Abstract
Hibiscus sabdariffa (HS) is an edible medicinal plant, indigenous to India, China and Thailand and is used in Ayurveda and traditional medicine. Alcoholic extract of HS leaves (HSEt) was studied for its anti-hyperammonemic and antioxidant effects in brain tissues of ammonium chloride-induced hyperammonemic rats. Oral administration of HSEt (250 mg kg−1 body weight) significantly normalizes the levels of ammonia, urea, uric acid, creatinine and non-protein nitrogen in the blood. HSEt significantly reduced brain levels of lipid peroxidation products such as thiobarbituric acid and reactive substances (TBARS) and hydroperoxides (HP). However, the administered extract significantly increased the levels of antioxidants such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and reduced glutathione (GSH) in brain tissues of hyperammonemic rats. This investigation demonstrates significant anti-hyperammonemic and antioxidant activity of HS.
Keywords: ammonia, antioxidants, creatinine, Hibiscus sabdariffa, hyperammonemia, lipid peroxidation, urea, uric acid
Introduction
The neurological complications of hyperammonemia in the central nervous system (CNS) are now receiving more attention. Ammonia is a neurotoxin that has been strongly implicated in the pathogenesis of hepatic encephalopathy (1). Ammonia has also been a major pathogenetic factor associated with inborn errors of urea cycle, Reye's syndrome, organic acidurias and disorders of fatty acid oxidation (2). Ammonia-induced neurotoxicity has been reported to include a dysfunction of multiple neurotransmitter system glutamate-mediated excitotoxicity, electrophysiological disturbances and defects in brain bioenergetics (1,3). In spite of extensive investigations, the precise mechanisms involved in ammonia neurotoxicity are not completely understood.
Oxidative stress is evolving concept in ammonia neurotoxicity. Its effect on the oxidative and nitrosative stress in the CNS has been recently reviewed (1). Recent studies have reported an increased production of free radicals in cultured astrocytes after treatment with pathophysiological concentrations of ammonia (4). A concurrent increase of superoxide production and a reduction in the activities of various antioxidant enzymes have been shown in animal models of acute ammonia toxicity (4,5). Oxidative stress-mediated lipid peroxidation was also shown as one of the characteristic features of hyperammonemia (5,6).
Hibiscus sabdariffa (Linn) (HS) (family Malvaceae), is an annual dicotyledonous herbaceous shrub popularly known as ‘Gongura’ in Hindi or ‘Pulicha keerai’ in Tamil. This plant is well known in Asia and Africa and is commonly used to make jellies, jams and beverages. In the Ayurvedic literature of India, different parts of this plant have been recommended as a remedy for various ailments such as hypertension, pyrexia, liver disorders and antidotes to poisoning chemicals (acids, alkali and pesticides) and venomous mushrooms (7). Anthocyanins, flavonols, protocatechuic acid (PCA), along with others, have been identified as contributors to the observed medicinal effect of HS (8). Anthocyanin and PCA have been shown to have antioxidant activity and to offer protection against atherosclerosis and cancer (9). Compared to common antioxidants such as ascorbate, anthocyanins were found to be much more potent antioxidants (10). It is well documented that most medicinal plants are enriched with phenolic compounds and bioflavonoids that represent potent antioxidants (11). There is currently a growing body of evidence that supplementing the human diet with antioxidants is of major benefit for human health and well-being.
Nowadays, the use of complementary/alternative medicine and especially the consumption of botanicals have been increasing rapidly worldwide, mostly because of the supposedly less frequent side effects when compared with modern Western medicine. Both in conventional and traditional medicines, plants continue to provide valuable therapeutic agents (12). Doubts about the efficacy and safety of currently available anti-hyperammonemic agents have prompted the search for safer and more effective alternatives (13). To our knowledge, this report is the first study to investigate the effect of alcoholic extract of HS leaves (HSEt) on brain lipid peroxidation and antioxidant status in ammonium chloride-induced hyperammonemic rats. Therefore, we made an attempt to bridge the information gap. The present study was undertaken to investigate the effect of HSEt on brain lipid peroxidation and antioxidant status in ammonium chloride-induced hyperammonemic rats.
