Abstract
Oral complications are a common side effect of cancer chemotherapy, as antineoplastic agents affect both the immune system and the oral mucosa. This study demonstrates preventive and therapeutic effects of dental treatment and regular use of Weleda Ratanhia-Mundwasser® (herbal mouthwash) and Weleda Pflanzen-Zahngel® (herbal toothgel) on oral mucositis during chemotherapy. Thirty-two female patients with breast cancer starting on chemotherapy were evaluated in this study. Plaque index, gingival index, degree of mucositis and 10 single symptoms were monitored once weekly for four consecutive weeks. After four weeks, plaque and gingival indexes were slightly decreased compared to baseline values. The degree of mucositis was increased by one grade in 15.6 % of the patients and over 70 % remained without symptoms. On the whole, single symptoms decreased from day 7 since beginning of chemotherapy to day 28. Mucositis symptoms were moderate in severity, and the results indicate a positive influence of using Weleda Ratanhia-Mundwasser and Weleda Pflanzen-Zahngel. Further studies might be promising.
Keywords: oral mucositis, chemotherapy (side effects), oral hygiene, herbal preparations, ratanhia
Background
Inflammatory changes of the oral mucosa are a common side effect of cancer chemotherapy. According to the guidelines for prophylaxis and therapy of mucositis (1) of 2004, the risk to develop a grade 3 (severe) or grade 4 (life threatening) oral mucositis lies between 2 and 66%, depending on the type of chemotherapy (without additional radiotherapy), and between 1 and 53% depending on the type of tumor. In more than one-third of the patients who develop a grade 3 or 4 oral or gastrointestinal mucositis, the start of the next chemotherapy cycle needs to be delayed (1), resulting in impaired cancer treatment.
Most of the cytostatic agents used for antitumor treatment exert their toxic properties especially on rapidly proliferating cell lines. Basal cells of the oral mucosa show a high rate of proliferation which is comparable to that of tumor cells. This is why chemotherapy affects both the oral mucosa by decreasing the regeneration of basal cells (2) and the immune system as a result of myelosuppression. Both factors together promote the development of diverse inflammatory changes in the oral mucosa under chemotherapy, will be summarized as ‘mucositis’ (3). Even if the underlying interactions on the level of molecular immunology are not yet completely understood, there is some evidence that e.g. the administration of GM-CSF (granulocyte-macrophage colony stimulating factor) results in clinically relevant improvement of mucositis (4). It has been shown that individual oral hygiene has considerable influence on symptom presence and severity of oral mucositis (5).
Common dental care products offer several different active ingredients to reduce bacterial counts and plaque. Fluorides have antibacterial (sodium fluoride, amine fluoride, tin fluoride) (6) and plaque decreasing effects (amine fluoride and tin fluoride). Chlorhexidine is antimicrobial and effective in inhibiting plaque growth (7). However, because of side effects (e.g. mucosal irritation) it is not advised for long-term use (7,8). The same holds true for sanguinarine, which is an antimicrobial alkaloid extracted from Canada Bloodroot or Paccoon plants (Sanguinaria canadensis) (9).
Kationic detergents such as cetylpyridiniumchloride have antimicrobial and plaque-inhibiting effects (8), and so do some volatile oils such as menthol, eucalyptol and thymol (10).
The principal active ingredients of the preparations used in this study are extracts from rathania root and myrrh, which are indicated as phytotherapeutics for local treatment of slight inflammations of the mucous membrane in mouth and larynx. In Germany, those extracts are approved by the authorities as ‘Standardzulassung’ (11). The primary effect of ratanhia (Krameria triandra) root extracts is tissue astringency. Myrrh tincture, an alcoholic solution of commiphora resins, shows additional antibacterial properties (12).
Further ingredients of Weleda Ratanhia-Mundwasser® (ratanhia mouthwash) are horse chestnut extract, volatile oils to stimulate the mouth mucosa and homeopathic components used in anthroposophic medicine to strengthen dental structure and to promote tooth enamel production (Fluoride of Calcium D10, Argentum vitr. D15, Sulfate of Magnesium D20, horse chestnut bark D20).
Weleda Pflanzen-Zahngel® (herbal dentifrice, toothgel) contains extracts from ratanhia roots, myrrh and chamomile, delivering a broad range of effects with primarily anti-inflammatory, antibacterial and lesion healing properties (13,14). Volatile oils (from peppermint, spearmint and fennel seeds) are supposed to stimulate the mucosa.
