Abstract
Pharmacological actions of Mokuboito and its constituents (Sinomenium acutum and sinomenine) on rat aorta were examined. Mokuboito and S. acutum at lower concentrations (0.03–1 mg ml−1) contracted the non-loaded aorta, but at higher concentrations (1–3 mg ml−1), reversed to dilate it. The vasoconstriction was blocked by phentolamine (10 μM). Sinomenine failed to exhibit the vasoconstriction. On the other hand, Mokuboito and S. acutum dilated the NE (5 μM)-induced vasoconstriction: at 3 mg ml−1, by 98.9 ± 2.5% (n = 6, P < 0.01) and 97.0 ± 4.8% (n = 6, P < 0.01). Vasorelaxation induced by Mokuboito and S. acutum was attenuated by indomethacin, L-NMMA and nicardipine. Propranolol decreased the vasorelaxation induced by Mokuboito, but not by S. acutum. Sinomenine also relaxed the constriction and at 100 μM, by 68.8 ± 5.1% (n = 7, P < 0.01). This vasorelaxation was attenuated by indomethacin, L-NMMA and nicardipine, and also by propranolol. Therefore, these results indicate that Mokuboito and its constituents exert both vasodilating actions mediated by endothelium-dependent mechanisms (PGI2 and NO from endothelium) and by endothelium-independent mechanisms (Ca2+ influx control on smooth muscle cells). Simultaneously, Mokuboito and S. acutum cause the vasoconstrictions mediated through α-adrenoceptor stimulation, but not sinomenine. Also, Mokuboito and sinomenine possess β-adrenoreceptor stimulating action, but not S. acutum.
Keywords: α- and β-adrenoceptors, Ca2+ channel, EDRF, endothelium, Mokuboito, PGI2, rat aorta, sinomenine, Sinomenium acutum, vasodilation
Introduction
Mokuboito (Mu-Fang-Yi-Tang), a kind of Kampo formulations, has clinically been used for heart failure (1). It has been shown that Mokuboito improves heart failure symptom, and reduces the level of New York Heart Association (NYHA) class and plasma brain natriuretic peptide (BNP) concentration (2).
Mokuboito consists of 4 g Sinomeni Caulis et Rhizoma (rhizome of Sinomenium acutum Rehdler et Wilson), 3 g Cinnamomi Cortex (bark of Cinnamomum cassia Blume), 3 g Ginseng radix (roots of Panax ginseng C. A. Meyer) and 10 g Gypsum Fibrosum. Cinnamon cortex and its constituent (cinnamic aldehyde) have been reported to have some pharmcological effects; antiplatelet and anti-inflammatory effects (3,4) and also antitumor activity (5). Panax ginseng possesses many pharmacological effects and exhibits some clinical efficicacies: care of general fatigue and stress (6,7). In addition, it possesses immunomodulating (8,9) and neuroprotective actions (10). Finally, Gypsum has been used as one of the crude drugs in traditional Kampo medicine. Its pharmacological is reasonably clear, but previous reports have shown that Gypsum prevents the thermogenesis effect induced by ephedrine at an ambient temperature of 22°C (11).
S. acutum has traditionally been considered as a modulator of body's fluid and been applied for disturbances of body fluids. Sinomenine is one of alkaloids extracted from S. acutum (12). As an antirheumatic and anti-inflammatory regulator, sinomenine is clinically used for the treatment of rheumatoid arthritis (13,14). In basic researches, sinomenine reduces the production of prostagrandin (PG) E2 and nitric oxide (NO) from macrophages (15). Sinomenine reduces inflammatory parameters and attenuates proliferation of synovial fibroblasts in rat adjuvant arthritis models (14). In addition, acute and chronic cardiac allograft ejections are blocked by the immunomodulatory effects of sinomenine (16). More recently, Satoh (17) has demonstrated that Mokuboito and its constituents exhibit cardiac electropharmacological actions and exert the conservative actions on cardiomyocytes. These findings indicate that Mokuboito, S. acutum and sinomenine exert effective pharmacological actions that can improve chronic heart failure via the recovery of cardiac functions.
