Abstract
Recent studies indicate that portions of ischemic and tumor neovasculature are derived by “neovasculogenesis”, whereby bone marrow (BM)-derived circulating endothelial progenitor cells (EPCs) home to sites of regenerative or malignant growth and contribute to blood vessel formation. Recent data from animal models suggest that a variety of cell types, including unfractionated BM mononuclear cells and those obtained by ex vivo expansion of human peripheral blood or enriched progenitors, can function as EPCs to promote tissue vasculogenesis, regeneration and repair when introduced in vivo. The promising preclinical results have led to several human clinical trials using BM as a potential source of EPCs in cardiac repair as well as ongoing basic research on using EPCs in tissue engineering or as cell therapy to target tumor growth.
Keywords: EPC, angiogenesis, vasculogenesis, cell therapy, tumor, ischemia
Introduction
The seminal observations by the late Jeffrey Isner and colleagues that established the existence of endothelial progenitor cells (EPCs) derived from the bone marrow (BM) and their participation in newly forming blood vessels after wounding altered our way of thinking about therapeutic angiogenesis[1]. Their study showed that BM cells were able to function as EPCs by homing from the peripheral circulation into ischemic foci and incorporating into microvasculature[1].
The finding that progenitor cells home to sites of neovascularization and differentiate into endothelial cells in situ is consistent with vasculogenesis, a mechanism previously believed to be restricted to development of blood vessels in the embryo [2]. The discovery of EPCs has changed that paradigm and introduced the notion of cellular therapy as a novel approach to therapeutic angiogenesis. The ability of EPCs to promote revascularization of injured and ischemic tissues or as cellular therapy to target cancer growth is being pursued in both preclinical models and, more recently, human trials to treat ischemia [3–8].
Additionally, the levels of circulating EPCs have been shown to be modulated during ischemic injury, disease states, aging and with certain pharmacologic agents [9–13]. Several studies have examined the numbers of circulating CD34+/VEGF-Receptor 2(R2)+ (also called flk-1) cells, as a marker for the endogenous EPCs, or enumerated the number of early outgrowth EPC colonies from human peripheral blood and have shown correlation of either assay with cardiovascular risk[11,14,15]. In this review, we will focus on the biological properties of EPCs, whereas the study and progress of using EPCs as a biomarker for disease progression or drug response will not be further discussed in this review.
Phenotype of the EPC
Initial studies identified EPCs by the expression pattern of cell-surface markers, which included VEGFR2, AC133, CXCR4, CD34, and c-Kit and the absence of markers of more committed hematopoietic lineages (lin-) [1,16,17]. Human-derived EPCs have been isolated from the CD34+/lin- or CD34+/VEGFR2+ (AC133 co-expression can also be used in lieu of, or in addition to, VEGFR2[17]) population; after administration to mice they have been shown to differentiate in vivo into endothelial cells associated with vessels that expressed the endothelial markers PECAM (CD31) or von Willebrand factor (vWF) [5]. Intravenous administration of mature endothelial cells (i.e. HUVECs) failed to result in engraftment into foci of neovascularization [1]. In this manner, mature endothelial cells were shown to be functionally distinct from BM-derived EPCs.
As with the hematopoietic or mesenchymal stem cells, several groups have attempted ex vivo expansion of EPCs [18–22]. At least two types of EPCs can be obtained by ex vivo culture of peripheral blood, an early and late outgrowth population (Figure 1). In most strategies, EPCs were generated by in vitro expansion of unmobilized human peripheral blood mononuclear cells (PB-MNCs) on fibronectin-coated tissue culture plates[18,21–26]. Human PB-MNCs or BM-MNCs cultured in endothelial growth media generated within 4–7 days spindle-shaped cells, which expressed endothelial markers such as VEGFR2 and VE-cadherin[18,23,26]. These early outgrowth, culture-expanded EPCs have limited proliferative capacity, can be maintained in culture for a short time (30–40 days) and co-express myeloid (e.g., CD14), pan-leukocytic (CD45) and endothelial markers (e.g., VE-cadherin and VEGFR2) [26,27]. However, continued culture of this population (>30 days) results in the loss of hemaotopoietic marker co-expression and uniform expression of endothelial lineage markers[26]. It has been proposed that this population resulted from the transdifferentiation of myeloid progenitors to endothelial-like cells; myeloid origination of early outgrowth EPCs has been demonstrated by multiple, independent laboratories [26–34]. Although there is controversy regarding whether both early and late myeloid precursors can acquire an endothelial phenotype, recent data suggest that this occurs with higher frequency with less mature or specific subsets of myeloid precursors [25,26,35]. Functionally, this population of early outgrowth EPCs homed and incorporated into both ischemic and tumor neovasculature after intravenous administration [26,28,36,37]. Moreover, this population was able to stimulate significant endogenous angiogenesis[5,18,29,37–39]. The combination of EPC contribution to neovasculature and their indirect stimulation of host blood vessel formation have resulted in significant therapeutic benefit in rodent hindlimb ischemia models[18,29,39–41]. The majority of the studies examining direct EPC contribution to neovasculature have analyzed early time points (<2 weeks) [18,39,41]. Although aspects such as persistence, remodeling and expansion of engrafted EPCs have not been directly addressed, the data in tumor models suggest that these early outgrowth, peripheral blood-derived EPCs engraft transiently in vivo [26,38].
