Abstract
Objective:
In children with Attention Deficit Hyperactivity Disorder (ADHD), clinical responses to the selective norepinephrine reuptake inhibitor atomoxetine (ATX) vary. We sought to determine in children with Tourette Syndrome (TS) whether clinical responses correlate with changes in short interval cortical inhibition (SICI).
Methods:
Fourteen children, ages 8 to 16, with ADHD and TS were treated open-label with ATX for one month. ADHD rating scale scores and SICI, measured with paired-pulse Transcranial Magnetic Stimulation (pTMS), were assessed blindly and independently at treatment onset and one month later.
Results:
Eleven children, mean ADHD Rating Scale scores 31.8 (SD 8.2) at onset completed the study. After one month, ADHDRS changes ranged from an increase of 4 points to a decrease (improvement) of 24 points (mean change -9.6, SD 9.1). The changes in ADHDRS scores correlated with reduction in SICI (r = .74, p = .010).
Conclusions:
In children with TS, one month of atomoxetine treatment appears to induce correlated improvements in ADHD and, paradoxically, further reductions in cortical inhibition.
Significance:
PTMS-evoked SICI in ADHD with TS may be a biomarker of both deficiency and compensatory changes within cortical interneuronal systems. Effective atomoxetine treatment may augment compensatory processes and thereby reduce SICI.
Keywords: Cortical inhibition, transcranial magnetic stimulation, Attention Deficit Hyperactivity Disorder, Tourette Syndrome, atomoxetine
INTRODUCTION
Understanding of the neurobiology of ADHD symptoms and treatment responses has been hampered by the lack of biological markers and the heterogeneity of genetic and environmentally-induced conditions that manifest with ADHD symptoms. To improve our understanding of ADHD symptoms and treatment responses, it may be helpful to focus biological studies within particular heritable and readily identifiable phenotypes, for example, to study ADHD within Tourette Syndrome (TS). ADHD is highly prevalent in TS and accounts for most motor and executive control deficits in TS (Denckla, 2006). To this end, we and others have used Paired-Pulse Transcranial Magnetic Stimulation (pTMS) to study neurophysiological parameters in ADHD in TS. One pTMS measure, GABAA-mediated, short interval cortical inhibition (SICI) (Kujirai et al., 1993), which represents inhibitory output of frontal cortex interneurons, has been most widely used. PTMS employs a hand-held magnet to provide low intensity magnetic “conditioning” pulses that activate inhibitory interneurons and high intensity “test” pulses that activate motor cortex, producing motor evoked potentials (MEPs). Comparing paired pulse MEP amplitudes to single pulse MEP amplitudes yields a quantitative measure of an inhibitory process in frontal cortex. SICI is expressed as a ratio of paired pulse-evoked MEP amplitudes to single pulse MEP amplitudes, where a ratio of 1.0 indicates no inhibition, and smaller ratios indicate greater levels of inhibition.
The inhibitory processes reflected in SICI are affected in a variety of neurologic and psychiatric conditions (Gilbert, 2006). SICI is significantly diminished (larger ratios) in children with ADHD (Moll et al., 2001). We have previously found consistent correlations between reductions in SICI and ADHD symptom severity in TS (Gilbert et al., 2004a, Gilbert et al., 2005). Moreover, many studies show that SICI changes in response to dopamine agonists, stimulants, and norepinephrine reuptake inhibitors (Gilbert et al., 2006a, Gilbert et al., 2006b, Ilic et al., 2003, Kirschner et al., 2003, Moll et al., 2000, Ziemann et al., 1997). Most of these pharmaco-physiological studies have measured effects of single doses in healthy adults.
We have shown that single doses of two ADHD medications, the stimulant methylphenidate and selective norepinephrine reuptake inhibitor (SNRI) atomoxetine, reduce SICI in adults. This reduction seems surprising, but is consistent with other healthy-adult studies of psychostimulants and of the SNRI reboxetine (Gilbert et al., 2006a). In children with ADHD, the effects on SICI of single doses of atomoxetine are more variable and may depend on dopamine transporter genotypes or on TS phenotypes (Gilbert et al., 2006b). To date, no published studies have assessed SICI changes and clinical ADHD responses to atomoxetine over longer time periods.