In general, the treatment of heart failure consists of (i) reducing workload of heart, (ii) protection of cardiomyocytes and (iii) restriction of sodium and fluid control. For reduction of the preload and afterload, the dilations of arterioles and veins are required in the case of elevated filling pressures and reduced cardiac output. We demonstrated that S. acutum dilated vasoconstrictions (18). Therefore, Mokuboito, S. acutum or sinomenine may treat heart failure not only by improvement of cardiac effects but also by controlling the tension of blood vessels. In these experiments, therefore, we wanted to elucidate in more detail the vascular pharmacology of Mokuboito, S. acutum and sinomenine, using rat aorta rings.
Methods
All experiments were carried out, according to the guidelines laid down by the Nara Medical University Animal Welfare Committee, and also under the terms of the Declaration of Helsinki.
Male Wistar rats (4–10 weeks old) were anesthetized with ether, and euthanized by exsanguination. The thoracic aorta was quickly removed, and the isolated aorta cut into rings of 3 mm in length. The rings were suspended between two triangular-shaped stainless steel stirrups in a jacketed organ chamber filled with 20 ml modified Krebs–Henseleit solution. The modified Krebs–Henseleit solution was comprised of (in mM); 118 NaCl, 4.6 KCl, 1.2 MgSO4, 1.2 KH2PO4, 11.1 glucose, 27.2 NaHCO3, 0.03 Na2-ethylenediaminetetraacetic acid (EGTA) and 1.8 CaCl2. The chamber solution was kept at 36.5°C and oxygenated with 95% O2 and 5% CO2. The lower stirrup was anchored and the upper stirrup was attached to a force-displacement transducer (Nihon Kohden TB-652T, Tokyo, Japan) to record the isometric force. All rings were stretched to generate a resting tension of 1.2 g, which was optimal for contractions with α-adrenergic receptor agonist. After 40 min of resting, norepinephrine (NE, 5 μM) was added to the tissue bath. After the contractile response became steady, the drugs were cumulatively administrated into the bath solution. The effects of each concentration of the drugs were measured 6–10 min after the responses became steady. The responses were analyzed as a percentage change from the value before the application of drugs.
Mokuboito, the kampo medicine used in this study, has been supplied from Tsumura Co., Ltd (Tokyo) as a spray-dried powder which was extracted with boiling water of a mixture of respective ground raw materials, 4 g of Sinomeni Caulis et Rhizoma (rhizome of S. acutum Rehdler et Wilson), 3 g of Cinnamomi Cortex (bark of C. cassia Blume), 3 g of Ginseng radix (roots of Panax ginseng C. A. Meyer) and 10 g of Gypsum Fibrosum. This Mokuboito is exactly equaled to TJ-36 provided by Tumura Co., Ltd. S. acutum extract was also prepared as a spray-dried powder extracted with boiling water of a ground raw material of Sinomeni Caulis et Rhizoma (rhizome of S. acutum Rehdler et Wilson).
The other drugs used were sinomenine (7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinae-6-one), indomethacin and staurosporine (Wako Chemical, Kyoto, Japan). NG-monomethyl-l-arginine acetate (L-NMMA), and nicardipine (Sigma Chemical Co., St Louis, MO, USA) were also used.
All values are represented as means ± SEM. The differences of data in mean values were analyzed by the Student's t-test and ANOVA followed by post hoc tests (Dunn–Bonferonii test), and a P-value of <0.05 was considered significant.
