation fails or as an addition to T cell activation. Elimination of CD4+CD25−FOX+ T cells (Nishioka et al., 2006) or CD8+CD28− T cells (Filaci et al., 2004) may enhance vaccine efficacy at older age.
Also approaches that may improve vaccine efficacy in general are important. For instance, multi-antigen vaccines may prevent escape of genetically unstable tumor cells that have lost antigen expression. Many tumors produce lymphokines or factors that may inhibit vaccine-induced T cells responses such as TGFβ, IL-6, cyclooxygenase-2 (COX-2) and its products prostaglandine E, PD1-ligand, or indolamine 2,3-dioxygenase (IDO)(Gajewski et al., 2006; Park et al., 2004). Reduction of these factors may enhance vaccine efficacy as well. Improved delivery systems resulting in improved expression of the vaccine antigen in vivo, not discussed in this review, will certainly improve vaccine efficacy in general.
Finally, prevention or elimination of metastases deserves more attention. In most cancers, metastases and not primary tumors contribute to morbidity and mortality. In contrast to primary tumors, metastases cannot be removed by surgery or radiation, and are usually chemoresistant (Pardal et al., 2003). It is therefore encouraging that vaccines such as Mage and Survivin proved especially effective against metastases. Further improvement of vaccines against metastases may dramatically improve the clinical outcome of cancer treatment.
Acknowledgments
This work was supported by the American Federation for Aging Research (AFAR) A000106, and NIA grant 1RO1 AG023096-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbasak A, Oldfield EH, Saris SC. Expression and modulation of major histocompatibility antigens on murine brain tumors in vitro. J Neurosurg. 1991;75:922–929. doi: 10.3171/jns.1991.75.6.0922. [DOI] [PubMed] [Google Scholar]
- Ali SA, Ahmed M, Lynam J, McLean CS, Entwisle C, Loudon P, Choolun E, McArdle SE, Li G, Mian S, Rees RC. Anti-tumor therapeutic efficacy of OX40L in murine tumor model. Vaccine. 2004;22:3585–3594. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clinical Cancer Research. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- Barrio MM, Motta PT, Kaplan J, von Euw EM, Bravo AI, Chacon RD, Mordoh J. A phase I study of an allogeneic cell vaccine (VACCIMEL) with GM-CSF in melanoma patients. J Immunother. 2006;29:444–454. doi: 10.1097/01.cji.0000208258.79005.5f. [DOI] [PubMed] [Google Scholar]
- Blieden TM, McAdam AJ, Foresman MD, Cerosaletti KM, Frelinger JG, Lord EM. Class-I MHC expression in the mouse lung carcinoma, line 1: a model for class-I inducible tumors. Int J Cancer Suppl. 1991;6:82–89. doi: 10.1002/ijc.2910470717. [DOI] [PubMed] [Google Scholar]
- Chan T, Sami A, El-Gayed A, Guo X, Xiang J. Her2/neu-gene engineered dendritic cell vaccine stimulates stronger HER2/neu-specific immune responses compared to DNA vaccination. Gene Ther. 2006;13:1391–1402. doi: 10.1038/sj.gt.3302797. [DOI] [PubMed] [Google Scholar]
- Chikkanna-Gowda CP, McNally S, Sheahan BJ, Fleeton MN, Atkins GJ. Inhibition of murine K-BALB and CT26 tumor growth using Semliki Forest virus vector with enhanced expression of IL-18. Oncol Rep. 2006;16:713–719. doi: 10.3892/or.16.4.713. [DOI] [PubMed] [Google Scholar]
- Cook AD, Braine EL, Hamilton JA. The phenotype of inflammatory macrophages is stimulus dependent: Implications for the nature of inflammatory response. J Immunol. 2003;171:4816–4823. doi: 10.4049/jimmunol.171.9.4816. [DOI] [PubMed] [Google Scholar]
- Corver WE, Koopman LA, van der Aa J, Regensburg M, Fleuren GJ, Cornelisse CJ. Four-color multiparameter DNA flow cytometry to study phenotypic intratumor heterogeneity in cervical cancer. Cytometry. 2000;39:96–107. [PubMed] [Google Scholar]
- Chou CK, Hung JY, Liu JC, Chen CT, Hung MC. An attenuated Salmonella oral DNA vaccine prevents the growth of hepato cellular carcinoma and colon cancer that express alpha-fetoprotein. Cancer Gene Ther. 2006;13:746–752. doi: 10.1038/sj.cgt.7700927. [DOI] [PubMed] [Google Scholar]
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancev D, Zhang A, Dahm P, Chao N, Gilbao E, Viewen J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees EC, McKinnon KP, Kuhns JJ, et al. Dendritic cells can be rapidly expanded ex vivo and safely administered in patients with metastatic breast cancer. Cancer Immunol Immunother. 2004;53:777–785. doi: 10.1007/s00262-004-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzojic H, Loskog A, Totterman TH, Essand M. Adenovirus-mediated CD40 ligand therapy induces tumor cell apoptosis and systemic immunity in the TRAMP-C2 mouse prostate cancer model. The Prostate. 2006;66:831–838. doi: 10.1002/pros.20344. [DOI] [PubMed] [Google Scholar]