Because the relationship between SICI and ADHD in TS remains consistent over a one-month period (Gilbert et al., 2005), it is feasible to study one-month ADHD treatment effects in children with TS. We selected the norepinephrine reuptake inhibitor ATX, which tends to reduce ADHD symptoms in children with TS without exacerbating tics (Allen et al., 2005), but has more gradual and variable clinical effects than stimulants. The aim of this study was to determine whether one-month clinical ADHD responses to ATX correlate with: 1) first-ATX-dose induced changes in SICI; or 2) one-month-ATX induced changes in SICI. We also assessed another pTMS measure, intracortical facilitation. Although ICF is not linked to ADHD, it is sensitive to effects of norepinephrine reuptake inhibitors (Gilbert et al., 2006a, Herwig et al., 2002, Plewnia et al., 2002). We report a novel result which may relate to ATX's mechanism of action in treating ADHD symptoms in TS.
METHODS AND MATERIALS
Subjects
Fourteen right-handed youth with ADHD and TS (with mild, non-impairing tics) were recruited through the TS Clinic at Cincinnati Children's Hospital Medical Center (CCHMC) and scheduled for visits separated by one month, an interval at which the relationship between ADHD and SICI is stable (Gilbert et al., 2005). Eleven subjects age 8 to 16 years (mean 12) completed the study. Some subjects had participated in a previous, single-dose study (Gilbert et al., 2006b). Diagnoses were based clinical interview, DSM-IV criteria (American Psychiatric Association, 2000), and DuPaul ADHD rating scale (ADHDRS) scores (DuPaul et al., 1998). The decision to initiate ATX for ADHD was made on clinical grounds, independent of study participation. We excluded children with conduct disorder or depression, screened with the childhood depression inventory (Kovacs, 1985). No concurrent behavioral treatments or other medical treatments for ADHD were administered during the study. Subjects and parents gave written informed consent, as approved by the CCHMC Institutional Review Board. Subjects were questioned for irregularities of sleep or general health on the day of the visit and none were identified.
Treatment and ADHD rating
ATX administration was open label at 0.8 to 1.0 mg/kg at treatment onset, increasing with a fixed titration the first week to 1.0 to 1.5 mg/kg (routine dosing). Clinical ratings were performed by an experienced rater. At each visit, ADHD symptoms for the past week were rated with direct parent interview using the ADHDRS (DuPaul et al., 1998). Clinical improvement was represented as a change score (ADHDRSone-month – ADHDRSbaseline). Parent and rater were blinded to TMS results, though not to treatment.
Motor Cortex Physiology
PTMS was performed, blinded to clinical ratings, twice at each visit – before and 90 minutes after (Witcher et al., 2003) ATX administration. All subjects were studied awake in a comfortable chair in the TMS laboratory at CCHMC, using two Magstim 200® stimulators (Magstim Co., New York, NY, USA) connected through a Bistim® module to a 90 mm circular coil. In order to ensure children were relaxed, the entire procedure was explained before-hand and the TMS pulses were demonstrated first on the investigator, then on the parent's arm, then on the child's arm. Low intensity pulses were given first on the scalp, to accustom the child to the feeling and sound of the TMS machine. Using this gentle approach, we have found most children cooperate well, do not express anxiety about the procedure (Gilbert et al., 2004b), and have robust test-retest reliability (Gilbert et al., 2005). Similar approaches using TMS for ADHD studies have found children rate the experience favorably (Garvey et al., 2001).
EMG was recorded with from the right abductor pollicis brevis (APB) with surface electrodes, amplified, filtered (100/1000 Hz) (Coulbourn Instruments, Allentown, PA) and stored using Signal® software and a Micro1401 interface (Cambridge Electronic Design, Cambridge, UK). The optimal coil location was located by moving the coil from the vertex in 1 cm increments. For the round coil, the optimal location is near the vertex, with a clockwise current direction for left motor cortex. The location was recorded and the coil stabilized with a wall-mounted clamp to increase consistency across pTMS sessions. As described in our previous studies (Gilbert et al., 2006a, Gilbert et al., 2006b), and consistent with convention, the resting motor threshold (RMT) was determined by increasing the coil stimulation intensity by increments of 10% of maximum stimulator output until a robust MEP was elicited. Then the intensity was decreased by 1% repeatedly until the lowest intensity able to produce MEPs of at least 50 uV on 5 out of 10 consecutive trials of stimulation was identified. Stimuli were delivered at least 5 s apart while the muscle was monitored for relaxation with visual inspection of the EMG and playback through a loudspeaker.