Figure 1.
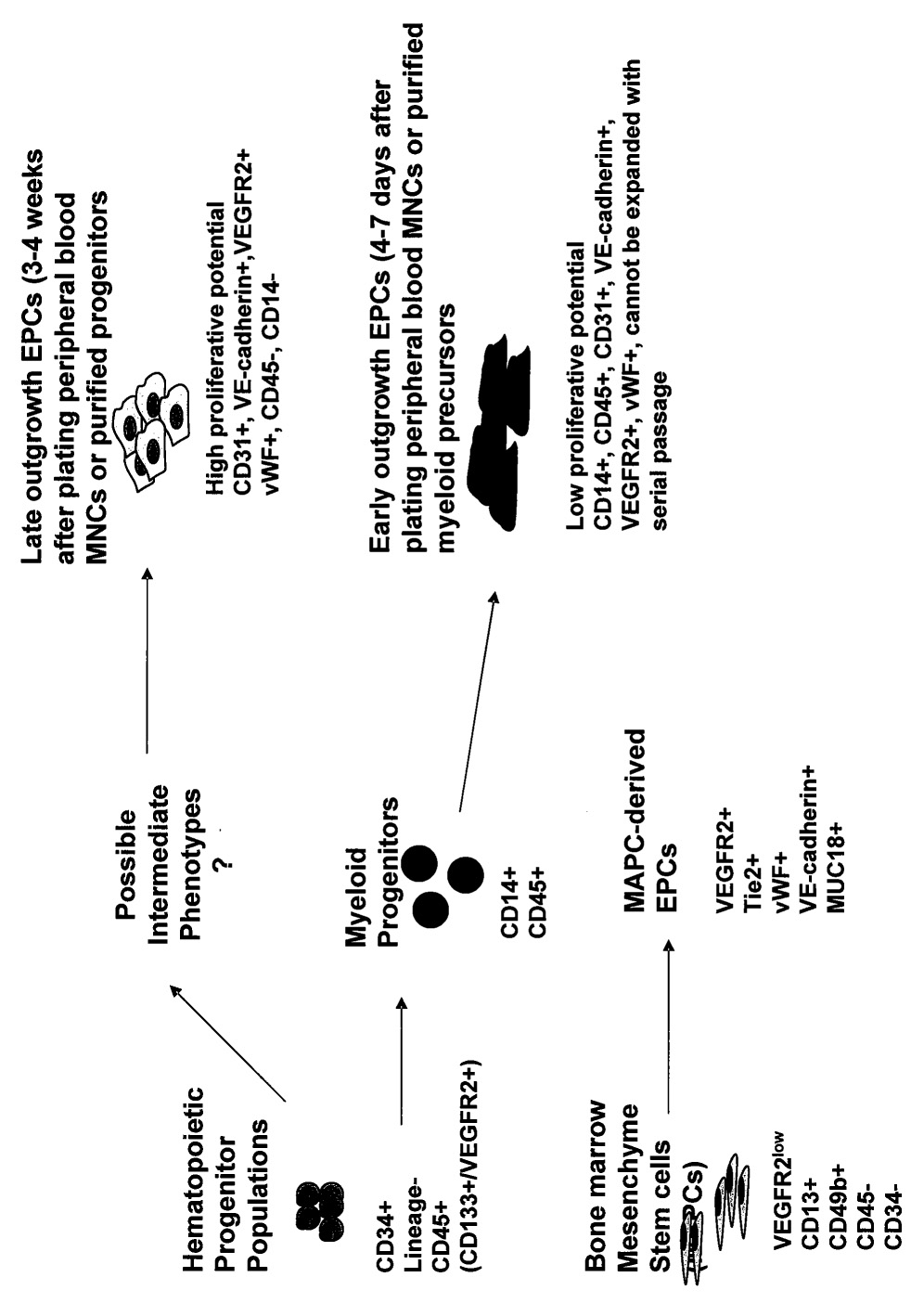
Model of characterized culture-expanded EPC populations. Early outgrowth EPCs co-express myeloid/pan-leukocyte and endothelial markers and cannot be expanded with serial passage in contrast to late outgrowth EPCs. Despite the heterogeneity in the origin and differentiation of these cell types, the in vivo vasculogenic and therapeutic efficacy appears similar.