Results
Significant Vasorelaxation Induced by Mokuboito
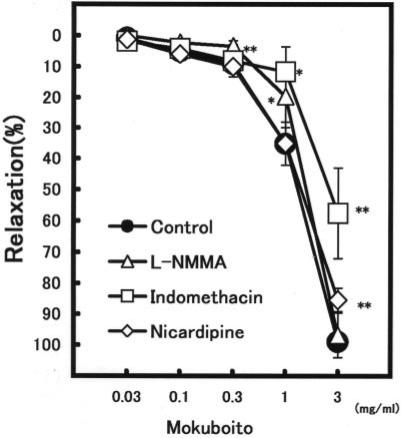
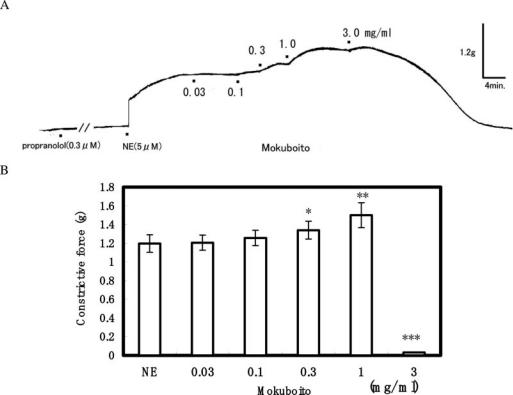
The aorta ring strip of rat exhibited a strong constriction after an initial application of 5 μM NE. Mokuboito applications (0.03–3 mg ml−1) markedly relaxed the constriction induced by NE in a concentration-dependent manner, as shown in Table 1. The significant relaxation was produced at concentrations of over 0.1 mg ml−1. Mokuboito at 3 mg ml−1 decreased it by 98.9 ± 2.5% (n = 7, P < 0.001). To examine the involvement with endothelium-dependent relaxation, 40 min pretreatment with L-NMMA (a non-selective NO synthesis inhibitor) was carried out. In the presence of L-NMMA (100 μM), the vasorelaxation induced by 0.03 and 0.1 mg ml−1 Mokuboito was significantly attenuated from 5.0 ± 0.5% (n = 7) and 9.2 ± 1.6% (n = 7) to 2.3 ± 1.3% (n = 5, P < 0.01) and 3.4 ± 1.7% (n = 5, P < 0.05), respectively (Fig. 1). Also, Mokuboito was applied in the presence of indomethacin (an inhibitor of prostanoid production). Indomethacin (10 μM) significantly reduced the vasorelaxation of Mokuboito at 1 and 3 mg ml−1 from 35.1 ± 7.1% (n = 7) and 98.9 ± 2.5% (n = 7) to 11.6 ± 8.1% (n = 5, P < 0.01) and 57.5 ± 14.4% (n = 5, P < 0.01), respectively (Fig. 1). In addition, the vasorelaxation was also examined in the presence of nicardipine to elucidate the involvement with Ca2+ channels on the vascular smooth muscle cells. Nicardipine (0.1 μM) attenuated the vasorelaxation at 3 mg ml−1 Mokuboito to 85.3 ± 3.7% (n = 5, P < 0.01). The involvement with β-adrenoreceptor stimulation (cAMP-dependence) was also examined. Mokuboito (0.03–1 mg ml−1) further constricted the NE-induced constrictions in the presence of 0.3 μM propranolol (Fig. 2A). However, additional application of 3 mg ml−1 Mokuboito reversely dilated the vasoconstriction to almost control level. The summarized data are presented in Fig. 2B.
Table 1.
Vasorelaxations induced by Mokuboito and Sinomenium acutum on NE-induced vasoconstriction: comparison with Mokuboito and a main constituent (S. acutum)
| n | 0.03 | 0.1 | 0.3 | 1 | 3 mg ml−1 | |
|---|---|---|---|---|---|---|
| Mokuboito | 7 | 0.90 ± 0.72 | 5.0 ± 0.5* | 9.2 ± 1.6* | 35.1 ± 7.1** | 98.9 ± 2.5*** |
| Sinomenium acutum | 6 | 1.76 ± 0.78* | 5.1 ± 0.54* | 13.4 ± 1.7* | 45.6 ± 7.1** | 97.0 ± 4.8*** |
Values (%) represent mean ± SEM.
*P < 0.05, **P < 0.01, ***P < 0.001, with respect to control value.
Figure 1.
Modulation of concentration-dependent relaxations of Mokuboito in the presence of several inhibitors. Symbols used are control (filled circles, n = 7) and the pretreatments with L-NMMA (triangles, n = 5), indomethacin (squares, n = 5) and nicardipine (diamonds, n = 5). Values (%) represent mean ± SEM. *P < 0.05, **P < 0.01, with respect to control value.
Figure 2.
Modulation of vasodilating action of Mokuboito by the pretreatment with propranolol. (A) Actual recording for the effects of Mokuboito in 0.3 μM propranolol. (B) Summary for the constrictive force at different concentrations of Mokuboito in the presence of propranolol. *P < 0.05, **P < 0.01, ***P < 0.001, with respect to control value.