The active motor threshold (AMT) was determined while the subject contracted the APB with auditory feedback to maintain a constant, moderate level of EMG. The stimulator output was decreased in increments of 1% from the RMT intensity until the point at which 10 rectified, averaged stimuli failed to show a MEP above background.
The suprathreshold “test” pulse was 10 to 20% above RMT, at a level which consistently elicited MEPs with 500-1500 uV amplitudes. This was confirmed at each TMS session, before and after each dose. The preceding conditioning pulse intensity was set at 1% below AMT, generally about 65% of RMT. Sixty trials consisting of 20 single and 20 paired pulses each at interstimulus intervals (ISI) of 3 msec (SICI) and 10 msec (intracortical facilitation – ICF) were delivered in random order, separated by 6 ± 10% seconds. By convention, SICI and ICF are expressed as ratios of mean paired to single pulse MEP amplitudes, with ratios relative to 1.0 (no change). Thus, for SICI, more motor cortex inhibition means smaller ratios; and for ICF, more motor cortex facilitation means greater ratios.
At each session, pre- and post-dose, RMT and the test pulse intensity were reassessed, by the same methods, prior to measuring SICI and ICF.
In order to assess for possible confounders, not related to ADHD, mood was assessed using a visual analog mood scale (Gilbert et al., 2004b) pre and post each TMS session to determine whether anxiety about the procedure might be present.
SICI changes were calculated separately for pre- and post-first ATX dose (SICIpost-dose1 – SICIpre-dose1), pre- and post- one-month ATX dose (SICIpost-dose1m – SICIpre-dose1m), and for baseline (pre-ATX) to one-month (pre-ATX), (SICIone-month – SICIbaseline). ICF changes were calculated in the same fashion.
Statistical Analysis
All analyses were performed with SPSSR version 14.0 (Chicago, IL). Prior to performing analysis of interest, univariate correlations of age and neurophysiological measures were performed to determine whether age needed to be included in a regression. Paired t test comparisons of single pulse TMS responses (resulting from pTMS) were performed to test whether, at each session, single pulse TMS responses differed. The mean single pulse TMS responses for each individual constitute the denominators for the SICI and ICF ratios and need to remain consistent for changes in SICI and ICF to be interpretable.
Primary outcomes
We assessed two Behavior vs. Neurophysiology correlations: 1) one-month ADHDRS change (ADHDRSone-month – ADHDRSbaseline) vs. First-dose SICI change (SICIpost-dose1 – SICIpre-dose1); and 2) one-month ADHDRS change (ADHDRSone-month – ADHDRSbaseline) vs. one-month SICI change (SICIone-month – SICIbaseline). Correlations were performed conservatively with non-parametric Spearman tests, with Bonferroni-adjusted p < .025 considered significant.
Secondary outcomes
We assessed ATX-related changes in SICI (pre vs. post 1st dose; pre 1st dose vs. pre one-month dose, pre vs. post one-month dose) with the Wilcoxon Signed Ranks Test for two related samples, with Bonferroni-adjusted p < .017 considered significant. We also analyzed changes in SICI vs. changes in the ADHDRS subscore domains for inattention and hyperactive/impulsive behavior. Finally, we assessed these ADHD changes in relation to changes in ICF.
Comparison with prior cohorts
The first-visit, pre-treatment SICI ratio in this cohort appeared to be lower than the SICI ratio in our prior single-dose study (Gilbert et al., 2006b), and closer to the baseline, pre-dose SICI in our study of healthy adults (Gilbert et al., 2006a). Therefore, we were concerned that this cohort might be atypical in some way and that the ATX-associated changes in SICI might not be generalizable. To address this, we compared the pre-first-dose-ATX SICI ratios: 1) current cohort vs. our prior ADHD TS cohort; and 2) current cohort vs. the healthy adult cohort. In addition, we performed the same group comparisons for the SICI difference scores pre- and post-ATX. Group comparisons were performed with the nonparametric Mann Whitney test.
