Abstract
The assembly of class I MHC molecules and their export from the endoplasmic reticulum is governed by chaperones and accessory proteins. We present evidence that the putative cargo receptor protein Bap31 participates in the transport and the quality control of human class I molecules. Transfection of the human adenocarcinoma cell line HeLa with YFP-Bap31 chimeras increased surface levels of class I in a dose-dependent manner, by as much as 3.7-fold. The increase in surface class I resulted from an increase in the rate of export of newly-synthesized class I molecules to the cell surface and from an increase in the stability of the exported molecules. We propose that Bap31 performs quality control on class I molecules in two distinct phases: first, by exporting peptide-loaded class I molecules to the ERGIC and second, by retrieving class I molecules which have lost peptides in the acidic post-ER environment. This function of Bap31 is conditional or redundant, since we find that Bap31 deficiency does not reduce surface class I levels. Overexpression of the Bap31 homolog, Bap29, decreases surface class levels in HeLa, indicating that it does not substitute for Bap31.
Keywords: MHC, trafficking, presentation
Introduction
Recognition of Class I MHC molecules by cytotolytic T lymphocytes (CTL), or by natural killer (NK) cells is key to the immune response to viral infection and malignant transformation. Class I molecules consist of three components: the β2-microglobulin protein (light chain), a type I transmembrane protein (heavy chain) with a highly polymorphic cleft, and a small, polymorphic peptide antigen (typically 8 or 9 residues) which binds in the cleft. The components are assembled in the lumen of the endoplasmic reticulum (ER) by several general-purpose chaperones, as well as by accessory molecules that are dedicated to class I assembly (reviewed in 1, 2). After class I heavy chains have folded and have bound β2-microglobulin, they may acquire peptides derived from cytosolic proteins at the peptide loading complex. Alternately, they may bind peptides generated by diverse processes in the lumen of the ER (reviewed in 3).Once assembled, class I molecules are exported from the ER to the cell surface via the Golgi complex.
It was initially surmised that class I molecules traffic by a non-selective, bulk-flow process (4). However, recent studies show that Class I molecules persist in the ER, even after they are fully assembled (5, 6). This indicates that the export of class I molecules from the ER is a regulated, presumably carrier-mediated, process.
Recently, our laboratory showed that mouse class I molecules can bind the putative cargo receptor, Bap31 (7). Bap31 is a 28-kilodalton, type III transmembrane protein consisting of an integral hydrophobic leader peptide, two additional hydrophobic transmembrane domains, and a cytoplasmic tail which contains two caspase cleavage sites (8–10). A slightly smaller homolog of mouse and human Bap31, Bap29, lacks the caspase cleavage sites; its function is unknown. Bap31 homologs are widely distributed in the biome; they are found in other mammals, birds, insects, worms, yeast and higher plants. In many of these, for example yeast, Bap31 homologs are represented by multiple genes (11).
Bap31 is abundant in the ER. Bap31 has also been seen in post-ER compartments in some studies (12, 13), though not in others (14). The effects of dominant-negative variants of Bap31 on the trafficking of cellubrevin (12) and mannosidase II (9) to postER compartments, the ER Golgi Intermediate compartment, ERGIC and the Golgi complex, are consistent with a role for Bap31 as an anterograde cargo receptor. In the absence of both Bap31 and its homolog, Bap29 (8), mouse class I MHC molecules fail to colocalize with the COP II marker for ER secretory vesicles, and are significantly delayed in their progress to the medial Golgi (15). However, cells lacking the two Bap proteins do maintain surface levels of class I comparable to those of controls.
The carboxy terminus of Bap31 contains a di-lysine (KKXX) motif, which has been implicated in retrieval of proteins from post-ER compartments via COP I vesicles (16), or alternately in direct ER retention (17). There is evidence that Bap31 can retain proteins in the ER, or retrieve them from post-ER compartments. The transmembrane form of the immunoglobulin D receptor (IgD) is not exported to the cell surface in the absence of the Ig-α and Ig-β molecules (18). Molecules which contain the transmembrane sequence from the mouse IgD heavy chain are exported to the cell surface when expressed in insect cells without Ig-α and Ig-β, but are retained when co-expressed with mouse Bap29 and Bap31 (19). Overexpression of wild-type Bap31 reduces the surface levels of the cystic fibrosis transmembrane conductance regulator (CFTR), a protein which is reported to mis-fold frequently; a malfunctioning CFTR mutant is retained more strongly still (20). Conversely, depletion of Bap31 increases surface levels of CFTR. Depletion of Bap31 also permits the cytochrome P450 2C2 protein to escape the ER and traffic to the nuclear membrane and the cell surface (60).
Here we show that Bap31 is associated with human class I MHC molecules, and that overexpression of Bap31 increases the amount of class I on the cell surface, in a dose-dependent manner. The rate at which newly-synthesized class I molecules reach the medial Golgi and the cell surface increases in the presence of excess Bap31. We also show that there is a higher fraction of long-lived class I molecules in cells which overexpress Bap31 than in control cells. The combination of a moderately increased export rate and increased stability of exported class I account for the increase in the steady-state levels of class I at the cell surface. However, neither siRNA-mediated reduction of Bap31 levels, nor the loss of a functional BAP31 gene, affected class I levels or stability. Thus, Bap31 must be a conditional or redundant participant in the class I maturation pathway, rather than being an essential component. We have also found that the overexpression of Bap29 reduces surface class I levels, indicating that it does not substitute for Bap31.
Materials and Methods
Cell lines
HeLa cells were obtained from the American Type Culture Collection (Manassas, VA). The normal human fibroblast cell lines 0498B and 5659C were obtained from the Coriell Institute Cell Repository (Camden, NJ). CADDS4 and CADDS5 are two human fibroblast lines from patients with the contiguous X-linked adrenoleukodystrophy / DXS1357E deletion syndrome (CADDS) genotype (S.J. Steinberg, unpublished results), similar to the cell lines described in (21). X-ALD 110362 and X-ALD 303617 are two human fibroblast cell lines from patients with ABCD1 gene mutations consistent with their clinical diagnosis of X-linked adrenoleukodystrophy (S.J. Steinberg, unpublished results), similar to the cell lines described in (22). The human Burkitt’s lymphoma cell line, Daudi, does not express class I MHC molecules on the cell surface because it lacks a functional β2-microglobulin protein (41).
Cell cultures were maintained in a humid 5% CO2 atmosphere at 37°C, and were grown in RPMI-1640 (Daudi) or Dulbecco’s modified Eagle medium (HeLa and fibroblasts) containing 2 mM L-glutamine. Culture medium for HeLa and Daudi cells was supplemented with 10% heat-inactivated fetal bovine serum. The medium for 5659C and 0498B cells was supplemented with 20% fetal bovine serum (not heat-inactivated), 1% non-essential amino acid solution, and 1% vitamin solution. X-ALD and CADDS culture medium was supplemented with 10% fetal bovine serum (not heat-inactivated), and an additional 2 mM L-glutamine for a total of 4 mM.
Construction of mammalian expression plasmids
Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Total RNA was prepared from HeLa cells using Trizol (Life Technologies, Gaithersburg MD). Single-strand cDNA was prepared from total RNA using the Advantage RT-PCR kit (Clontech, Palo Alto, CA). Taq DNA polymerase and Pfu DNA polymerase were purchased from Stratagene (La Jolla, CA), and were typically combined for PCR, at 0.05 U/μl and 0.025 U/μl, respectively.
For general cloning and mammalian expression use, we have prepared the plasmid pN3-noGFP, a variant of the plasmid pEGFP-N3 (Clontech) from which we have deleted the EGFP gene. pEGFP-N3 was cut with Kpn I and BsrG I. The large fragment was isolated and self-ligated. The YFP-tapasin construct has been described previously (23). The pEYFP-N3 plasmid is a variant of pEGFP-N3 in which EGFP was replaced by EYFP (6). The plasmid pA2-YFP-N3, which expresses a full-length human class I MHC, HLA-A2, fused to the yellow fluorescent protein, was described in the same reference.