Materials and Methods
Plant Material and Extraction
The mature green leaves of Hibiscus sabdarifa were collected from Chidambaram, Cuddalore District, Tamil Nadu, India. The plant was identified and authenticated at the Herbarium of Botany Directorate in Annamalai University. A voucher specimen (No. 3648) was deposited in the Botany Department of Annamalai University. The shade-dried and powdered leaves of Hibiscus subdariffa were subjected to extraction with 70% ethanol under reflux for 8 h and concentrated to a semi-solid mass under reduced pressure (Rotavapor apparatus, Buchi Labortechnik AG, Switzerland). The yield was about 24% (w/w) of the starting crude material. In the preliminary phytochemical screening, the ethanolic extract of HSEt gave positive tests for glycosides, anthocyanins, polyphenols and flavones (14). The residual extract was dissolved in sterile water and used in the investigation.
Rats
Male albino Wistar rats weighing 180–200 were used for the study. They were housed in polycarbonate cages under standard conditions (22 ± 2°C, humidity of 45–64%, 12 h light/dark cycles). They were given standard pellet diet (Hindustan Lever Ltd, Mumbai, India) and water ad libitum. All animal experiments were approved by the ethical committee (Vide. No. 273/2004), Annamalai University, India, and were in accordance with the guidelines of the National Institute of Nutrition (NIN), Indian Council of Medical Research (ICMR), Hyderabad, India. Ammonium chloride was purchased from Sisco Research Laboratories, Mumbai, India. All other chemicals used in the study were of analytical grade.
Experimental Design
Hyperammonemia was induced in Wistar rats by daily intraperitoneal injections of ammonium chloride at a dose of 100 mg kg−1 body weight for eight consecutive weeks (13). Rats were divided into four groups, eight animals each. Group 1: control rats. Group 2: rats orally administered with HSEt (250 mg kg−1 body weight) (15). Group 3: rats intraperitoneally treated with ammonium chloride (100 mg kg−1 body weight) (13). Group 4: rats treated with ammonium chloride (100 mg kg−1) + HSEt (250 mg kg−1). At the end of 8 weeks, the animals were killed by decapitation and the blood samples were used for the estimation of circulatory ammonia, urea, uric acid, creatinine and non-protein nitrogen. Whole brain was dissected out and washed in ice-cold phosphate buffered saline. The brains were weighed and 10% tissue homogenates were prepared in 0.025 M Tris–HCl buffer, pH 7.5, and used to measure the activities of thiobarbituric acid reactive substances (TBARS), hydroperoxides (HP) and glutathione peroxidase (GPx). Enzyme activity was assayed in 10% brain homogenates prepared in 0.2 M phosphate buffer, pH 8.0.
Biochemical Determinations
Ammonia levels were estimated in the blood where blood, triethanolamine and NADPH/GLDH/buffered substrates were well-mixed and the absorbance was read at 470 nm using UV spectrophotometer-Hitachi 912. This procedure is detailed elsewhere (16). Levels of urea, uric acid and non-protein nitrogen were measured in the plasma according to standard methods stated elsewhere (17). Creatinine levels were estimated in serum following an alkaline picrate method [detailed in ref. (17)]. The absorbance of each of the previous parameters was read at 480 nm using UV spectrophotometer-Hitachi 912. Blank and a series of standards were processed similarly.
Estimation of Lipid Peroxidation Products
Lipid peroxidation was estimated colorimetrically in brain by assessing TBARS and HP according to standard methods (18,19). In brief, for the estimation of TBARS the supernatant of tissue homogenate was treated with TBA–TCA–HCl reagent and mixed thoroughly. The mixture was kept in boiling water bath for 15 min. After cooling, the tubes were centrifuged for 10 min and the supernatant was taken for measurement. The developed color was read at 535 nm using UV spectrophotometer-Hitachi 912 against the reagent blank. For the estimation of HP, the supernatant of tissue homogenate was treated with Fox reagent (19) and incubated at 37°C for 30 min. The developed color was read at 560 nm using UV spectrophotometer-Hitachi 912 against reagent blank. TBARS and HP were expressed as mM per 100 g tissue.
