Abstract
Background and purpose:
Pathological cardiac hypertrophy is associated with the expression of a gene profile reminiscent of foetal development. The non selective β-adrenoceptor antagonist propranolol is able to blunt cardiomyocyte hypertrophic response in pressure-overloaded hearts. It remains to be determined whether propranolol also attenuates the expression of hypertrophy-associated foetal genes.
Experimental approach:
To address this question, the foetal gene programme, of which atrial natriuretic peptide (ANP), the β-isoform of myosin heavy chain (β-MHC), and the α-skeletal muscle isoform of actin (skACT) are classical members, was induced by thoracic aortic coarctation (TAC) in C57BL/6 mice, or by phenylephrine, a selective α1-adrenoceptor agonist, in cultured rat neonatal cardiomyocytes.
Key results:
In TAC mice, the left ventricular weight-to-body weight (LVW/BW) ratio increased by 35% after 2 weeks. Levels of ANP, β-MHC and skACT mRNA in the left ventricles increased 2.2-fold, 2.0-fold and 12.1-fold, respectively, whereas α-MHC and SERCA mRNA levels decreased by ≈ 50%. Although propranolol blunted cardiomyocyte growth, with approximately an 11% increase in the LVW/BW ratio, it enhanced the expression of ANP, β-MHC and skACT genes (10.5-fold, 27.7-fold and 22.7-fold, respectively). Propranolol also enhanced phenylephrine-stimulated ANP and β-MHC gene expression in cultured cardiomyocytes. Similar results were obtained with metoprolol, a selective β1-adrenoceptor antagonist, but not with ICI 118551, a β2-adrenoceptor antagonist.
Conclusions and implications:
Propranolol enhances expression of the hypertrophy-associated foetal genes mainly via the β1-adrenoceptor blockade. Our results also suggest that, in pressure-overloaded hearts, cardiomyocyte growth and foetal gene expression occur as independent processes.
Keywords: propranolol, phenylephrine, pressure overload, hypertrophy, heart, gene expression
Introduction
Cardiac hypertrophy is a common condition that often develops as a consequence of chronic haemodynamic overload. Although the increase in the myocardial mass seems to be a necessary adaptive process to accommodate the increased workload, cardiac hypertrophy is associated with an enhanced risk of ventricular dysfunction and heart failure (Brown et al., 2000). Despite intense investigation, our understanding of the cellular mechanisms that are responsible for the initiation and the maintenance of this adaptation is largely incomplete and prevention or regression of cardiac hypertrophy is a major challenge.
Pathological myocardial hypertrophy is characterized by the increase in cardiomyocyte size associated with the re-expression of the so-called foetal gene programme. This programme includes increased β-myosin heavy chain (β-MHC), skeletal α-actin (skACT) and atrial natriuretic peptide (ANP) gene expression. In conjunction with these changes, a decrease in the adult cardiac muscle-specific genes, α-myosin heavy chain (α-MHC) and sarcoplasmic reticulum Ca2+-ATPase (SERCA) also occurs (Izumo et al., 1988; Komuro et al., 1989; van den Bosch et al., 2006). ANP, β-MHC and skACT genes are normally expressed in late-foetal and early-neonatal heart tissues and are extinguished in adult ventricular myocardium. Although the significance of these changes has still not been fully understood, the re-expression of foetal genes could be one facet of the complex adaptive system that serves to reduce energy demand under increased haemodynamic burden. For example, β-MHC would allow cardiac muscle to work more efficiently when chronically overloaded because it contracts and relaxes more slowly than α-MHC (Izumo et al., 1988), while ANP would contribute to limit cardiac energy consumption through its natriuretic and anti-hypertrophic properties (Holtwick et al., 2003).
In the aortic banding-induced pressure-overload model of cardiac hypertrophy, the administration of the β-adrenoceptor antagonist propranolol markedly attenuates the development of cardiomyocyte hypertrophy (Ostman-Smith, 1995; Marano et al., 2002, 2003), without impairing cardiac performance (Marano et al., 2003). It is unknown, however, whether propranolol also blunts the expression of the hypertrophy-associated foetal genes.