The chimera consisting of YFP fused to the transmembrane region and cytoplasmic tail of HLA-A2 was prepared from pA2-YFP-N3, and pGFP-gpi (24) which was kindly provided by Dr. Stephen Lacey (University of Texas-Southwestern Medical Center, Dallas TX). First, pA2-YFP-N3 was digested with Bbs I; then, the single-stranded overhangs were removed with mung bean nuclease; and finally, the product was digested with EcoR I. We retained the large fragment, which includes the C-terminus of HLA-A2, up to the 10 amino acids immediately preceding the transmembrane region (EPSSQPTIPI). The GFP-gpi plasmid was digested sequentially with Ban I, mung bean nuclease, and EcoR I. The small fragment from pGFP-gpi, containing the human folate receptor signal sequence, followed by the myc antibody epitope tag (EQKLISEEDL), and 11 amino acids from GFP (MSKGEELFTGV), was ligated to the large fragment from pA2-YFP-N3.
The BAP29-YFP expression plasmid was also prepared from pA2-YFP-N3. Using total HeLa cDNA as a template, an 861-bp DNA fragment was prepared by PCR. This 861-bp fragment was used as a template, producing a 756-bp fragment carrying BAP29 with a deleted stop codon, and two silent mutations to remove internal restriction sites. Both the pA2-YFP-N3 plasmid and the 756-bp BAP29 fragment were cut with EcoR I and BamH I. The BAP29 fragment was ligated to the large plasmid fragment.
Primary BAP31 cDNA was prepared by RT-PCR, using the oligonucleotides BAP31.F0 and BAP31.R0. The YFP-BAP31 expression plasmid was assembled in several steps. PCR was performed using the primary BAP31 cDNA as a template. The nested PCR product and pN3-noGFP were both cut with Bgl II and Nhe I, then ligated together. In this intermediate construct, BAP31 was inserted into pN3-noGFP in the anti-sense orientation. Error-free clones were identified by sequencing. A BamH I - Bgl II fragment containing YFP plus a 25 amino-acid linker sequence, SSMTGGQQMGGDLYDDDDGDPPAGS (25), was inserted into the Bgl II site of the pN3-BAP31 plasmid. A Sal I - BsrG I fragment containing the folate receptor leader sequence, the myc tag, and most of the YFP sequence was ligated to the large Sal I -BsrG I fragment of the pN3-YFP-BAP31 plasmid. Finally, the entire leader-myc-YFP-linker-BAP31 construct was excised from its plasmid with Nhe I and Sal I, then inserted in the sense orientation into pN3-noGFP which had been cut with Sal I and Xba I.
For the preparation of the BAP31-YFP expression plasmid, nested PCR was performed on the primary BAP31 cDNA. The nested PCR product and the pEYFP-N3 plasmid were both cut with EcoR I and Nhe I, then ligated. Error-free clones were identified by sequencing. The sequence of the 18-amino acid linker between Bap31 and YFP in this construct, SGENSAVDGTAGPGSIAT, is mostly determined by the multiple cloning site of pEYFP-N3. The coding regions of all completed constructs were confirmed to be error-free by sequencing. Details of the primers for all the constructions can be supplied by the authors.
Plasmid transfections
Approximately 5 x 106 HeLa cells were trypsinized, centrifuged, suspended in 0.50 ml OptiMEM culture medium (Invitrogen, Carlsbad CA) at 37°C containing 15 μg plasmid DNA, and placed in a 4-mm gap electroporation cuvette. Cells were electroporated with a BTX model 600 electroporator (Harvard Apparatus, Holliston MA) using the following settings: 240 V, 1500 μF, and 129 Ω. Cells were immediately diluted with 1.0 ml DMEM + 10% FBS at 37°C, then diluted as desired for subsequent cell culture. Transient transfectants were typically analyzed 2 days after electroporation. Stable YFP/Bap31 and YFP-tapasin transfectants were obtained by culturing cells for approximately three weeks in DMEM + 10% FBS + 0.60 mg/ml active G418 sulfate (Invitrogen). High-expressors were enriched by fluorescence-activated cell sorting. Single-cell clones were prepared by limiting dilution. We selected three YFP-Bap31 clones, which expressed varying levels of the chimeric protein. In increasing order of expression (Figure 3A), these clones were named B8, C1, and E2. We only obtained one Bap31-YFP clone (1D5), which expressed comparatively low levels of chimera. Therefore, most of our studies focused on the YFP-Bap31 transfectants.
Figure 3.
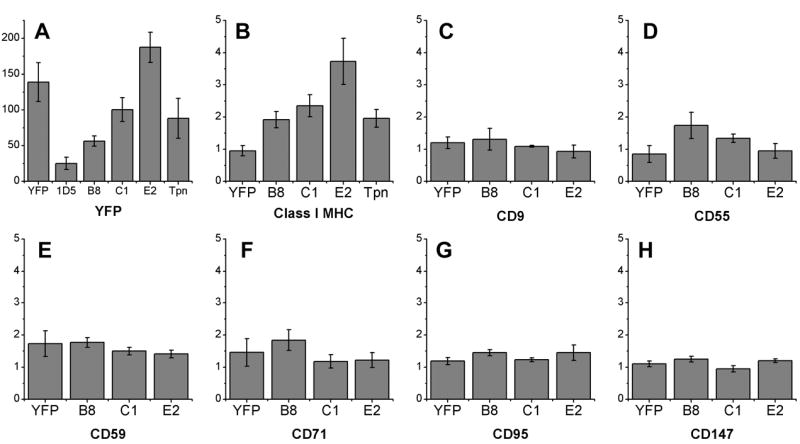
The effect of Bap31 and tapasin overexpression on the steady-state levels of transmembrane proteins found on the surface of HeLa cells. Cells were transfected with free YFP, Bap31-YFP (clone 1D5), YFP-Bap31 (clones B8, C1, and E2), or YFP-tapasin. A) Yellow fluorescence levels, which provide an estimate of the level of the transfected protein expressed. B) Levels of surface class I MHC, labeled by KE2-Cy5, increase with increasing expression of YFP-Bap31. The expression of YFP-Bap31 does not affect surface levels of seven other proteins: C) CD9, a type III protein D) CD55 and E) CD59, gpi-linked proteins F) CD71, a type II protein G) CD95 and H) CD147, type I transmembrane proteins. In B-H expression levels are normalized to the level of expression found on untransfected HeLa cells.
Antibodies
The rat anti-human Bap31 monoclonal antibodies, CC-1 and CC-4 (26), were obtained from Affinity Bioreagents (Golden, CO). Polyclonal rabbit antiserum which recognizes the cytoplasmic tail of human Bap31 was prepared by SynPep (Dublin, CA). Rabbits were immunized with the peptide CLEEHAKLQAAVDGPMDKKEE, conjugated via the N-terminal cysteine to a keyhole limpet hemocyanin carrier protein. With the exception of the initial cysteine residue, the peptide sequence is identical to the C-terminus of the human Bap31 protein, and is known to contain the CC-1 epitope. In our hands, CC-1 and the rabbit antiserum were interchangeable (results not shown). Anti-Bap29 antibody was the kind gift of Gordon Shore (McGill University, Montreal, Canada).
The mouse monoclonal antibody KE2 recognizes fully-assembled human HLA-A, -B, and -C molecules, and is cross-blocked by the W6/32 antibody (27, and M. Edidin, unpublished results). The mouse monoclonal antibody HC10 recognizes free human class I MHC heavy chains (28).
Anti-calnexin rabbit serum and anti-GFP mouse monoclonal antibody were purchased from Stressgen Biotechnologies (Victoria, British Columbia). The mouse monoclonal antibody G1/93, which recognizes ERGIC-53, was the generous gift of Dr. Hans-Peter Hauri (Biozentrum, University of Basel).
The anti-CD9 antibody MM 2/57 (Biosource Inc., Camarillo CA), and the anti-CD95 antibody CH11 (Coulter Immunotech, Miami FL) were kindly shared by Dr. Bruce Bochner (Johns Hopkins University School of Medicine, Baltimore MD). A hybridoma expressing the anti-CD55 antibody MEM43, samples of the anti-CD59 antibody MEM118, and samples of the anti-CD147 antibody MEM M6/1 were all generous gifts from Dr. Vaclav Horejsi (Institute of Molecular Genetics, Czech Academy of Sciences, Prague, Czech Republic). The monoclonal antibody L5.1 recognizes the human transferrin receptor (29).
