Abstract
An important but controversial class of hypotheses concerning the evolution of female preferences for extreme male mating displays involves “indirect selection.” Even in the absence of direct fitness effects, preference for males with high overall fitness can spread via a genetic correlation that develops between preference alleles and high fitness genotypes. Here we develop a quantitative expression for the force of indirect selection that (i) applies to any female mating behavior, (ii) is relatively insensitive to the underlying genetics, and (iii) is based on measurable quantities. In conjunction with the limited data now available, it suggests that the evolutionary force generated by indirect selection on preferences is weak in absolute terms. This finding raises the possibility that direct selection on preference genes may often be more important than indirect selection, but more data on the quantities identified by our model and on direct selection are needed to decide the question.
Keywords: sexual selection, good genes, runaway process, direct selection
Female preferences have caused the evolution of extreme male mating displays throughout the animal kingdom (1–3). One mechanism that might establish these preferences is indirect selection, which occurs when preference genes are associated (that is, in linkage disequilibrium) with other genes that are spreading under selection. Indirect selection is invoked by two types of theories (2). The first are “good genes” theories, which interpret male mating displays as indicators of high viability. In this view, the expression of the male display is correlated with the presence of alleles that increase viability. Because associations naturally develop between preference genes and male trait genes, as the high viability alleles spread by natural selection, preference alleles for extreme displays also spread. The second class of theories that postulates indirect selection involves a “runaway process.” Here alleles that exaggerate a male mating display decrease survival but nevertheless spread because they enhance male mating success. Genes for extreme preferences can then be established as the result of their associations with these spreading male trait genes.
Alternatively, extreme mating preferences can be established by selection acting directly on the preference genes themselves, without any indirect selection whatever (2, 4). One way in which direct selection occurs is when preference alleles alter mating behavior in a way that affects female survival or fertility. This can happen, for example, when females receive help raising offspring from their mates and when females experience search costs while looking for mates. Direct selection on preference genes also can happen as the result of pleiotropy, when genes that affect female mating behavior also affect other characters that are under selection. This type of direct selection occurs, for example, when elements of the sensory system that bias mate choice also experience natural selection in other contexts (4, 5).
Understanding the relative importance of direct and indirect selection in preference evolution is perhaps the central challenge of current research on sexual selection. Highly simplified genetic models have established qualitatively that both mechanisms can work in principle (2, 3). The strengths of direct and indirect selection, however, have never been measured empirically for any mating preference in nature.
A surrogate approach to measuring these forces is to calculate their strength using a model. That strategy is taken here to estimate the strength of indirect selection. We have two aims. First, we want to quantify indirect selection in a way that is independent of many of the underlying genetic and behavioral details, since these are so poorly understood in most natural populations. Second, we wish to frame the results in terms of quantities that have been or can be measured. The theoretical approach taken here allows much more biologically plausible assumptions than those in previous models and relates parameters in the models to observable quantities.
We consider a “preference,” P, to be any measurable component of a female’s behavior that influences which male she mates. It could refer to the value of the male trait that is most preferred, for example, or the average number of males that a female inspects before choosing. This general view of preferences departs from earlier models, which assume females mate according to some simple and stereotyped behavioral rule.
The impact of indirect selection on the preference is conveniently measured by ΔI, defined as the change in the mean preference across one generation, measured in units of the preference’s phenotypic standard deviation:
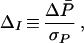 |
1 |
where ΔP̄ is the change in the preference mean and σP2 is the preference’s phenotypic variance. One appeal of this measure is that it is dimensionless, allowing comparisons across species and sensory modalities. Furthermore, the definition holds regardless of the underlying genetics of the preference. Our objective now is to calculate ΔI.
Indirect selection requires that there be heritable variation for total fitness. Here we simply assume the existence of this variation, without making assumptions about its sources. These may include coevolution with pathogens (6, 7) or deleterious mutation (8–11), as envisioned by some good genes theories, or a runaway process (12). Results developed below will give the precise definition of “fitness” that is relevant to indirect selection.
In the next section, we outline the calculation of ΔI. Readers who are mainly interested in the biological conclusions may wish to skip to the following section, where the major results and their implications are presented.
The Model
An expression for ΔI can be calculated under these general assumptions regarding the preference and fitness using methods introduced in ref. 13. The genetic model allows for any type of interaction between fitness loci, arbitrary recombination rates, any strength of selection on the phenotypes, and any number of loci and alleles per locus. For simplicity, the calculation here assumes haploid individuals, two alleles per locus, and autosomal inheritance, but these assumptions can be relaxed. In this section, we outline the calculation for ΔI in terms of only the preference and fitness; in the next section, we consider how the results are affected by properties of the male trait that females actually use to choose their mates.