RESULTS
Demographics, adverse events, dropouts
Eleven children age 8-16 years (mean 12), 9 male, completed the study. Three were removed by the investigator (non-compliance, illicit stimulant use, marijuana use). Results are for study-completers. The median weight was 52 kg (27-75 kg). The median ATX doses were 40 mg (0.8 mg/kg) at baseline and 60 mg (1.2 mg/kg) at one month. All side effects of pTMS and ATX were mild and transient, for example, transient headaches, as has been previously reported (Garvey et al., 2001, Gilbert et al., 2004b).
There was no correlation between age and any neurophysiological measures. Therefore, correlations were performed as planned, without regression over age.
Consistency of Thresholds, Stimulation Intensities, and single pulse TMS MEP amplitudes across sessions
Resting and Active Motor Thresholds did not change significantly pre- and post-ATX at either visit, or across visits. The mean (SD) RMTs at visit 1 were 60.2 (10.4) prior to ATX and 59.6 (10.6) after ATX. The mean (SD) RMTs at visit 2 were 59.2 (11.2) prior to ATX and 59.7 (11.6) after ATX. The mean (SD) AMTs at visit 1 were 37.5 (7.5) prior to ATX and 37.7 (8.3) after ATX. The mean (SD) AMTs at visit 2 were 38.3 (9.4) prior to ATX and 40.3 (8.8) after ATX.
Settings for the test and control pulses did not vary significantly pre- and post-ATX at either visit or across visits. The test pulse intensity was selected to induce MEP amplitudes from 500 to 1500 uV. The mean (SD) test pulse intensities at visit 1 were 69.8 (11.5) prior to ATX and 69.2 (11.0) after ATX. The mean (SD) test pulse intensities at visit 2 were 69.9 (11.8) prior to ATX and 70.3 (11.9) after ATX. The mean (SD) conditioning pulse intensities at visit 1 were 37.2 (7.7) prior to ATX and 36.9 (7.8) after ATX. The mean (SD) conditioning pulse intensities at visit 2 were 38.3 (9.1) prior to ATX and 38.3 (9.1) after ATX.
The test pulse MEP amplitudes induced during pTMS did not change significantly across TMS sessions. The mean (SD) single-pulse TMS MEP amplitudes at visit 1 were 800 (300) uV before ATX and 760 (300) after ATX. The mean (SD) single-pulse TMS MEP amplitudes at visit 2 were 840 (370) uV before ATX and 990 (420) after ATX. Paired t-test comparisons of these MEP amplitudes did not demonstrate any changes (t10 p values ranged from .13 to .80).
Effects of one month of ATX on ADHDRS
Baseline and one-month ADHD scores are shown in table 1. Mean total ADHDRS scores dropped 30%. Changes ranged incrementally from a worsening of 4 points to an improvement of 24 points, with a near normal distribution. There was no meaningful cut-off that could create categories of responders vs. non-responders, so clinical responses were analyzed only as continuous change scores.
Table 1.
Changes in ADHD, SICI, and ICF
| Baseline Visit | One-month Visit | P value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| ADHD Rating Scale Score | 32 (8) | 22 (9) | .010* |
| Attention Score | 21 (5) | 14 (6) | .006* |
| Hyperactivity Score | 11 (7) | 8 (5) | .126 |
| SICI | |||
| Pre-ATX | .36 (.18) | .42 (.22) | .062 |
| Post-ATX | .33 (.12) | .43 (.20) | - |
| P value | .477 | .929 | |
| ICF | |||
| Pre-ATX | 1.25 (.25) | 1.39 (.32) | .182 |
| Post-ATX | 1.57 (.52) | 1.28 (.30) | - |
| P value | .006* | .155 |
ATX = atomoxetine. SICI = motor cortex short interval cortical inhibition. ICF = motor cortex intracortical facilitation. SD = standard deviation. All p values from Wilcoxon Signed Ranks Tests for 2 related samples.
= significant after multiple test correction.
Effects of first dose and one-month ATX on SICI and ICF
Resting and active motor thresholds did not change throughout the study. MEP amplitudes after single pulse TMS also did not change. Thus, changes in SICI and ICF ratios were due to the numerators of these ratios, reflecting the responses to the subthreshold, conditioning pulse.