Direct fluorescent antibody conjugates were prepared using the FluoroLink Cy5-NHS ester conjugation kit (Amersham Biosciences, Piscataway NJ), and the Alexa Fluor 546 monoclonal antibody labeling kit (Molecular Probes, Eugene OR). Donkey anti-rabbit Ig F(ab’)2 conjugated to Cy3 was purchased from Jackson ImmunoResearch (West Grove, PA). Goat anti-mouse IgG and anti-mouse IgM antisera conjugated to Cy3, Cy5, or R-phycoerythrin (R-PE) were purchased from Molecular Probes.
siRNA transfections
siRNA was purchased from Qiagen (Germantown, MD). The sequences will be provided by the authors on request. Transfections were performed using Oligofectamine (Invitrogen) as described previously (30). We administered a single 240 pmol dose of siRNA duplex to a 12-well dish of HeLa cells at 50% confluency. Maximal siRNA effects were observed three days after transfection. Transfection efficiencies of approximately 70% were achieved, as measured by flow cytometry.
Immunoprecipitation, Western blots, and endoglycosidase H assay
For the analysis of the co-precipitation of Bap31 and class I molecules (Figure 1), one protease inhibitor tablet (Roche Diagnostics, Indianapolis IN) was added to each 10 ml of cell lysis buffer (0.15 M NaCl, 50 mM Tris-HCl pH 7.5, 0.5% Triton X-100, 1 mM PMSF). Immunoprecipitations and Western blotting were otherwise performed as described previously (7). The endoglycosidase H assay for assessing the arrival of class I molecules at the medial Golgi was performed as described previously (7). Cells were pulsed for 20 minutes with medium containing 35S-labeled cysteine and methionine, then chased for varying amounts of time with unlabeled amino acids. Class I molecules were precipitated from cell lysates using KE2. Proteins were resolved by SDS-PAGE using 10% acrylamide/bis-acrylamide gel.
Figure 1.

Co-immunoprecipitation of Bap31 with class I MHC molecules. A) Western blotting of Bap31 with mAb CC-1. Fully-assembled class I MHC from HeLa cells was serially immunoprecipitated with mAb KE2 (top row). Cleared supernatant (top row, right lane) possesses additional Bap31. Free class I heavy chains were serially immunoprecipitated with mAb HC10 (middle row); associated Bap31 was identified as for KE2. Bap31 also associates with free heavy chains in Daudi cells (bottom row). B) HeLa cells were transfected with constructs expressing the transmembrane and cytoplasmic domains of HLA-A2 fused to YFP (left), or with pA2-YFP-N3, which expresses the full-length HLA-A2 fused to YFP (right). YFP-tagged molecules were immunoprecipitated with anti-GFP antibody. Blots were probed with anti-GFP (top row, center row), or with CC-1 (bottom row). C) HeLa cells transfected with constructs expressing YFP-Bap31 (top row) or Bap31-YFP (bottom row). Class I was serially immunoprecipitated with KE2 and blots were probed with anti-GFP. Chimeric proteins run in proximity to a non-specific band (*) which may be IgH; this band is absent from whole-cell lysate (not shown) and cleared supernatant (right column).
Flow cytometry
Fluorescence-activated cell sorting was performed on either an EPICS (Beckman Coulter, Fullerton CA) or a MoFlo (DakoCytomation, Fort Collins CO) fluorescence-activated cell sorter. For analysis, we used a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with a 488-nm argon laser and a 647-nm diode laser. For most assays, cells were harvested with a PBS solution containing trypsin, collagenase, and EDTA. Trypsin was omitted when measuring surface CD71 levels, because the extracellular domain of CD71 is readily cleaved by trypsin (31).
We used 1 – 2 x 105 cells per sample. Each sample was stained with 2.5 – 5 og of primary antibody in <100 μl. Secondary antibody, if needed, was added in kind. This ratio of antibody to antigen is higher than generally used in flow cytometry, and subjectively qualifies as “saturating” for several of the antibodies we used, including KE2 (data not shown). Even so, the coefficient of variance (CV) of absolute fluorescence intensity measurements of replicate samples was approximately 30%, which was inadequate for our class I MHC kinetics assays. We suspect that the variation in staining results from two causes: first, that the amount of antibody required to saturate antigens is generally underestimated in the literature; second, the limited control over the volume of fluid remaining after aspiration in the wash steps. To improve precision, we added untreated HeLa cells to each test sample to serve as an internal reference. Prior to mixing, the reference cells were marked by staining with MEM M6/1, then with goat anti-mouse IgG conjugated to R-PE. The test cells and reference cells were then stained simultaneously with KE2-Cy5. KE2-Cy5 staining was recorded as the ratio between the fluorescence of the test cells and the reference cells. Using this approach, we reduced our replicate CV to 4% (data not shown). This ratiometric method has the added advantage that cell counts need not be equal between tubes to take a consistent measurement, allowing us to dispense with hemacytometer counting.
Dead cells were identified by their uptake of 7-aminoactinomycin D (Sigma Chemical, St. Louis MO), and were excluded from analysis.
Data analysis of the transfections in Figure 7 was performed by converting flow cytometry list-mode data to a spreadsheet format using FCSExtract version 1.02 by E. F. Glynn (Stowers Institute for Medical Research, Kansas City, MO). Graphs and least-squares regression fits were then prepared using OriginPro version 6.1 (OriginLab Corp., Northampton MA).
Figure 7.

Bap29-YFP expression reduces surface class I levels. A) HeLa cells were transiently transfected with Bap29-YFP and analyzed by two-color flow cytometry. Transfected cells were identified by high levels of yellow fluorescence (rectangle, upper right). B) Expansion of the selected region from panel A; least-squares linear regression fit shows a negative correlation between surface class I levels and Bap29-YFP expression. C) Transient transfection of Bap31-YFP. D) Transfection of equal amounts of BAP29-YFP and BAP31-YFP plasmids.
Brefeldin A, measurement of surface class I stability
Cells at 50% confluency in 12-well dishes were incubated for up to 36 hours in 0.75 ml culture medium containing 5 og/ml brefeldin A (Sigma). Cells were detached from culture dishes as described above, then stained with KE2-Cy5. Surface class I levels were measured by flow cytometry. Untreated HeLa cells were used as a reference, as described above.
Papain, measurement of class I export
We modified a method for the depletion of class I molecules from the surface of living cells using papain (32). Briefly, a 10-cm dish of cells at 50% confluency was washed once with Hanks’ balanced salt solution (HBSS), then overlaid with 2.5 ml HBSS containing 2 mM L-cysteine, 1 mM EDTA, and 2 U/ml papain (Sigma). Cells were treated with papain for 3 hours in a tissue culture incubator. Typically, cells were 60% viable after treatment, and surface class I levels were depleted five-fold (data not shown). Following papain treatment, cells were washed twice with DMEM, returned to DMEM + FBS, and cultured for up to 60 hours. Cells were harvested and analyzed for surface class I as described for the brefeldin A assay, above.
Confocal fluorescence microscopy
Except as noted here, cells were prepared for intracellular staining as described previously (7). HeLa cells and Bap31 transfectants were used in most experiments. We also used X-ALD fibroblasts in some experiments since they are very flat and well-spread, reducing artifacts from superposition of fluorescence from different volumes in the cytoplasm. Cover slips were fixed for 30 min at room temperature in PBS containing 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield PA). Cells were washed with 0.25% NH4Cl in PBS, then permeabilized with PBS containing 0.2% saponin and 1% bovine serum albumin. Antibodies were diluted in permeabilization buffer. Anti-Bap31 serum was diluted 300X; CC-4 was diluted 500X; anti-calnexin serum was diluted 200X; G1/93 was diluted 1000X. Fluorescent secondary antibodies were diluted to 10 ng/ol. Prior to staining, antibodies were airfuged at 120,000 g for 1 hour. Stained cover slips were protected from photobleaching using SlowFade mounting medium (Molecular Probes), and sealed to glass slides. Samples were imaged on a Zeiss LSM 510 confocal laser scanning microscope, using the 63X oil immersion lens. Images were acquired in multi-track mode in order to eliminate fluorescence cross-talk.
Quantitative colocalization used the auto-thresholding algorithm of Costes et. al. (33), as realized in the NIH MIPAV (Medical image processing, analysis and visualization) software package (http://mipav.cit.nih.gov).