It is the aim of this prospective study to document the effects of dental treatment and regular use of Weleda ratanhia mouthwash and herbal dentrifice on incidence and severity of oral mucositis under chemotherapy.
Methods
This study was conducted at the Poliklinik für Zahnerhaltungskunde with patients of the Klinikum für Frauenheilkunde (Clinic for female health) of Johannes Gutenberg-University in Mainz, Germany. All participants were scheduled for individual adjuvant chemotherapy after surgery of breast cancer. The study was designed as a controlled trial with a patient's choice at study entry for participation in the medication or in the control group.
Study conduct
Each patient underwent a total of five dental investigations by the same treating dentist. A baseline visit was scheduled immediately before the beginning of the first chemotherapy cycle, followed by three investigations under treatment in weekly intervals and a study termination visit at week four.
At the baseline visit, the patients' ages, types of chemotherapy and their usual intake of alcohol and nicotine were registered. A dental examination established baseline DMF-T index (Diseased, Missing, Filled Teeth, max. 28), plaque index (after to Silness and Löe, grades 0–3, buccal tooth surfaces) and gingiva index (after Löe and Silness, grades 0–3, 4 points of measurement, highest grade per tooth). Moreover, a visual assessment method (mucositis grading grades 0–3, after Carl and Emrich) was used to describe the extent of oral tissue damage (Table 1). Only the Ramfjord teeth (nos. 16/21/24/36/41/44) were evaluated for the parameters depicting oral hygiene status (plaque index and and gingiva index).
Table 1.
Grading of indices
| Plaque index according to Silness and Löe | ||
| Grade 0 | No plaque (visual inspection and periodontal probing) | |
| Grade 1 | Thin plaque attachments at the gingival margins on probing | |
| Grade 2 | Moderate plaque attachments at the gingival margins, visible to the eye, interdental spaces free | |
| Grade 3 | Marked plaque at the gingival margins, interdental spaces filled with plaque | |
| Gingiva index according to Löe and Silness | ||
| Grade 0 | Normal gingiva, no inflammation, no reddening, no bleeding | |
| Grade 1 | Slight inflammation, slight reddening, no bleeding | |
| Grade 2 | Moderate inflammation, redness, edema, bleeding on probing | |
| Grade 3 | Severe inflammation, redness, edema, tendency to spontaneous bleeding, ulceration left | |
| Mucositis index according to Carl und Emrich | WHO / NCI | |
| Grade 0 | None | None |
| Grade 1 | Mild | Mild |
| Grade 2 | Moderate | Moderate |
| Grade 3 | Severe | Severe |
| Grade 4 | — | Life threatening |
| Grade 5 | — | Fatal |
Before iniating the study, all patients were subjected to a baseline professional tooth cleaning. Each patient was given a soft bristle toothbrush and the study products, Weleda Pflanzen-Zahngel and Weleda Ratanhia-Mundwasser (herbal dentrifice and herbal mouthwash, as described above). Only these products were to be used for oral care, three times daily, during the four week study period. In order to ensure patient compliance they were asked to choose their preferred treatment.
Assessments
After start of chemotherapy treatment, weekly dental investigations were scheduled to determine grade of mucositis, plaque index and gingival index. Furthermore, the following symptoms were evaluated in patient interviews and visual examinations: desquamation, mucosal lesions and ulcerations, gum bleeding, mucosal redness, irritation/ burning sensations at mucosa and tongue, dry mouth, diminished tasting sense, problems with food intake and herpes simplex virus infections.
The signs and symptoms were classified as ‘yes’, ‘no’ or ‘slightly’. All above assessments—indices and symptom severity evaluations—were repeated at the week four study termination visit. Moreover, the patients were asked to self-assess the severity changes of their symptoms in the course of the observation period, their experience of symptom mitigation immediately after mouthwash application, and total symptom mitigation. They were also asked to comment on the taste of the mouthwash and if they would continue to use the products. Finally they were asked whether they would advocate intensified dental assistance during and after chemotherapy.
Statistics
All data were subjected to statistical analysis using descriptive statistics i.e. mean, median, standard deviation, minimum, maximum, using the computer program SPSS. Due to the exploratory approach, no null hypothesis was set to be tested.
Results
Since all 49 patients who were eligible insisted on participation in the active group, no control group could be established. This bias had unfortunately to be accepted, since blinding was impossible due to the astringent effects and the typical taste of the herbal ingredients, and active compliance of the patients was required to ensure correct administration.
Due to their poor overall condition (cancer patients), only 32 of the 49 patients enrolled in the medication phase were able to attend to all five study visits. Only these patients were included in the analysis (Table 2).
