Abstract
Drug treatment of functional dyspepsia is often unsatisfactory. We assessed the efficacy of a bicarbonate-sulphate-calcium thermal water cycle of 12 days, in patients with functional dyspepsia. Patients with functional dyspepsia were sent by their general practitioners to 12 days of treatment with thermal water, 200–400 ml in the morning, at temperature of 33°C (91.4 F) and were evaluated on a strict intention to treat basis. Four efficacy endpoints were analyzed as follows: (i) reduction of the global symptoms score, (ii) reduction of intensity to a level not interfering with everyday activities, (iii) specific efficacy on ulcer-like or dysmotility-like dyspepsia and (iv) esophageal or abdominal-associated symptoms. Statistical significance was reached for all three primary outcomes after the first 29 consecutive patients. Thermal water reduced the global symptom score, reduced intensity of symptoms to a level not interfering with everyday activity, but was unable to completely suppress all symptoms. A parallel effect emerged for ulcer-like and dyspepsia-like subgroups. The effect on heartburn and abdominal symptoms was not significant, suggesting a specific effect of the water on the gastric and duodenal wall. The Roma II criteria identify a natural kind of dyspepsia that improves with thermal water. Ulcer-like and dysmotility-like are not therapeutically distinguishable subgroups. Patients with dominant esophageal or abdominal symptoms should receive a different therapy. Sequential methods are very effective for the evaluation of traditional care practices and should be considered preliminary and integrative to randomized controlled trials in this context.
Keywords: balneotherapy, functional dyspepsia, functional gastrointestinal disorders, hydrotherapy, sequential methods, spa, thermal care
Introduction
Science, broadly defined, is also the knowledge obtained by study of traditional practices after a careful trial showing the predictability of their effects in identifiable groups of patients.
Today, when functional dyspepsia is diagnosed, the drug treatment is often unsatisfactory (1), many patients are advised to drink different waters and types of wine, to change their diet (2) and introduce vitamins or are referred to a spa without much effort to individualized care (3–5). Without clear descriptions of patient symptoms and syndromes, it is difficult for the primary physicians to lump together typical groups of patients in order to test their response to specific treatments and transform a traditional art in explicit and public scientific knowledge (6–8).
In the past three decades, following the example of psychiatrists and rheumatologists, symptom-based diagnostic criteria for functional gastrointestinal disorders have been suggested (9–11). When they allow the identification of natural groups of patients responsive to a specific kind of treatment (12), they are extremely useful in the daily medical practice, considering that functional gastrointestinal disorders represent a large proportion—more than 50%—of gastroenterologists' referral, at least in the rich countries (13,14).
Since one in every four persons in these societies has symptoms compatible with one of the component functional diagnoses, these syndromes have been known for centuries, albeit with variable expressions of the primary symptoms. Rich Etruscans and Romans spent all day at the Terme and the Chianciano water is known for its gastroduodenal healing effects since then.
Why have we accrued so little knowledge of the specific type of patients that can take advantage and relief from this traditional style of treatment? There are both historical and scientific explanations: for centuries to spend days or months at the Terme was a luxury for few privileged riches, a sign of social distinction and a status symbol. After the Second World War, in both West and East Europe, thermal care was reimbursed by the Welfare State, but was considered a kind of non-specific panacea against any kind of undefined stress, or as a benefit for tired workers and exhausted housewives. No clear definition of functional bowel diseases existed, nor was there any recognition that patients should be selected in order to benefit from this traditional kind of therapy (15–18).
As a result, thermal care turned out to be considered non-scientific and its popularity declined with the economical restrictions of the eighties. Today spas are coming back with a revenge—they sono di moda—partly because a growing number of patients affected by functional disorders is dissatisfied with the few available drugs (19,20).
Methods
Study Design and Patient Population
A group of general practitioners of the Tuscany Region was trained in the use of the Rome II—and a preliminary draft of the Roma III criteria (21)—for dyspepsia and other functional gastrointestinal disorders, in Continuing Medical Education courses (Educazione Continua in Medicina, ECM) focused on the classification of clinical cases (22).
Patients considered to have functional dyspepsia on the basis of the Rome II criteria were eligible for the trial. Functional dyspepsia was diagnosed if persistent or recurrent upper abdominal pain or discomfort was the dominant complaint (23). Pain consisted of epigastric pain or burning; discomfort was characterized by the presence of one or more symptoms that included postprandial fullness, early satiety, gastric distension, belching, nausea or vomiting. Symptoms had to be present for at least 12 weeks within the previous 12 months.
Since it is well known that many patients have clinical pictures characterized by the overlap of different functional disorders, to increase the specificity and precision of the trial, only patients with predominant functional dyspepsia were included in the study.