Results
Co-precipitation of human Bap31 and class I MHC
Recently, it was shown that mouse class I MHC molecules co-precipitate with Bap31 (7, 15). Human Bap31 also co-precipitates with fully-assembled human class I MHC in HeLa cells (Figure 1A), though most Bap31 molecules are not associated with class I MHC. Unlike mouse Bap31, human Bap31 also co-precipitates with free class I heavy chains. Some Bap31 also co-precipitates with free class I MHC heavy chains in the β2m-negative cell line, Daudi (Figure 1A).
The transmembrane domain of the immunoglobulin D molecule is sufficient to mediate the binding of IgD to Bap31 (34). We transfected cells with a plasmid expressing a tagged, truncated class I molecule consisting of YFP fused to the transmembrane and cytoplasmic domains of HLA-A2. Bap31 co-precipitates with this truncated class I molecule, as well as with a full-length HLA-A2-YFP chimera (Figure 1B). The results imply that, as with IgD, Bap31 binds to the transmembrane region of class I heavy chains.
For subsequent studies, we constructed chimeric plasmids, comprising YFP fused to the N-terminus or the C-terminus of BAP31. HeLa cells were transfected with YFP-BAP31 or BAP31-YFP. Both YFP-Bap31 and Bap31-YFP chimeric proteins co-precipitate with fully-assembled class I molecules (Figure 1C).
Subcellular location of human Bap31
We used qualitative and quantitative fluorescence microscopy to localize endogenous Bap31, YFP-Bap31, and Bap31-YFP in transfected cells. The pattern of YFP fluorescence of the transfectants was compared to that of endogenous Bap31 labeled with anti-Bap31 antiserum. The patterns of fluorescence were similar, though not identical, for all forms of Bap31. A significant fraction of Bap31 was found in the endoplasmic reticulum, as defined by colocalization with the ER marker, calnexin (Figures 2A–C). Colocalization between Bap31 and calnexin was extensive, but incomplete. Bap31 was often absent from peripheral, calnexin-rich regions of the cell. Also, Bap31 was enriched in a calnexin-poor, perinuclear region (arrowheads in Figure 2C), which may include ERGIC and a novel ER-derived quality control compartment described recently (35). This localization was not an artifact of the YFP tag, since endogenous Bap31, visualized with antibody, was also found in this region (data not shown). Similar colocalization patterns were observed between Bap31 and protein disulfide isomerase (PDI), another ER marker (12, and data not shown). Bap31 clearly did not colocalize with giantin or with mannosidase II, two markers for the Golgi complex (data not shown).
Figure 2.

Sub-cellular localization of Bap31 by confocal fluorescence microscopy. In HeLa cells, both A) anti-calnexin antibody staining and B) YFP-Bap31 fluorescence show a reticulated staining pattern characteristic of the endoplasmic reticulum. C) Overlay of panels A and B show that co-localization of calnexin and Bap31 is extensive but incomplete, with Bap31 enriched in a calnexin-poor, peri-nuclear region. In HeLa, D) anti-ERGIC-53 staining and E) YFP-Bap31 fluorescence are F) not strongly correlated by eye, but the correlation is apparent on two-dimensional fluorescence correlation histogram (Inset) (33). In this histogram red and green pixel intensity are plotted on horizontal and vertical axes, respectively; least-squares-fit (diagonal yellow line) is used to search for a pair of thresholds (yellow horizontal and vertical lines) which intersect at the diagonal and which lie just above the subset of data for which the correlation coefficient is zero. G, H, I) Same as D, E, F, respectively, but with X-ALD fibroblast cells. Correlation coefficients and % colocalization are given in Table I, along with values for negative and positive controls.
In HeLa cells and fibroblasts cultured under normal conditions, colocalization between ERGIC-53 (Figures 2D, 2G) and Bap31 (Figures 2E, 2H), read as yellow fluorescence, was not immediately apparent by eye (Figures 2F, 2J). A recently-developed quantitative colocalization algorithm (33) confirmed that some Bap31 colocalizes with ERGIC-53. Results of the quantitation are summarized in Table I. The correlation coefficients (r) for Bap31 and ERGIC-53 are nearly as high as that determined for HLA-A2 molecules labeled with both CFP and YFP in tandem (6), and significantly higher than the correlation observed between Bap31 and giantin, or Bap31 and mannosidase II. The fluorescence correlation results did not depend on the method that we used to label Bap31 (not shown).
Table I.
Quantitative correlation fluorescence microscopy (33) of Bap31 versus ERGIC-53. The correlation coefficient (r) is for the subset of data which lies above automatically-defined thresholds (see Figure 2 insets).
| cells | labeled proteins | correlation coefficient (r) | %ERGIC colocalized with Bap31 | notes |
|---|---|---|---|---|
| HeLa | Bap31,anti-giantin; Bap31, anti-mannosidase II | 0.35 | N/A | negative control, minimally correlated |
| HLA-A2 CFP-YFP tandem | 0.93 ± 0.03 | N/A | positive control, maximally correlated | |
| anti-Bap31, anti-ERGIC-53 | 0.81 ± 0.01 | 60% | ||
| X-ALD | anti-Bap31, anti-ERGIC-53 | 0.90 ± 0.01 | 70% |
Our findings agree with those of Annaert et. al. (12) who identified a fraction of hamster Bap31 in a perinuclear, post-ER compartment by microscopy. Furthermore, in their hands density centrifugation yielded a cell fraction which contained significant amounts of both Bap31 and ERGIC-53.
Bap31 overexpression specifically increases surface class I levels
Quantitative measurements of mature surface class I molecules on HeLa cells and transfectants were performed by flow cytometry, using a universal anti-class I MHC antibody, KE2, conjugated to Cy5. Bap31 expression levels were assessed by YFP fluorescence. The only stable BAP31-YFP transfectant that we obtained was dim, and did not measurably increase surface class I. However, transient transfection of BAP31-YFP did increase surface class I levels, in proportion to the amount of Bap31 expressed (Figure 7C). We obtained several stable clones which expressed YFP-Bap31 to different extents. Surface class I was increased in these clones with YFP-Bap31 expression, in a dose-dependent manner (p < 0.05) (Figures 3A, B). In clone E2, which expresses the most YFP-Bap31, we measured a 3.7-fold increase in surface class I levels, relative to untransfected HeLa cells.
To assess the breadth of the effect of Bap31 overexpression, we compared the expression levels of other surface proteins on HeLa cells and on the YFP-Bap31 transfectants. Bap31 does not appear to induce a general up-regulation of HeLa cell surface proteins. None of six proteins – CD9, CD55, CD59, CD71, CD95, and CD147 – were affected by overexpression of Bap31 (Figures 3C – H). Our results agree with a report that the trafficking of CD71 is unaffected by Bap31 (12), but contrast somewhat with a report that deletion of the BAP31 gene, or caspase cleavage of Bap31, can impair the export of CD9 in mouse embryonic stem cells (37).
As a negative control, we transfected HeLa cells with free YFP (Figure 3A, B). The surface levels of class I were unchanged in these cells, confirming that the increase in surface class I observed in the YFP-BAP31 transfectants was caused by the Bap31 portion of the chimeric protein. Stable HeLa transfectants expressing YFP-tapasin (23) were also prepared, and were intended to serve as a second negative control. In contrast to a previous report that the overexpression of tapasin in HeLa does not increase surface class I levels (38), we observed a two-fold increase in our YFP-tapasin transfectant.
We were not able to isolate stable clones of HeLa transfected with either YFP-Bap29 or Bap29-YFP.
Bap31 overexpression increases the export rate of nascent class I MHC molecules
If Bap31 functions as a cargo receptor for class I MHC proteins in the ER, and is normally limiting, then the rate at which nascent class I molecules are exported to the medial-Golgi should increase in cells expressing excess Bap31. This was the case. The rate of traffic of newly-synthesized class I molecules to the medial Golgi, measured in terms of maturation of oligosaccharides to endo H resistance, was 24±6 minutes (n = 4) in untransfected HeLa cells. The rate of maturation increased in proportion to the level of Bap31 expressed, up to 1.5-fold over controls (Figures 4A–C).
Figure 4.