Mokuboito Produced Vasoconstriction at Lower Concentrations
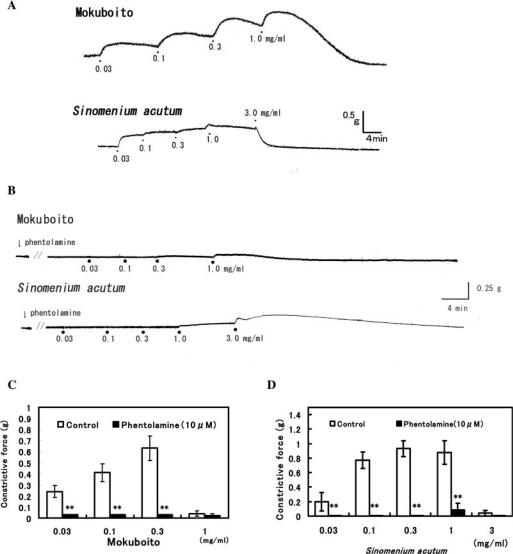
Using a non-loaded aorta (not pretreated with NE), Mokuboito was cumulatively administrated to examine whether it caused vasoconstriction (Fig. 3A). Mokuboito at lower concentrations of 0.03–0.3 mg ml−1 produced a vasoconstriction on the non-loaded aorta. At 1 mg ml−1, however, Mokuboito reversed to the vasorelaxation. Furthermore, the vasoconstriction induced by Mokuboito (at 0.03–0.3 mg ml−1) was blocked by 10 μM phentolamine (α-adrenoceptor blocker) (Fig. 3B and C). Mokuboito involved with β-adrenoceptor stimulation, as mentioned above. These results indicate that Mokuboito has both pharmacological characteristics for a vasorelaxation via β-adrenoreceptor stimulation and a vasoconstriction via α-adrenoreceptor stimulation.
Figure 3.
Vasomodulation induced by Mokuboito and Sinomenium acutum of the non-loaded (resting state of) aortic smooth muscle. (A) Vasoconstriction and vasodilation induced by the compounds, administrated cumulatively. (B) Blockade of vasoconstriction of Mokuboito and S. acutum by 10 μM phentolamine. (C) Modulation by phentolamine of the vasoconstriction induced by Mokuboito. The bars are Mokuboito in the absence (white column, n = 7) and in the presence of 10 μM phentolamine (black column, n = 5). Values (g) represent mean ± SEM. **P < 0.01, with respect to control value. (D) Effects of phentolamine on the vasoconstriction induced by S. acutum. The bars mean S. acutum alone (white column, n = 5) and S. acutum in 10 μM phentolamine (black column, n = 5). Values (g) represent mean ± SEM. **P < 0.01, with respect to control value.
S. acutum Induced a Concentration-Dependent Vasorelaxation
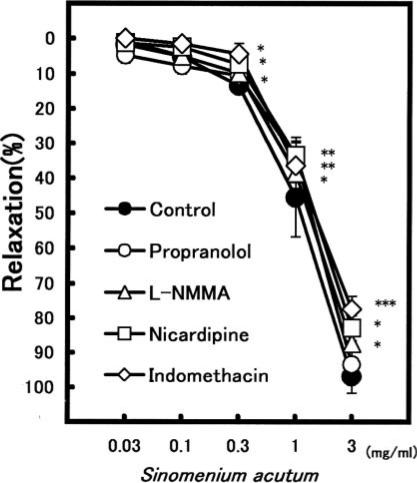
S. acutum also caused a concentration-dependent vasorelaxation on the NE-induced vasoconstriction (Table 1). S. acutum at 3 mg ml−1 dilated the constriction by 97.0 ± 4.8% (n = 6). In the presence of L-NMMA (100 μM), S. acutum (at 0.1–3 mg ml−1) also significantly decreased the relaxation (Fig. 4). The vasorelaxation at 3 mg ml−1 was attenuated from 97.0 ± 4.8% (n = 6) to 84.9 ± 1.9% (n = 5, P < 0.05). Indomethacin (10 μM) and nicardipine (0.1 μM) also attenuated the S. acutum-induced relaxation. However, propranolol did not affect it.
Figure 4.
Modulation of the vasorelaxation induced by Sinomenium acutum in the pretreatment with several inhibitors. Symbols used are control (filled circles, n = 6), propranolol (open circles, n = 6), L-NMMA (triangles, n = 6), nicardipine (squares, n = 6) and indomethacin (diamonds, n = 6). Values (%) represent mean ± SEM. *P < 0.05, with respect to control value.