SICI and ICF changes are shown in table 1. The SICI reduction (−.061; 95% CI −.14 to .0042) (MEP amplitude ratio increase) from baseline to one month approached significance (t10 −2.10; p = .062). SICI values did not change significantly from pre- to post-ATX at either visit.
Correlations between changes in ADHDRS and SICI and ICF
One-month changes in SICI and ICF correlated with improvement in the total ADHD rating scale score, with similar correlations for the ADHDRS subscales (figure 1, table 2). First-dose ATX-induced changes in SICI and ICF did not correlate with one-month ADHDRS changes (data not shown).
Figure 1.
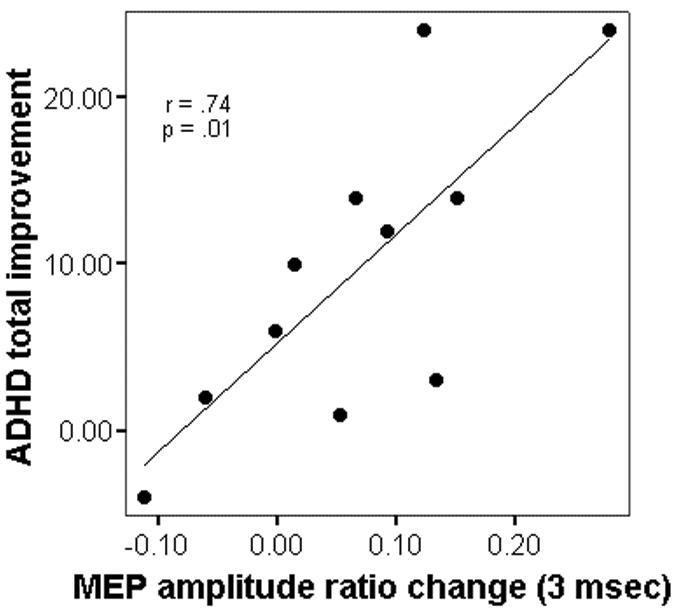
Changes in Motor Cortex SICI and ADHDRS scores from baseline to one month of treatment with atomoxetine (ATX). For ADHD (y axis), a change score (ADHDRSone-month – ADHDRSbaseline), a positive change represents improvement. For the SICI change score (x axis), a positive change indicates an increase in the paired pulse to single pulse MEP amplitude ratios, i.e. a decrease in SICI (see methods).
Table 2.
Correlations between changes in Short Interval Cortical Inhibition, Intracortical Facilitation and changes in ADHD scores after one month of Atomoxetine
| SICI | ICF | |||
|---|---|---|---|---|
| ADHD Scores | r | p value | r | p value |
| Total ADHDRS | .74 | .01 | .71 | .01 |
| Attention Subscore | .52 | .10 | .56 | .07 |
| Hyperactive/Impulsive Subscore | .61 | .05 | .61 | .05 |
ADHDRS = ADHD rating scale. SICI = motor cortex short interval cortical inhibition. ICF = motor cortex intracortical facilitation. All r and p values from Spearman correlations.
Comparison with prior ADHD/TS child and normal adult studies
Mean pre-ATX SICI in this cohort was 0.36, vs. SICI of 0.23 (p = .067) in the prior healthy adult study (Gilbert et al., 2006a) and 0.55 (p = .044) in the prior ADHD/TS study (Gilbert et al., 2006b) . Thus pre-dose SICI in the present study was intermediate between the previously studied cohorts.
Pre- to post-ATX SICI changes in this cohort were similar to the prior ADHD/TS cohort and differed from the changes in the healthy adults. The mean first dose pre- to post-ATX change in this cohort was −.030, significantly different than the 0.19 for the healthy adults (p=.002). The change of .0044 for the prior ADHD/TS cohort was not different (p = .73)
DISCUSSION
In this study, we have assessed, for the first time, one-month changes in ADHD symptoms and Paired-Pulse Transcranial Magnetic Stimulation (pTMS)-evoked motor cortex inhibition in children taking the selective norepinephrine reuptake inhibitor atomoxetine (ATX). The primary outcome, the finding that decreasing short interval cortical inhibition (SICI) correlated with improving ADHD in TS, was unexpected.