Table 2.
Patient baseline demography
| No. | Percentage | Mean | Median | SD | Min. | Max. | n | |
|---|---|---|---|---|---|---|---|---|
| Patients(total) | 49 | |||||||
| Dropouts | 17 | |||||||
| PP | 32 | 100 | ||||||
| Females | 32 | 100 | 32 | |||||
| Age (years) | 58.9 | 8.9 | 34 | 72 | 32 | |||
| DMF-T Index | 18.87 | 19.00 | 5.96 | 5.00 | 28.00 | 32 | ||
| Plaque index | 0.87 | 0.92 | 0.50 | 0 | 2.20 | 30 | ||
| Gingiva index | 0.90 | 0.74 | 0.70 | 0 | 2.60 | 30 | ||
| Mucositis grading | ||||||||
| Grade 0 | 29 | 90.6 | ||||||
| Grade 1 | 1 | 3.1 | ||||||
| Grade 2 | 2 | 6.3 | ||||||
| Grade 3 | 0 | 0.0 |
Baseline characteristics: more than 90% of the patients had intact mucosae without pathological alterations
The mean age was 58.9 (±8.9) years. The majority of patients were classified as low consumers of alcohol and nicotine; 81.3% were non-smokers; 9.4% consumed no alcohol, 43.8% seldom and 40.6% occasionally; 15.6% of the patients conceded to regular consumption of tobacco products and 3.1% to regular consumption of alcoholic beverages. One patient refused to comment.
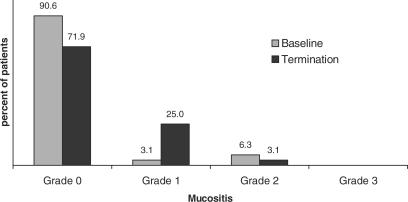
The drugs used for the underlying breast cancer chemotherapy were carboplatin, cyclophosphamide, epirubicin and taxol, which were administered either as single agents or in combination chemotherapy. The mean DMF-T index of the patients when the study began was 18.87 (±5.96). Before the beginning administration of chemotherapy, >90% of the patients presented intact mucosae without pathological alterations at the baseline visit (Fig. 1).
Figure 1.
Prevalence and severity of mucositis, grades 0–3, at baseline and termination examinations, (n = 32).
Efficacy: Both plaque and gingival index were reduced compared to baseline values
At study termination, >70% were free of symptoms and 8 of 32 patients (25%) were diagnosed with grade 1 mucositis. Of those, 5 had progressed from grade 0, and 2 had regressed from baseline grade 2. Thus, mucositis severity was increased by one grade in 15.6% of all patients. During the observation period, no patient developed grade 3 oral mucositis.
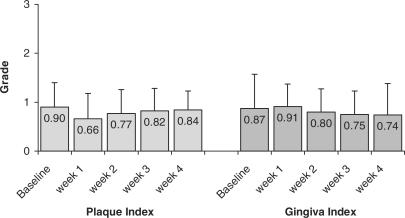
Both plaque index and gingival index were reduced after the study period compared to baseline values. Plaque index first declined from 0.90 (±0.50) at baseline to 0.66 (±0,53), to then continually increase over time up to 0.84 (±0.39). Mean gingival index declined to 0.74 (±0.64) from 0.87 (±0.70) after an intermittent slight increase (Fig. 2).
Figure 2.
Changes in mean values of Plaque index (P) and Gingiva index (G) over time grades 0 to 3 (Baseline examination; week 1, 2, 3 = study week visits; week 4 = final examination).
Tolerability: Adverse symptoms markedly improved by day 28
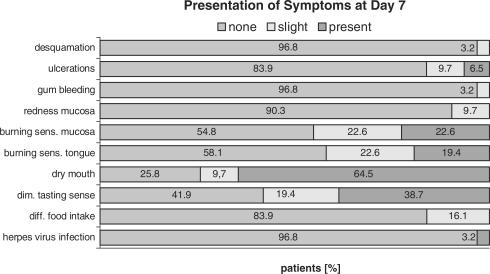
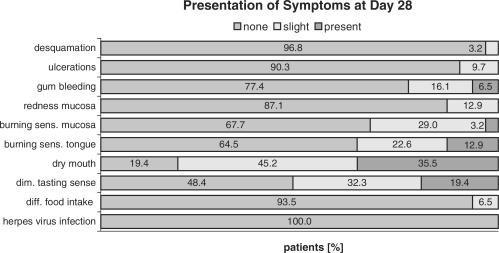
None of the adverse events that developed during the first week of chemotherapy was present at the baseline visit (Fig. 3). The adverse events that were most common at day 7 visit included dry mouth (64.5%), impaired sense of taste (38.7%), burning sensations at the mucosa (22.6%) and at the tongue (19.7%). At the day 28 study termination visit these symptoms were markedly improved (Fig. 4). Only 35.5% of the patients still complained about dry mouth, the others experienced only slight or no discomfort. A similar development was noted with burning sensations, diminished sense of taste and ulcerations.