As shown in Fig. 3A, similar vasoconstriction on the non-loaded rat aorta was produced by 0.03–1 mg ml−1 S. acutum. At 3 mg ml−1 S. acutum reversed to dilate the vasoconstriction, like Mokuboito and the vasoconstriction is completely blocked by 10 μM phentolamine (Fig. 3B–D).
Sinomenine Never Produced Vasoconstriction
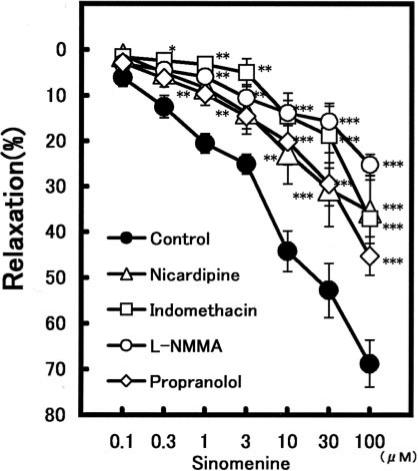
Sinomenine caused no effects on non-loaded aorta and never produced vasoconstrictive effect. Sinomenine (0.1–100 μM) potently relaxed the constriction induced by NE in a concentration-dependent manner, as shown in Table 2. Under the pretreatment with 100 μM L-NMMA, the vasorelaxation induced by 100 μM sinomenine was attenuated from 68.8 ± 5.1% (n = 6) to 25.3 ± 2.3% (n = 5, P < 0.01) (Fig. 5). Indomethacin (10 μM) also strongly reduced it to 37.1 ± 9.3% (n = 5, P < 0.001). The relaxation of sinomenine (0.1–100 μM) was significantly attenuated by nicardipine; at 100 μM sinomenine from 68.8 ± 5.1% (n = 6) to 35.5 ± 6.9% (n = 5, P < 0.001). Propranolol (0.3 μM) also significantly attenuated the sinomenine (1–100 μM)-induced relaxation. The vasorelaxation at 100 μM sinomenine was attenuated to 45.2 ± 4.2% (n = 5, P < 0.01).
Table 2.
Modulation by sinomenine of the relaxation on NE-induced vasoconstriction: pharmacology of a major constituent, sinomenine
| Sinomenine | |||||||
|---|---|---|---|---|---|---|---|
| n | 0.1 | 0.3 | 1 | 3 | 10 | 30 | 100 μM |
| 6 | 6.1 ± 2.0* | 12.5 ± 2.5** | 20.5 ± 2.2** | 25.1 ± 2.2** | 44.2 ± 4.4*** | 52.8 ± 6.0*** | 68.8 ± 5.1*** |
Values (%) represent mean ± SEM.
*P < 0.05, **P < 0.01, ***P < 0.001, with respect to control value.
Figure 5.
Modulation of the vasorelaxations induced by sinomenine in the presence of some inhibitors. Symbols used are control (filled circles, n = 7), propranolol (open circles, n = 5), L-NMMA (triangles, n = 5), nicardipine (squares, n = 5) and indomethacin (diamonds, n = 5). Values (%) represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, with respect to control value.
Discussion
The results from the present experiments were as follows: (i) Mokuboito, S. acutum and sinomenine exhibited concentration-dependent vasorelaxation on NE-induced vasoconstriction, (ii) L-NMMA and indomethacin significantly attenuated the vasorelaxation induced by Mokuboito, S. acutum and sinomenine, (iii) nicardipine also decreased them, (iv) Mokuboito and S. acutum at lower concentrations caused vasoconstrictions on the non-loaded aorta, but at high concentrations oppositely dilated the vasoconstriction induced by lower concentrations, (v) sinomenine never caused the vasoconstriction on the non-loaded aorta, (vi) in the presence of propranolol, Mokuboito at lower concentrations reinforced further the NE-induced vasoconstriction, but at 3 mg ml−1 it was reversed to decrease the vasoconstriction and (vii) propranolol also attenuated sinomenine-induced vasorelaxation, but caused no effects on the vasorelaxation induced by S. acutum.