We begin by defining genetic models for the preference and male fitness. Consider a preference determined by genes with additive effects:
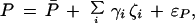 |
2 |
where P̄ is the mean preference, γi is the effect on the preference of locus i, and ɛP is a random environmental contribution to a female’s preference phenotype. Assuming additivity here is restrictive but does include the special case of a single preference locus. Having defined the preference in a way that is biologically appropriate to the species being studied, the phenotype of an individual female can be measured, for example, by repeated choice tests (e.g., ref. 14).
A male’s relative fitness, also referred to as his “genetic quality,” can be represented in general as
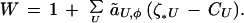 |
3 |
The coefficient ãU,φ is a measure of the overall force of natural and sexual selection acting on the set of loci U, averaged over the two sexes, and (ζU∗ − CU) represents the genetic deviation of the male from the population average at loci U (see equation 6 of ref. 13). The summation is over all sets and subsets of loci U in the genome exclusive of the preference loci; each permutation of the elements in a set is counted separately. Fitness has been scaled so that the population’s mean is 1. Conceptually, W is the average lifetime fitness that would be measured for the male’s genotype if it was replicated and expressed in a large number of males and females; we will see shortly that this is the definition of fitness that is relevant to indirect selection. The variance in W is denoted GW.
Under indirect selection, we find using equation 16 of ref. 13 that
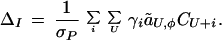 |
4 |
CU+i is the linkage disequilibrium among the preference locus i and the fitness loci U caused by preferential matings. Explicit expressions for the ãU,φ and CU+i values can be calculated for any model of natural and sexual selection and any set of genotype frequencies using the methods described in ref. 13.
Eq. 4 shows that indirect selection on the mating preference depends on the force of selection acting on individual loci and sets of loci that affect lifetime fitness, the ã values, and the genetic associations between those loci and the genes that affect the preference, the C values. These associations, in turn, evolve in response to selection and recombination. Our next task is to find expressions for the C and ã values that can be related to observable quantities.
To calculate the C values, we assume that the effects of individual alleles are small enough that their frequencies change slowly compared with the time needed for the loci to reach a state of “quasi-linkage equilibrium,” or QLE (13, 15, 16). Genetic associations between genes then change at a much slower rate than the allele frequencies, making it possible to calculate the C values. [If selection coefficients fluctuate in sign, as they do under some theories (6, 7), then the genetic correlation between preference and fitness alleles will be weakened, and our results will generally overestimate the impact of indirect selection.] Equation 25 of ref. 13 gives an approximation for CU+i at QLE. That expression involves the coefficients ãU,i, which measure the strength of sexual selection favoring associations between preference alleles at locus i and fitness alleles at the loci in set U. These coefficients can be calculated using a linear approximation for the effects of the preference genes. The frequency of matings between females with preference P and males with fitness W, relative to a random mating population, is
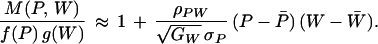 |
5 |
On the left, M(P, W) is the
frequency of matings between P females and W
males, and f( ) is the frequency of the preference and
g( ) of fitness. On the right are three macroscopic
quantities: ρPW, which is the correlation across mated pairs
of the female’s preference phenotype and the male’s genetic quality;
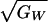 , which is the genetic coefficient
of variation for fitness (10); and σP, which is the
phenotypic standard deviation of the preference P. By
substituting the definitions for P and W from
Eqs. 2 and 3 into Eq. 5, then equating
terms with equation 6 of ref. 13, we find
, which is the genetic coefficient
of variation for fitness (10); and σP, which is the
phenotypic standard deviation of the preference P. By
substituting the definitions for P and W from
Eqs. 2 and 3 into Eq. 5, then equating
terms with equation 6 of ref. 13, we find
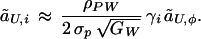 |
6 |
The factor of 2 emerges from the calculation as a result of the assumption that only one sex exercises mate choice. Substituting ãU,i into equation 25 of ref. 13 yields the value of CU+i at QLE, which is then substituted back into Eq. 4.
