Abstract
Moutan Cortex (MCE) has been used in traditional medicine to remove heat from the blood, promote blood circulation and alleviate blood stasis. This study was conducted to evaluate the effects of MCE on regulatory mechanisms of cytokines and nitric oxide (NO) involved in immunological activity of Raw264.7 cells. Cells were pretreated with methanolic extracts of MCE, and further cultured for an appropriate time after lipopolyssacharide (LPS) addition. During the entire experimental period, 0.1 and 0.3 mg ml−1 of MCE had no cytotoxicity. In these concentrations, MCE inhibited the production of NO and prostaglandin E2 (PGE2), the expression of inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2) and phosphorylated inhibitor of κBα (p-IκBα), and the activation of nuclear factor κB (NF-κB). MCE also reduced the concentration of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in the Raw264.7 cells that were activated by LPS. These results demonstrate that MCE has anti-inflammatory effects through the inhibition of iNOS and COX-2 expression by suppressing the phosphorylation of I-κBα and the activation of NF-κB.
Keywords: LPS, Moutan Cortex, NF-κB, nitric oxide
Introduction
Moutan Cortex (MCE), the root cortex of Paeonia suffruticosa Andrews, is a traditional Korean herb with various biological activities. It has been used in oriental medicine to remove heat from the blood, promote blood circulation and remove blood stasis (1). Moutan Cortex has been shown to have antimicrobial actions against Escherichia coli, typhoid, paratyphoid bacilli and Cholera vibrio, and antihypertensive efficacy including analgesic, sedative and anticonvulsant effects. Moutan Cortex exerts a protective effect against acetaminophen-induced hepatotoxicity by attenuating GSH depletion, cytochrome P450 2E1 activity and hepatic DNA damage (2,3), and scavenging effects on the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical, superoxide anion radical (4), hydroxy radicals (5) and intracellular ROS in oxidative condition (6).
Lipopolyssacharide (LPS)-activated macrophages have usually been used for evaluating the anti-inflammatory effects of various materials. LPS is a principle component of the outer membrane of Gram-negative bacteria, is an endotoxin that induces septic shock syndrome and stimulates the production of inflammatory mediators such as nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukins, prostanoids and leukotrienes (7–9). Therefore, LPS plays a key role in not only eliciting an inflammatory response but also causing septic shock during a Gram-negative bacterial infection. Inflammatory responses are advantageous for eradicating bacteria, as long as they are under control. However, when out of control, deregulated inflammation leads to the massive production of proinflammatory cytokines such as TNF-α, interleukin-1 (IL-1) and interleukin-6 (IL-6) by macrophages (10,11), which can cause tissue injury and multiple organ failure (12).
Recently, there have been many studies concerning natural products with anti-inflammatory activity, for example, Polygonum tinctorium (13), Melia azedarach (14), Cyperus rotundus (15,16), Cudrania tricuspidata (17), Ginsenoside Rg3 (18), sauchinone (19,20), curcuma rhizomes (21) and Farfarae Flos (22). Although Moutan Cortex has been used as an anti-inflammatory agent in oriental medicine, the mechanisms of anti-iflammatory activity of Moutan Cortex have not been studied scientifically. Paeonol is a major component of Moutan Cortex that may be largely responsible for the therapeutic effect of Moutan Cortex. Paeonol regulated histamine, IgE and TNF-α (23). However, it is not enough to explain the anti-inflammatory activity of paeonol and Moutan Cortex. There is no evidence to explain the mechanism on the inflammatory cytokine regulation of Moutan Cortex. Including paeonol, there are many known chemical components of Moutan Cortex such as paenoside, paeonolide, paeoniflorin, oxypaeonolniflorin, benzoylpaeoniflorin, benzoyl-oxy-paeoniflorin and apiopaeonoside. Therefore, this study evaluated the effect of methanol extract of Moutan Cortex (MCE) on the regulatory mechanism of NO, cytokines and prostaglandin E2 (PGE2) in the LPS-activated Raw264.7 cells.