- Dorigo D, Shawler DL, Royton I, Sobol RE, Berek RE, Fakhrai H. Combination of transforming growth factor antisense and interleukin-2 gene therapy in the murine ovarian teratoma model. Gynecolog oncology. 1998;71:204–210. doi: 10.1006/gyno.1998.5151. [DOI] [PubMed] [Google Scholar]
- Effros RB. Replicative senescence of CD8 T cells: effect on human aging. Exp Ger. 2004;39:517–24. doi: 10.1016/j.exger.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2006;7 doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, Brenci S, Contini P, Olive D, Ghio M, Setti M, Accolla RS, Puppo F, Indiveri F. Nonantigen-specific CD8+ suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T cell proliferation and CTL function. Hum Immunol. 2004;65:142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Meng Y, Harlin H. Immune suppression in tumor microenvironment. J Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/s0167-5699(96)10075-x. [DOI] [PubMed] [Google Scholar]
- George AJT, Ritter MA. Thymic involution with ageing: obsolescence or good houskeeping? Immunol Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- Gravekamp C, Bontenbal M, Ronteltap CPM, van Duivenbode D, Bolhuis RLH. In vitro and in vivo activation of CD4+ lymphocytes by autologous tumor cells. Int. J. Cancer. 1990;46:152–154. doi: 10.1002/ijc.2910460127. [DOI] [PubMed] [Google Scholar]
- Gravekamp C. Tailoring cancer vaccines to the elderly: the importance of suitable mouse models. MAD. 2001;122:1087–1105. doi: 10.1016/s0047-6374(01)00252-4. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B. Changes in the aging immune system. Biologicals. 1997;25:205–208. doi: 10.1006/biol.1997.0085. [DOI] [PubMed] [Google Scholar]
- Guthmann MD, Castro MA, Cinat G, Venier C, Koliren L, Bitton RJ, Vazquez AM, Fainboim L. Cellular and humoral immune response to N-glycolyl-GM3 elicited by prolonged immunotherapy with an anti-idiotypic vaccine in high risk and metastatic breast cancer patients. J Immunother. 2006;29:215–223. doi: 10.1097/01.cji.0000188502.11348.34. [DOI] [PubMed] [Google Scholar]
- Inoges S, Rodriguez-Calvillo M, Zabalegui N, et al. Clinical benefir associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;20:1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- Koyama S, Maruyama T, Adachi S, Nozue M. Expression of costimulatory molecules, B7-1 and B7-2 on human gastric carcinoma. J Can Res Clin Oncol. 1998;124:383–388. doi: 10.1007/s004320050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang H, Yang Z, Kon T, Zhu J, Cao Y, Li F, Kirkpatrick J, Nichitta CV, Li CY. Enhancement of cancer radiation therapy by use of adenovirus-mediated secretable glucose-regulated protein 94/gp96 expression. Cancer Res. 2005;65:9126–9131. doi: 10.1158/0008-5472.CAN-05-0945. [DOI] [PubMed] [Google Scholar]
- Loo DT, Chalupny NJ, Bajorath J, Shuford WW, Mittler RS, Aruffo A. Analysis of 4-IBBL and laminin binding to murine 4-IBB, a member of the tumor necrosis factor receptor superfamily, and comparison with human 4-IBB. J Biol Chem. 1997;272:6448–6456. doi: 10.1074/jbc.272.10.6448. [DOI] [PubMed] [Google Scholar]
- Loukinov D, Ghochikyan A, Mkrtichyan M, Ichim TE, Lobanenkov VV, Cribbs DH, Agadjanyan MG. Antitumor efficacy pf DNA vaccination to the epigenetically acting tumor promoting transcription factor BORIS and CD80 molecular adjuvant. J Cell Biochem. 2006;98:1037–1043. doi: 10.1002/jcb.20953. [DOI] [PubMed] [Google Scholar]
- Lung TL, Sauerwein-Teissl M, Parson W, Schonitzer D, Grubeck-Loebenstain B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–1612. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- Lusgarten J, Dominguez AL, Thomas M. Aged mice develop protective antitumor responses with appropriate costimulation. J Immunol. 2004;173:4510–4515. doi: 10.4049/jimmunol.173.7.4510. [DOI] [PubMed] [Google Scholar]
- Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, Alvarez FB, Bautista OM, Jansen KU, Barr E. Efficacy of human papilloma virus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- Markovitc SN, Suman VJ, Ingle JN, Kauer JS, Pitot HC, Loprinzi CL, Rao RD, Creagan ET, Pittelkow MR, Allred JB, Nevala WK, Celis E. Peptide vaccination of patients with metastatic melanoma: improved clinical outcome in patients demonstrating effective immunization. Am J Clin Oncol. 2006;29:352–360. doi: 10.1097/01.coc.0000217877.78473.a4. [DOI] [PubMed] [Google Scholar]