Estimation of Antioxidants
Catalase (CAT) was assayed colorimetrically at 620 nm and was expressed as μmoles of H2O2 consumed per min per mg protein as described by Sinha (1972) (20). Superoxide dismutase (SOD) was assayed utilizing the technique of Kakkar et al. (1984) (21). A single unit of enzyme was expressed as 50% inhibition of NBT (nitroblue tetrazolium) reduction per min per mg protein. GPx activity was measured according to the method described by Rotruck et al. (1973) (22). Briefly, reaction mixture contained 0.2 ml of 0.4 M Tris–HCl buffer, pH 7.0, 0.1 ml of 10 mM sodium azide, 0.2 ml of tissue homogenate (homogenized in 0.4 M Tris–HCl buffer, pH 7.0), 0.2 ml glutathione and 0.1 ml of 0.2 mM hydrogen peroxide. The contents were incubated at 37°C for 10 min. The reaction was stopped by 0.4 ml of 10% TCA, and centrifuged. Supernatant was assayed for glutathione content by using Ellmans reagent (19.8 mg of 5,5'-dithiobisnitro benzoic acid in 100 ml of 0.1% sodium nitrate). Reduced glutathione (GSH) was determined by the method of Ellman (1959) (23). One milliliter of supernatant was treated with Ellman's reagent and phosphate buffer (0.2 M, pH 8.0). The absorbance was read at 412 nm. The activities of GPx and GSH were expressed as μg of GSH consumed per min per mg protein and as mg per 100 g of tissue, respectively.
Statistical Analysis
Data analysis was carried out by analysis of variance (ANOVA) and the groups were compared using Duncan's Multiple Range Test (DMRT).
Results
Blood Ammonia, Urea, Uric Acid and Creatinine were Restored to Normal Levels in Treated Rats
Table 1 shows the levels of blood ammonia, urea, uric acid, non-protein nitrogen and creatinine of control and experimental groups. Levels of blood ammonia, urea, uric acid, non-protein nitrogen and creatinine increased significantly in ammonium chloride-treated rats. These levels were significantly restored to near normal upon the administration of HSEt (Table 1). There is no significant change in bodyweight of animals in the experimental groups when compared with controls (Table 1).
Table 1.
Effect of HSEt on changes in the body weight, blood ammonia, urea, uric acid, non-protein nitrogen and serum creatinine of normal and experimental rats (mean ± SD, n = 6)
| Group | Changes in body weight (g) | Blood ammonia (μmol l−1) | Blood urea (mg dl−1) | Uric acid (mg dl−1) | Creatinine (mg dl−1) | Non-protein nitrogen (mg dl−1) | |
|---|---|---|---|---|---|---|---|
| Initial | Final | ||||||
| Control | 181.03 ± 13.78 | 195.29 ± 14.87a | 88.01 ± 6.70a | 10.79 ± 0.82a | 1.69 ± 0.13a | 0.80 ± 0.06a | 23.58 ± 1.80a |
| Control + HSEt (250 mg kg−1) | 189.59 ± 14.44 | 197.71 ± 15.06a | 82.30 ± 6.27a | 11.60 ± 0.88a | 1.71 ± 0.13a | 0.81 ± 0.06a | 23.95 ± 1.82a |
| NH4Cl treated (100 mg kg−1) | 185.03 ± 14.09 | 205.68 ± 15.66a | 342.67 ± 26.09b | 22.18 ± 1.69b | 2.31 ± 0.18b | 1.40 ± 0.11b | 49.96 ± 3.80b |
| NH4Cl + HSEt | 184.68 ± 14.06 | 197.03 ± 15.00a | 136.70 ± 12.46c | 13.00 ± 0.99c | 1.78 ± 0.14a | 0.88 ± 0.07a | 30.12 ± 2.29c |
ANOVA followed by DMRT. Values not sharing a common superscript letter differ significantly at P < 0.05 (DMRT).
Duncan procedure: ranges for the levels are 2.91, 3.06, 3.16, 3.22.
Antioxidants in the Brain Restored to Near Normal upon the Administration of HSEt
The levels of lipid peroxidation products and antioxidants in brain of control and experimental groups are shown in Table 2. The levels of TBARS and HP were significantly higher and the levels of SOD, CAT, GSH and GPx were significantly lower in the brain of ammonium chloride-treated rats. These levels were significantly restored to near normal upon the administration of HSEt (Table 2).