Esophageal reflux disorder, other functional esophageal disorders, irritable bowel syndrome and functional abdominal pain identified by the application of the Rome II criteria were excluded when presenting as the predominant problem.
Biliary disorders, Helicobacter pilori infection (24), structural lesions or clinically significant biochemical abnormalities were excluded by recent available documentation or ad hoc ecotomography, blood tests including fasting blood sugar and liver function tests and gastrointestinal endoscopy (25,26).
Patients were excluded if they were already taking other medications that may alter gastric function on a regular basis and if they were known by the general practitioners as heavy drinkers, heavy smokers or habitual drinkers of more than 2–3 cups of coffee every day (27).
From a methodological perspective, traditional clinical trials are too expensive and difficult to apply to thermal care. In controlled clinical trials for the comparison of two drugs and for registration, the number of observation is decided in advance and is not affected by the observed results of treatment after each patient has completed therapy. Very often, when assessing a traditional practice of care, the efficacy is unpredictable before its start but depends on the observed results in a series of individual patients. The decision to stop the investigation depends on the results. A study of this type is called sequential (28), following Wald (29), Armitage (30) and Whitehead (31). The main reason for using sequential methods are as follows: (i) economy emerging from the possibility to reduce the total amount of experimentation depending on the efficacy of the treatment under study and the results obtained in the patients already completed, (ii) the possibility to achieve a specified 0.05 sensitivity and 80% power of the study without being forced to anticipate a numerical estimate of treatment effect, (iii) ethical considerations preclude random allocation or the use of placebo when there is strong historical and anecdotal prior evidence—or common belief—in the efficacy of a traditional form of treatment and (iv) for the same reason it would be undesirable to continue following the tradition when the treatment is shown by sequential medical trials to be no better than the tossing of a balanced coin.
The ethical committee approved the research and each patient knew of his involvement in a study protocol and signed a written informed consent of agreement to using resulting information for medical publications; no candidate patient refused the prescription of 12 days in Chianciano or to participate in the study.
Characteristics of the Thermal Water
The protocol consisted of 200–400 ml of thermal water every morning, before breakfast, for 12 days. The thermal source has a constant temperature of 33°C (91.4 F) and in this form is assumed by the patients going to Chianciano.
It is also available in glass bottles but in this case it is not classified as thermal water, but as a bicarbonate-sulphate-calcium mineral water, is usually drunk at ambient temperature, and in our experience does not have the same therapeutic efficacy as the thermal mother source for the patient population involved in this research. The chemical composition of the water is reported in Table 1.
Table 1.
Chemical characteristic of the Chianciano thermal water
| Ion | g l−1 |
|---|---|
| SO42−1 | 1.8400 |
| HCO3−1 | 0.7300 |
| Ca2+ | 0.8400 |
| Mg2+ | 0.1800 |
| Na+ | 0.0410 |
| Cl−1 | 0.0294 |
| Sr2+ | 0.0001 |
| K+ | 0.0070 |
| F−1 | 0.0020 |
| Fe2+ | 0.0008 |
| Br2+ | 0.0002 |
| Free CO2 | 537 ml l−1 |
Assessments
A standardized questionnaire based on the Leeds Dyspepsia Questionnaire (32) and already tested for validity by our group in a previous study of mineral water (33) was filled by each patient before going to Chianciano and in the first week after the end of treatment.
For clarity symptoms were divided into (i) specific of functional dyspepsia and (ii) associated. The specific were epigastric pain, epigastric burning, postprandial fullness, early satiety, gastric distension, nausea and vomiting. Heartburn, regurgitation, functional dysphagia, chest pain, abdominal pain and abdominal distension, were considered as associated and in no case they dominated the clinical picture of the individual patient.
For each symptom frequency was rated on a 4 levels ordinal scale as follows: (i) occasional or regular but no more than 1 day for week, (ii) presenting 2–3 days every week, (iii) 4–6 days a week and (iv) almost continuous. The intensity was rated as follows: (i) no modification of everyday activity, (ii) interfering with everyday activities, (iii) induced modification of everyday activities and (iv) forced in bed when symptoms were present.
Statistical Analysis
Sequential Trial with Closed Plan
A sequential trial was the statistical model applied in the design and analysis of the study. The sensitivity chosen for the boundary conditions was α = 0.05 and the power θ = 0.80. To avoid the possibility of exceptionally large sample sizes while preserving the required statistical characteristics of the study, the plan was truncated at 38 patients by applying the upper, lower and middle boundaries of a Restricted Sequential Plan (RSP) by Armitage (30).