The late-outgrowth EPCs do not emerge until 2–3 weeks of culture of peripheral blood or BM and rapidly grow out thereafter to greater than 100 population doublings until eventually undergoing senesensce[27,38,42,43]. Interestingly, the culture of highly purified BM hematopoietic progenitor populations (e.g. CD34+/lin- or AC133+/VEGFR2+) generated attached cells expressing endothelial markers with similar kinetics, supporting the notion that the late outgrowth EPCs were derived from the CD 14-(non-myeloid) population and resulted from aggressive expansion of the early progenitor (CD34+/AC133+/VEGFR2+/lin-) populations[26,27,38,42,43]. Similar to direct administration of purified progenitor populations or early outgrowth EPCs, administration of the late outgrowth human EPCs to immunodeficient, ischemic mice resulted in their incorporation into new vessels, and promoted overall angiogenesis[18,19,44–49]. Although there have been no direct comparisons reported of therapeutic outcome among purified progenitors with the various culture-expanded populations, a recent study compared the therapeutic efficacy of early and late outgrowth EPCs. The data suggested that while intramuscular injection of either population of culture expanded EPCs was able to promote neovascularization, the administration of a mixture of the two resulted in enhanced engraftment of both populations and improved neovascularization of hindlimb ischemia in immunodeficient mice[50]. Since only small populations of CD34 (∼2%) or AC 133 (∼0.5%) progenitors are present in BM, and even lower numbers (<20–100 fold) in peripheral circulation [5], ex vivo expanded EPCs have been widely used in many experimental studies and may be a feasible source of EPCs for clinical cell therapy [7,23,51]. Some studies suggested that other BM stroma-derived cells, such as multipotent adult progenitor cells (MAPCs) (Figure 1) or mesenchymal stem cells (MSCs) can also be differentiated into endothelial-like cells in culture that function as EPCs in mouse models of ischemia or tumor growth[45,48,52]. Despite greater elucidation of the phenotype of the distinct EPC populations derived after ex vivo culture expansion, our understanding of their relative efficacy in tissue vasculogenesis/repair as well as the nature of the endogenous EPC population(s) remains incomplete.
The Role of EPCs in Ischemic Injury
A growing body of preclinical data suggest that introduction of exogenous vascular progenitors, either unselected BM-MNCs, enriched AC133+ progenitors or ex vivo expanded early outgrowth EPCs, may promote revascularization and improve organ function[53]. Vascular trauma (e.g., limb ischemia, myocardial infarct) releases signals into the circulation such as VEGF and SDF-1 that promote the mobilization of EPCs into the circulation[53]. Moreover, local concentrations of VEGF and SDF-1 are elevated in ischemic foci and contribute to EPC recruitment from the circulation to the site of injury[54,55]. Other cytokines such as GCSF and GMCSF also promote EPC mobilization and downstream endothelial regeneration [53,56]. Hence, these agents may be developed to augment both the circulating levels as well as tissue recruitment of endogenous BM-derived vascular progenitors to promote organ repair.
Intravenous administration of both early and late outgrowth culture-expanded EPCs after ischemic injury resulted in their selective recruitment to ischemic sites [18,50,57]. However, the efficiency of recruitment is reduced if cells are introduced into the systemic venous circulation due to sequestration in the lung and other tissues[26,50,58,59]. Data suggested that a more targeted delivery, e.g., into the coronary arteries for cardiac therapy, improved cell engraftment [26,50,58,59]. This approach may also help avert EPC recruitment to unwanted sites of neovascularization.