Table 2.
Effects of HSEt on changes in the levels of TBARS, HP, SOD, CAT, GPX and GSH in brain of normal and experimental rats (mean ± SD, n = 6)
| Group | SOD (UA) | CAT (UB) | GPx (UC) | GSH (mg per 100 g tissue) | TBARS (mM per 100 g tissue) | HP (mM per 100 g tissue) |
|---|---|---|---|---|---|---|
| Control | 7.75 ± 0.39a | 3.21 ± 0.23a | 3.43 ± 0.20a | 36.01 ± 2.69a | 1.09 ± 0.07 a | 113.17 ± 4.39a |
| Control + HSEt (250 mg kg−1) | 7.01 ± 0.27a | 3.30 ± 0.29a | 3.81 ± 0.18a | 37.52 ± 2.72a | 0.91 ± 0.09 a | 109.01 ± 5.10a |
| NH4Cl treated (100 mg kg−1) | 5.11 ± 0.43b | 0.79 ± 0.06b | 1.15 ± 0.07b | 15.27 ± 1.43b | 2.01 ± 0.14 b | 132.70 ± 2.54b |
| NH4Cl + HSEt | 7.32 ± 0.46c | 2.74 ± 0.19c | 2.61 ± 0.14c | 27.01 ± 2.32c | 1.21 ± 0.06c | 118.42 ± 2.75c |
ANOVA followed by DMRT. Values not sharing a common superscript (a, b, c) differ significantly at P ≤ 0.05.
Duncan procedure: ranges for the levels are 2.91, 3.06, 3.16, 3.22.
AAmount of enzyme required to inhibit 50% of NBT reduction per mg protein.
BMicromoles of H2O2 consumed per min per mg protein.
CMicromoles of GSH utilized per gram protein.
Discussion
This work is one of the series of studies showing that chronic hyperammonemia causes an imbalance in the oxidative status of nervous tissue and that the resulting free radicals damage the brain through a peroxidative mechanism. The elevated levels of ammonia and urea might vindicate hyperammonemic condition in rats treated with ammonium chloride (4,6,13). The reduction in levels of ammonia, urea, uric acid, creatinine and non-protein nitrogen during HSEt treatment shows significant anti-hyperammonemic activity of this plant. This is probably indicative of antioxidant efficacy of this plant (24). Phenolic compounds and flavonoids have the ability to remove excess ammonia, urea, uric acid and creatinine during hyperammonemic and nephrotoxic conditions and offer protection against hyperammonemia (11,13). Our results corroborate these previous findings.
Many studies have shown that oxidative stress and free radical production-mediated lipid peroxidation could be involved in the mechanism of ammonia toxicity (4–6,24,25). Elevated levels of ammonia in blood and brain result in derangement of cerebral function (4–6). A marked elevation in the concentration of TBARS and HP are observed in the brain of hyperammonemic rats. Excess ammonia induces nitric oxide synthase, which leads to enhanced production of nitric oxide and other toxic free radicals as well as thiobarbituric acid-positive compounds in brain and leads to oxidative stress and tissue damage (5,6,25,26). Administration of HSEt significantly decreased brain levels of TBARS and HP in Group 4 rats. HSEt could reduce levels of circulatory lipid peroxidation products during hyperammonemia (24), which may corroborate our present findings. This may be due to the free radical scavenging property of HSEt and has been previously reported (27,28). In addition, a marked nitric oxide scavenging activity was observed for the alcoholic extract of HS flowers supporting the plant's potent antioxidant property (15,27–29).