As we know that 40–45% of patients may improve simply because of a placebo effect or as a regression to the mean phenomenon, in order to avoid the need for a control group, the comparison was done assuming a fixed standard (30) much higher than the conventional null hypothesis. In fact we required the efficacy to be predictable in 80% of patients in fixing the boundaries of the sequential trial instead of 50%.
To test the inefficacy of water on heartburn and abdominal syndrome, an open sequential trial with the same power and requirements was applied, as Armitage showed this design to be more efficient to prove that the null hypothesis is in fact true (30). Following the sequential trial terminology, each patient at the end of the trial can be classified as follows: (i) a success, (ii) a failure and (iii) unchanged or not responsive to this treatment. Only successes and failures are considered preferences and are included in the statistical analysis, but for the clinician the number unchanged is also of great relevance.
Many different successful criteria of the trial were tested as follows: (i) a reduction of the global score for both specific and accessory symptoms of at least 3 points (clinically significant), (ii) a reduction of the global score for symptoms specific of functional dyspepsia of at least 3 points and (iii) the presence of no symptoms with an intensity greater then 2 (no compromise in everyday activity) or greater than 1 (complete resolution). All other outcomes were counted as no change or insufficient change and cumulated as failures for the statistical analysis.
Epigastric pain and epigastric burning were grouped together as ulcer-like syndrome. Postprandial fullness, early satiety, gastric distension, nausea and vomiting were considered part of the dysmotility-like syndrome. The reduction in both the ulcer-like and the dysmotility-like syndrome was tested with the criteria of at least 3 points reduction of intensity. Although accessory in the clinical picture of patients, both heartburn and abdominal distension and pain were analyzed separately for the reduction of intensity of at least 3 points.
Results
Study Population
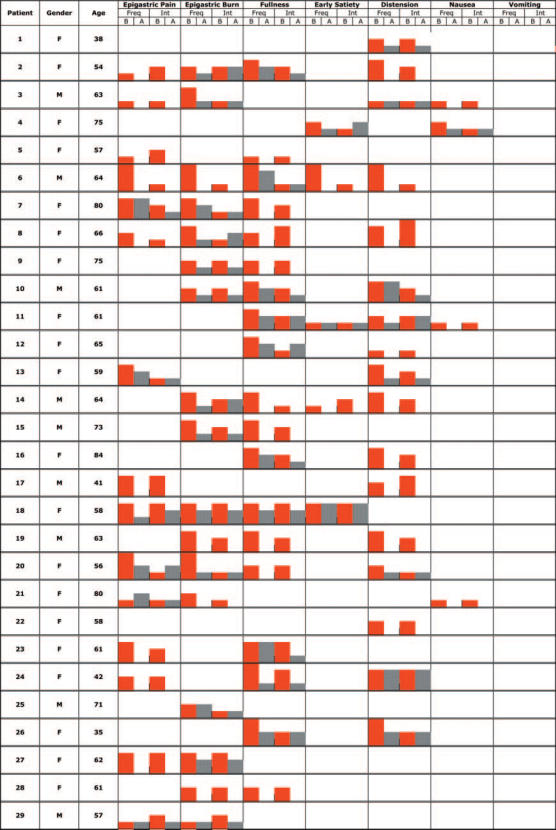
The characteristics of each individual patient and the frequency and intensity of each symptom before (B) and after (A) treatment are reported for specific symptoms in Table 2 and for accessory symptoms in Table 3. Since the trial focused on individual patients, no use has been done of averages or other summary statistics calculated on the whole group (the availability of complete raw data make it easy to do it if the reader consider it more informative).
Table 2.
Specific symptoms
B: before treatment; A: after treatment; Freq: frequency score; Int: intensity score
Table 3.