To address this issue, the foetal gene programme was induced by two well-characterized hypertrophic stimuli, thoracic aortic coarctation (TAC)-induced pressure overload and phenylephrine. The former causes cardiomyocyte hypertrophy associated with the re-expression of foetal genes via mechanical and neurohumoral factors. Phenylephrine, an α1-adrenoceptor agonist, also induces cell growth as well as the foetal gene programme in cultured neonatal cardiomyocytes via activation of the Gq protein-coupled signalling cascade. Using these experimental approaches, we found that propranolol attenuates cardiac hypertrophy but causes a paradoxical enhancement of the foetal gene response to hypertrophic stimuli mainly via the β1-adrenoceptor blockade.
Methods
Animals
Male C57Bl/6 12-week-old mice (Harlan, S Pietro al Natisone, Italy) were used in the present study and maintained in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Primary cardiomyocytes cultures
Cardiomyocytes were obtained from 1- to 3-day-old neonatal Wistar rat ventricles as described (Sadoshima et al., 1992) and plated at a density of ≈5.0 × 105 cells per well in six-well tissue culture plates. Cardiomyocytes were cultured in Dulbecco's modified Eagle's medium (DMEM)-Medium 199 (4:1) supplemented with 5% horse serum and 5% foetal calf serum. Three days after plating, cardiomyocytes were incubated in a serum-free medium supplemented with insulin (1 μg ml−1) and transferrin (2 μg ml−1) for 24 h. To induce foetal gene expression, cardiomyocytes were exposed to phenylephrine (25 μM) for 24 h in the presence or absence of propranolol (1 μM), metoprolol (1 μM), ICI 118551 (1 μM) or prazosin (2 μM). In a separate series of experiments, cells were treated with noradrenaline (10 μM) for 24 h in the presence or absence of propranolol (1 μM).
Mouse model of left ventricle pressure overload
Pressure overload on the left ventricle (LV) was induced by TAC as reported previously (Rockman et al., 1991), except for the anaesthetic used (isoflurane 1.5–2.0% in 100% oxygen) and the lesser degree of aortic stenosis (55–60% instead of 65–70%). A concurrent group of mice was subjected to a sham operation in which an identical surgical procedure was performed but the ligature was not tightened. On the surgery day, mice received either propranolol (Pro-TAC and Pro-Sham groups) or vehicle (Veh-TAC and Veh-Sham groups) (Figure 1a). To quantify the haemodynamic load imposed on the mouse LV after aortic banding, left ventricle systolic pressure (LVSP) was measured with a 1.4 Fr micromanometer-tipped catheter (Millar Instruments, mod. SPR 839, Houston, TX, USA) by direct catheterization of the LV. In addition, the systolic pressure gradient was also measured by selective cannulation of left and right carotid arteries in three mice for each TAC group 2 weeks after aortic surgery.
Figure 1.
LV mass, cardiomyocyte size and interstitial fibrosis. (a) Experimental design of the study. Veh-Sham, sham-operated mice treated with vehicle; Pro-Sham, sham-operated mice treated with propranolol; Veh-TAC, banded mice treated with vehicle; Pro-TAC, banded mice treated with propranolol. Propranolol (10 mg kg−1 day−1) or vehicle (0.9% NaCl) was administered through osmotic minipumps. (b) Assessment of left ventricular weight to body weight (LVW/BW) ratio. TAC-induced cardiac hypertrophy was significantly blunted in Pro-TAC group compared to Veh-TAC (n=5–7 per group). (c) Morphometric analysis of cardiomyocytes. A marked increase in the cross-sectional area was observed in Veh-TAC group but not in Pro-TAC group. The cross-sectional areas of individual cardiomyocytes were measured using the Metamorph image analysis programme; ≈100 cells were analysed from each LV (n=5–7 per group). (d) Assessment of interstitial fibrosis. No difference was found in cardiac fibrosis among groups. Interstitial fibrosis was calculated as a percentage of total microscopic area (10–12 fields per section, 4–5 sections per ventricle) (n=5–7 per group). *P<0.05, TAC vs corresponding control group; ‡P<0.05, propranolol vs vehicle within TAC or sham-operated groups. LV, left ventricle; TAC, thoracic aortic coarctation.