In preclinical studies, direct EPC contribution to vessels ranged from 1–25%, depending on the model system or disease[60]. However, the rate of total vascular density (angiogenesis) was also increased. These observations suggest that incorporated progenitor cells may also promote neovasculariation and tissue regeneration by releasing factors which act in a paracrine manner to support local angiogenesis, mobilize tissue residing progenitor cells, and/or improve host cell survival, thereby creating a permissive environment that enables rapid revascularization, proliferation, and survival of damaged cells[59].
The encouraging results using EPCs in animal models to stimulate angiogenesis and improve recovery of ischemic tissue led to a number of clinical studies. The human trials have utilized two general designs: intracoronary delivery to patients with recent myocardial infarctions or catheter-based intramyocardial injections into patients with chronic ischemic disease[53]. Due to the relative scarcity of the hematopoietic stem cell (CD34+/AC133+/VEGFR2+), the majority of the recently completed studies have used unfractionated BM-MNCs[8] in small scale, uncontrolled and non-randomized clinical trials. Most of the trials found a moderate improvement in heart function, documented by an increase in left ventricular ejection fraction, a decrease in end-systolic volume, shrinkage of the infarct size, thickening of the LV wall and improvement in exercise capacity without serious adverse events, such as inflammation (as measured by leukocyte count or serum C-reactive protein levels), emboli, arrhythmia, or death[52,53,61,62].
The encouraging results from the first, small-scale clinical studies led to larger, randomized, placebo-controlled, or double blind trials. Patient numbers ranged from 40 to 200 per study with average age between 55 to 60 years old. Three trials used intracoronary infusion of 0.7–2×108 autologous, bone marrow-derived MNCs 4–8 days after acute MI [63–65], whereas one trial enrolled patients with stable ischemic heart disease who had MI at least 3 months before cell therapy[66]. The remaining trials aimed on mobilizing endogenous stem cells by subcutaneous injection of G-CSF over a time period of 5–6 days after percutaneous coronary intervention [67–69]. Most trials showed no substantial benefits, except a modest increase in ejection fraction³,7 or infarct size and reperfusion¹ indicating that straightforward administration of unfractionated BM-MNCs, or mobilization of autologous BM stem cells might not be effective in the clinic [52,53,61,62,64,66,67,70]. It is also possible that the patient group, timing, cell dose or enhanced engraftment might be critical parameters in cell therapy.
EPCs Target Angiogenic Sites Within Growing Tumors
Thus far, the study of tumor neovascularization has focused primarily on the angiogenesis mechanism [71]; however, a growing body of studies has demonstrated that vasculogenesis may be an alternate, important mechanism of the tumor angiogenic switch[26,51,55,58,72–76]. The study that first identified a potential role of EPCs in tumor neovascularization utilized an adult immunodeficient mouse model engrafted with BM from mice carrying a transgene expressing β-galactosidase (lac Z) from endothelial-specific promoters [77]. In this model, donor-derived cells stained positive with X-gal at sites of tumor xenotransplants. Rafii and colleagues further studied the role of BM-derived EPCs in tumor growth utilizing Id-deficient (Id1+/−Id3−/−) mice that were unable to support growth of implanted tumors due to defects in angiogenesis[78]. However, susceptibility of Id mice to tumor challenge was restored after BM transplantation from wild-type mice [78]. Examination of these tumors revealed that >90% of the endothelium was BM-derived, suggesting that, at least in this model, vasculogenesis played an overwhelming role in tumor neovascularization. However, estimation of the physiologic role of vasculogenesis in tumor neovascularization in this mouse was confounded by the genetic impairment of angiogenesis in Id deficient mice [78].
Other studies in wild-type hosts confirmed that EPCs generated from mouse embryos or embryonic stem cells, culture-expanded EPCs or human BM-derived mesenchymal cells, preferentially home and contribute to tumor vasculature[26,55,72,79,80].