Methods
Preparation of Methanol Extract of Moutan Cortex
Moutan Cortex (300 g, Wolsung, Daegu, Korea) was extracted with 1000 ml of methanol at room temperature for 24 h. The extract was filtered through a 0.2 μm filter (Nalgene, New York, NY, USA), lyophilized and stored at −20°C until needed. The amount of the extract was estimated by dividing the dried weight of Moutan Cortex into the lyophilized MCE. The yield of MCE from Moutan Cortex was 13.2%.
Cell Culture
Raw264.7 cell, which is a murine macrophage cell line (KCLRF, Korean Cell Line Research Foundation, Seoul, Korea), was cultured in Dulbecco's modified Eagle's medium (DMEM, Cambrex Bio Science, MD, USA) containing 10% fetal bovine serum (FBS), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. For all experiments, the cells were grown to 80–90% confluence, and were subjected to no more than 20 cell passages. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. The Raw264.7 cells were plated at a density of 2–3 × 106 per ml and preincubated at 37°C for 24 h. After serum starvation for 12 h, the cells were exposed to either LPS (1 μg ml−1) or LPS + MCE for the indicated time periods (6–24 h). MCE was dissolved in a medium (EMEM, Cambrex Bio Science, MD, USA) and added to the incubation medium 1 h prior to adding the LPS.
Reagents
LPS (E. coli 026:B6) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoleum (MTT) were obtained from Sigma (St Louis, MO, USA). The FBS and antibiotics were purchased from Gibco/BRL (Eggenstein, Germany). The antibodies were obtained from BD Bioscience (USA), Cayman (USA) and Zymed (USA), and the NC paper used was Schleicher & Schuell (USA). The TNF-α, IL-6 and interleukin-1β (IL-1β) ELISA kits were purchased from Pierce endogen (Rockford, IL, USA).
Assay of NO Production
The level of NO production was monitored by measuring the nitrite concentration in the cultured medium. Briefly, the samples were mixed with Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine dihydrochloride and 2.5% phosphoric acid) and incubated for 10 min at room temperature. The absorbance was measured at 540 nm using a Titertek Multiskan Automatic ELISA microplate reader (Model MCC/340, Huntsville, AL).
Cell Viability (MTT assay)
The Raw264.7 cells were plated at a density of 5 × 104 cells per well in a 96-well plate to determine the cytotoxic concentrations of MCE. The cells were exposed to MCE at concentrations of 0.1 and 0.3 mg ml−1 at 37°C under 5% CO2. After incubating the cells in the presence of MCE, the viable cells were stained with MTT (0.5 mg ml−1) for 4 h. The media were then removed and the formazan crystals produced in the wells were dissolved in 200 μl of dimethylsulfoxide (DMSO). The absorbance was measured at 540 nm using a Titertek Multiskan Automatic ELISA microplate reader (Model MCC/340, Huntsville, AL). The cell viability was defined as the % of untreated control cells [i.e. viability (% control) = 100 × {(absorbance of MCE-treated sample)/(absorbance of control)}].
Immunoblot Analysis
The cells were lysed in the buffer containing 20 mM Tris (pH 7.5), 1% Triton X-100, 137 mM sodium chloride, 10% glycerol, 2 mM EDTA, 1 mM sodium orthovanadate, 25 mM beta-glycerophosphate, 2 mM sodium pyrophosphate, 1 mM phenylmethylsulfonylfluoride and 1 mg ml−1 leupeptin. The total cell lysate was prepared by centrifuging the cells at 10 000× g for 10 min and collecting the supernatant. The expression of iNOS, cyclooxygenase-2 (COX-2) and phosphorylated inhibitor of κBα (p-IκBα) was immunochemically monitored with the total lysate fraction using anti-mouse iNOS, COX-2 and p-IκBα antibodies, respectively. The bands for the iNOS, COX-2 and p-IκBα proteins were visualized using ECL western blotting detection reagents (Amersham Biosciences, NJ, USA) according to the manufacturer's instructions.