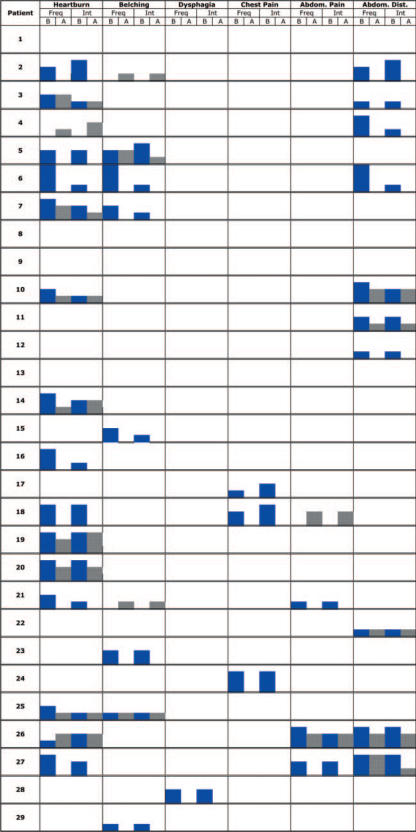
Accessory symptoms
Legend B: before treatment; A: after treatment; Freq: frequency score; Int: intensity score
Response to Treatment
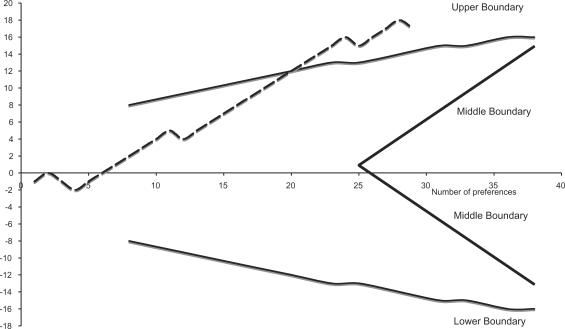
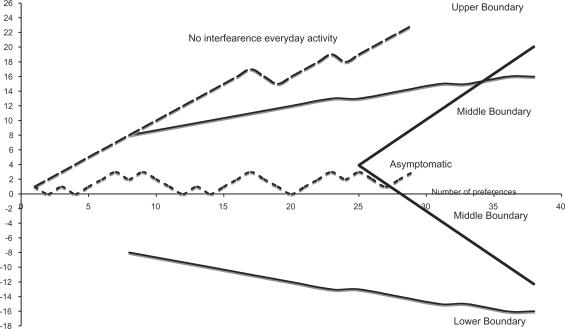
Figure 1 shows the results of the sequential trial when a reduction of the global score for all symptoms (specific plus accessory) of at least 3 points (clinically relevant) as the effect of treatment is considered. The horizontal axis shows the number of patients; the vertical axis the excess preferences or successes. The continuous thick lines are the upper, lower and middle boundaries typical of a sequential plan for the required sensitivity and power of the trial. The efficacy of treatment on each patient of the series is analyzed sequentially. As soon as the effect on a new patient is known, the line move up one unit if the case is a success, move down one unit if it is a failure.
Figure 1.
Global score for all symptoms including specific and accessory symptoms.
When the series of data crosses the upper boundary the treatment is a statistically significant success, when it crosses the lower boundary is a statistically significant failure. If the series crosses the median boundaries the treatment is not effective in more than 80% of patients (null hypothesis). In our case, after the first 20 preferences the upper boundary was crossed and the efficacy of water in more than 80% of patients was confirmed.
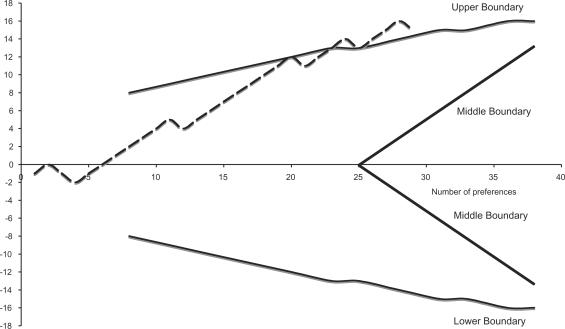
Figure 2 shows results for score based on symptoms specific for functional dyspepsia. A definitive significance was reached after 23 preferences.
Figure 2.
Specific score for functional dyspepsia.
Figure 3 shows the number of patients with no impairment of everyday activities as a result of treatment (symptoms with an intensity lower or equal 2), or asymptomatic—intensity ≤1—after treatment. A vast majority of patients benefited from an improvement of symptoms to the range of intensity not interfering with activities of daily living and statistical significance was obtained after nine preferences. On the contrary complete suppression of symptoms is not a realistic outcome of treatment and the series shows no statistical significance after 27 preferences.
Figure 3.
Intensity of symptoms interfering with everyday activity.