- Maurer CA, Friess H, Kretschmann B, Wildi S, Muller C, Graber H, Schilling M, Buchler MW. Over-expression of ICAM-1, VCAM-1 and ELAM-1 might influence tumor progression in colorectal cancer. Int J Cancer. 1998;79:76–81. doi: 10.1002/(sici)1097-0215(19980220)79:1<76::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Meneilly GS, Lechelt KE, Bleackley RC. Split-virus influenza vaccines: do they provide adequate immunity in the elderly? Gerontol. 1994;49:M37–43. doi: 10.1093/geronj/49.2.m37. [DOI] [PubMed] [Google Scholar]
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Helstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-IBB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Muss HB. Factors used to select adjuvant therapy of breast cancer in the United States: an overview of age, race, and socioeconomic status. J Natl Cancer Inst Monogram. 2001;30:52–55. doi: 10.1093/oxfordjournals.jncimonographs.a003461. [DOI] [PubMed] [Google Scholar]
- Nardin A, Levebre ML, Labroquere K, Faure O, Abastado JP. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Current Cancer Drug Targets. 6:123–133. doi: 10.2174/156800906776056473. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Shimizu J, Yamazaki S, Sakaguchi S. CD4+CD25+Fox3+ T cells and CD4+CD25−Fox3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the priciples of stem-cell biology to cancer. Nat rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Park SJ, Nakgawa T, Kitamura H, Atsumi T, Kamon H, Sawa SI, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–54. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- Pedrinaci S, Algarra I, Garcia LA, Gaforio JJ, Perez M, Garrido F. Selective upregulation of MHC class I expression in metastatic colonies derived from tumor clones of a murine fibrosarcoma. Int J Clin Lab Res. 1999;29:166–173. doi: 10.1007/s005990050085. [DOI] [PubMed] [Google Scholar]
- Peoples GE, Gurney JM, Hueman MT, Woll MM, Rvan GB, Storrer CE, Fisher C, Shriver CD, Ioannides CG, Ponniah S. Clinical trials results of a HER2/neu (E75) vaccine to prevent recurrence in high risk breast cancer patients. J Clin Oncol. 2005;23:7536–75–45. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Perambakam S, Hallmeyer S, Reddy S, Mahmud N, Bressier L, DeChristopher P, Mahmud D, Nunez R, Sosman JA, Peace DJ. Induction of specific T cell immunity in patients with prostate cancer by vaccination with PSA146-154 peptide. Cancer Immunol Immunother. 2006;55:1033–1042. doi: 10.1007/s00262-005-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Thomas S, Li W, Briggs JJ, Dieu J, Mule JJ, Riker AI. Immunostimulatory effect of CpG-ODN upon dendritic cell-based immunotherapy in a murine melanoma model. J Immunol. 2006;29:381–387. doi: 10.1097/01.cji.0000199199.20717.67. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Argentati K, Tibaldi A. Efficacy of cancer gene therapy in aging: adenocarcinoma cells engineered to release IL-2 are rejected but do not induce tumor specific immune memory in old mice. Gene Ther. 2000;7:624–632. doi: 10.1038/sj.gt.3301131. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Smorlesi A, Donnini A, Bartozzi B, Amici A. Low effectiveness of DNA vaccination against HER2/neu in aging. Vaccine. 2003;21:843–848. doi: 10.1016/s0264-410x(02)00530-3. [DOI] [PubMed] [Google Scholar]
- Quyang Q, Cicek G, Westendorp RGJ, Cools HJM, ven der Klis RJ, Remarque EJ. Reduced IFNγ production in elderly people following in vitro stimulation with influenza vaccine and endotoxin. Mech Ageing Dev. 2000;121:131–137. doi: 10.1016/s0047-6374(00)00204-9. [DOI] [PubMed] [Google Scholar]
- Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo M, Pupa SM, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musiani P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammary carcinomas in Her2/neu transgenic mice. Cancer Research. 2004;64:2858–2864. doi: 10.1158/0008-5472.can-03-2962. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Rocjwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Rock KL, Rothstein L, Gamble S, Fleischacker C. Characterization of antigen presenting cells that present exogenous antigens in association with class I MHC molecules. J Immunol. 150:438–446. [PubMed] [Google Scholar]