Gel Retardation Assay
A double-stranded DNA probe for the consensus sequence of nuclear factor κB (NF-κB) (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was used for the gel shift analyses after end labeling the probes with [γ-32P]ATP and T4 polynucleotide kinase. The reaction mixtures contained 2 μl of 5× binding buffer (20% glycerol, 5 mM MgCl2, 250 mM NaCl, 2.5 mM EDTA, 2.5 mM dithiothreitol, 0.25 mg ml−1 poly dI-dC and 50 mM Tris–Cl, pH 7.5), 2 μg of the nuclear extracts and sterile water in a total volume of 10 μl. The reactions were initiated by adding 1 μl of the probe (106 c.p.m.) and preincubating the resulting mixture for 10 min. Incubation was continued for 20 min at room temperature. The samples were loaded onto 4% polyacrylamide gels at 140 V. The gels were removed, fixed and dried, followed by autoradiography.
Measurement of Cytokines Production
For the cytokine immunoassays, the cells (1 × 106 per ml) were preincubated with MCE for 1 h and further cultured for 6 or 12 h with 1 μg ml−1 of LPS in 6-well plates. The supernatants were removed at the allotted times and the level of TNF-α, IL-6 and IL-1β production was quantified using an ELISA kit (Pierce endogen, Rockford, IL, USA) according to the manufacturer's instructions. Briefly, 50 μl of biotinylated antibody reagent and the samples were added to the anti-mouse TNF-α, IL-6 and IL-1β precoated 96-well strip plates. The plates were covered and kept at room temperature for 2 h and washed three times in a prepared washing buffer. This was followed by the addition of 100 μl of Streptavidin–HRP concentrate. After 30 min incubation at room temperature, the wells were washed three times, and 100 μl of TMB substrate solution was then added and developed in the dark at room temperature for 30 min. The reaction was quenched by adding 100 μl of TMB stop solution, and the absorbance of the plates was at 450 nm to 550 nm using an automated microplate ELISA reader. A standard curve was run on each assay plate using recombinant TNF-α, IL-6 and IL-1β in serial dilutions. Each kit was specific to TNF-α, IL-6 or IL-1β and does not measure other cytokines.
Measurement of PGE2 Production
MCE was added to the culture medium 1 h before the addition of 1 μg ml−1 LPS. LPS-treated cells were further cultured with vehicle or MCE for 24 h. The cultured medium was collected and assayed with ELISA kit (RnD Systems, Minneapolis, MN, USA). Cultured medium was incubated in goat anti-mouse IgG coated plate with acetylcholinesterase linked to PGE2 and PGE2 monoclonal antibody for 18 h at 4°C. The plate was emptied and rinsed five times with wash buffer contained in the kit. And then, 200 μl of substrate reagent was added to each well and incubated for 1 h at 37°C. The developed plate was read at 405 nm and the PGE2 concentration of each sample was determined according to the standard curve.
Statistical Analysis
The data were expressed as a mean ± SD of the results obtained from a number (n) of experiments. One-way analysis of variance (ANOVA) was used to assess the significant differences between the treatment groups. For each significant effect of treatment, the Newman–Keuls test was used to compare the multiple group means. A P-value <0.05 was considered significant.
Results
MCE Inhibits LPS-Activated NO, Cytokines and PGE2 Release
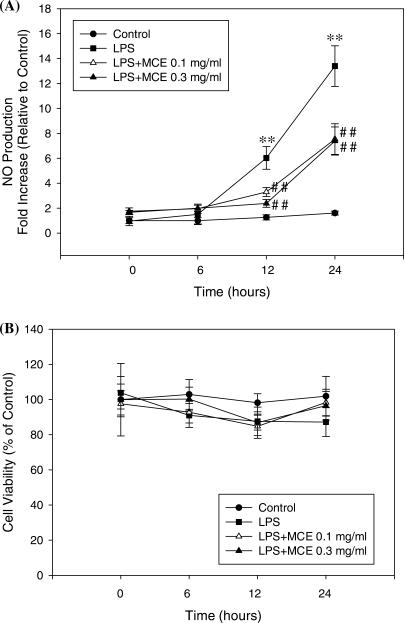
The inhibition of NO production by MCE was investigated by measuring the level of NO production in Raw264.7 cells treated with 0.1 and 0.3 mg ml−1 of MCE. As shown in Fig. 1, in the LPS plus MCE groups, the level of NO production decreased in a concentration and time-dependent manner compared with the LPS only group. In the 0.1 and 0.3 mg ml−1 of MCE group, the level of NO was significantly inhibited at 12 h and 24 h (Fig. 1A).