This finally gives us an expression for the impact of indirect selection:
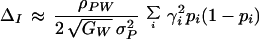 |
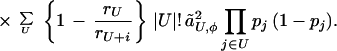 |
7 |
Here rA is the recombination frequency for loci in set A (that is, the average fraction of an individual’s gametes that carry a mixture of maternally and paternally inherited alleles at those loci), pi is the frequency of either allele at preference locus i, pj is the frequency of either allele at fitness locus j, and |U|! is the factorial of the number of loci in set U. Eq. 7 shows that recombination which breaks apart sets of coadapted fitness alleles (represented by the term rU) decreases the force of indirect selection.
The approximation of Eq. 7 is correct up to and including terms of order ã2; the simplification was achieved by dropping terms that are of order ã3 and smaller. The size of the error involved with the approximation can be determined for any specific genetic system using the methods of ref. 13, which explains how the ã values are calculated in terms of conventional selection coefficients and preference intensities. Generally, the ã values are of the same order of magnitude as those parameters. Thus if individual alleles have at most a 10% effect on their characters, for example, then the individual terms that were dropped from Eq. 7 will be at most 10% as large as those that were retained. Comparisons of our approximation with results from two detailed simulation models (including that described in ref. 11) show agreement within 10% for a wide range of parameter values once the population has reached QLE (results not shown).
Results
We now examine the implications of this model. First consider the simplest situation, when the nonadditive component of genetic variation in fitness is negligible. In that case, it can be shown that the inner summation of Eq. 7 reduces to GW and the outer summation to the genetic variance for the preference. The force of indirect selection is therefore simply
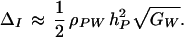 |
8 |
Again, ρPW is the correlation between the female’s
preference phenotype and the male’s genetic quality across breeding
pairs and 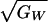 is the genetic
coefficient of variation for fitness, while
hp2 is the heritability of the
preference.
is the genetic
coefficient of variation for fitness, while
hp2 is the heritability of the
preference.
Robertson’s secondary theorem of natural selection (17, 18) links genetic variation in fitness to the force of direct selection on a character. Eq. 8 plays an analogous role in preference evolution by relating fitness variation to the force of indirect selection. Remarkably, the result is independent of the genetic details underlying the preference and male fitness, such as the number of loci and the recombination rates between them.
What are the consequences of nonadditive genetic variation in fitness? Eq. 7 shows that a preference can spread if it favors mating with males who carry coadapted sets of alleles. Consider, for example, an additive polygenic trait that is under natural selection for an intermediate optimum. The high fitness genotypes are those that carry trait alleles in repulsion, say + − and − +. Genetic variation in fitness can be generated by a balance between selection, which builds up repulsion genotypes, and recombination, which breaks them apart. A preference for intermediate males will then spread: in Eq. 7, ãU,φ measures the selection favoring the high fitness repulsion genotypes, and ρPW the strength of the preference for them. The force of indirect selection in this situation is always smaller than in Eq. 8, however, because the term in braces in Eq. 7, which represents recombination between the coadapted fitness loci, must be less than unity. The amount by which ΔI falls short of Eq. 8 when indirect selection is driven by epistatically interacting fitness genes depends on these recombination rates.
Females do not have direct information about a male’s fitness, of course, but rather choose their mates on the basis of phenotypic traits. How do male display traits mediate the relation between the preference and the fitness genes, and therefore the impact of indirect selection? Again assume that genetic variation is mainly additive, and further that the relation between a male’s trait phenotype T and his genotypic fitness W is approximately linear. A regression argument and Eq. 8 then show that
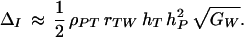 |
9 |
Here, ρPT is the phenotypic correlation across breeding pairs between the female preference and the male display trait, rTW is the genetic correlation between the male trait and total fitness, and hT is the square root of the heritability of the male trait.
Eq. 9 appears to be the first estimate for the strength of
indirect selection on preferences based on quantities that in principle
can be measured. Previously, the force of indirect selection has either
been calculated under highly simplified assumptions regarding behavior
and genetics, or simply viewed as an unknown parameter. Eq.
9, by contrast, holds regardless of the mating system or how
females choose their mates, and is relatively insensitive to the
underlying genetics. Further, it has a simple intuitive interpretation:
the fitness advantage that preference genes gain by indirect selection
is proportional to the coefficient of variation of male genetic
quality, 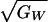 , and the accuracy with
which preference genes can associate themselves with fitness genes,
ρPTrTWhThP2.
, and the accuracy with
which preference genes can associate themselves with fitness genes,
ρPTrTWhThP2.