Figure 3.
Prevalence and severity of the symptoms on day 7 (n = 31).
Figure 4.
Prevalence and severity of the symptoms on day 28 (n = 31).
A slight increase was observed in gingival bleeding and mucosal redness. Slight or no changes were observed for desquamation, difficulties with food intake, and herpes virus infections.
Subjective impressions: 93% of patients considered continuing using the intervention after the study
Asked for their subjective assessments, 13.3% of the patients stated an overall improvement in total signs and symptoms between day 7 and day 28, whereas 86.7% indicated no change. Immediately after administration of the mouthwash, about half of them experienced subjective mitigation of their individual complaints. On the whole, more than a third of them (36.7%) assessed the mitigating effects of the mouthwash as ‘good’ and more than half (56.7%) as ‘satisfactory’. 6.7% noted less gingival bleeding. The mouthwash's taste was termed ‘refreshing’ (46.7%) or ‘neutral’ (40.0%) by most patients, resulting in an overall opinion of ‘good’ (about half of the patients) or ‘satisfactory’ (another 30%) taste. Almost all patients (93.3%) considered continuing use of Weleda Pflanzen-Zahngel and Weleda Ratanhia-Mundwasser after termination of the study. Intensified dental care and assistance during chemotherapy was unequivocally judged as helpful.
Discussion
Our analysis was purely documentary in intention, not interventive. Additionally, incidence and severity of oral complications under chemotherapy strongly depend on the type of agents used, the therapeutical regimen administered, and on the patients' individual baseline conditions (5). Still, considering the extensive and detailed representation of mucositis in the abovementioned guidelines (1), a relative appraisal of the results of this study is deemed essential.
Professional tooth cleaning procedures immediately after the baseline visit served to provide equal and comparable dental baseline conditions for all participants. In order to ensure consistency in the evaluation of the dental parameters and indices, all investigations were conducted by the same treating dentist. Considering the mean patient age of 58.9 years, the baseline extent of caries as delineated by the DMF-T index was well within the normal range. Average values in Germany were listed as 16.1 for 35–44 year old persons and as 23.6 for 65–74 years of age in 1997 (15).
Both plaque and gingiva indexes were below baseline values after four weeks of treatment and thus demonstrated good oral hygiene effectiveness of thrice daily administration of Weleda Rantanhia mouthwash and Weleda herbal tooth gel.
As expected, the patients showed various signs of damage of oral mucosal cells during the first week of chemotherapy. This is a common fact with cytostatic agents that are known to harm all kinds of mucosal cells. Until the final observation visit after four weeks, they tended to recede in spite of continued chemotherapy with the exceptions of gingival bleeding and mucosal redness, rendering an overall positive development.
More than 70% of the patients remained asymptomatic of mucositis even after four weeks. In only 15.6% of all patients mucositis was increased in severity by one degree, only one patient presented grade 2 severity and none grade 3.
On the whole, the symptoms could be described as moderate in severity. A basis for comparison is found which names a relative risk for developing grade 3 or 4 mucositis of 3 to 13% after administration of the cytostatic drugs that were observed (1). The overall risk for breast cancer patients was mentioned as being 8%.
Since for these data, as for non-study patients, basic common oral hygiene can be safely assumed, the observed mildly positive effects are probably due to thrice daily astringent mouthwash administration, which is not part of everyday dental care routine.
On the other hand, it is highly improbable that the observed symptoms of oral mucositis were caused by the mouthrinse itself. It is generally known that oral mucositis is an almost inevitable side effect of cancer chemotherapy. On the other hand, this mouthwash is widely sold all over Europe for normal daily oral hygiene and has not been reported to cause such side effects in thousands of administrations.
In their self-assessments, none of the patients complained about worsening symptoms between day 7 and day 28 of chemotherapy. Most patients (86.7%) judged the severity as unchanged, and 13.3% experienced some degree of relief. Almost all patients (93.3%) wanted to continue the use of the herbal mouthwash and tooth gel, and all of them voted in favor of professional dental assistance under chemotherapy.