Figure 1.
NO release and the cytotoxicity by MCE in LPS-activated Raw264.7 cells. The NO concentration (A) in the culture medium and cytotoxicity (B) were measured over a 6–24 h period. The data represent the mean ± SD of eight separate experiments. One-way ANOVA was used to compare the multiple group means followed by Newman–Keuls test (*: significant compared with the control, **P < 0.01, #: significant compared with the LPS alone, ##P < 0.01).
Cell viability was measured at different MCE concentrations for different times using an MTT assay in order to determine if the decrease in NO production was caused by a decrease in the cell population as a result of MCE-induced cytotoxicity. The results showed that MCE concentration dose revealed no cytotoxicity over a 6 to 24 h period (Fig. 1B).
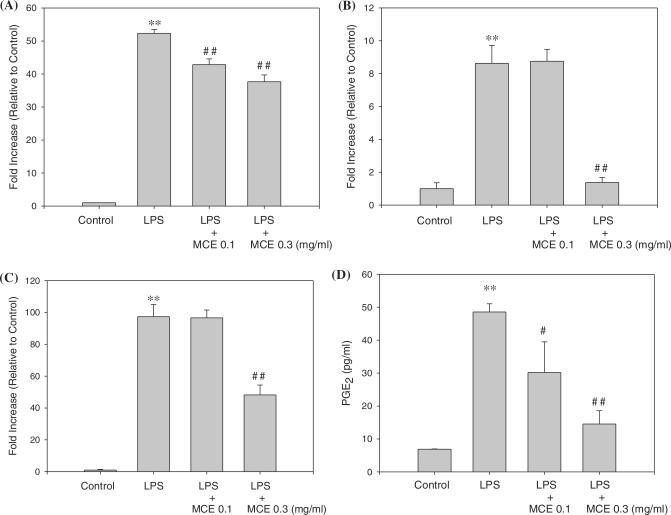
We treated LPS to Raw264.7 cells, collected media every 3 h for 24 h, detected cytokines from the collected media, and then determined appropriate time for each cytokine assay. TNF- α, IL-1β and IL-6 were highly induced by LPS at 12, 12 and 6 h, respectively (data are not shown). TNF-α, IL-1β and IL-6 from the culture media were analyzed at the appropriate time. As shown in Fig. 2, 0.3 mg ml−1 of MCE significantly reduced the level of TNF-α (Fig. 2A), IL-1β (Fig. 2B) and IL-6 (Fig. 2C) at 12, 12 and 6 h incubation. LPS-activated PGE2 also decreased by MCE as compared to control (Fig. 2D). PGE2 per 1 ml medium was 6.89 pg in control and increased to 48.6 pg in LPS group. However, PGE2 was reduced to 30.2 and 14.5 pg ml−1 in 0.1 and 0.3 mg ml−1 of MCE, respectively.
Figure 2.
Inhibition of LPS-activated cytokines and PGE2 production by MCE. Raw264.7 cells were cultured with LPS (1 μg ml−1) in the presence or absence of MCE for 12 h to determine the level of TNF-α (A) and IL-1β (B). For IL-6 (C), the cells were incubated for 6 h under same conditions. For PGE2 (D), the cells were incubated for 24 h under same conditions. The cultured medium was collected and directly assayed for cytokines and PGE2. Data represent the mean ± SD of eight separate experiments. One-way ANOVA was used to compare the multiple group means followed by a Newman–Keuls test (*: significant as compared to control, **P < 0.01, #: significant as compared to LPS alone, #P < 0.05, ##P < 0.01).