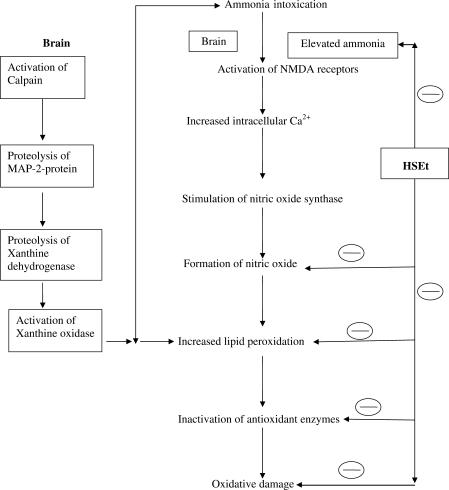
The level of lipid peroxidation in cells is controlled by various cellular defense mechanisms consisting of enzymatic and non-enzymatic scavenger systems (30), the levels of which are altered in hyperammonemia (6,26). This might have decreased levels of antioxidants in brain such as SOD, CAT, GPx and GSH in ammonium chloride-treated group rats. Increased superoxide production and reduced activities of antioxidant enzymes have been reported in brains of rats subjected to acute ammonia toxicity (5). In our investigations, the levels of both enzymatic and non-enzymatic antioxidants, which declined in the brain of hyperammonemic animals, were significantly restored to near normal after treatment with the HSEt extract. This restoring potential of HSEt might be due to active principles of HS. Preliminary phytochemical screening of HS showed the presence of flavanoids and phenolic compounds (like anthocyanins, glycosides, PCA and hydroxycitric acid) (31), which have been reported to have antioxidant activity. It is believed that phenolic antioxidants can scavenge harmful free radicals and thus inhibit their oxidative reactions with vital biological molecules (32) and prevent development of many pathophysiological conditions, which can manifest into disease. Previous reports have shown that the calyces extract of HS decreased lipid peroxidation and cell damage (27–29). Previous studies show that the plant extracts and their active constituents have the ability to improve the antioxidant status and reduce the levels of urea, creatinine during various disease conditions (33,34). Therefore, it is possible that the mechanism by which the HSEt modulates brain lipid peroxidation and antioxidant status during hyperammonemic condition could be attributed to the presence of natural antioxidants, ammonia lowering effect and its free radical scavenging properties (Fig. 1). But the exact mechanism is still under investigation and isolation of contributing active constituents is required.
Figure 1.
Proposed mechanism of action on HSEt against hyperammonemia (mechanism of ammonia intoxication proposed by Kosenko et al. (1997) (5) (inhibition).
References
- 1.Norenberg MD, Rama Rao KV, Jayakumar AR. Ammonia neurotoxicity and the mitochondrial permeability transition. J Bioenerg Biomembr. 2004;36:303–7. doi: 10.1023/B:JOBB.0000041758.20071.19. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi IA, Rama Rao KV. Decreased brain cytochrome C oxidase activity in congenitally hyperammonemic spf mice: effects of acetyl-l-carnitine. In: Mardini RL, editor. Advances in Hepatic Encephalopathy and Metabolism in Liver disease. UK: Ipswich Book Company; 1997. pp. 385–93. [Google Scholar]
- 3.Rama Rao KV, Jayakumar AR, Norenberg MD. Differential response of glutamine in cultured neurons and astrocytes. J Neurosci Res. 2005;79:193–9. doi: 10.1002/jnr.20295. [DOI] [PubMed] [Google Scholar]
- 4.Murthy CR, Rama Rao KV, Bai G, Norenburg MD. Ammonia induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:282–8. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- 5.Kosenko E, Kaminsky A, Valencia M, Lee L, Hermenegildo C, Felipo V. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res. 1997;27:637–44. doi: 10.3109/10715769709097867. [DOI] [PubMed] [Google Scholar]
- 6.Lena PJ, Subramanian P. Effects of melatonin on the levels of antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Pharmazie. 2004;59:636–9. [PubMed] [Google Scholar]
- 7.Chifundera K, Balagizi K, Kizungu B. Les empoisonnements et leurs antidotes en me decine traditionnelle au Bushi, Zaire. Fitoterapia. 1994;65:307–13. [Google Scholar]
- 8.Seca AML, Silva AMS, Silvestre AJD, Cavaleiro JAS, Domingues FMJ, Neto CP. Phenolic constituents from the core of kenaf (Hibiscus cannabinus) Phytochemistry. 2001;56:759–67. doi: 10.1016/s0031-9422(00)00473-8. [DOI] [PubMed] [Google Scholar]
- 9.Satue-Gracia MT, Heinonen M, Frankel EN. Anthocyanins as antioxidants on human low-density lipoprotein and lecithin-liposome systems. J Agric Food Chem. 1997;45:3362–7. [Google Scholar]