Prior studies employing pTMS have in motor cortex have shown that SICI is decreased in ADHD (Gilbert et al., 2004a, Moll et al., 2001), that SICI correlates consistently over one month with ADHD symptom severity in TS (Gilbert et al., 2004a), and that SICI changes in response to single doses of both stimulants and norepinephrine reuptake inhibitors (Gilbert et al., 2006b, Moll et al., 2000). No prior studies have used pTMS to assess longer-term ATX-induced SICI changes and their relationship to clinical benefit. Although we had anticipated that ADHD improvements would correlate with normalization (increase) in SICI, we found the opposite – in TS children, one-month improvements in ADHD symptoms correlated significantly with further reduction of SICI.
The results of this study are surprising when considered in light of prior ADHD and TMS studies in children. The reduction in pTMS-evoked SICI in ADHD, consistently identified in several independent neurophysiological laboratories, has been presumed to represent a cortical deficiency (Moll et al., 2001). The finding that a single, low dose of a psychostimulant partially normalized SICI in children with ADHD (Moll et al., 2000) supported this interpretation. This simple interpretation also seems plausible: persons with deficient inhibitory behavior control might have deficient inhibitory neurophysiological function in frontal cortex. Given this background, we had anticipated that effective norepinephrine reuptake inhibitor treatment of ADHD would increase, or normalize, SICI, although perhaps more slowly – not at the first dose.
However, to complicate this prediction, previous studies of ADHD medications in healthy adults seemed to contradict the findings in ADHD children. In normal adults, we and others had found that single doses of both psychostimulants (Gilbert et al., 2006a, Kirschner et al., 2003, Moll et al., 2003) and norepinephrine reuptake inhibitors (Gilbert et al., 2006a, Herwig et al., 2002, Plewnia et al., 2002) reduced (“worsened”) SICI and increased ICF. It was unclear how to harmonize studies showing that a medication could increase/normalize SICI in one situation and decrease/worsen it in another.
All these referenced studies have fairly small sample sizes, thus one possibility is that some report spurious findings. However, the presence of some consistency across laboratories in the baseline pTMS measures in ADHD and TS, demonstrated in our meta-analysis of single dose, SNRI-induced changes in SICI and ICF (Gilbert et al., 2006a) make this less likely. Determining whether the differences with child-ADHD vs. normal adult studies relates to age or ADHD diagnosis is not currently possible. Perhaps one difference between pediatric and adult studies might relate to the degree of myelination of cortical-cortical fibers. However, we speculate that pTMS measures in motor cortex in ADHD reflect both intrinsic underlying deficiencies and compensatory processes. In some types of ADHD, both the deficient and the compensatory process might reduce SICI. Then a medication that primarily counteracts a deficiency would increase SICI, but one that augments a compensatory process would decrease it. Applying that idea to the present study, ATX, when clinically effective in TS+ADHD, may have differentially affected a compensatory process that further reduced SICI.
It should also be pointed out that the relationship between changes in behavior and changes in SICI could reasonably differ for ATX and the psychostimulants or might vary depending on the presence of TS or other phenotypes. Although early clinical trials showed clinical responses to methylphenidate and ATX appeared to be equivalent (Kratochvil et al., 2002), more recent studies comparing ATX to long-acting stimulants (Gibson et al., 2006) as well as clinical experience suggest ATX induces much more variable clinical responses and may differ in other important ways. It would also be of interest to compare ATX effects on other measures, such as transcallosal inhibition, with those of psychostimulants (Buchmann et al., 2006).
The clinical meaning of the changes in ICF remains unclear. ICF has not been shown in any prior study to correlate with ADHD presence or severity, but we were interested in one-month changes because single doses of SNRIs atomoxetine and reboxetine both increase ICF (Gilbert et al., 2006a). Our finding that ICF changes at one month also correlated with clinical improvement may mean that both ICF and SICI measures reflect a SNRI-sensitive process.