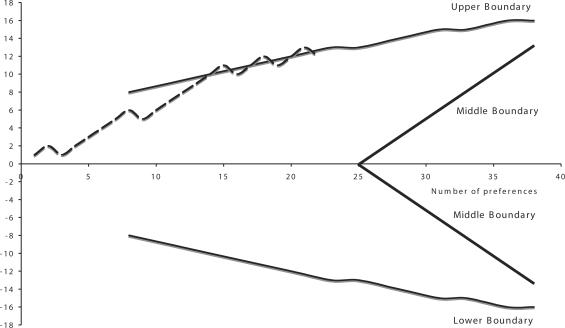
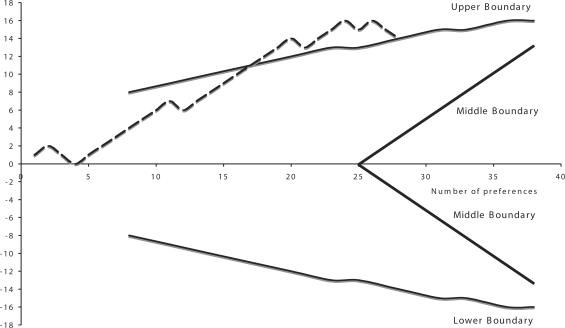
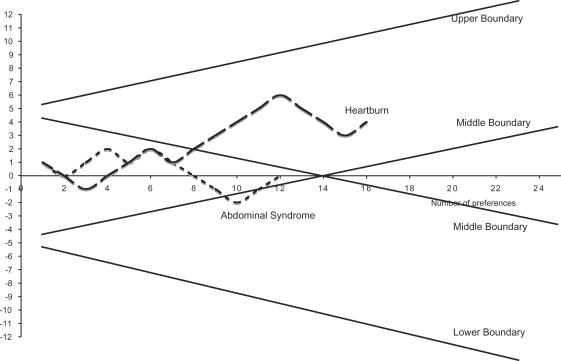
Figure 4 shows the effect of water on the ulcer-like syndrome with a clinical success defined as a reduction of intensity of at least 3 points of symptoms as before. After 15 preferences the significance was reached. Figure 5 shows the effect on the dysmotility-like syndrome using the same criteria. The significance was reached after 17 preferences. For the associated symptoms, Fig. 6 shows the non-significant effect of the water on heartburn and the abdominal syndrome. The sequential analysis is somewhat different from the previous ones because we wanted the middle boundaries to be very sensitive to the negative results suggested by previous experience (30).
Figure 4.
Ulcer-like syndrome.
Figure 5.
Dysmotility-like syndrome.
Figure 6.
Heartburn and abdominal syndrome.
Discussion
The Chianciano thermal water care is an effective short-term therapy for the specific and associated symptoms of functional dyspepsia in a carefully selected group of patients. Parallel results are obtained when only the specific symptoms are analyzed (Figs 1 and 2). For practicing physicians the most relevant result is the very small number of individual patients showing no improvement and the significant number benefiting from a marked clinical improvement. This is confirmed by the statistically significant number of patients in whom we saw a change with the disappearance of impairment in everyday activities because of symptoms intensity (Fig. 3). Vice versa, the complete disappearance of symptoms is not what physicians should promise to patients, as shown by the non-significant series in Fig. 3 where no symptoms with an intensity ≤1 was the required outcome.
If the pattern of response to a specific therapy is accepted as evidence of the underlying disease mechanism and classification validity, thermal care emerges from the trial as a specific treatment for functional dyspepsia and associated symptoms, but does not confirm the possibility to differentiate between ulcer-like and dysmotility-like subgroups. On the contrary, the marked parallelism among the series of patients for the two syndromes suggests that as far as therapy is involved, they should be classified together (Figs 4 and 5) (34,35).
Thermal water and care has no significant effects on heartburn and the abdominal syndrome (Fig. 6), suggesting a specific effect of therapy at the gastroduodenal level and the possibility of different mechanisms for therapies targeted at the esophageal and abdominal walls (36–39).
The trial confirms the validity and practical utility of the Roma II criteria for identification of patients affected by functional dyspepsia and its inclusion among the gastroduodenal disorders, but does not confirm the more specific subgroups of ulcer-like and dysmotility-like dyspepsia as a criterion that makes a difference for treatment (40–42). Both functional esophageal disorders and abdominal disorders need a different and more specific type of therapy (43–46).
The study confirms the great practicality of the sequential trial approach to test the efficacy of traditional kinds of care on individual patients. If treatment is very efficacious, as in this case, a small number of carefully selected patients are sufficient to test the many hypotheses emerging from traditional wisdom or previous experience while avoiding the ethical, practical and economic difficulties of applying the more standard fixed-number trial approach.
The major weak point of the sequential approach is that the evidence for efficacy is often reached with very few patients; as expected, the confidence limits for the percentage of success obtained are wide. This is not critical when the efficacy of the treatment is great, but limit their application to the comparison of drugs or remedies when a small difference is expected (for example in the comparison of two statins or proton pump inhibitors).
As far as the practical physician is concerned, the sequential approach gives information at the individual level, avoiding the abstract presentation of table of averages and percentages without any precise indication of the concrete type of person that can take advantage of the tested practice of care. For this reason, the complete database of individual data is provided as part of the results and no summary measure has been calculated. The concrete individualized approach should help the physician in the application of the results to the precisely described natural kind of patient they can encounter in everyday ambulatory practice (47).