The size of the genetic correlation between a preference and a male display trait is important to both the good genes and the runaway forms of indirect selection. The same argument that leads to Eq. 9 implies that when the preference and male trait are both determined by genes of mainly additive effect, the genetic correlation between them will be
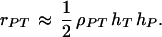 |
10 |
This relation holds regardless of how females choose their mates. It is also independent of the recombination rates between the preference and fitness loci, consistent with previous analyses of sexual selection (12, 13, 19).
These theoretical results lead to three conclusions with empirical
consequences. First, Eq. 9 identifies the parameters that
must be measured to quantify the force of indirect selection. Most have
yet to be estimated with precision. The current state of knowledge can
be briefly summarized as follows. The correlation between a female
preference and a male trait among mated pairs, ρPT, has not
been measured in any natural population. It is expected to be strongest
when females have acute discrimination abilities and free access to
many potential mates, as in some lekking species. One might expect the
many factors that affect expression of a male display trait to make the
genetic correlation between the male trait and total fitness,
rTW, small. Nevertheless, field studies have
suggested that the genetic correlation between male traits and certain
components of fitness may be high in some populations (refs. 20–23,
but see also refs. 24 and 25). The heritabilities of male display
traits and female preferences have been recently reviewed (26, 27). The
median estimate for hT is 0.69 (range: 0.2–1.2) and
the median for hP2 is 0.38
(range: 0.10–0.65). Estimates for 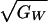 from natural and laboratory populations range from 0 to 0.45 (10, 28,
29). The values in natural populations are uncertain but may typically
fall between 0.1 and 0.3 (28).
from natural and laboratory populations range from 0 to 0.45 (10, 28,
29). The values in natural populations are uncertain but may typically
fall between 0.1 and 0.3 (28).
A second conclusion from Eq. 9 is that even with limited
data an upper bound can be placed on the force of indirect selection.
For example, if we accept the values suggested by the data
above of 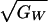 = 0.25,
hT = 0.7, and
hP2 = 0.4, then the maximum
possible value for ΔI is 0.035. This outcome would require
the most favorable conditions: a display trait that indicates breeding
value for fitness with complete fidelity (rTW = 1),
and a perfect correlation between the preference and male trait among
mated pairs (ρPT = 1). Even then, indirect selection changes
the mean preference by only 3.5% of the preference’s standard
deviation per generation. The actual value will be much smaller if the
correlations ρPT and rTW are appreciably
less than 1, as seems likely. The general impression that emerges is
that the covariance between preference genes and fitness produced by
sexual selection is not large.
= 0.25,
hT = 0.7, and
hP2 = 0.4, then the maximum
possible value for ΔI is 0.035. This outcome would require
the most favorable conditions: a display trait that indicates breeding
value for fitness with complete fidelity (rTW = 1),
and a perfect correlation between the preference and male trait among
mated pairs (ρPT = 1). Even then, indirect selection changes
the mean preference by only 3.5% of the preference’s standard
deviation per generation. The actual value will be much smaller if the
correlations ρPT and rTW are appreciably
less than 1, as seems likely. The general impression that emerges is
that the covariance between preference genes and fitness produced by
sexual selection is not large.
Our third conclusion is that ΔI allows indirect selection to be compared quantitatively with other forces acting on preferences. One important alternative force is direct natural selection acting on preference genes, caused either by their effects on mating per se or by their pleiotropic effects (2, 4). Its impact can be measured by ΔD, defined as for ΔI but with ΔP̄ now reflecting the change in the mean preference caused by direct selection. Estimates of ΔD are not available for any preference. Direct selection on other kinds of characters, however, can cause values of ΔD more than an order of magnitude larger than ΔI = 0.035. [This follows from observed rates of evolution (30, 31) and the relation ΔD = 10−4 d/CVP, where d is the character’s evolutionary rate measured in darwins (30), and CVP is its phenotypic coefficient of variation expressed as a percentage, and assuming one generation per year on average.) Thus direct selection on preference genes may overwhelm indirect selection. On the other hand, even weak indirect selection will be important if preference genes are virtually selectively neutral, that is, free of direct selection.
Discussion
Earlier theoretical studies showed qualitatively that indirect selection can work (e.g., refs. 7, 10–13, 19, 28, 32, 33). They made simplified assumptions regarding behavior and genetics, however, making it unlikely that their results can be applied quantitatively to nature. Further, previous models were framed in terms of parameters about which we have little or no empirical information. Those limitations made it impossible to gauge the impact of indirect selection in nature. The main point of this paper is to show how theory can be used to this end. The model is based on much more general and biologically plausible assumptions than earlier models, and happily the major results are independent of many unknown genetic and behavioral details such as the number of loci and the decision rules used in mate choice. These developments encourage us to estimate the force of indirect selection for the first time. Since the data presently available are limited, the estimate is tentative. We hope that by identifying the key parameters that govern indirect selection, this model will stimulate further empirical studies.