Mokuboito possessed the vasoconstrictive effect on the non-loaded rat aorta. Mokuboito at 0.03–0.3 mg ml−1 caused vasoconstriction, but at 1 mg ml−1 decreased the vasoconstriction induced by the lower concentrations and then recovered to control level. Since the vasoconstriction was blocked by phentolamine, Mokuboito produced the vasoconstriction mediated through α-adrenoceptors. Under the pretreatment with propranolol, also, Mokuboito at lower concentrations (0.03–1 mg ml−1) reinforced further the NE-induced vasoconstrictions. Therefore, these results indicate that Mokuboito possesses both β- and α-adrenoceptor stimulating actions. At lower concentrations of Mokuboito, α-adrenoceptor stimulating action overcomes its β-adrenoceptor stimulating action, resulting in an appearance of only vasoconstriction on the non-loaded rat aorta. At higher concentrations, the other actions including β-adrenoceptor stimulation of Mokuboito were exerted, and as a result, vasodilation would be produced.
Both L-NMMA and indomethacin attenuate vasorelaxation induced by Mokuboito. Thus, Mokuboito exerts the vasorelaxation by the secretions of endothelium-derived relaxation factor (EDRF, that is NO) and also of PGI2. Furthermore, nicardipine attenuated the vasorelaxation induced by Mokuboito, resulting from Ca2+ channel inhibitory action.
S. acutum also possesses the vasoconstrictive effect on the non-loaded rat aorta; and Sinomenine acutum at 3 mg ml−1 reversed to decrease the vasoconstriction. Similarly, the vasoconstriction was attenuated by phentolamine, and the vasorelaxation was reduced by propranolol. S. acutum never caused vasoconstriction in the presence of propranolol. Therefore, these results indicate that S. acutum also possesses both β- and α-adrenoceptor stimulating actions. However, α-adrenoceptor stimulating action is not as strong as Mokuboito, and the vasoconstriction does not appear even under the pretreatment with propranolol.
Mokuboito has β- and α-stimulating actions. Since both α- and β-adrenoceptors are widespread in the whole body, Mokuboito might stimulate both adrenoceptors. In our study, it is still unclear whether Mokuboito, S. acutum and sinomenine have more selective affinity to α- and β-adrenoceptors. The α-stimulating effects of Mokuboito and S. acutum might be mediated through α1-adrenoceptor on adrenergic nerve terminals.
Sinomenine possesses the strong vasodilating action (19). However, sinomenine never causes the vasoconstrictive effect. The vasorelaxation was similarly attenuated by L-NMMA and indomethacin. These results indicate that sinomenine also produces EDRF and PGI2 releases. Furthermore, since nicardipine inhibited the sinomenine-induced vasorelaxation, the vasorelaxation is responsible for Ca2+ channel inhibition.
The EDRF and PGI2 releases are activated by an increase of cellular Ca2+ ([Ca2+]i) in endothelium cells (20). The detailed mechanisms for the endothelium-dependent relaxations are not yet unclear. However, Mokuboito and the constituents would increase [Ca2+]i in endothelium cells and then activate NOS activity and PGI2 release. Thus, we conclude that Mokuboito, S. acutum and sinomenine involve with EDRF and PGI2, and regulate the Ca2+ influx via L-type Ca2+ channels on the cell membrane of aortic smooth muscle.
Mokuboito consists of S. acutum (4 g), Cinnamon cortex (3 g), Ginseng radix (3 g) and Gypsum (10 g). Sinomenine is one of the constituents of S. acutum. It is estimated that ∼66 μM sinomenine is contained in 1 mg ml−1 of S. acutum, corresponding to 2.2% (Pharmaceutical Institute of Tsumura Co.). S. acutum at 1 mg ml−1 caused the vasorelaxation by ∼46%. On the other hand, sinomenine at 66 μM estimated vasorelaxation by ∼60%. The relaxing strength of S. acutum and sinomenine is not so different. Since both compounds may cause the vasorelaxation by similar mechanisms, sinomenine may play a main role for the vasorelaxation induced by S. acutum. In the presence of propranolol, however, there were differences between the effects of S. acutum and sinomenine. S. acutum causes vasoconstriction, whereas sinomenine has no effect. This difference cannot be explained now, but there could be a component in S. acutum different from sinomenine.