Echocardiography
Two weeks after banding, in mice intubated and anaesthetized with isoflurane (1% in 100% of oxygen), echocardiographic examination was performed with a SONOLINE G50 (Siemens AG, Erlangen, Germany) equipped with a 13-MHz imaging transducer. After good-quality 2D short-axis images of the LV were obtained, M-mode freeze frames were printed on common echocardiographic paper and digitized. Left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), and posterior wall end-diastolic thickness were measured by an image-analysis system (Metamorph, Universal Image Corporation, Dowingtown, PA, USA). Percent fractional shortening (FS) was calculated as ((LVEDD−LVESD)/LVEDD) × 100.
Haemodynamic analysis
LV haemodynamic measurements were obtained by direct LV catheterization as described (Marano et al., 2004). Briefly, a 1.4-Fr, four-electrode pressure-volume catheter (model SPR-839, Millar Instruments) was inserted into the LV through the LV apex in the open chest anaesthetized animal (isoflurane 1.5–2% in 100% oxygen) and advanced along the long axis with proximal electrode just within the wall of the LV apex. The pressure-volume catheter was connected to an MPCU-200 unit (Millar Instruments). Correct catheter positioning was confirmed by on line visualization of the pressure-volume loops. For volume measurements, the conductance catheter was calibrated with known volumes of heparin-treated mouse blood according to the manufacturer's instructions. All pressure-volume loop data were analysed with cardiac pressure-volume analysis programme (IOX 1.7; EMKA Technologies, Paris, France), and the heart rate (HR), maximal LVSP, left ventricular end-diastolic pressure (LVEDP), maximal slope of LV pressure increment (+dP/dt) or fall (−dP/dt) and end-systolic elastance (Ees, a load-independent parameter of cardiac contractility) were computed. To change the cardiac preload, occlusion of the inferior vena cava was produced over 3 s. All pressure-volume loops were acquired while pulmonary ventilation was temporarily suspended. The data were recorded as a series of 10–20 pressure-volume loops.
Histological analysis
Histological analysis was performed as reported previously (Marano et al., 2004). Briefly, LV sections were cut and stained with haematoxylin and eosin for measurement of myocyte cross-sectional area or with the Sirius red/picric acid method to determine LV fibrosis by quantitative morphometry (Metamorph 6.1).
Chronic administration of propranolol
Propranolol was administered in a dose of 10 mg kg−1 day−1 for 14 consecutive days. The dosage was chosen on the basis of literature data (Boluyt et al., 1995; Nishio et al., 2003). The β-blocker was administered through osmotic minipumps (Alzet model 2002, Cupertino, CA, USA) implanted subcutaneously on the right side of the back.
RNA isolation and quantification
Total RNA was extracted from LVs of individual mice and from cultured neonatal rat cardiomyocytes by using SV Total RNA Isolation System (Promega, Madison, WI, USA). cDNA out of total ventricular RNA was synthesized by using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA). RNA expression levels for ANF, β-MHC, skACT, α-MHC and SERCA2 after banding experiments in the LV or in cultured neonatal cardiomyocytes were quantified with real-time TaqMan reverse transcriptase (RT)-PCR using 7500 Real-Time PCR system (Applied Biosystems). Taqman reactions were carried out in 96-well plates using cDNA, Taqman universal PCR mastermix, pre-designed and pre-optimized TaqMan Gene expression assays including specific primers and fluorescent probes (ANP, Mm01255747_g1; β-MHC, Mm00600555_m1; α-MHC, Mm00440354_m1; SERCA, Mm00437634_m1; skACT, Mm00808218_g1; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Mm99999915_g1), and water to a final volume of 50 μl according to manufacturer's instructions. GAPDH mRNA was used as an endogenous control. The codes for primers and probes were obtained from the Applied Biosystems catalogue for quantitative gene expression analysis. No RT and no template controls were used to monitor for any contaminating amplification. The ΔCt was used for statistical analysis, and 2−ΔΔCt for data presentation (Livak and Schmittgen, 2001; Yuan et al., 2006).