Under biologically plausible assumptions, the prediction for the force of indirect selection has a simple form given by Eq. 9. The data available suggest that ΔI is small in natural populations, perhaps not greater than 3.5% and perhaps much less than that. This number might be revised upward if future empirical work discovers that parameter values in some populations are very different than those studied to date. Sexually selected species might, for example, have unusually high levels of heritable variation for fitness. If the variation is generated by an ongoing good genes process, this proposition should be empirically testable. On the other hand, if the fitness variation results from an unstable runaway process, the episode of exaggeration might be brief and therefore difficult to observe (12).
What could generate the heritable variation in fitness that drives indirect selection? A number of well known processes maintain genetic variation for total fitness, including changing abiotic environments, coevolution, migration, deleterious mutation, and advantageous mutation (10, 28). Sexual selection itself produces variation in total fitness during a runaway process. Perhaps as the result of an overzealous reading of Fisher (34), it is sometimes thought that generally there is no heritable variation for fitness in natural populations. The real issue is not if there is heritable variation for fitness, but rather how much there is.
What measure of fitness is relevant to indirect selection of preferences? The model shows that the lifetime fitness effects of single alleles and sets of alleles, averaged over their expression in males and females, are what drives indirect selection of preferences (the ãU,φ values that appear in Eqs. 4 and 7). Ideally, GW would be measured by the variance in lifetime fitness between genotypes that were replicated in a large number of males and females. This is not quite the same as variation in the contribution that a male makes to his offspring’s fitness (that is, his “breeding value” for fitness) because recombination breaks up gene combinations in offspring. The difference will be small if the nonadditive component of fitness variation is small, however, as suggested by some experimental results (10). Thus standard quantitative genetic methods offer one approach to estimating GW. Other approaches have also been suggested (28).
Several suggestions have been made regarding how a genetic correlation between the male trait and total fitness, rTW, might arise (2–3). The conceptually simplest possibility is that females might choose males on the basis of traits that are evolving directionally. This is what happens in a runaway process, where the female preferences themselves are responsible for the continuing evolution of the male trait (12). Alternatively, the genetic quality of males may affect expression of their display traits, for example, when high quality males are able to divert more energy to their mating displays. With male displays of this sort (sometimes called “conditional handicaps” or “revealing handicaps”), the trait–fitness correlation results from development, physiology, and behavior. A third possibility is that extreme traits exact a smaller viability cost from high quality males than from low quality males (“epistatic handicaps”); then selection causes rTW to build up. Our results apply to all these cases, showing that indirect selection operates regardless of the cause of the genetic correlation between lifetime fitness and the male trait. Earlier conclusions that the epistatic handicap mechanism cannot work in principle (32, 33, 35, 36) therefore appear to be in error. It seems likely that epistatic handicaps may generally produce small values for rTW, however, because that correlation is incessantly being eroded away by recombination (unlike the correlation produced by conditional and revealing handicaps). Epistatic handicaps may therefore result in a particularly weak form of indirect selection.
A second (and quite separate) question involving epistasis arises if the underlying variation in fitness is caused by epistatic selection. Eq. 7 establishes that indirect selection can operate in this situation. There is some experimental evidence, however, suggesting that the bulk of genetic variation for fitness is additive (10). That evidence implies that the simpler results of Eqs. 8 and 9 may give reasonable approximations for natural populations. In any event, those equations provide an upper bound for ΔI in cases where nonadditive fitness variation is not negligible.
We have suggested that the force of indirect selection on mating preferences is small in absolute terms. Whether it is small or large compared with direct selection and the other forces acting on preference genes is an empirical question. While it seems unlikely that genes influencing such a major behavioral character as a mating preference would be completely free of all direct selection, the issue cannot be settled until more empirical studies focus on the consequences of variation among females and their preferences.
Acknowledgments
We thank J. J. Bull, M. J. Ryan, M. Wade, B. Walsh, G. C. Williams, and an anonymous reviewer for discussions and suggestions. This research was supported by National Science Foundation Grant DEB94–07969, Biotechnology and Biological Sciences Research Council Grants GR/H/09928 and GR/J/76057, and a travel grant from the Burroughs-Wellcome Fund.