Several limitations of this study bear mention. First and foremost, we assessed a clinical ADHD response and a neurophysiological response to a medication without an active comparator or placebo treatment group. Thus, both the one-month clinical improvements and neurophysiological changes observed may be due to time or other factors, and not ATX. First to second-visit neurophysiological changes might be due to a reduction in anxiety from the first visit to the second visit, or some other factor inducing test-retest variability. However, if a confounder, not ATX, is responsible for these results, this confounder would have had to produce changes that led to correlations between two independent measures. Also, it is possible that SICI changes are an epiphenomenon, not related to behavioral changes. However, since clinical responses varied widely and pTMS was performed blinded to this data, it seems unlikely that that time or placebo-response effects would cause the result we found: larger SICI changes in children with larger improvements and ADHD symptoms, and small or no SICI changes in children with small or no improvement in ADHD (the correlation in figure 1). As a final note, with regard to the use of placebo, our study is consistent with most TMS-pharmacology literature, as reviewed elsewhere (Ziemann, 2004), which has not used a placebo comparator.
Second, we relied on parent ratings to judge clinical responses. Parent ratings may be subjective or inaccurate, or ADHD symptoms might be better rated by teachers. However, any error introduced by biased or unreliable parent reporting should be independent of our neurophysiological studies, and thereby should have skewed our pTMS correlational results toward the null hypothesis. Third, with regard to generalizability, this study's sample was small and limited to TS+ADHD children. Although, the clinical response rate in this study is consistent with those in large clinical trials of ATX in children with tics (Allen et al., 2006) and without tics (Michelson et al., 2002), it would be premature to generalize the results from this study to non-TS ADHD. In addition, this TS sample had a somewhat lower SICI ratio than our prior cohorts, for unclear reasons. Fourth, we did not account for tic severity in this analysis. The reason for this is that we had previously found that SICI correlated with the tic frequency score (Gilbert et al., 2004a) of the Yale Global Tic Severity Scale (Leckman et al., 1989), but in this study we had no patients with high tic frequency ratings at baseline.
In conclusion, pTMS-evoked SICI appears to directly or indirectly measure a cortical property that is sensitive, at one month, to the degree of clinical benefit produced by the selective norepinephrine reuptake inhibitor ATX. Longer-term studies involving genetic determinants may help validate and further elucidate mechanisms of treatment response.
Acknowledgement
We are grateful to our patients who participated in this study.
Funding sources: This research was supported by an investigator-initiated grant from Lilly Research Laboratories, a Division of Eli Lilly and Company (Indianapolis, IN) (DLG); by NINDS K23 NS41920 (DLG); and by the NARSAD Young Investigator Award (ZW); by a Child Neurology Foundation Summer Medical Student Fellowship (NN); and by the University of Cincinnati Summer Undergraduate Research Foundation program (JB). No reprints available.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AJ, Kurlan RM, Gilbert DL, Coffey BJ, Linder SL, Lewis DW, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology. 2005. 2005 December 27;65(12):1941–1949. doi: 10.1212/01.wnl.0000188869.58300.a7. [DOI] [PubMed] [Google Scholar]
- Allen AJ, Spencer TJ, Dunn DW, Gilbert DL, Milton DR, Feldman PD. Atomoxetine Treatment of ADHD in Children with Comorbid Tourette Syndrome. Neurology. 2006 doi: 10.1177/1087054707306109. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Buchmann J, Gierow W, Weber S, Hoeppner J, Klauer T, Wittstock M, et al. Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH) Neurosci Lett. 2006;405(12):14–18. doi: 10.1016/j.neulet.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Attention deficit hyperactivity disorder: the childhood co-morbidity that most influences the disability burden in Tourette syndrome. Adv Neurol. 2006;99:17–21. [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklist, norms, and clinical interpretations. Guilford Press; New York: 1998. [Google Scholar]
- Garvey MA, Kaczynski KJ, Becker DA, Bartko JJ. Subjective reactions of children to single-pulse transcranial magnetic stimulation. J Child Neurol. 2001;16(12):891–894. doi: 10.1177/088307380101601205. [DOI] [PubMed] [Google Scholar]