Inhibitory Activities of MCE on LPS-Activated iNOS and COX-2 Expression
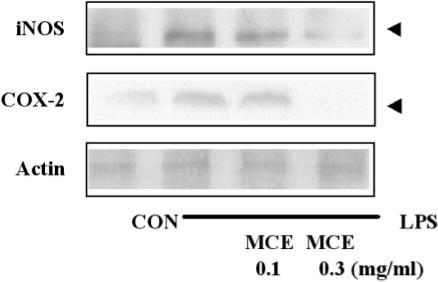
The expression level of the iNOS protein in the cytosol fraction was next examined using immunoblotting analysis. The iNOS protein was strongly induced by LPS. The groups of 0.1 and 0.3 mg ml−1 of MCE showed a decrease in iNOS protein expression in a concentration-dependent manner. As shown in Fig. 3, 0.3 mg ml−1 of MCE with LPS strongly suppressed the induction of iNOS.
Figure 3.
Reduction of LPS-activated iNOS and COX-2 expression by MCE. The level of iNOS and COX-2 was monitored 12 h after treating cells with LPS (1 μg ml−1) with or without MCE pretreatment (i.e. 1 h before the LPS treatment). The amount of protein loaded in each lane was confirmed by actin protein expression.
COX-2 plays a key role in the development of inflammation (24,25). MCE was further investigated to determine if it affects the COX-2 expression levels. As shown in this experiment, the COX-2 protein was strongly induced by LPS. 0.1 mg ml−1 of MCE slightly suppressed the induction of COX-2, but 0.3 mg ml−1 of MCE with LPS strongly suppressed the induction of COX-2 (Fig. 3).
Inhibitory Activities of MCE on IκBα Phosphorylation and NF-κB Activation
In order to determine if MCE can directly affect p-IκBα expression in macrophage cells, the level of p-IκBα protein expression was assessed immunochemically in Raw264.7 cells incubated with or without MCE. LPS increased the p-IκBα level. However, 0.1 mg ml−1 of MCE reduced the LPS-inducible p-IκBα expression level, and 0.3 mg ml−1 of MCE markedly reduced the protein levels of p-IκBα expression in a dose-dependent manner (Fig. 4A).
Figure 4.
Inhibition of LPS-activated p-IκBα protein expression and NF-κB activation by MCE. The level of the p-IκBα protein (A) was monitored for 15 min after treating the cells with LPS (1 μg ml−1) with or without the MCE pretreatment (i.e. 1 h before LPS treatment). NF-κB activation (B) from the nuclear extracts was analyzed by gel shift assay. Each lane contained 5 μg of the nuclear extracts. The results were confirmed by repeated analyses.
NF-κB is activated in cells challenged with LPS, and is involved in the transcriptional activation of the responsive genes. Gel shift analysis was carried out to determine if MCE alters the NF-κB DNA binding activity. LPS (1 μg ml−1, 1 h) increased the binding activity of the nuclear extracts to the NF-κB DNA consensus sequence. Although the treatment of macrophages with 0.1 mg ml−1 of MCE for 1 h prior to the addition of LPS slightly inhibited the LPS-inducible increase in the band intensity of NF-κB binding, a pretreatment with 0.3 mg ml−1 of MCE significantly (<50%) suppressed the band intensity that was increased by LPS (Fig. 4B).
Discussion
Inflammation is the first response of the immune system to infection or irritation. It is caused by cytokines such as TNF-α, IL-1 and IL-6 (10), and by eicosanoid such as PGE2 (26). Thus, inhibitors of these cytokines have been considered as a candidate of an anti-inflammatory drug. Moutan Cortex has been used to diminish inflammation in oriental medicine. However, few studies have been conducted to evaluate the effects of Moutan Cortex on inflammation. Herbal medicine, keishibukuryogan (Gui-Zhi-Fu-Ling-Wan) containing Moutan Cortex, was reported to decrease disease activity evaluated by levels of inflammatory cytokines (27). In this study, we show that MCE could modulate the regulatory mechanism of NO, cytokines and PGE2 in the LPS-activated Raw264.7 cells. MCE inhibited the level of NO, PGE2, TNF-α, IL-1β and IL-6, and the expression of iNOS and COX-2 activated by LPS. These inhibitory effects were mediated through the inhibition of IκBα phosphorylation and NF-κB activation.