Footnotes
Abbreviation: QLE, quasi-linkage equilibrium.
References
- 1.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 2.Kirkpatrick M, Ryan M J. Nature (London) 1991;350:33–38. [Google Scholar]
- 3.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 4.Kirkpatrick M. Annu Rev Ecol Syst. 1987;18:43–70. [Google Scholar]
- 5.Ryan M. In: Behavioral Ecology: An Evolutionary Approach. 4th Ed. Krebs J R, Davies N B, editors. Oxford: Blackwell; 1997. pp. 179–202. [Google Scholar]
- 6.Hamilton W D, Zuk M. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 7.Pomiankowski A. Proc R Soc London Ser B. 1987;231:123–145. [Google Scholar]
- 8.Partridge L. In: Mate Choice. Bateson P, editor. Cambridge, U.K.: Cambridge Univ. Press; 1983. pp. 227–255. [Google Scholar]
- 9.Kondrashov A S. J Theor Biol. 1988;131:487–496. doi: 10.1016/s0022-5193(88)80043-2. [DOI] [PubMed] [Google Scholar]
- 10.Charlesworth B. In: Sexual Selection: Testing the Alternatives. Bradbury J W, Andersson M B, editors. Chichester, U.K.: Wiley; 1987. pp. 21–40. [Google Scholar]
- 11.Kirkpatrick M. Evolution (Lawrence, Kans) 1996;50:2125–2140. doi: 10.1111/j.1558-5646.1996.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 12.Lande R. Proc Natl Acad Sci USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton N H, Turelli M. Genetics. 1991;127:229–255. doi: 10.1093/genetics/127.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner W E, Murray A-M, Cade W H. Anim Behav. 1995;49:1269–1281. [Google Scholar]
- 15.Kimura M. Genetics. 1965;52:875–890. doi: 10.1093/genetics/52.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagylaki T. Genetics. 1976;83:583–600. [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson A. Anim Prod. 1966;8:95–108. [Google Scholar]
- 18.Price G R. Nature (London) 1970;227:520–521. doi: 10.1038/227520a0. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick M. Evolution (Lawrence, Kans) 1982;36:1–12. doi: 10.1111/j.1558-5646.1982.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 20.Norris K. Nature (London) 1993;362:537–539. [Google Scholar]
- 21.Møller A P. Sexual Selection and the Barn Swallow. Oxford: Oxford Univ. Press; 1994. [Google Scholar]
- 22.Petrie M. Nature (London) 1994;371:598–599. [Google Scholar]
- 23.Moore A J. Behav Ecol Sociobiol. 1994;35:235–241. [Google Scholar]
- 24.Boake C R B. Science. 1985;227:1061–1063. doi: 10.1126/science.227.4690.1061. [DOI] [PubMed] [Google Scholar]
- 25.Boake C R B. Am Nat. 1986;127:654–666. [Google Scholar]
- 26.Pomiankowski M, Møller A P. Proc R Soc London Ser B. 1995;260:21–29. [Google Scholar]
- 27.Bakker T C M, Pomiankowski A. J Evol Biol. 1995;8:129–171. [Google Scholar]
- 28.Burt A. Evolution (Lawrence, Kans) 1995;49:1–8. doi: 10.1111/j.1558-5646.1995.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 29.Hughes K A. Evolution (Lawrence, Kans) 1995;49:521–537. doi: 10.1111/j.1558-5646.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 30.Gingerich P D. Science. 1983;222:159–161. doi: 10.1126/science.222.4620.159. [DOI] [PubMed] [Google Scholar]
- 31.Stearns S C. The Evolution of Life Histories. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 32.Pomiankowski A. J Theor Biol. 1987;128:195–218. doi: 10.1016/s0022-5193(87)80169-8. [DOI] [PubMed] [Google Scholar]
- 33.Iwasa Y, Pomiankowski A, Nee S. Evolution (Lawrence, Kans) 1991;45:1431–1442. doi: 10.1111/j.1558-5646.1991.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RA. The Genetical Theory of Natural Selection. 2nd Ed. New York: Dover; 1958. [Google Scholar]
- 35.Kirkpatrick M. Am Nat. 1986;127:222–240. [Google Scholar]
- 36.Maynard Smith J. Trends Ecol Evol. 1991;6:146–151. doi: 10.1016/0169-5347(91)90055-3. [DOI] [PubMed] [Google Scholar]