- Gibson AP, Bettinger TL, Patel NC, Crismon ML. Atomoxetine versus stimulants for treatment of attention deficit/hyperactivity disorder. Ann Pharmacother. 2006;40(6):1134–1142. doi: 10.1345/aph.1G582. [DOI] [PubMed] [Google Scholar]
- Gilbert DL. Motor Cortex Inhibitory function in Tourette Syndrome, Attention Deficit Disorder, and Obsessive Compulsive Disorder - studies using transcranial magnetic stimulation. Adv Neurol. 2006;99:107–114. [PubMed] [Google Scholar]
- Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T, et al. Association of Cortical Disinhibition with tic, ADHD, and OCD Severity in Tourette Syndrome. Mov Disord. 2004a;19:416–425. doi: 10.1002/mds.20044. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin Neurophysiol. 2004b;115(8):1730–1739. doi: 10.1016/j.clinph.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Ridel KR, Sallee FR, Zhang J, Lipps T, Wassermann E. Comparison of the inhibitory and excitatory effects of ADHD medications methylphenidate and atomoxetine on motor cortex. Neuropsychopharmacology. 2006a;31:442–449. doi: 10.1038/sj.npp.1300806. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Sallee FR, Zhang J, Lipps TD, Wassermann EM. TMS-evoked cortical inhibition: a consistent marker of ADHD scores in Tourette Syndrome. Biol Psychiatry. 2005;57:1597–1600. doi: 10.1016/j.biopsych.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Wang Z, Ridel KR, Merhar SL, Sallee FR, Zhang J, et al. Dopamine transporter genotype influences the physiological response to medication in ADHD. Brain. 2006b;129:2038–2046. doi: 10.1093/brain/awl147. [DOI] [PubMed] [Google Scholar]
- Herwig U, Brauer K, Connemann B, Spitzer M, Schonfeldt-Lecuona C. Intracortical excitability is modulated by a norepinephrine-reuptake inhibitor as measured with paired-pulse transcranial magnetic stimulation. Psychopharmacology (Berl) 2002 Nov;164(2):228–232. doi: 10.1007/s00213-002-1206-z. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Korchounov A, Ziemann U. Methylphenidate facilitates and disinhibits the motor cortex in intact humans. Neuroreport. 2003 Apr 15;14(5):773–776. doi: 10.1097/00001756-200304150-00023. [DOI] [PubMed] [Google Scholar]
- Kirschner J, Moll GH, Fietzek UM, Heinrich H, Mall V, Berweck S, et al. Methylphenidate enhances both intracortical inhibition and facilitation in healthy adults. Pharmacopsychiatry. 2003;36(2):79–82. doi: 10.1055/s-2003-39049. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression, Inventory (CDI) Psychopharmacol Bull. 1985. 1985;21(4):995–998. [PubMed] [Google Scholar]
- Kratochvil CJ, Heiligenstein JH, Dittmann R, Spencer TJ, Biederman J, Wernicke J, et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry. 2002;41(7):776–784. doi: 10.1097/00004583-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993. 1993 Nov;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. 501-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989. 1989 Jul;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. American Journal of Psychiatry. 2002;159(11):1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Rothenberger A. Methylphenidate and intracortical excitability: opposite effects in healthy subjects and attention-deficit hyperactivity disorder. Acta Psychiatr Scand. 2003;107(1):69–72. doi: 10.1034/j.1600-0447.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott G, Wirth S, Bock N, Rothenberger A. Children with Comorbid Attention-Deficit-Hyperactivity Disorder and Tic Disorder: Evidence for Additive Inhibitory Deficits Within the Motor System. Ann Neurol. 2001. 2001;49:393–396. [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett. 2000. 2000;284(12):121–125. doi: 10.1016/s0304-3940(00)00980-0. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Hoppe J, Hiemke C, Bartels M, Cohen LG, Gerloff C. Enhancement of human cortico-motoneuronal excitability by the selective norepinephrine reuptake inhibitor reboxetine. Neurosci Lett. 2002 Sep 27;330(3):231–234. doi: 10.1016/s0304-3940(02)00803-0. [DOI] [PubMed] [Google Scholar]
- Witcher JW, Long A, Smith B, Sauer JM, Heilgenstein J, Wilens T, et al. Atomoxetine pharmacokinetics in children and adolescents with attention deficit hyperactivity disorder. Journal of Child & Adolescent Psychopharmacology. 2003;13(1):53–63. doi: 10.1089/104454603321666199. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115(8):1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr Clin Neurophysiol. 1997. 1997 Dec;105(6):430–437. doi: 10.1016/s0924-980x(97)00050-7. [DOI] [PubMed] [Google Scholar]