Efforts to quantify tumor reliance on vasculogenesis suggested wide variation in tumor vasculogenesis in mouse models, ranging from 0 to >90% [73,78,81]. Most murine orthotopic tumor models, however, suggested a low level (less than 5%) of vasculogenic-derived vessels[3,51,78,81,82]. The wide variation in the degree of tumor vasculogenesis may be the result of different model systems, e.g., different reporter constructs and types of tumor, or in the method of tissue sampling (e.g. vasculogenic vessels are not uniformly distributed but occur in ‘hotspots’) [81,83,84]. The role of vasculogenesis in human tumors has recently been assessed in one study of 6 patients who developed tumors after BM transplantation from donors of the opposite sex by colocalizing sex-chromosome specific probes with markers of endothelial cells [74]. The mean contribution of BM-derived endothelial cells to human tumors was 4.8% and ranged from 1–12%[74]. Future studies to examine location and numbers of BM-derived cells during tumor progression are necessary to further our understanding of the importance of tumor vasculogenesis. However, current evidence suggests that although angiogenesis is overwhelmingly the most widely studied process, it is not the only mechanism of blood vessel formation within a tumor.
Various types of exogenous EPCs have been shown to preferentially target tumor tissues[58,79]. These observations, as well as reports which suggest that EPCs demonstrated a predilection for ischemic vasculature, led to the hypothesis that EPCs may be utilized as a delivery vehicle for tumor gene and/or cell therapy[18,85,86]. Wei and colleagues demonstrated that mouse embryonic EPCs stably expressing a suicide gene were able to reduce tumor volume and the number of lung metastases[58]. Furthermore, their degree of tumor inhibition supported a role for EPC-directed bystander cytotoxicity[58]. It will be important to establish if human genetically modified, ex vivo-expanded EPCs can have similar anti-tumor effects.
The phenotype of the endogenous, tumor-infiltrating EPCs is unclear. Several reports have observed that myeloid subsets can co-express endothelial markers, such as VE-cadherin, P1H12, or Tie2, and constitute significant leukocyte populations within tumors[26,75,82,87,88]. These bi-phenotypic “vascular leukocytes” were shown to contribute to vasculogenesis in some model systems [26,75,82,88], whereas in other instances they did not directly contribute to tumor vessels but promoted angiogenesis in a paracrine manner[87,89]. Interestingly, a recent study suggested that BM-derived lymphatic EPCs contributed to new lymphatic vessels and may have differentiated from the inflammatory myeloid cells that circumscribe areas of new lymphatic growth[90]. This hypothesis was supported by demonstrating that CD14+ myeloid cells upregulated a number of specific markers of lymphatic endothelium such as VEGF-C, podoplanin, and LYVE-1 during in vitro culture to generate lymphatic endothelium[90]. These studies further strengthened the observations that myeloid populations can be induced to express markers of blood vessel endothelium in vitro and in vivo and that myeloid to endothelial transdifferentiation may contribute to tumor progression. These bi-phenotypic myeloid/endothelial (i.e. vascular leukocytes) populations may be isolated from circulation or derived ex vivo for use as tumor cell therapy[26,87,88]. Alternatively, this proangiogenic vascular leukocyte population may itself be a target to selectively retard tumor neovasculature [87].
Other Potential Applications
Another direction of cell therapy may be the application of EPCs in the construction of endothelial-coated vascular grafts in the context of tissue engineering. The poor patency rate of small diameter prosthetic bypass grafts has been largely attributed to thrombosis caused by delayed endothelialization of their lumen[91–93]. Autologous, vessel-derived endothelial cells have been used to seed these grafts, but lack of sufficient number of cells has limited its clinical utility [94].
It had been shown that BM-derived cells served as a significant source of endothelialization of Dacron vascular grafts in dogs [95]. Furthermore, seeding grafts with CD34+, BM-derived progenitor cells dramatically increased endothelialization[96]. Supporting this observation was the report that GCSF treatment, which mobilized leukocytes (including CD34+ cells) from the BM into the peripheral circulation, enhanced spontaneous endothelialization of vascular grafts[97]. More recently, sheep peripheral blood late outgrowth EPCs were used to seed decellularized porcine iliac vessels [4]. Seeded grafts remained patent for 130 days, >8-times longer than non-seeded grafts[4]. EPC-seeded grafts demonstrated contractile properties and nitric-oxide-mediated relaxation similar to native vessels [4]. Further studies are needed to compare the utility of different leukocyte subsets or culture expanded EPC populations in promoting vessel endothelialization in vitro and in vivo.