Bap31 overexpression accelerates the maturation of class I MHC. HeLa cells and YFP-Bap31 transfectants were pulsed with [35S] Cys + Met, chased with unlabeled amino acids for 0–120 minutes, lysed, and immunoprecipitated with the anti-MHC antibody KE2. Half of each sample was treated with endoglycosidase H, so that nascent, endo H-sensitive class I, which has not reached the medial-Golgi was distinguishable from more mature, endo H-resistant class I. A) Typical autoradiographs. Untransfected HeLa cells and YFP-Bap31 clone E2 are shown. Four bands are observed in lanes treated with endo H. Lower two bands represent endo H-sensitive (endoS) class I; upper two bands, endo H-resistant (endoR). B) Maturation rates computed from quantitative densitometry of autoradiographs in panel A. First-order exponential curves were fit to the data. EndoS-class I in untransfected HeLa (squares, thin line) has a half-life of 20.1 minutes; in clone E2 (circles, thick line), 13.4 minutes. C) Summary of maturation rates measured in all pulse-chase experiments, normalized to maturation rate of untransfected HeLa cells. Circles and squares indicate rates computed from experiments performed on two separate days. Height of solid bar indicates mean relative maturation rate. D) Effect of Bap31 overexpression on reappearance of class I molecules after stripping surface class I with papain. First-order exponential curves were fit to the data. One set of measurements from untransfected HeLa (squares, thin line) and one from clone E2 (circles, thick line) are shown. E) Summary of class I recovery rates measured after papain treatment, normalized to rate of recovery in untransfected HeLa cells. Circles, squares and solid bars are as described for C). Both YFP-Bap31 and YFP-tapasin transfectants export class I molecules to the cell surface more rapidly than untransfected HeLa.
A 1.5-fold increase in the rate of ER-to-Golgi transport is inadequate to explain the 3.7-fold increase in the steady-state surface levels of class I. In considering this discrepancy, we had two concerns about the pulse-chase assay: first, that our autoradiography was insufficiently quantitative; second, that the manipulations required for the pulse-chase assay delayed the maturation of class I. To address these concerns, we depleted class I molecules from the surface of HeLa cells and Bap31 transfectants by papain digestion, then monitored class I recovery by flow cytometry (Figure 4D). Recovery rates were computed from first-order exponential fits to the data, normalized to an untransfected HeLa cell reference (Figures 4D, 4E). The rate at which class I reappeared on the cell surface increased with increasing expression of Bap31. In clone E2, which expresses the most YFP-Bap31, we observe a 2.2-fold increase in the export rate. This increase is comparable to the increase in the rate at which nascent class I molecules acquire endo H resistance. However, it still cannot account for the increase in steady-state surface class I levels induced by the overexpression of YFP-Bap31.
Overexpression of Bap31 increases the stability of surface class I
The steady-state level of a surface protein is determined by a balance between the rate of delivery of newly-synthesized molecules to the cell surface, and the rate of loss of resident surface molecules by shedding, endocytosis or other processes. To assess the effect of Bap31 on the lifetime of class I molecules on the cell surface, we blocked the secretory pathway for varying lengths of time using brefeldin A (39), then measured surface class I levels by flow cytometry.
Class I molecules disappear more quickly from the surface of untransfected HeLa cells than from HeLa cells overexpressing either YFP-Bap31 or a positive-control, YFP-tapasin. Single-exponential fits to the data (Figure 5A, thin line) consistently underestimated early data points, and overestimated middle-to-late data points. Bi-exponential fits yielded much better approximations to the data (Figure 5A, thick line), as was previously reported in a study of the surface stability of the mouse class I MHC molecule, H-2Kb (40).
Figure 5.
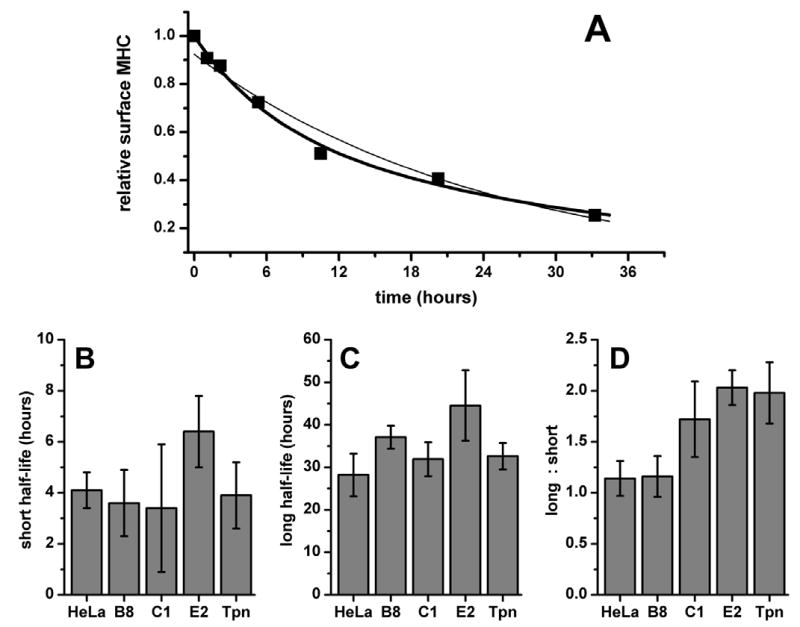
Bap31 over-expression increases the stability of surface class I MHC molecules. Export of nascent class I to the cell surface was halted by culturing cells in medium containing brefeldin A. Surface class I loss was monitored over a 36-hour period by flow cytometry using KE2-Cy5. A) Loss of surface class I as a function of time in control, untransfected HeLa cells. The curves shown are the best first-order exponential decay fit (thin line) and the best second-order fit (thick line). The equation for the second-order curve fit describing the surface concentration of class I MHC (y) as a function of time (t) is y = x1−t/t1 + x2−t/t2. B) Half-life (mean ± SEM) of the unstable class I population (t1), C) Half-life of the stable class I (t2), and D) the ratio of stable to unstable class I (r = x1/x2).
Bi-exponential decay curves may be described by three independent variables: the half-life of the short-lived, relatively unstable population (which we designate here as t1); the half-life of the long-lived, more stable population (t2); and the ratio of the number of molecules in the long-lived population to the number in the short-lived population (r). In order to characterize the increase in surface class I stability in greater detail, we examined each of the three variables separately (Figures 5B – 5D). In untransfected HeLa, t1 = 4.1 hours, and t2 = 33 hours. Long-lived class I molecules slightly outnumber their unstable counterparts, r = 1.1. These results are comparable to those reported for the stability of H-2Kb molecules on mouse lymphocytes (40). With the possible exception of clone E2, which appears to exhibit somewhat longer half-lives for each population (t1 and t2 = 6.4 and 44 hours, respectively), no trend is observed in t1 or t2 as a function of the amount of Bap31. However, a consistent, dose-dependent response is observed in r (p < 0.05). In clone E2, which expresses the most YFP-Bap31, there are twice as many stable class I molecules as there are unstable molecules, r = 2.0. HeLa cells which express YFP-tapasin also present twice as many stable class I molecules as unstable molecules. There is no apparent change in t1 or t2.
Bap31 deficiency does not reduce levels of surface class I
Since the overexpression of Bap31 increases surface class I levels, we investigated whether depletion of Bap31 would reduce surface class I by transfecting cells with short inhibiting RNA (siRNA). We prepared siRNA duplexes which correspond to human BAP31 cDNA and, as a positive control for class I loss, human B2M cDNA (41). A nonsense siRNA, which corresponds to no known human cDNA sequence, served as a negative control. The efficacy of the BAP31 siRNA was confirmed by the reduction of fluorescence (~3.5-fold) from cells expressing YFP-Bap31 (Figure 6A). The B2M siRNA reduced surface class I levels roughly five-fold (Figure 6B). However, cells which had been transfected with the BAP31 siRNA showed no change in surface class I levels when compared to the fraction of cells in the B2M siRNA treatment which remained untransfected (Figure 6B), or when compared to cells treated with the nonsense siRNA control (data not shown).
Figure 6.