NO is a free radical produced from l-arginine by nitric oxide synthases (NOSs), and an important cellular second messenger (28). The modulation of iNOS-mediated NO release is one of the major contributing factors during the inflammatory process (18). At adequate concentrations, NO can generate or modify intracellular signals, thereby affecting the function of immune cells, as well as tumor cells and resident cells of different tissues and organs. However, its uncontrolled release can cause inflammatory destruction of target tissue during an infection (29–31). Moutan Cortex has cytoprotective effects against NO-mediated neuronal cell death in cultured cerebellar granule cells (CGCs) (32). In our study, MCE reduced the level of iNOS expression in a concentration-dependent manner.
PGE2 is generated by the sequential metabolism of arachidonic acid by cyclooxygenase and it is associated with inflammation (26). Cyclooxygenase exists in two isoforms; COX-1 and COX-2. COX-1 is a constitutively expressed enzyme with general housekeeping function. COX-2 is an inducible isoform of cyclooxygenase (33,34), and its most important role is in inflammation. Herb medicine containing Moutan Cortex acts as a potent inhibitor of COX-1 and COX-2 (35). Moutan Cortex also showed analgesic effect on the PGF2α-induced allodynia in mice (36). We found that MCE could suppress the induction of COX-2 by LPS, and consequently reduce the production of PGE2 in a dose-dependent manner.
NF-κB has been implicated in the expression of iNOS and COX-2 protein. Activation of the NF-κB signaling cascade results in the complete degradation of I-κB via phosphorylation and ubiquitination. Paeonol showed regulatory effects on inflammatory cytokines-related anaphylactic reaction (37), and cerebral disorder (38). Recently, paeonol was reported to reduce TNF-α-stimulated iNOS protein and mRNA expression via inhibiting NF-κB activation (39). In this study, MCE inhibited the phosphorylation of I-κBα and LPS-inducible increase in the band intensity of NF-κB binding. Thus, it seems that anti-inflammatory effect of MCE is partly responsible for paeonol. To evaluate the anti-inflammatory activity of paeonol in LPS-induced macrophage, studies on the effects of paeonol on the mechanism of LPS-induced NO, cytokines and PGE2 are needed.
In conclusion, we determined that the MCE can have anti-inflammatory activity by suppressing the phosphorylation of I-κBα and the activation of NF-κB, and by inhibiting the expression of iNOS and COX-2 in LPS-activated Raw264.7 cells.
Acknowledgments
This study was supported by a grant from the Oriental Medicine R&D project, Ministry of Health & Welfare, Republic of Korea (B050035-AM0815-05N1-00020B).