Paralleling these results, the mucositis guidelines stress the necessity of basic oral hygiene and the efficacy of oral care under professional guidance (oral care protocols and patient guideline, evidence level III b) (16).
The results of this study suggest that the use of Weleda Ratanhia mouthwash and Weleda herbal dentrifice may have a positive influence on the oral side effects of cancer chemotherapy, and that further investigations might be desirable.
References
- 1.Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology. Mucositis—Perspectives and Clinical Practice Guidelines—Perspectives on Cancer Therapy-Induced Mucosal Injury [online] URL: http://www3.interscience.wiley.com/cgi-bin/fulltext/108069519. [Google Scholar]
- 2.Hornecker E, Stolpe M, Mausberg R. ZWR. 1999;11:678–684. (german) [Google Scholar]
- 3.Carrega G, Castagnola E, Canessa A, Argenta P, Haupt R, Dini G. Herpes simplex virus and oral mucositis in children with cancer. Support Care Cancer. 1994;2:266–9. doi: 10.1007/BF00365734. [DOI] [PubMed] [Google Scholar]
- 4.Chiapelli F. The molecular immunology of mucositis: implications for evidence-based research in alternative and complementary treatments. Evid Based Complement Alternat Med. 2005;2:489–94. doi: 10.1093/ecam/neh129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toth BB, Martin JW, Fleming TJ. Oral complications associated with cancer therapy. J Clin Periodontol. 1990;17:508–15. doi: 10.1111/j.1365-2710.1992.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 6.Strübig W, Gülzow HJ. Bakterienstoffwechsel unter dem Einfluss von Aminfluorid/Zinnfluorid. In: Flores-de-Jacoby L, editor. Möglichkeiten der Plaque- und Gingivitisprävention. Berlin, Chicago: Quintessenz Verlag; 1991. pp. 19–26. (german) [Google Scholar]
- 7.Axelson P, Öslund L, Hontwedt M, Paulaner J. The effect of three different mouthrinses on plaque formation rate and salivary levels of mutans streptococci and lactobacilli-results after 4 months. Proceedings of the Fourth World Congress on Preventive Dentistry WCPD; Umea, Schweden. p. 58. [Google Scholar]
- 8.David G. Antimikrobielle Wirkstoffe in der Zahnheilkunde. Bzb bayer zahnarztebl. 1997;12:36–8. (german) [Google Scholar]
- 9.Baumann MA. Köln: Hundt. 1995. Grundlagen der Zahnerhaltungskunde; pp. 33–70. (german) [Google Scholar]
- 10.Perdok JF, Busscher HJ, Weerkamp AH, Arends J. The effect of an aminfluoride—stannous fluoride—containing mouthrinse on enamel surface free energy and the development of plaque and gingivitis. Clin Prev Dent. 1988;5:3–9. [PubMed] [Google Scholar]
- 11.St. Zul.1179.99.99 (Ratanhiawurzel) St. Zul. 6699.99.99 (Myrrhentinktur) Standardzulassungen. In: Braun R, editor. Text und Kommentar. Stuttgart: Deutscher Apotheker Verlag; 2002. (german) [Google Scholar]
- 12.Czygan FC, Hiller K, Myrrha M. In: Teedrogen und Phytopharmaka, edn 4, revised. Wichtl M, editor. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2002. pp. 404–406. (german) [Google Scholar]
- 13.Willuhn G. Matricariae flos. In: Teedrogen und Phytopharmaka, edn 4, revised. Wichtl M, editor. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2002. pp. 369–373. (german) [Google Scholar]
- 14.St. Zul. 7999.99.99 (Kamillenblüten). Standardzulassungen. Text und Kommentar. In: Braun R, editor. Stuttgart: Deutscher Apotheker Verlag; 2002. (german) [Google Scholar]
- 15.Michaelis W, Reich E. Köln: Deutscher Ärzte-Verlag; 1999. Dritte Deutsche Mundgesundheitsstudie (DMS III). Ergebnisse, Trends und Problemanalysen auf der Grundlage bevölkerungsrepräsentativer Stichproben in Deutschland 1997; pp. 23–24. (german) [Google Scholar]
- 16.Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology. 2005. Mucositis—Perspectives and Clinical Practice Guidelines—Clinical Practice Guidelines for the Prevention and Treatment of Cancer Therapy-Induced Oral and Gastrointestinal Mucositis [online] Url: http://www3.interscience.wiley.com/cgi-bin/fulltext/108069518. [Google Scholar]