Introduction
The Diabetes Control and Complications Trial (DCCT) confirmed the importance of tight glucose control in limiting the development of microvascular complications, and established a standard for measuring A1c levels using high-performance liquid chromatography (HPLC).(1) Several studies demonstrated the benefit of rapid A1c testing in the clinic while face-to-face with the patient/family.(2-4) The DCA2000® Analyzer (Bayer, Inc., Tarrytown, NY) uses an immunoassay method certified by the National Glycohemoglobin Standardization Program (NGSP).(5) It is frequently used to provide a rapid (6-min) A1c result, enhancing the ability to optimize therapy in a timely fashion. We recently reported that the DCA2000 correlated highly with an HPLC reference (the DCCT standard) (r=0.94, p<0.001), although there was a slight bias with DCA2000 values being on average 0.2% higher than lab values.(6)
The A1cNow® (Metrika, Inc., Sunnyvale, CA) was developed as a single-use, disposable test for measuring A1c at home. It is small, about the size of a pager, requires one drop of blood, and also uses an immunoassay. Results are displayed in approximately 8-min. However, there has been only one published study(7) to date assessing the accuracy of the A1cNow, and that study did not compare A1cNow values to the DCA2000. Furthermore, older generation A1cNow devices that were not NGSP-certified were used in that study. We therefore designed the current study to compare the accuracy of updated, NGSP-certified A1cNow devices and corresponding DCA2000 levels with the DCCT/EDIC Laboratory reference values when used at home and during a clinic visit in children with type 1 diabetes (T1D).
Research Design and Methods
The study was conducted at the five DirecNet clinical centers in 32 children with T1D. IRB approval and informed consent were obtained for the study. A1c was measured four times using the A1cNow, twice by the subject or parent at home and twice the following day by site staff at a DirecNet study visit. Commercially available A1cNow monitors were used. Subjects were given the instructions provided by the manufacturer with no additional instructions provided by clinical center staff. At the clinic visit, A1c was measured using the DCA2000 and a fingerstick blood sample was obtained, frozen at -70°C, and shipped to the DCCT/DirecNet Central Laboratory where measurements were performed using cation-exchange HPLC methodology (Tosoh).(1) At the same time as each A1cNow test at home and in the clinic, the subjects’ blood glucose concentration (reported in plasma equivalents) was measured using a Freestyle® meter (Abbott Diabetes Care, Alameda, CA).
Least squares repeated measures regression was used to compare the accuracy (absolute value of the difference between the device and laboratory A1c value) of the A1cNow versus DCA2000. Additional repeated measures regressions were run to assess the impact of A1c (laboratory value) and glucose concentration on A1cNow accuracy.
Results
The average age was 15 years; 41% were female and 94% Caucasian. The mean Central Laboratory A1c was 7.5%±0.9%, with 28% of values <7.0%, 41% between 7.0% and 7.9%, and 31% ≥8.0%. The DCA2000 was considerably more accurate than the A1cNow (P<0.001). Thirty-two percent of the A1cNow values differed from the reference value by more than 0.5% compared with only 3% of the DCA2000 values (Table). There were no clinically significant differences in accuracy between subject/parent and staff measurements. Accuracy of the A1cNow did not vary with A1c level (P=0.22); the A1cNow was within 0.5% in 74% of reference values ≥8.0% and 67% of reference values <8.0%. Glucose concentration at the time of the A1cNow also did not impact accuracy (P=0.18). For the 25 cases in which two simultaneous A1cNow measurements were made at home and the 29 cases at the clinic, 32% and 34%, respectively, of the two measurements differed by more than 0.5%. Scatterplots of the A1cNow and DCA2000 against the reference with the regression lines (Figure) also demonstrate a much tighter relationship with the DCA2000.
Table.
A1cNow and DCA2000 Accuracy for 32 Subjects
| Comparison | N* | Median Difference | Median Abs Diff | Within±0.1 | Within±0.3 | Within±0.5 |
|---|---|---|---|---|---|---|
| DCA2000 vs. Laboratory | 32 | +0.2 | 0.2 | 25% | 84% | 97% |
| A1cNow (subject) vs. Laboratory | 55 | 0.0 | 0.4 | 15% | 42% | 69% |
| A1cNow (staff) vs. Laboratory | 61 | 0.0 | 0.4 | 13% | 41% | 67% |
| A1cNow (pooled) vs. Laboratory | 116 | 0.0 | 0.4 | 14% | 41% | 68% |
| 2 Subject A1cNow Values | 25 | 0.3 | 16% | 56% | 68% | |
| 2 Staff A1cNow Values | 29 | N/A | 0.4 | 17% | 45% | 66% |
Includes multiple pairs per subject. A1cNow value missing for 9 subject measurements and 3 clinic staff measurements.
Figure 1.