Effect of Bap31 depletion on steady-state levels and stability of surface class I MHC molecules. A) YFP-Bap31 clone B8 was treated with siRNA against BAP31 (black line) or with a nonsense siRNA (gray fill). Loss of yellow fluorescence shows the efficacy of the BAP31 siRNA. B) siRNA depletion of BAP31 to about 1/3 of steady-state level did not reduce surface class I levels (black line). siRNA against B2M is a positive control for class I depletion, with approximately 70% of HeLa cells showing a five-fold reduction in surface class I levels (gray fill). Surface class I levels on untreated HeLa cells, and on HeLa treated with a nonsense siRNA control (not shown) were identical to those obtained with the BAP31 siRNA. C) Normalized steady-state surface class I levels on wild-type human fibroblasts 0498B and 5659C, X-ALD fibroblasts 110362 and 303617, and CADDS fibroblasts CADDS4 and CADDS5.
Humans with a natural deletion across the BAP31 locus (also known by its genetic designation, DXS1357E) have been recently described (21). These BAP31-negative individuals were identified as a subset of individuals who also lack an adjacent gene, ABCD1, an ABC type lipid transporter. The loss of a functional ABCD1 gene results in X-linked adrenoleukodystrophy (X-ALD) (42). The absence of both ABCD1 and BAP31 has been designated CADDS, for contiguous ABCD1/DX1357E deletion syndrome (21).
In order to establish that our failure to decrease class I levels with BAP31 siRNA was not a consequence of some unknown, compensatory siRNA effect, we examined the steady-state levels of surface class I on CADDS fibroblasts. Polymorphism at the human class I heavy chain loci is high; many of these polymorphisms affect the interaction of class I with other proteins of the antigen presentation pathway (43–45). We wished to control for any effects that class I polymorphism, or the absence of ABCD1, might have on class I expression levels in our experiments. Therefore, we examined fibroblasts from two normal individuals, and two X-ALD individuals, as controls for two CADDS lines. Consistent with our findings for HeLa cells treated with BAP31 siRNA, steady-state class I MHC levels were not depleted in CADDS cells (Figure 6C). In fact, the surface levels of class I on X-ALD and CADDS cells were modestly higher than those on the two normal cell lines.
Lastly, we examined the kinetics of class I loss under brefeldin A treatment. No significant trends were noted in the half-lives of class I molecules, regardless of the status of the ABCD1 or BAP31 genes. In general, the half-life of the unstable surface class I on all fibroblasts was shorter than that of HeLa (t1 averages 3.3 hours). The half-life of the stable fraction of class I generally exceeded that of HeLa (t2 averages 51 hours). The ratio of stable class I to unstable class I varied widely between cell lines (r ranges from 1.3 to 2.4). This variation did not correlate with presence or absence of BAP31, and may reflect class I polymorphism.
Overexpression of Bap29 reduces surface levels of class I
Since Bap31 deficiency does not reduce surface class I levels (Figure 6), we considered the possibility that Bap31 is redundant with Bap29 in the class I maturation pathway. Western blotting indicated that the Bap31 homolog, Bap29, is found in CADDS cells at levels comparable to those in wild-type cells (data not shown). Deletion of both BAP29 and BAP31 in mouse embryonic stem cells was reported to impair the export of class I (15). HeLa cells were transfected with Bap29-YFP, and changes in surface class I MHC levels were assessed. We were surprised to observe that surface class I levels decreased in proportion to the level of Bap29-YFP expression (Figures 7A, 7B). Bap31-YFP transfection increased surface class I MHC levels (Figure 7C), and its effect was co-dominant with the effect of Bap29-YFP; in cells expressing both plasmids there was no change in surface class I levels with increasing YFP expression (Figure 7D). Transfection of free cytosolic YFP into HeLa had no effect on surface class I levels (Figure 3). Finally, we found that surface class I levels in CADDS 5 cells were not affected by the transient transfection of Bap29-YFP, Bap31-YFP, or free YFP (data not shown).
Discussion
Recent findings indicate that class I molecules pass through additional intracellular regulatory steps, subsequent to dissociating from Tap. Fully-assembled class I molecules are not immediately exported from the ER (5, 6). Providing an excess of high-affinity peptide accelerates the dissociation of class I molecules from the Tap complex, but still has no effect on the rate at which class I progresses to the medial Golgi (7). These findings imply that an active process is required to export fully-assembled class I molecules from the ER. We recently reported that class I co-precipitates with Bap31 (7), a putative anterograde cargo receptor for cellubrevin (12) and for mannosidase II (9). Subsequently, Paquet et. al. have reported that the simultaneous deletion of BAP31 BAP29, slightly impairs the export of class I from the ER (15).
The central finding in our present report is that over-expression of Bap31 increases the amount of fully-assembled class I MHC on the cell surface. This is due to effects of Bap31 on the rate of forward traffic of class I MHC molecules, and on the stability of these molecules at the cell surface. The effect on anterograde traffic is consistent with a function for Bap31 in exporting newly-synthesized class I MHC molecules from the ER.
The effect of Bap31 on the lifetime and stability of surface class I is consistent with a role in quality control – for example, promoting the binding of high-affinity peptides to nascent class I molecules (40). The free class I heavy chains that we found in association with Bap31 are not necessarily all newly-synthesized molecules. Instead, these free heavy chains may be components of previously-assembled class I which have lost their peptides, triggering a concurrent dissociation from β2m (46–48). The dissociation of β2m triggers conformational changes across the transmembrane region of the MHC heavy chain, which is the putative site of Bap31 binding, and into the cytoplasmic tail (48, 53). Bap31 may therefore function in quality control by acting as a retrograde transporter, retrieving class I molecules which have lost their peptides in post-ER compartments. The acidic pH of the secretory pathway could play a role in destabilizing peptide-MHC bonds (49, 50). Retrieval of class I molecules would be mediated by the binding of Bap31’s C-terminal di-lysine motif to COP I. Bap31 has been shown to prevent the export of unassembled IgD molecules (19) and mis-folded CFTR proteins (20), processes which could also, in principle, involve retrograde transport.
A similar quality-control mechanism has been proposed for HLA-G, a non-classical human class I MHC molecule with limited expression (51). Unlike classical class I molecules, HLA-G possesses a C-terminal di-lysine motif which could bind COP I directly. HLA-G’s di-lysine motif has been demonstrated to function in the retrieval of HLA-G molecules which were bound to lower-affinity antigenic peptides. HLA-G’s quality-control mechanism would presumably be functional in the absence of Bap31, but the purpose and the method of retrieval are the same as what we propose here for classical class I.
Whatever the underlying mechanism, our results show that Bap31 is a limiting factor in the maturation and traffic of the class I MHC molecules of HeLa cells. Despite this, it is not essential. Reduction of Bap31 levels to roughly one-third of steady-state levels with siRNA did not affect levels of class I molecules on the surface of HeLa cells; class I molecules of cells lacking a functional BAP31 gene appeared normal in amount and stability. We also investigated the possibility that the Bap31 homolog, Bap29, provides a redundant function in the class I export pathway. Overexpression of Bap29 in HeLa cells decreased surface levels of class I, rather than enhancing them. Our results exclude the possibility that Bap29 can substitute for Bap31 in every circumstance, and raise the possibility that Bap29 can function as a negative regulator of class I surface expression. Our results also appear to contrast with the finding that deletion of both BAP29 and BAP31 in differentiated mouse ES cells modestly decreased the amount of class I that colocalizes with COP II, and retarded the transport of class I MHC molecules into the medial-Golgi (15). Finally, overexpression of Bap29 in human CADDS cells did not decrease class I levels as we observed in HeLa. Any effect that Bap29 may have on the class I pathway may therefore depend on the presence of Bap31.
In parallel to our findings for Bap31, we showed that overexpression of YFP-tapasin causes an increase in surface class I levels, rate of export, and lifetime. Like Bap29 and Bap31, tapasin possesses a C-terminal di-lysine retrieval motif. Taken alone, the results in the present report might suggest that tapasin, rather than Bap29, functions as a redundant cargo receptor for class I (54). However, our results can also be explained by other well-established functions for tapasin (reviewed in 55), such as the promotion of a peptide-receptive conformation in class I molecules; recruitment of class I to the Tap complex; stabilizing the expression of Tap; and promoting peptide translocation by Tap. Reports from our laboratory (6) and others (54) disagree as to whether tapasin can be found in post-ER compartments. Tapasin’s di-lysine retrieval motif is at least partially dispensable to tapasin’s role in class I maturation (56). The extent to which class I molecules require the presence of tapasin depends upon polymorphisms in class I heavy chain sequences (43–45). While Tap and tapasin greatly facilitate the loading of class I molecules with peptides, some class I molecules can acquire peptides in their absence. Similarly, it is possible that only certain alleles of class I, or certain specific unfolded states, require Bap31 or Bap29 for quality control. Therefore, the functional overlap between Bap31, Bap29, and other potential cargo receptors in the class I export pathway may be incomplete.