Although sinomenine may be considered as a key constituent of S. acutum, we cannot necessarily explain the entire pharmacological effects of Mokuboito; because sinomenine is only 2.2% in S. acutum, and only 0.6% in Mokuboito. Thus, there may be a great difference between the pharmacological potencies of Mokuboito and sinomenine included in Mokuboito. S. acutum include only 20% in Mokuboito, but the potencies of Mokuboito and S. acutum are almost similar. Cinnamon cortex and Ginseng radix also possess vasodilating effects (21,22). Therefore, the complex interactions among S. acutum, Cinnamon cortex and Ginseng radix would produce the pharmacological effects of Mokuboito as a whole. The whole effect as a Mokuboito is absolutely not a simple sum of each effect induced by its constituents.
Mokuboito is traditionally used for dyspnea and edema (1,2). Thus, Mokuboito may be expected as one of the therapeutic drugs for heart failure. Most recently, Satoh (17) has demonstrated the effective modulation of cardiac ion channels by Mokuboito, S. acutum and sinomenine. Sinomenine inhibits ICa, and simultaneously produces the Ik decrease in cardiomyocytes, resulting in the APD prolongation. And sinomenine also exhibits an antiarrhythmic action on dysrhythmias under Ca2+-overloaded conditions. The antiarrhythmic effect would be due to the APD prolongation and the [Ca2+]i reduction by the inhibitions in ICa and INa channels.
Mokuboito, S. acutum and sinomenine have multiple vasodilating mechanisms. The vasodilating actions are expected as one of the great useful tools for heart failure, and can regulate the preload and afterload of cardiovascular systems. The reduction of both loads is the essential therapeutic strategies for heart failure. Therefore, the vasodilating actions by Mokuboito, S. acutum and sinomenine can sufficiently improve cardiac functions by reduction of the preload and afterload against heart failure.
In this study, Mokuboito, S. acutum and sinomenine may regulate the Ca2+ influx via L-type Ca2+ channel, consistent with the previous report (17). This may be a Ca2+ channel inhibitor, because the effect of sinomenine was attenuated under the conditions of low Ca2+ extracellular concentration (19), as well as in the presence of nicardipine. Under ischemia and heart failure, cellular Ca2+ overload of heart muscles elicits arrhythmias and dysfunctions (23,24). The Ca2+ influx control may regulate cellular Ca2+, overload cardiomyocytes and produce the protective actions for Ca2+-overloaded cells (23). Thus, Mokuboito, S. acutum and sinomenine might protect cell damages of heart muscles via regulation of [Ca2+]i, and as a result, would exert a cardioprotective action. Further experiments are needed to elucidate in more detail the mechanisms for pharmacological actions.
Acknowledgments
The authors wish to express thanks for the supply of Mokuboito and S. acutum extracts (Tsumura Co.). This work was in part supported by the grants from Tsumura Co.
References
- 1.Inaki K. Diseases and Kampo. In: Sato Y, Hanawa T, Arai M, Cyong J, Fukuzawa M, Mitani K, Ogihara Y, Sakiyama T, Shimada Y, Toriizuka K, Ymamada T, editors. Introduction to Kampo. Japan: Elsevier; 2005. p. 125. [Google Scholar]
- 2.Yakubu S, Kinoshita Y, Arakawa Y, Takahashi M, Kitanaka S. Clinical evaluation of Moku-boi-to (Mu-Fang-Yi-Tang): a Japanese and Chinese traditional medicine for heart failure. J Trad Med. 2002;19:159–63. [Google Scholar]
- 3.Hirai A. Study on the mechanism of anti-atherosclerotic effects of Sino-Japanese traditional medicines: focusing arachidonic acid cascade. J Trad Med. 1994;11:16–28. [Google Scholar]
- 4.Kubo M, Ma S, Wu J, Matsuda H. Anti-inflammatory activities of 70% methanolic extract from Cinnamomi Cortex. Biol Pharm Bull. 1996;19:1041–5. doi: 10.1248/bpb.19.1041. [DOI] [PubMed] [Google Scholar]
- 5.Haranaka R, Kosoto H, Hirama N, Hanawa T, Hasegawa R, Hyun JS, et al. Antitumor activities of Zyuzen-taiho-to and Cinnamoni Cortex. J Trad Med. 1987;4:49–58. [Google Scholar]