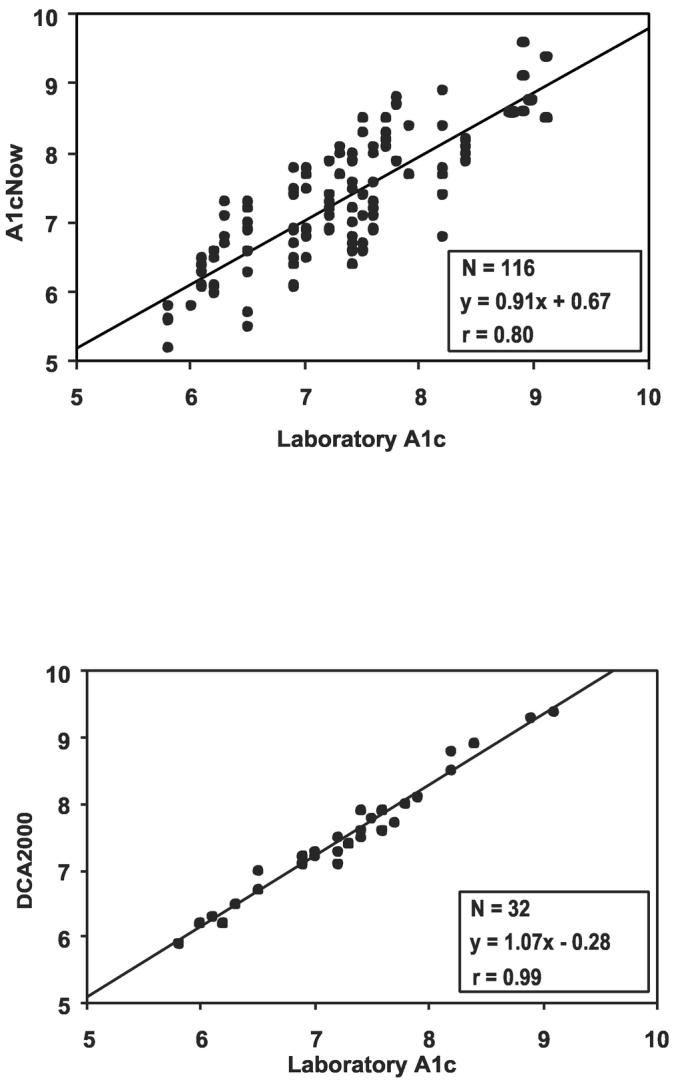
Scatterplots of A1cNow (A) and DCA2000 (B) vs. laboratory reference. The regression line for A1cNow (A) accounts for repeated measurements per subject.
Conclusions
The A1cNow is not as accurate as the DCA2000. A substantial proportion of measurements differ from a reference value by >0.5%, whereas only 3% of DCA2000 values differed from the reference value by >0.5%, consistent with our previous report.(6) Furthermore, and probably more importantly, there were marked differences in values when two simultaneous measurements were made, either at home by the parents or in the clinic setting by experienced clinical center staff. Thus, variability amongst simultaneous values does not appear to reflect errors in performing the tests by untrained parents and patients, but instead to problems inherent to the A1cNow, even though the kits we used were NGSP-certified. Our A1cNow results were consistent with those published by Kennedy and Herman.(7) Using data from 6,231 subjects, they reported 32% of values differed >0.75% from the laboratory reference (Bio-Rad Variant ion exchange) and 20% were >1.0% discrepant. At present, the routine use of the A1cNow in children with T1D cannot be recommended.
Footnotes
Supported by NIH/NICHD Grants HD041919, HD041915, HD041890, HD041918, HD041908, HD041906; GCRC Grants RR00069, RR00059, RR06022, RR00070.
References
- 1.Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer H-M, Rohlfing C, England J, Bucksa J, Nowicki M. the DERG: Hemoglobin A1c Measurements over Nearly Two Decades: Sustaining Comparable Values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Clin Chem. 2005;51:753–758. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in Type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22:1785–1789. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 3.Thaler LM, Ziemer DC, Gallina DL, Cook CB, Dunbar VG, Phillips LS, El-Kebbi IM. Diabetes in urban African-Americans. XVII. Availability of rapid HbA1c measurements enhances clinical decision making. Diabetes Care. 1999;22:1415–1421. doi: 10.2337/diacare.22.9.1415. [DOI] [PubMed] [Google Scholar]
- 4.Miller CD, Barnes CS, Phillips LS, Ziemer DC, Gallina DL, Cook CB, Maryman SD, El-Kebbi IM. Rapid A1c availability improves clinical decision-making in an urban primary care clinic. Diabetes Care. 2003;26:1158–1163. doi: 10.2337/diacare.26.4.1158. [DOI] [PubMed] [Google Scholar]
- 5.Little RR. Glycated hemoglobin standardization--National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41:1191–1198. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Research in Children Network (DirecNet) Study Group Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes. 2005;6:13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy L, Herman WH. Glycated hemoglobin assessment in clinical practice: comparison of the A1cNow Point-of-Care Device with Central Laboratory Testing (GOAL A1C Study) Diabetes Technol Ther. 2005;7:907–912. doi: 10.1089/dia.2005.7.907. [DOI] [PubMed] [Google Scholar]