Similar questions about non-essential, partially-overlapping and antagonistic functions have arisen in studies of the YET genes, homologs of the mammalian BAP genes found in Saccharomyces cerevisiae (59; V. Goh, M. Edidin and K. Cunningham, unpublished results). No essential function was impaired by the deletion of YET1, YET2, YET3, or combinations thereof. Disruption of YET1 moderately accelerated yeast cell growth in liquid culture, while disruption of YET3 exhibited slower growth and reduced secretion of the invertase enzyme. A YET1-YET3 double mutant returned to normal growth rates. The redundancy of proteins in the Bap family remains a paradox.
The fate of class I heavy chains which might be retrieved from post-ER compartments is unknown. The class I assembly pathway in HeLa cells is not saturated with heavy chains, as evidenced by the fact that class I heavy chain overexpression increases antigen presentation far more readily than the overexpression of β2m, Tap, or tapasin (38). While many proteins that fail a quality-control step are exported to the cytosol for degradation (52), it is attractive to speculate that retrieved class I heavy chains might be recovered, even if the peptides to which they were initially bound were labile. Recycling these heavy chains, if possible, would be an efficient use of an unsaturated class I antigen presentation system.
Acknowledgments
We are grateful to Taiyin Wei, Karen Chadwick, and Dr. Kostas Konstantoupoulos for flow cytometry resources, and for providing assistance with fluorescence-activated cell sorting. We also thank Dr. Elias Spiliotis for providing technical assistance, and valuable discussions.
Footnotes
This work was supported by a grant from the National Institutes of Health, AI-14584 (M.E.). J.J.L. was supported in part by a training grant T32AI007247 (Mark Soloski, p.i.)
Abbreviations used in this paper: ER, endoplasmic reticulum; CFTR, cystic fibrosis transmembrane conductance regulator; YFP, yellow fluorescent protein; CFP, cyan fluorescent protein; siRNA, small interfering RNA; ERGIC, ER/Golgi intermediate compartment; X-ALD, X-linked adrenoleukodystrophy; CADDS, contiguous X-linked adrenoleukodystrophy / DXS1357E deletion syndrome.
References
- 1.Paulsson K, Wang P. Chaperones and folding of MHC class I molecules in the endoplasmic reticulum. Biochim Biophys Acta. 2003;1641:1–12. doi: 10.1016/s0167-4889(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 2.Williams A, Peh CA, Elliott T. The cell biology of MHC class I antigen presentation. Tissue Antigens. 2002;59:3–17. doi: 10.1034/j.1399-0039.2002.590103.x. [DOI] [PubMed] [Google Scholar]
- 3.Bouvier M. Accessory proteins and the assembly of human class I MHC molecules: a molecular and structural perspective. Mol Immunol. 2003;39:697–706. doi: 10.1016/s0161-5890(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 4.Jackson MR, Cohen-Doyle MF, Peterson PA, Williams DB. Regulation of MHC class I transport by the molecular chaperone, calnexin (p88, IP90) Science. 1994;263:384–387. doi: 10.1126/science.8278813. [DOI] [PubMed] [Google Scholar]
- 5.Marguet D, Spiliotis ET, Pentcheva T, Lebowitz M, Schneck J, Edidin M. Lateral diffusion of GFP-tagged H2Ld molecules and of GFP-TAP1 reports on the assembly and retention of these molecules in the endoplasmic reticulum. Immunity. 1999;11:231–240. doi: 10.1016/s1074-7613(00)80098-9. [DOI] [PubMed] [Google Scholar]
- 6.Pentcheva T, Edidin M. Clustering of peptide-loaded MHC class I molecules for endoplasmic reticulum export imaged by fluorescence resonance energy transfer. J Immunol. 2001;166:6625–6632. doi: 10.4049/jimmunol.166.11.6625. [DOI] [PubMed] [Google Scholar]
- 7.Spiliotis ET, Manley H, Osorio M, Zuniga MC, Edidin M. Selective export of MHC class I molecules from the ER after their dissociation from TAP. Immunity. 2000;13:841–851. doi: 10.1016/s1074-7613(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 8.Kim KM, Adachi T, Nielsen PJ, Terashima M, Lamers MC, Kohler G, Reth M. Two new proteins preferentially associated with membrane immunoglobulin D. EMBO J. 1994;13:3793–3800. doi: 10.1002/j.1460-2075.1994.tb06690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maatta J, Hallikas O, Welti S, Hilden P, Schroder J, Kuismanen E. Limited caspase cleavage of human BAP31. FEBS Lett. 2000;484:202–206. doi: 10.1016/s0014-5793(00)02159-1. [DOI] [PubMed] [Google Scholar]
- 10.Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, Shore GC. p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuppig S. Molecular Immunology. Max-Planck Institute for Immunobiology. Freiburg; Germany: 2001. Generation and Characterization of BAP-deficient Cell Lines; p. 136. [Google Scholar]
- 12.Annaert WG, Becker B, Kistner U, Reth M, Jahn R. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J Cell Biol. 1997;139(6):1397–1410. doi: 10.1083/jcb.139.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell AW, Ward MA, Blackstock WP, Freeman HN, Choudhary JS, Lewis AP, Chotai D, Fazel A, Gushue JN, Paiement J, Palcy S, Chevet E, Lafreniere-Roula M, Solari R, Thomas DY, Rowley A, Bergeron JJ. Proteomics characterization of abundant Golgi membrane proteins. J Biol Chem. 2001;276:5152–5165. doi: 10.1074/jbc.M006143200. [DOI] [PubMed] [Google Scholar]
- 14.Breuza L, Halbeisen R, Jeno P, Otte S, Barlowe C, Hong W, Hauri HP. Proteomics of ERGIC membranes from brefeldin A-treated HepG2 cells identifies ERGIC-32, a new cycling protein that interacts with human Erv46. J Biol Chem. 2004;279:47242–47253. doi: 10.1074/jbc.M406644200. [DOI] [PubMed] [Google Scholar]
- 15.Paquet ME, Cohen-Doyle M, Shore GC, Williams DB. Bap29/31 influences the intracellular traffic of MHC class I molecules. J Immunol. 2004;172:7548–7555. doi: 10.4049/jimmunol.172.12.7548. [DOI] [PubMed] [Google Scholar]
- 16.Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- 17.Andersson H, Kappeler F, Hauri HP. Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J Biol Chem. 1999;274:15080–15084. doi: 10.1074/jbc.274.21.15080. [DOI] [PubMed] [Google Scholar]
- 18.Wienands J, Reth M. Glycosyl-phosphatidylinositol linkage as a mechanism for cell-surface expression of immunoglobulin D. Nature. 1992;356:246–248. doi: 10.1038/356246a0. [DOI] [PubMed] [Google Scholar]
- 19.Schamel WW, Kuppig S, Becker B, Gimborn K, Hauri HP, Reth M. A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2003;100:9861–9866. doi: 10.1073/pnas.1633363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert G, Becker B, Schreiber R, Boucherot A, Reth M, Kunzelmann K. Control of cystic fibrosis transmembrane conductance regulator expression by BAP31. J Biol Chem. 2001;276:20340–20345. doi: 10.1074/jbc.M011209200. [DOI] [PubMed] [Google Scholar]
- 21.Corzo D, Gibson W, Johnson K, Mitchell G, LePage G, Cox GF, Casey R, Zeiss C, Tyson H, Cutting GR, Raymond GV, Smith KD, Watkins PA, Moser AB, Moser HW, Steinberg SJ. Contiguous deletion of the X-linked adrenoleukodystrophy gene (ABCD1) and DXS1357E: a novel neonatal phenotype similar to peroxisomal biogenesis disorders. Am J Hum Genet. 2002;70:1520–1531. doi: 10.1086/340849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins PA, Gould SJ, Smith MA, Braiterman LT, Wei HM, Kok F, Moser AB, Moser HW, Smith KW. Altered expression of ALDP in X-linked adrenoleukodystrophy. Am J Hum Genet. 1995;57:292–301. [PMC free article] [PubMed] [Google Scholar]