- Sauerwein-Teissl M, Schonitzer D, Grubeck-Loebenstin B. Dendritic cell responsiveness to stimulation with influenza vaccine is unimpaired in old age. Exp Ger. 1998;33:625–631. doi: 10.1016/s0531-5565(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Salcedo M, Bercovici N, Taylor R, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogenic tumor cell lysate. Cancer Immunol Immunother. 2006;55:819–829. doi: 10.1007/s00262-005-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Domiguez AL, Lusgarten J. Aging affect the anti-tumor potential of dendritic cell vaccination, but it can be overcome by co-stimulation with anti-OX40 or anti-4-1BB. Exp Ger. 2006;41:78–84. doi: 10.1016/j.exger.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Schreiber H. Fundamental Immunology. 4. Paul, W; Lippencott-Raven: 1998. Tumor Immunology; pp. 1237–1270. [Google Scholar]
- Singh R, Dominiecki ME, Jaffe EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of Her2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- Spadaro M, Ambrosino E, Iezzi M, Di Carlo E, Sachetti P, Curcio C, Amici A, Wei WZ, Musiani P, Lollini PL, Cavallo F, Forn G. Cure of mammary carcinoma in Her2/neu transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clinical Cancer Research. 2005;11:1941–952. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- Sprecher E, Becker Y, Kraal G, Harrison D, Shultz LD. Effect of aging on the epidermal dendritic cell population in C57Bl/6J mice. J Invest Dermatol. 1990;94:247–253. doi: 10.1111/1523-1747.ep12874586. [DOI] [PubMed] [Google Scholar]
- Stacy S, Infante AJ, Wall K, Krolick K, Kraig E. Recall immune memory: a new tool for generating late onset autoimmune myasthenia gravis. Mech Ageing Dev. 2003;124:931–940. doi: 10.1016/s0047-6374(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Laden H, Luger TA. Aging and the skin immune system. Arch Dermatolog. 1997;133:1256–1262. doi: 10.1001/archderm.1997.03890460078009. [DOI] [PubMed] [Google Scholar]
- Svane IM, Pedersen AE, Johnsen HE, Nielsen D, Kamby S, Claesson MH. Vaccination with p53-peptide-pulsed dendritic cells of patients with advanced breast cancer: report from a phase I study. Cancer Immunol Immunother. 2004;53:633–641. doi: 10.1007/s00262-003-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypniewska RK, Hoflack L, Tarango M, Gauntt S, Leal BZ, Reddick RL, Gravekamp C. Prevention of metastases with Mage-b vaccine in a mouse breast tumor model: potential for breast cancer therapy. Breast Cancer Research Treatment. 2005;91:19–28. doi: 10.1007/s10549-004-6454-7. [DOI] [PubMed] [Google Scholar]
- Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 20000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naïve T cells. Proc Natl Acad Sci. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inmatsu T, Suzuki K, Hashimoto W, Sato K. Differential age-change in the number of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- Van Elsas A, Hurwitz AA, Allison P. Combination of immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-1) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;3:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack A, Cossarizza A, Heltai S, Barbieri D, D’Addato S, Fransceschi C, Dellabona P, Casorati G. Age-related modifications of the human alphabeta T cell repertoire due to different clonal expansions in the CD4+ and CD8+ subsets. Int Immunol. 1998;10:1281–88. doi: 10.1093/intimm/10.9.1281. [DOI] [PubMed] [Google Scholar]
- Weber JS, Rosenberg SA. Effects of murine tumor class I major histocompatibility complex expression on antitumor activity of tumor-infiltrating lymphocytes. J Natl Cancer Inst. 1990;82:755–761. doi: 10.1093/jnci/82.9.755. [DOI] [PubMed] [Google Scholar]
- Wobster M, Keikavoussi P, Kunzman V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastases of pancreatic cancer under vaccination with HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Okada N, Tsujino M, Gao JQ, Hayashi A, Tsutsumi Y, Mayuni T, Yamamoto A, Nakawaga S. Vaccine efficacy of fusogenic liposomes containing tumor cell-lysate against murine B16BL6 melanoma. Biol Phar Bull. 2006;29:100–104. doi: 10.1248/bpb.29.100. [DOI] [PubMed] [Google Scholar]
- Zou W. Regulatory T cell, tumour immunity and immunotherapy. Nature reviews/Immunology. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]