References
- 1.Huang KC. New York: CRC Press; 1999. The pharmacology of Chinese herbs; pp. 403–4. [Google Scholar]
- 2.Shon YH, Nam KS. Protective effect of moutan cortex extract on acetaminophen-induced cytotoxicity in human Chang liver cells. Biol Pharm Bull. 2002;25:1427–31. doi: 10.1248/bpb.25.1427. [DOI] [PubMed] [Google Scholar]
- 3.Shon YH, Nam KS. Protective effect of Moutan Cortex extract on acetaminophen-induced hepatotoxicity in mice. J Ethnopharmacol. 2004;90:415–9. doi: 10.1016/j.jep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa M, Ohta T, Kawaguchi A, Matsuda H. Bioactive constituents of Chinese natural medicines. V. Radical scavenging effect of Moutan Cortex. (1): Absolute stereostructures of two monoterpenes, paeonisuffrone and paeonisuffral. Chem Pharm Bull (Tokyo) 2000;48:1327–31. doi: 10.1248/cpb.48.1327. [DOI] [PubMed] [Google Scholar]
- 5.Okubo T, Nagai F, Seto T, Satoh K, Ushiyama K, Kano I. The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of Moutan Cortex and Paeoniae Radix. Biol Pharm Bull. 2000;23:199–203. doi: 10.1248/bpb.23.199. [DOI] [PubMed] [Google Scholar]
- 6.Rho S, Chung H-S, Kang M, Lee E, Cho C, Kim H, et al. Inhibition of production of reactive oxygen species and gene expression profile by treatment of ethanol extract of Moutan cortex radicis in oxidative stressed PC12 cells. Biol Pharm Bull. 2005;28:661–6. doi: 10.1248/bpb.28.661. [DOI] [PubMed] [Google Scholar]
- 7.Hewett JA, Roth RA. Hepatic and extrahepatic pathobiology of bacterial lipopolysaccharides. Pharmacol Rev. 1993;45:382–411. [PubMed] [Google Scholar]
- 8.Watson WH, Zhao Y, Chawla RK. S-adenosylmethionine attenuates the lipopolysaccharide-induced expression of the gene for tumour necrosis factor alpha. Biochem J. 1999;342:21–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–8. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- 10.Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides. 2003;37:355–61. doi: 10.1016/j.npep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: A review. Evid Based Complement Alternat Med. 2005;2:301–8. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 2004;12:186–92. doi: 10.1016/j.tim.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Kunikata T, Tatefuji T, Aga H, Iwaki K, Ikeda M, Kurimoto M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur J Pharmacol. 2000;410:93–100. doi: 10.1016/s0014-2999(00)00879-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee BG, Kim SH, Zee OP, Lee KR, Lee HY, Han JW, et al. Suppression of inducible nitric oxide synthase expression in RAW264.7 macrophages by two-carboline alkaloids extracted from Melia azedarach. Eur J Pharmacol. 2000;406:301–9. doi: 10.1016/s0014-2999(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 15.Seo WG, Pae HO, Oh GS, Chai KY, Kwon TO, Yun YG, et al. Inhibitory effects of methanol extract of Cyperus rotundus rhizomes on nitric oxide and superoxide production by murine macrophage cell line, RAW264.7 cells. J Ethnopharmacol. 2001;76:59–64. doi: 10.1016/s0378-8741(01)00221-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee YS, Han OK, Shin SW, Park JH, Kwon YK. Effects of water-extracted Cyperus Rotundus on the nitric oxide production and cytokine gene expression. Korean J Oriental Physiol Pathol. 2003;17:771–6. [Google Scholar]
- 17.Seo WG, Pae HO, Oh GS, Chai KY, Yun YG, Kwon TO, et al. Inhibitory effect of ethyl acetate fraction from Cudrania tricuspidata on the expression of nitric oxide synthase gene in RAW264.7 macrophages stimulated with interferon-and lipopolysaccharide. Gen Pharmacol. 2000;35:21–8. doi: 10.1016/s0306-3623(01)00086-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim ND, Kim EM, Kang KW, Cho MK, Choi SY, Kim SG. Ginsenoside Rg3 inhibits phenylephrine-induced vascular contraction through induction of nitric oxide synthase. Br J Pharmacol. 2003;140:661–70. doi: 10.1038/sj.bjp.0705490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AK, Sung SH, Kim YC, Kim SG. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by sauchinone effects on I-κBα phosphorylation, C/EBP and AP-1 activation. Br J Pharmacol. 2003;139:11–20. doi: 10.1038/sj.bjp.0705231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang BY, Lee JH, Jung HS, Kim KS, Nam JB, Hong YS, et al. Sauchinone, a lignan from Saururus chinensis, suppresses iNOS expression through the inhibition of transactivation activity of RelA of NF-kappaB. Planta Med. 2003;69:1096–101. doi: 10.1055/s-2003-45189. [DOI] [PubMed] [Google Scholar]