Statistical analysis
Group means (±s.e.m.) were calculated for all relevant variables. Statistical analysis was performed by analysis of variance with Bonferroni's multiple comparison test for post hoc analyses. A value of P<0.05 was considered to be statistically significant.
Materials
The chemicals used were propranolol, metoprolol, ICI 118551, phenylephrine, noradrenaline, prazosin, insulin, transferrin (Sigma-Aldrich, Milan, Italy); DMEM-Medium 199, horse serum and foetal calf serum (GIBCO, Rockville, USA); isoflurane (Abbott, Pomezia, Italy).
Results
Propranolol attenuates TAC-induced cardiac hypertrophy
Two weeks after surgery, a significant increase in LVSP was observed in Veh-TAC group compared to Veh-Sham one (108±4 vs 65±3 mm Hg, respectively), but no significant difference was observed between Veh-TAC and Pro-TAC groups (108±4 and 106±5 mm Hg, respectively). The transstenotic systolic pressure gradient was also found to be similar in both TAC groups (38±3 and 36±4 mm Hg, respectively). Pressure overload was tolerated well by all TAC groups with no signs of cardiovascular compromise or postoperative increased mortality. As expected, left ventricular weight to body weight (LVW/BW) ratio significantly increased by ≈35% in Veh-TAC group (Figure 1b). In contrast, cardiac hypertrophic growth was blunted significantly in Pro-TAC mice, with approximately an 11% increase in the LVW/BW ratio (Figure 1b). Furthermore, microscopic analysis of histological sections revealed that the increase in the cardiomyocyte cross-sectional area was reduced significantly in propranolol-treated mice (a 19% increase in TAC vs sham) compared with vehicle-treated ones (a 51% increase in TAC vs sham), confirming the gross pathological data (Figure 1c). No difference was found in cardiac fibrosis between TAC and sham animals (Figure 1d).
To evaluate the functional consequences of propranolol treatment, cardiac function and ventricular size were assessed by transthoracic echocardiography and, invasively, by using a 1.4 Fr conductance microcatheter. In anaesthetized mice, propranolol reduced HR, but not significantly (Table 1). With the exception of its ability to attenuate the increase in the indices of hypertrophy (PWT and echo-derived LV mass), propranolol had no significant effect on ventricular function (FS, +dP/dt and Ees) and chamber size (LVEDD) in both TAC and sham groups (Table 1).
Table 1.
Echocardiography and haemodynamic measurements 2 weeks after surgery
|
Sham |
TAC |
|||
|---|---|---|---|---|
| Vehicle | Propranolol | Vehicle | Propranolol | |
| n | 6 | 5 | 5 | 6 |
| Body weight (g) | 26.5±0.5 | 26.0±0.4 | 26.3±0.3 | 25.6±0.6 |
| Heart rate (beats min−1) | 455±11 | 422±8 | 462±13 | 419±9 |
| LVEDP (mm Hg) | 3±0.4 | 4±0.5 | 5±0.4 | 4±0.3 |
| −dP/dt (mm Hg s−1) | 4296±187 | 3965±41 | 5882±265* | 6475±468* |
| LVEDD (mm) | 3.2±0.2 | 3.3±0.3 | 3.1±0.2 | 3.0±0.3 |
| PWT (mm) | 0.61±0.06 | 0.60±0.05 | 0.91±0.04* | 0.69±0.03‡ |
| eLV mass (mg) | 81±3 | 80±3 | 114±4* | 91±3‡ |
| FS (%) | 39±2 | 38±3 | 37±4 | 37±2 |
| +dP/dt (mm Hg s−1) | 4765±87 | 4511±95 | 7550±235* | 7362±259* |
| Ees (mm Hg μl−1) | 5.8±1.1 | 5.0±0.6 | 10.9±1.0* | 9.9±0.9* |
Abbreviations: +dP and –dP, maximum rate of left ventricular pressure rise or fall; Ees, end-systolic elastance; eLV mass, echo-derived LV mass=1.05[(IVS+LVEDD+PWT)3−(LVEDD)3]; FS, fractional shortening; IVS, interventricular septal thickness; LVEDP, left ventricular end-diastolic pressure; LVEDD, left ventricular end-diastolic diameter; PWT, posterior wall thickness; TAC, thoracic aortic coarctation.