Nobody can believe that 12 days of care, although very effective, can have positive effects lasting forever on functional gastrointestinal disorders that we know for their chronic course. The next studies should answer the many questions that the positive results reported make urgent: how long the positive effects of 12 days in Chianciano last? How often should the cycle be repeated in order to optimize the improvements? Is the practice best for prevention in mildly affected patients, or is it better to prescribe it when the clinical pattern is more severe, as in these patients? Does the availability of a regular and individualized schedule in each case improve the quality of life and reduce the direct and indirect costs of functional dyspepsia? Do these patients need the association of other kinds of treatment or is thermal care enough by itself for the long-term care of functional dyspepsia? Why thermal care is more effective than the equivalent mineral water based care? Is any extramolecular mechanism involved? (48)
We believe that this study is only the first step toward a more scientific approach to the evaluation of many types of traditional care now di moda and reimbursed by both the welfare state and insurance companies in many countries.
The emerging specificity of thermal care for a easily identified group of patients, affected by a specific functional disorder confirm our assumption that many kinds of traditional care are not panacea or placebos, but show specific activity at precise levels or organs of the body and on specific and recognizable symptoms, patterns and syndromes.
The results of the study suggest that it is important to test the efficacy of different practices of thermal and other traditional care on different disorders, upon different levels of the gastrointestinal tract and on different organs and systems of the body. This can be done easily and scientifically by the application of the sequential trial approach. Stimulated by the results of this study, a randomized controlled trials (RCT) is under way to compare the efficacy, direct and indirect costs, and duration of the positive effect of thermal water compared to standard treatments.
Why in our experience does thermal water and mineral water, with the same chemical composition give different results in the same group of patients? The available literature and the regulation for reimbursement are focused mainly or exclusively on the molecular effect of the minerals—chemical analysis—while in our experience other three factors should be integrated in future research as follows: (i) the temperature at the moment of ingestion, (ii) a possible homeopathic effect of thermal water but not mineral water due to the passage on diluted minerals and (iii) the relaxing effect of 12 days at a spa, that is missing when the patients drinks the mineral water at home. No data at the moment are available to estimate the integrated contribution of each of these three additional factors.
The traditional classification of therapeutic waters is based on the chemical characteristics, but experience with patients suggests that other factors should be taken into account in future research. The Rome II and Rome III definition of functional dyspepsia consider these symptoms as specific of the syndrome. Most patients affected by functional dyspepsia are affected also by other symptoms that do not dominate the clinical picture but are accessory and often present.
Thermal care is effective in reducing the global score for all symptoms in more than 80% of patients after only 20 trials. It targets the specific symptoms for functional dyspepsia and is effective in 80% of patients after only 23 trials. Thermal care is effective in reducing symptoms to a level not interfering with everyday activities—significant after nine patients—but is generally unable to completely suppress the symptoms—non-significance after 27 trials.
Rome II criteria defines ulcer-like syndrome as a subgroup of functional dyspepsia, but Figs 4 and 5 show that they are not therapeutically distinguishable. The results are similar to those obtained with patients affected by ulcer-like dyspepsia, suggesting they are not therapeutically distinguishable. The Chianciano thermal care is ineffective against esophageal and abdominal symptoms, showing a specific effect on the wall of the stomach and duodenum.
References