Finally, the observation that EPCs can be incorporated into growing vasculature introduces the theoretical possibility of correcting congenital diseases. Using BM transplant models, it was shown that vasculogenesis occurred during normal organ growth only in the early neonatal period[6]. The absence of vasculogenesis during adult organ growth was also confirmed by others[98]. However, it has not yet been reported if ex vivo expanded cell populations can engraft into growing neovasculature. Further work is necessary to define the window of organ vasculogenesis after birth, the efficacy of various EPC populations, and persistence of engrafted cells.
Vasculogenesis may be an essential cascade for tissue and organ regeneration following pathological damage in various diseases. EPCs can participate directly in neoangiogenesis by differentiating in situ into endothelial cells, but growing data suggest that they also affect tissue repair through paracrine mechanisms that are not well defined. The ease of obtaining human peripheral blood EPCs after ex vivo expansion as well as unfractionated BM cells as a source of EPCs make it possible to aggressively pursue efforts to develop them as cellular therapy to potentially augment vascularization in ischemic areas (i.e. after myocardial infarction), in bioengineering, or alternatively, to carry an anti-tumor gene to sites of tumor neo vascularization.
Acknowledgements
This work was supported by American Heart Association Grant (PPY), Veterans Affairs (PPY) and NIH grant HL083958 (AKH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, Van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 3.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Siefert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–735. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 4.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JEJ. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nature Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raffi S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nature Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 6.Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs) into vasculature of newborn murine recipients. Proc Natl Acad Sci, USA. 2002;99:11951–11956. doi: 10.1073/pnas.182215799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahlmann FH, Groot K, Spandau J, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 10.Haeshen C, Aicher A, Lehmann R, Fichtlsherer S, Vasa M, Urbich C, Rildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 11.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endlthelial progenitor cells vascular function, and cardiovascular risk. N Engl J Med. 2003;248:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 12.Isner JM. Tissue responses to ischemia: local and remote responses for preserving perfusion of ischemic muscle. J Clin Invest. 2000;106:615–620. doi: 10.1172/JCI10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amango H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Raffi S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemagiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circ. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 16.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi, Norihisa, Nishikawa SI. Maturation of embyronic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;4:1253–1263. [PubMed] [Google Scholar]
- 17.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MAS, Rafii S. Expression of VEGFR-2 and AC 133 by circulating human CD34+ cells identifies a population of funcitonal endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 18.Kalka C, Masuda H, Tomono T, Kalka-moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularizaition. Proc Natl Acad Sci, USA. 1999;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schatteman G, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y, Chang L, Solovey A, Healey PL, Hebbel RP. Use of blood outgrowth endothelial cells for gene therapy for hemophilia A. Blood. 2002;99:457–462. doi: 10.1182/blood.v99.2.457. [DOI] [PubMed] [Google Scholar]
- 21.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetes exhibit impaired proliferation, adhesion and incorporation into vascular structures. Circ. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 22.Boyer M, Townsend LE, Vogel M, Falk J, Reitz-Vick D, Trevor KT, Villalba M, Bendick PJ, Glover JL. Isolation of endothelial cells and their progenitor cells from human peripheral blood. J Vasc Surg. 2000;31:181–189. doi: 10.1016/s0741-5214(00)70080-2. [DOI] [PubMed] [Google Scholar]
- 23.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 24.Asakage M, Tsuno NH, Kitayama J, Kawai K, Okaji Y, Yazawa K, Kaisaki S, Osada T, Watnabe T, Takahashi K, Nagawa H. Early-outgrowth of endothelial progenitor cells can function as antigen-presenting cells. Cancer Immunol Immunother. 2006;55:708–716. doi: 10.1007/s00262-005-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Manzzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S. CD14+CD34low cells with stem cell phenotype and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–322. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe EE, Teleron AA, Li B, Price J, Sands MS, Alford K, Young PP. The origin and in vivo significance of murine and human culture expanded endothelial progenitor cells (CE-EPCs) Am J Pathol. 2006;168:1710–1720. doi: 10.2353/ajpath.2006.050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 28.Harraz MC, Jiao C, Hanlon HD, Harley RS, Schatteman GC. CD34-blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–312. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 29.Rehman J, Jingling L, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circ. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 30.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 31.Loomans CJ, Wan H, de Crom R, van Haperen R, de Boer HC, Leenen PJ, Drexhage HA, Rabelink TJ, van Zonneveld AJ, Staal FJ. Angiogenic murine endothelial progenitor cells are derived from a myeloid bone marrow fraction and can be identified by endothelial NO Synthase expression. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000229243.49320.c9. [DOI] [PubMed] [Google Scholar]