- 23.Pentcheva T, Spiliotis ET, Edidin M. Tapasin is retained in the endoplasmic reticulum by dynamic clustering and exclusion from endoplasmic reticulum exit sites. J Immunol. 2002;168:1538–1541. doi: 10.4049/jimmunol.168.4.1538. [DOI] [PubMed] [Google Scholar]
- 24.Conrad PA, Smart EJ, Dougherty D, Anderson RGW, Bloom GS, Lacey SW. GPI anchors target green fluorescent proteins on the plasma membrane to caveolae. Mol Biol Cell. 1996;7-S:1597. [Google Scholar]
- 25.Heim R, Tsien RY. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 26.Manley HA, Lennon VA. Endoplasmic reticulum membrane-sorting protein of lymphocytes (BAP31) is highly expressed in neurons and discrete endocrine cells. J Histochem Cytochem. 2001;49:1235–1243. doi: 10.1177/002215540104901005. [DOI] [PubMed] [Google Scholar]
- 27.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of Monoclonal Antibodies to Group A Erythrocytes, HLA, and Other Human Cell Surface Antigens - New Tools for Genetic Analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 28.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 29.Lebman D, Trucco M, Bottero L, Lange B, Pessano S, Rovera G. A monoclonal antibody that detects expression of transferrin receptor in human erythroid precursor cells. Blood. 1982;59:671–678. [PubMed] [Google Scholar]
- 30.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber KTT. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 31.Rutledge EA, Mikoryak CA, Draper RK. Turnover of the transferrin receptor is not influenced by removing most of the extracellular domain. J Biol Chem. 1991;266:21125–21130. [PubMed] [Google Scholar]
- 32.Galati G, Arcelloni C, Paroni R, Heltai S, Rovere P, Rugarli C, Manfredi AA. Quantitative cytometry of MHC class I digestion from living cells. Cytometry. 1997;27:77–83. doi: 10.1002/(sici)1097-0320(19970101)27:1<77::aid-cyto10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi T, Schamel WW, Kim KM, Watanabe T, Becker B, Nielsen PJ, Reth M. The specificity of association of the IgD molecule with the accessory proteins BAP31/BAP29 lies in the IgD transmembrane sequence. EMBO J. 1996;15:1534–1541. [PMC free article] [PubMed] [Google Scholar]
- 35.Kamhi-Nesher S, Shenkman M, Tolchinsky S, Fromm SV, Ehrlich R, Lederkremer GZ. A novel quality control compartment derived from the endoplasmic reticulum. Mol Biol Cell. 2001;12:1711–1723. doi: 10.1091/mbc.12.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klumperman J, Schweizer A, Clausen H, Tang BL, Hong W, Oorschot V, Hauri HP. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J Cell Sci. 1998;111:3411–3425. doi: 10.1242/jcs.111.22.3411. [DOI] [PubMed] [Google Scholar]
- 37.Stojanovic M, Germain M, Nguyen M, Shore GC. BAP31 and its caspase cleavage product regulate cell surface expression of tetraspanins and integrin-mediated cell survival. J Biol Chem. 2005;280:30018–30024. doi: 10.1074/jbc.M501306200. [DOI] [PubMed] [Google Scholar]
- 38.Johnson DR, Mook-Kanamori B. Dependence of elevated human leukocyte antigen class I molecule expression on increased heavy chain, light chain (beta 2-microglobulin), transporter associated with antigen processing, tapasin, and peptide. J Biol Chem. 2000;275:16643–16649. doi: 10.1074/jbc.M910035199. [DOI] [PubMed] [Google Scholar]
- 39.Nebenfuhr A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su RC, Miller RG. Stability of surface H-2Kb, H-2Db, and peptide-receptive H-2Kb on splenocytes. J Immunol. 2001;167:4869–4877. doi: 10.4049/jimmunol.167.9.4869. [DOI] [PubMed] [Google Scholar]
- 41.Seong RH, Clayberger CA, Krensky AM, Parnes JR. Rescue of Daudi cell HLA expression by transfection of the mouse beta 2-microglobulin gene. J Exp Med. 1988;167:288–299. doi: 10.1084/jem.167.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:682–683. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 43.Peh CA, Burrows SR, Barnden M, Khanna R, Cresswell P, Moss DJ, McCluskey J. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity. 1998;8:531–542. doi: 10.1016/s1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- 44.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 45.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16:509–520. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 46.Danliczyk UG, Delovitch TL. Beta 2-microglobulin induces a conformational change in an MHC class I H chain that occurs intracellularly and is maintained at the cell surface. J Immunol. 1994;153:3533–3542. [PubMed] [Google Scholar]
- 47.Gakamsky DM, Bjorkman PJ, Pecht I. Peptide interaction with a class I major histocompatibility complex-encoded molecule: allosteric control of the ternary complex stability. Biochemistry. 1996;35:14841–14848. doi: 10.1021/bi961707u. [DOI] [PubMed] [Google Scholar]
- 48.Smith MH, Barber BH. The conformational flexibility of class I H-2 molecules as revealed by anti-peptide antibodies specific for intracytoplasmic determinants: differential reactivity of beta 2-microglobulin "bound" and "free" H-2Kb heavy chains. Mol Immunol. 1990;27:169–180. doi: 10.1016/0161-5890(90)90112-d. [DOI] [PubMed] [Google Scholar]
- 49.Stryhn A, Pedersen LO, Romme T, Olsen AC, Nissen MH, Thorpe CJ, Buus S. pH dependence of MHC class I-restricted peptide presentation. J Immunol. 1996;156:4191–4197. [PubMed] [Google Scholar]
- 50.Weisz OA. Acidification and protein traffic. Int Rev Cytol. 2003;226:259–319. doi: 10.1016/s0074-7696(03)01005-2. [DOI] [PubMed] [Google Scholar]
- 51.Park B, Lee S, Kim E, Chang S, Jin M, Ahn K. The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity. 2001;15:213–224. doi: 10.1016/s1074-7613(01)00179-0. [DOI] [PubMed] [Google Scholar]
- 52.Romisch K. Surfing the Sec61 channel: bidirectional protein translocation across the ER membrane. J Cell Sci. 1999;112:4185–4191. doi: 10.1242/jcs.112.23.4185. [DOI] [PubMed] [Google Scholar]
- 53.Little A-M, Nößner EE, Parham P. Dissociation of beta 2- microglobulin from HLA class I heavy chains correlates with acquisition of epitopes in the cytoplasmic tail. J Immunol. 1995;154:5205–5215. [PubMed] [Google Scholar]
- 54.Paulsson KM, Kleijmeer MJ, Griffith J, Jevon M, Chen S, Anderson PO, Sjogren HO, Li S, Wang P. Association of tapasin and COP I provides a mechanism for the retrograde transport of major histocompatibility complex (MHC) class I molecules from the Golgi complex to the endoplasmic reticulum. J Biol Chem. 2002;277:18266–18271. doi: 10.1074/jbc.M201388200. [DOI] [PubMed] [Google Scholar]
- 55.Momburg F, Tan P. Tapasin-the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol Immunol. 2002;29:217–233. doi: 10.1016/s0161-5890(02)00103-7. [DOI] [PubMed] [Google Scholar]
- 56.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- 57.Skerra A. Phosphorothioate primers improve the amplification of DNA sequences by DNA polymerases with proofreading activity. Nucleic Acids Res. 1992;20:3551–355. doi: 10.1093/nar/20.14.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 59.Toikkanen JH, Fatal N, Hildén P, Makarow M, Kuismanen E. YET1, YET2 and YET3 of Saccharomyces cerevisiae encode BAP31 homologs with partially overlapping functions. J Biol Sci. 2006;6:446–456. [Google Scholar]
- 60.Szczesna-Skorupa E, Kemper B. BAP31 is involved in the retention of cytochrome P450 2C2 in the endoplasmic reticulum. J Biol Chem. 2006;281:4142–4148. doi: 10.1074/jbc.M509522200. [DOI] [PubMed] [Google Scholar]