- 1.Longstreth GF. Functional dyspepsia—managing the conundrum. N Engl J Med. 2006;354:791–3. doi: 10.1056/NEJMp058326. [DOI] [PubMed] [Google Scholar]
- 2.Saito YA, Locke GR, 3rd, Weaver AL, Zinsmeister AR, Talley NJ. Diet and functional gastrointestinal disorders: a population-based case-control study. Am J Gastroenterol. 2005;100:2743–8. doi: 10.1111/j.1572-0241.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 3.Chrubasik S, Chrubasik C, Torda T, Madisch A. Efficacy and tolerability of potato juice in dyspeptic patients: a pilot study. Phytomedicine. 2006;13:11–5. doi: 10.1016/j.phymed.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Panteris V, Karamanolis DG. Different aspects in functional dyspepsia. Hepatogastroenterology. 2005;52:1782–91. [PubMed] [Google Scholar]
- 5.Oikawa T, Ito G, Koyama H, Hanawa T. Prokinetic effect of a Kampo medicine, Hange-koboku-to (Banxia-houpo-tang), on patients with functional dyspepsia. Phytomedicine. 2005;12:730–4. doi: 10.1016/j.phymed.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA, RJ, Talley NJ, Thomson WG, Corazziari E, Whitehead WE. The Functional Gastrointestinal Disorders. Boston: Little Brown and Company; 1994. [Google Scholar]
- 7.Wicks D, Wright J, Rayment P, Spiller R. Impact of bitter taste on gastric motility. Eur J Gastroenterol Hepatol. 2005;17:961–5. doi: 10.1097/00042737-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Thijs JC, Kleibeuker JH. The management of uninvestigated dyspepsia in primary care. Minerva Gastroenterol Dietol. 2005;51:213–24. [PubMed] [Google Scholar]
- 9.Schurman JV, Friesen CA, Danda CE, Andre L, Welchert E, Lavenbarg T, et al. Diagnosing functional abdominal pain with the Rome II criteria: parent, child, and clinician agreement. J Pediatr Gastroenterol Nutr. 2005;41:291–5. doi: 10.1097/01.mpg.0000178438.64675.c4. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45:II-37–42. doi: 10.1136/gut.45.2008.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vakil N, van Zanten SV, Chang L, Toth G, Sherman J, Fraser M, et al. Comprehension and awareness of symptoms in women with dyspepsia. Aliment Pharmacol Ther. 2005;22:1147–55. doi: 10.1111/j.1365-2036.2005.02699.x. [DOI] [PubMed] [Google Scholar]
- 12.Hansen AN, Bergheim R, Fagertun H, Lund H, Wiklund I, Moum B. Long-term management of patients with symptoms of gastro-oesophageal reflux disease—a Norwegian randomised prospective study comparing the effects of esomeprazole and ranitidine treatment strategies on health-related quality of life in a general practitioners setting. Int J Clin Pract. 2006;60:15–22. doi: 10.1111/j.1368-5031.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 13.Van den Heuvel-Janssen HA, Borghouts JA, Muris JW, Koes BW, Bouter LM, Knottnerus JA. Chronic non-specific abdominal complaints in general practice: a prospective study on management, patient health status and course of complaints. BMC Fam Pract. 2006;7:12. doi: 10.1186/1471-2296-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–80. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Gan AP. Clinical and psychological assessment on xinwei decoction for treating functional dyspepsia accompanied with depression and anxiety. Am J Chin Med. 2005;33:249–57. doi: 10.1142/S0192415X05002801. [DOI] [PubMed] [Google Scholar]
- 16.Zhang WD, Wei BH, Chen ZS. [Schedule for diagnosis and treatment of functional indigestion syndrome with intergrative Chinese and Western medicine]. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi/Chinese Journal of Integrated Traditional and Western Medicine/Zhongguo Zhong Xi Yi Jie He Xue Hui, Zhongguo Zhong Yi Yan Jiu Yuan Zhu Ban. 2005;25:559–61. [PubMed] [Google Scholar]
- 17.Vasil'ev Iu V, Masharova AA, Ianova OB. [New alternative drug therapy for reducing symptoms associated with the functional gastric disorders] Eksp Klin Gastroenterol. 2005:4:39–41. [PubMed] [Google Scholar]
- 18.Talley NJ, Vakil N. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–37. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 19.Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. New Engl J Med. 2006;354:832–40. doi: 10.1056/NEJMoa052639. [DOI] [PubMed] [Google Scholar]
- 20.Otaka M, Jin M, Odashima M, Matsuhashi T, Wada I, Horikawa Y, et al. New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline. Aliment Pharmacol Ther. 2005;21(Suppl 2):42–6. doi: 10.1111/j.1365-2036.2005.02473.x. [DOI] [PubMed] [Google Scholar]
- 21. See www.romecriteria.org for a comparison between Rome II and Rome III criteria.