- 32.Kim SY, Park SY, Kim JM, Kim MY, Yang JH, Kim JO, Choi KH, Kim SB, Ryu HM. Differentiation of endothelial cells from human umbilical cord AC133-CD14+ cells. Ann Hematol. 2005;84:417–422. doi: 10.1007/s00277-004-0988-y. [DOI] [PubMed] [Google Scholar]
- 33.Rhode E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 34.Pujol BF, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte M, Adamkiewicz J, Elsasser H, Muller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 35.Elsheikh E, Uzunel M, He Z, Holgersson J, Nowak G, Sumitran-Holgersson S. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–2355. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Sharpe EE, Teleron AA, Pyle AL, Carmeliet P, Young PP. VEGF and P1GF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at site of tumor neovascularization. FASEB J. 2006 doi: 10.1096/fj.05-5137fje. in press. [DOI] [PubMed] [Google Scholar]
- 37.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation and tubilization of circulating bone marrow-derived cells. Blood. 2005;105:1068–1077. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 38.Ingram DA, Mead LE, Tanaka H, Meade V, Fonoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 39.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper OM, Gravereaux E, Pieczek A, Iw H, Hayashi S, Isner JM, Asahara T. Vascular endothelial growth factor165 gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 40.Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circ. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circ. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 42.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Hypertension. 2004;24:1–6. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant MB, May WS, Cabellero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;2002:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 45.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawamoto A, Gwon HCI H, Yamguchi JL, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circ. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 47.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urbich C, Dimmeler S. Endothelial progenitor cells characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 49.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 50.Lee N, Thorne T, Losordo DW, Yoon YS. Repair of ischemic heart disease with novel bone marrow-derived multipotent stem cells. Cell Cycle. 2005;4:861–864. doi: 10.4161/cc.4.7.1799. [DOI] [PubMed] [Google Scholar]
- 51.Moore XL, Lu J, Sun L, Zhu CJ, Tan I, Wong MC. Endothelial progenitor cells' "homing" specificity to brain tumors. Gene Therapy. 2004 doi: 10.1038/sj.gt.3302151. doi:10.1038/sj.gt.3302151:1–8. [DOI] [PubMed] [Google Scholar]
- 52.Zimmet JM, Hare JM. Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy. Basic Res Cardiol. 2005;100:471–481. doi: 10.1007/s00395-005-0553-4. [DOI] [PubMed] [Google Scholar]
- 53.Bengel FM, Schachinger V, Dimmeler S. Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging. 2005;32:S404–S416. doi: 10.1007/s00259-005-1898-5. [DOI] [PubMed] [Google Scholar]
- 54.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1946. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006 doi: 10.1007/s00441-005-0144-6. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Takamiya M, Okigaki MJD, Takai S, Nozawa Y, Adachi Y, Urao N, Tateishi K, Nomura T, Zen K, Ashihara E, Miyazaki M, Tatsumi T, Takahashi T, Matsubara H. Granulocyte colony-stimulating factor-mobilized circulating c-Kit+/Flk-1+ progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Aterioscler Thromb Vasc Biol. 2006;26:751–757. doi: 10.1161/01.ATV.0000205607.98538.9a. [DOI] [PubMed] [Google Scholar]
- 57.Ricousse-Roussanne S, Barateau V, Contreres J, Boval B, Kraus-Berthier L, Tobelem G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc Res. 2004;62:176–184. doi: 10.1016/j.cardiores.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Wei J, Blum S, Unger M, jarmy G, Lamparter M, Geishauser A, Vlastos GA, Chan G, Fischer K, Rattat D, Debatin KM, Hatzopoulos AK, Beltinger C. Embryonic endothelial progneitor cells armed with a suicide gene target hypoxic lung metastasis after intravenous delivery. Cancer Cell. 2004;5:477–488. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 59.Kupatt C, Hinkel R, Lamparter M, von Bruhl ML, Pohl T, Horstkotte J, Beck H, Muller S, Delker S, Gildehaus FJ, Buning H, Hatzopoulos AK, Boekstegers P. Retrofusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT kinase. Circ. 2005;112:I117–I122. doi: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 60.Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth facotr-induced neovascularization in vivo. Exp Hematol. 2002;30:967–972. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 61.Amado LC, Saliaris AP, Schuleri KH, St. John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchmal stem cells after myocardial infarction. Proc Natl Acad Sci, USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laflamme MA, Murry CE. Regenerating the heart. Nature Biotech. 2005;7:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 63.