- 21.Tohda C, Nakayama N, Hatanaka F, Komatsu K. Comparison of anti-inflammatory activities of six Curcuma rhizomes: A possible curcuminoid-independent pathway mediated by curcuma phaeocaulis extract. Evid Based Complement Alternat Med. 2006;2:255–60. doi: 10.1093/ecam/nel008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon TG, Byun BH, Kwon TK, Suh SI, Byun SH, Kwon YK, et al. Inhibitory effect of Farfarae Flos water extract on COX-2, iNOS expression and Nitric Oxide production in lipopolysaccharide-activated RAW264.7 cells. Korean J Oriental Physiol Pathol. 2004;18:908–13. [Google Scholar]
- 23.Kim SH, Kim S-A, Park M-K, Kim S-H, Park Y-D, Na H-J, et al. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-α. Int Immunopharmacol. 2004;4:279–87. doi: 10.1016/j.intimp.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001;480–481:243–68. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 25.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40:1091–7. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 26.Tannenbaum H, Davis P, Russell AS, Atkinson MH, Maksymowych W, Huang SH, et al. An evidence-based approach to prescribing NSAIDs in musculoskeletal disease: a Canadian consensus. Can Med Assoc J. 1996;155:77–88. [PMC free article] [PubMed] [Google Scholar]
- 27.Nozaki K, Hikiami H, Goto H, Nakagawa T, Shibahara N, Shimada Y. Keishibukuryogan (Gui-Zhi-Fu-Ling-Wan), a kampo formula, decreases disease activity and soluble vascular adhesion molecule-1 in patients with rheumatoid arthritis. Evid Based Complement Alternat Med. 2006;3:359–64. doi: 10.1093/ecam/nel025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 29.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 30.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 31.Miljkovic D, Trajkovic V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004;15(1):21–32. doi: 10.1016/j.cytogfr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Shimada Y, Yokoyama K, Goto H, Sekiya N, Mantani N, Tahara E, et al. Protective effect of keishi-bukuryo-gan and its constituent medicinal plants against nitric oxide donor-induced neuronal death in cultured cerebellar granule cells. Phytomedicine. 2004;11:404–10. doi: 10.1016/j.phymed.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Lim BV, Lee CY, Kang JO, Kim CJ, Cho SH. Bee venom suppresses ischemia-induced increment of apoptosis and cell proliferation in hippocampal dentate gyrus. Korean J Oriental Physiol Pathol. 2004;18:236–42. [Google Scholar]
- 35.Park WH, Lee SK, Oh HK, Bae JY, Kim CH. Tumor initiation inhibition through inhibition COX-1 activity of a traditional Korean herbal prescription, Geiji-Bokryung-Hwan, in human hepatocarcinoma cells. Immunopharmacol Immunotoxicol. 2005;27:473–83. doi: 10.1080/08923970500241311. [DOI] [PubMed] [Google Scholar]
- 36.Tatsumi S, Nabuchi T, Abe T, Xu L, Minami T, Ito S. Analgesic effect of extracts of Chinese medicinal herbs moutan cortex and coicis semen on neuropathic pain in mice. Neurosci Lett. 2004;370:130–4. doi: 10.1016/j.neulet.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Kim SA, Park MK, Kim SH, Park YD, Na HJ, et al. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-α. Int Immunopharmacol. 2004;4:279–87. doi: 10.1016/j.intimp.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh CL, Cheng CY, Tsai TH, Lin IH, Lin CH, Chiang SY, et al. Paeonol reduced cerebral infarction involving the superoxide anion and microglia activation in ischemia-reperfusion injured rats. J Ethnopharmacol. 2006;106:208–15. doi: 10.1016/j.jep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Ishiguro K, Ando T, Maeda O, Hasegawa M, Kadomatsu K, Ohmiya N, et al. Paeonol attenuates TNBS-induced colitis by inhibiting NF-κB and STAT1 transactivation. Toxicol Appl Pharmacol. 2006;217:35–42. doi: 10.1016/j.taap.2006.07.002. [DOI] [PubMed] [Google Scholar]