- 10.Wang H, Cao G, Prior RL. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997;45:302–9. [Google Scholar]
- 11.Shirwaikar A, Malini S, Kumari SC. Protective effect of Pongamia pinnata flowers against cisplatin and gentamicin induced nephrotoxicity in rats. Indian J Exp Biol. 2003;41:58–62. [PubMed] [Google Scholar]
- 12.Hu X, Sato J, Oshida Y, Yu M, Bajotto G, Sato Y. Effect of Goshajinki-gam on insulin resistance in STZ induced diabetic rats. Diabetes Res Clin Pract. 2003;59:101–3. doi: 10.1016/s0168-8227(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 13.Essa MM, Subramanian P, Suthakar G, Manivasagam T, Dakshayani KB. Protective influence of Pongamia pinnata (Karanja) on blood ammonia and urea levels in ammonium chloride-induced hyperammonemia. J Appl Biomed. 2005;3:133–8. [Google Scholar]
- 14.Trease CE, Evan VC. Pharmacopoeial and Related Drugs of Biological Origin. Part V Pharmacognosy. London: Saunders; 1959. pp. 161–466. [Google Scholar]
- 15.Odigie IP, Ettarh RR, Adigun S. Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. J Ethnopharmacol. 2003;86:181–5. doi: 10.1016/s0378-8741(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 16.Wolheim DF. Preanalytical increase of ammonia in blood specimens from healthy subjects. Clin Chem. 1984;30:906–8. [PubMed] [Google Scholar]
- 17.Varley H, Gowenlock AH, Bell M. Practical Clinical Biochemistry. 4th edn. Vol. 1. CBS Publishers; 1998. pp. 161–210. [Google Scholar]
- 18.Niehaus WG, Samuelson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 19.Jiang ZY, Hunt JV, Wolff SP. Detection of lipid hydroperoxides using the Fox reagent. Anal Biochem. 1992;202:384–9. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 20.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;22:130–2. [PubMed] [Google Scholar]
- 22.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical roles as components of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 23.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Essa MM, Subramanian P, Suthakar G, Manivasagam T, Dakshayani KB, Sivaperumal R, et al. Influence of Hibiscus sabdariffa (gongura) on the levels of circulatory lipid peroxidation products and liver marker enzymes in experimental hyperammonemia. J Appl Biomed. 2006 (in press) [Google Scholar]
- 25.Kosenko E, Kaminsky Y, Lopata O, Muravyov N, Kaminsky A, Hermenegildo C, et al. Nitroarginine, an inhibitor of nitric oxide synthase, prevents changes in superoxide radical and antioxidant enzymes induced by ammonia intoxication. Metab Brain Dis. 1998;13:29–41. doi: 10.1023/a:1020626928259. [DOI] [PubMed] [Google Scholar]
- 26.Kosenko E, Kaminsky Y, Stavroskaya IG, Felipo V. Alteration of mitochondrial calcium homeostasis by ammonia-reduced activation of NMDA receptors in rat brain in vivo. Brain Res. 2000;880:139–46. doi: 10.1016/s0006-8993(00)02785-2. [DOI] [PubMed] [Google Scholar]
- 27.Amin A, Hamza AA. Hepatoprotective effects of Hibiscus, Rosmarinus and Salvia on azathioprine-induced toxicity in rats. Life Sci. 2006 doi: 10.1016/j.lfs.2004.09.048. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Wang J, Chu C, Cheng M, Tseng T. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem Toxicol. 2002;40:635–41. doi: 10.1016/s0278-6915(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 29.Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411–6. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutterridge JMC. Lipid peroxidation, oxygen radicals, cell damage and antioxidant therapy. Lancet. 1994;1:1396–7. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 31.Osman AM, El-Garby Younes M, Mokhtar A. Sitosterol—galactoside from Hibiscus sabdariffa. Phytochemistry. 1975;14:829–30. [Google Scholar]
- 32.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 33.Punitha ISR, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padmavathi B, Rath PC, Rao AR, Singh RP. Roots of Withania somnifera inhibit forestomach and skin carcinogenesis in mice. Evid Based Complement Alternat Med. 2005;2:99–105. doi: 10.1093/ecam/neh064. [DOI] [PMC free article] [PubMed] [Google Scholar]