- 6.Bentler SE, Hartz AJ, Kuhn EM. Prospective observational study of treatments for unexplained chronic fatigue. J Clin Psychiatry. 2005;66:625–32. doi: 10.4088/jcp.v66n0513. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–62. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 8.Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003;2:247–67. doi: 10.1177/1534735403256419. [DOI] [PubMed] [Google Scholar]
- 9.See DM, Broumand N, Sahl L, Tillers JG. In vitro effects of echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology. 1997;35:229–35. doi: 10.1016/s0162-3109(96)00125-7. [DOI] [PubMed] [Google Scholar]
- 10.Rudakewich M, Ba F, Benishin CG. Neurotrophic and neuroprotective actions of ginsenosides Rb(1) and Rg(1) Planta Med. 2001;67:533–7. doi: 10.1055/s-2001-16488. [DOI] [PubMed] [Google Scholar]
- 11.Yuan D, Sunouchi H, Sakurai T, Saito K, Kano Y. Pharmacological properties of traditional medicines (XXVII). Interaction between Ephedra herb and Gypsum under hyperthermal conditions in rats. Biol Pharm Bull. 2002;25:872–74. doi: 10.1248/bpb.25.872. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Cui S, Cheng Y, Chen X, Hu Z. Application of nonaqueous capillary electrophoresis for quantitative analysis of quinolizidine alkaloids in Chinese herbs. Anal Chim Acta. 2004;508:17–22. [Google Scholar]
- 13.Yamasaki H. Pharmacology of sinomenine, an anti-rheumatic alkaloids from sinomenium acutum. Acta Med Okayama. 1976;30:1–20. [PubMed] [Google Scholar]
- 14.Liu L, Buchner E, Beitze D, Schmidt-Weber CB, Kaever V, Emmrich F, et al. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine. Int J Immunopharmacol. 1996;18:529–43. doi: 10.1016/s0192-0561(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Riese J, Resch K, Kaever V. Impairment of macrophage eicosanoids and nitric oxide production by alkaloid from Sinomenium acutum. Arzneimittelforschung. 1994;44:1223–6. [PubMed] [Google Scholar]
- 16.Mark W, Schneeberger S, Seiler R, Stroke DM, Amberger A, Offner F, et al. Sinomenine blocks tissue remodeling in a rat model of chronic cardiac allograft rejection. Transplantation. 2003;15:940–5. doi: 10.1097/01.TP.0000056610.22062.03. [DOI] [PubMed] [Google Scholar]
- 17.Satoh H. Electropharmacology of sinomeni caulis et rhizome and its constituents in cardiomyocytes. Am J Chin Med. 2005;33:967–79. doi: 10.1142/S0192415X05003569. [DOI] [PubMed] [Google Scholar]
- 18.Nishida S, Satoh H. Pharmacological actions of sinomenine acutum, Kampo medicine, on rat aorta. J Pharmacol Sci. 2004;94(Suppl. 1):212. [Google Scholar]
- 19.Nishida S, Satoh H. In vitro pharmacological actions of sinomenine on the smooth muscle and the endothelial cell activity in rat aorta. Life Sci. 2006;79:1203–6. doi: 10.1016/j.lfs.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Quignard JF, Feleton M, Thollon C, Vilaine JP, Duhault J, Vanhoutte PM. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br J Pharmacol. 1999;127:27–37. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashihara Y, Goto H, Shimada Y, Sekiya N, Yang Q, Terasawa K. Inhibitory effects of Cinnamomi Cortex and cinnamaldehyde on oxygen-derived free radical-induced vasocontraction in isolated aorta of spontaneously hypertensive rats. J Trad Med. 2002;19:51–7. [Google Scholar]
- 22.Fukuda K, Kido T, Miura N, Yamamoto M, Komatu Y. Increase in nitric oxide synthase and cyclic GMP in vascular smooth muscle cells by treatment with aqueous extracts of Astragali Radix, Ginsen radix and Scutellariae radix. J Trad Med. 1995;12:38–44. [Google Scholar]
- 23.Satoh H, Sperelakis N. Review of some actions of taurine on ion channels of cardiac muscle cells and others. Gen Pharmacol. 1998;30:451–63. doi: 10.1016/s0306-3623(97)00309-1. [DOI] [PubMed] [Google Scholar]
- 24.Satoh H. [Ca2+]-dependent actions of taurine in spontaneously beating rabbit sino-atrial nodal cells. Eur J Pharmacol. 2001;424:19–25. doi: 10.1016/s0014-2999(01)01128-1. [DOI] [PubMed] [Google Scholar]