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantizi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 64.Lunde K, Solheim S, Aakhus S, Amesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschu A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 65.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM Investigators R-A. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 66.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 67.Englemann I, Madisch I, Pommer H, Heim A. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 68.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, Johnsen HE, Kober L, Grande P, Kastrup J. Stem cell mobilization induced by subcutaneous granuloctye-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circ. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 69.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, Meisetschlager G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schomig A Investigators R- Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 70.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circ. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 71.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 72.Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Englehardt B, Vestweber D, Hatzopoulos AK. Multistep nature of microvascular recruitment of ex vivo expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197:1755–1765. doi: 10.1084/jem.20021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Annabi B, Naud E, Lee Y, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–1158. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- 74.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, Kinzler K, Lengauer C. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nature Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 75.Conejo-Garcia JR, Benencia F, Courreges M, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a b-defensin contribute to vasculogenesis under the influence of VEGF-A. Nature Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 76.Duda DG, Cohen KS, Koszin SV, Perentes JY, Fukumura D, Scadden DT, Jain RK. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood. 2006;107:2774–2776. doi: 10.1182/blood-2005-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 78.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin DJ, Zhu Z, Hackett NR, Crystal RG, Moore MAS, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 79.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchumal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 80.Yurugi-Kobayashi T, Itoh H, Yamashita J, Yamahara K, Hirai H, Kobayashi T, Ogawa M, Nishikawa S, Nishikawa S, Nakao K. Effective contribution of transplanted vascular progenitor cells derived from embryonic stem cells to adult neovascularizaiton in proper differentiation stage. Blood. 2003;101:2675–2678. doi: 10.1182/blood-2002-06-1877. [DOI] [PubMed] [Google Scholar]
- 81.Larrivee B, Niessen K, Pollet I, Corbel SY, Long M, Rossi FM, Olive PL, Karsan A. Minimal contribution of marrow-derived endothelial precursors to tumor vasculature. J Immunol. 2005;175:2890–2899. doi: 10.4049/jimmunol.175.5.2890. [DOI] [PubMed] [Google Scholar]
- 82.Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges M, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679–681. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 83.Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 84.Weissleder R. Bone marrow-derived lin(-)c-kit(+)sca-1+ stem cells do not contribute to vasculogenesis in Lewis lung carcinoma. Neoplasia. 2005;7:234–240. doi: 10.1593/neo.04523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via "imported" nitric oxide synthase activity. Circ. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 86.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Basitidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIP-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 87.de Palma M, Vanneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 88.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 89.De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Kerjaschki D, Hurttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 91.Chard RB, Johnson DC, Nunn GR, Cartmill TB. Aorta-coronary bypass grafting with polytetrafluoroethylene conduits. Early and late outcome in eight patients. J Thorac Cardiovasc Surg. 1987;94:132–134. [PubMed] [Google Scholar]
- 92.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 93.LHeureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. Faseb J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 94.Deutsch M, Meinhart J, Fischlein T, Preiss P, Zilla P. Clinical autologous in vitro endothelialization of infrainguinal ePTFE grafts in 100 patients: a 9-year experience. Surgery. 1999;126:847–855. [PubMed] [Google Scholar]
- 95.Inoue M, Ono I, Tateshita T, Kuoryanagi Y, Shioya N. Effect of a collagen matrix containing epidermal growth factor on wound repair. Wound Repair Regen. 1998;6:213–222. doi: 10.1046/j.1524-475x.1998.60307.x. [DOI] [PubMed] [Google Scholar]
- 96.Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, Storb RF, Sauvage LR, Hammond WP, Wu MH. Enhanced endothelializatin and microvessel formation in polyester grafts seeded with CD34+ bone marrow cells. Blood. 2000;95:581–585. [PubMed] [Google Scholar]
- 97.Shi Q, Bhattacharya V, Hong-De WM, Sauvage LR. Utilizing granulocyte colony-stimulating factor to enhance vascular graft endothelialization from circulating blood cells. Annals of Vascular Surgery. 2002;16:314–320. doi: 10.1007/s10016-001-0238-x. [DOI] [PubMed] [Google Scholar]
- 98.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2003;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]