- 22.Drossman D, editor. The Functional Gastrointestinal Disorders. Rome II and the Multinational Working Teams. McLean: Degnon Associates; 2000. [Google Scholar]
- 23.Tack J, Caenepeel P, Arts J, Lee KJ, Sifrim D, Janssens J. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut. 2005;54:1370–6. doi: 10.1136/gut.2004.053355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H, Masaoka T, Sakai G, Ishii H, Hibi T. Improvement of gastrointestinal quality of life scores in cases of Helicobacter pylori-positive functional dyspepsia after successful eradication therapy. J Gastroenterol Hepatol. 2005;20:1652–60. doi: 10.1111/j.1440-1746.2005.04039.x. [DOI] [PubMed] [Google Scholar]
- 25.Moayyedi P, Talley NJ, Fennerty MB, Vakil N. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566–76. doi: 10.1001/jama.295.13.1566. [DOI] [PubMed] [Google Scholar]
- 26.Talley NJ. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129:1753–5. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Veldhuyzen van Zanten SJ, Bradette M, Chiba N, Armstrong D, Barkun A, Flook N, et al. Evidence-based recommendations for short- and long-term management of uninvestigated dyspepsia in primary care: an update of the Canadian Dyspepsia Working Group (CanDys) clinical management tool. Can J Gastroenterol. 2005;19:285–303. doi: 10.1155/2005/674607. [DOI] [PubMed] [Google Scholar]
- 28.Armitage P, Berry G. Statistical Methods in Medical Research. Oxford: Blackwell; 1994. [Google Scholar]
- 29.Wald A. Sequential Analysis. New York: Wiley; 1947. [Google Scholar]
- 30.Armitage P. Sequential Medical Trials. New York: Wiley; 1975. pp. 42–8. 66, 157–8. [Google Scholar]
- 31.Whitehead J. The Design and Analysis of Sequential Clinical Trials. Horwood: Chichester; 1992. [Google Scholar]
- 32.Moayyedi P, Duffett S, Braunholtz D, Mason S, Richards ID, Dowell AC, Axon AT. The Leeds Dyspepsia Questionnaire:a valid tool for measuring the presence and severity of dyspepsia. Aliment Pharmacol Ther. 1998;12:1257–62. doi: 10.1046/j.1365-2036.1998.00404.x. [DOI] [PubMed] [Google Scholar]
- 33.Bertoni M, Olivieri F, manghetti M, Boccolini E, Bellomini MG, Blandizzi C, et al. Effects of a bicarbonate-alkaline mineral water on gastric functions and functional dyspepsia: a preclinical and clinical study. Pharmacol Res. 2002;46:525–31. doi: 10.1016/s1043661802002323. [DOI] [PubMed] [Google Scholar]
- 34.Delgado-Aros S. [Gastric emptying and functional dyspepsia] Gastroenterol Hepatol. 2006;29:34–9. doi: 10.1157/13083250. [DOI] [PubMed] [Google Scholar]
- 35.Camilleri M, Bharucha AE. Drawing a blank in functional dyspepsia? Gastroenterology. 2006;130:593–6. doi: 10.1053/j.gastro.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri M. Dyspepsia is distinguishable from heartburn. Gut. 2006;55:746–7. [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenberghe J, Vos R, Persoons P, Demyttenaere K, Janssens J, Tack J. Dyspeptic patients with visceral hypersensitivity: sensitisation of pain specific or multimodal pathways? Gut. 2005;54:914–9. doi: 10.1136/gut.2004.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vakil N. Dyspepsia and GERD: breaking the rules. Am J Gastroenterol. 2005;100:1489–90. doi: 10.1111/j.1572-0241.2005.50317.x. [DOI] [PubMed] [Google Scholar]
- 39.Sarnelli G, Grasso R, Ierardi E, De Giorgi F, Savarese MF, Russo L, et al. Symptoms and pathophysiological correlations in patients with constipation and functional dyspepsia. Digestion. 2005;71:225–30. doi: 10.1159/000087047. [DOI] [PubMed] [Google Scholar]
- 40.Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology. 2006;130:296–303. doi: 10.1053/j.gastro.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Tack J, Lee KJ. Pathophysiology and treatment of functional dyspepsia. J Clin Gastroenterol. 2005;39:S211–6. doi: 10.1097/01.mcg.0000156109.97999.d1. [DOI] [PubMed] [Google Scholar]
- 42.Tack J, Kindt S. Pathogenesis and therapy for idiopathic dyspepsia. Curr Gastroenterol Rep. 2005;7:437–44. doi: 10.1007/s11894-005-0073-2. [DOI] [PubMed] [Google Scholar]
- 43.Waldum HL, Martinsen TC, Brenna E. Nonulcer dyspepsia and proton pump inhibitors. Gastroenterology. 2005;128:805. doi: 10.1053/j.gastro.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, Barkun A, Thomson A, Smyth S, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in Helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477–88. doi: 10.1111/j.1572-0241.2005.40280.x. [DOI] [PubMed] [Google Scholar]
- 45.Vakil N. Toward a simplified strategy for managing dyspepsia. Postgrad Med. 2005;117:13–6. doi: 10.3810/pgm.2005.06.1651. [DOI] [PubMed] [Google Scholar]
- 46.Smith ML. Functional dyspepsia pathogenesis and therapeutic options-implications for management. Dig Liver Dis. 2005;37:547–58. doi: 10.1016/j.dld.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Sanft T, Jones MP. Functional dyspepsia: subgroups, history and outcomes. Minerva Gastroenterol Dietol. 2005;51:225–34. [PubMed] [Google Scholar]
- 48.Ventura C. CAM and cell fate targeting: molecular and energetic insights into cell growth and differentiation. Evid Based Complement Alternat Med. 2005;2:277–83. doi: 10.1093/ecam/neh100. [DOI] [PMC free article] [PubMed] [Google Scholar]