P<0.05, TAC vs corresponding sham group.
P<0.05, propranolol vs vehicle within TAC or sham-operated groups.
Propranolol enhances TAC-induced foetal gene expression
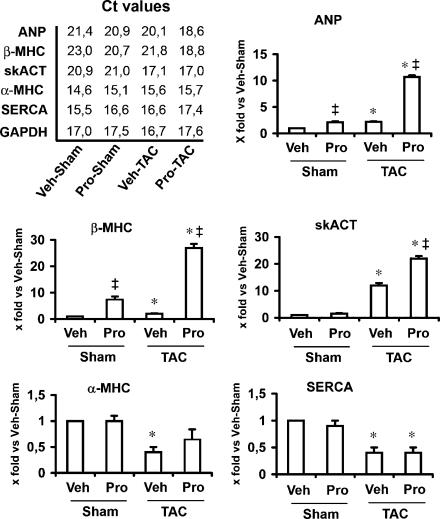
We used real-time quantitative RT-PCR to assess the foetal gene expression in LVs. As expected, expression of ANP, β-MHC and skACT genes increased, whereas α-MHC and SERCA genes significantly decreased 2 weeks after TAC (Figure 2). The foetal gene response to TAC was largely enhanced by propranolol treatment (Figure 2). Given that propranolol treatment alone was sufficient to upregulate ANP and β-MHC gene expression (Figure 2), the foetal gene induction in TAC groups was also evaluated as fold increase in respective sham controls. We found that levels of ANP, β-MHC and skACT mRNA increased 2.2-, 2.0- and 12.1-fold, respectively, in the untreated LVs, whereas increased 5.0-, 3.4- and 22.7-fold, respectively, in propranolol-treated ones. Taken altogether, these results indicate that propranolol causes an enhancement of the foetal gene response in pressure-overloaded hearts.
Figure 2.
Foetal gene induction after TAC. Real-time TaqMan quantitative PCR was used to assess the expression of the foetal genes in LVs. The Ct (Cycle threshold) values for each gene are taken from a single experiment. ΔCt for each gene target is then calculated by subtracting the Ct number of GAPDH gene from that of target gene. ΔΔCt is calculated by subtracting the ΔCt of control (Veh-Sham group) from that of the remaining groups. Data for each single gene are presented as the fold change relative to the expression levels of Veh-Sham mice (2−ΔΔCt). Summary data for ANP, β-MHC, skACT, α-MHC and SERCA from 3 to 4 separate experiments performed in triplicate are shown. In Pro-TAC group, induction of the foetal genes by pressure overload was largely enhanced (10.5-, 27.7- and 22.7-fold, respectively). Propranolol treatment alone was sufficient to upregulate ANP and β-MHC gene expression (2.1- and 8.1-fold, respectively). *P<0.05, TAC vs corresponding control group; ‡P<0.05, propranolol vs vehicle within TAC or sham groups. TAC, thoracic aortic coarctation; LV, left ventricle; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ANP, atrial natriuretic peptide; β-MHC, β-myosin heavy chain; skACT, skeletal α-actin; α-MHC, α-myosin heavy chain; SERCA, sarcoplasmic reticulum Ca2+-ATPase.
Propranolol enhances the expression of foetal genes in cultured cardiomyocytes via the blockade of β1-adrenoceptors
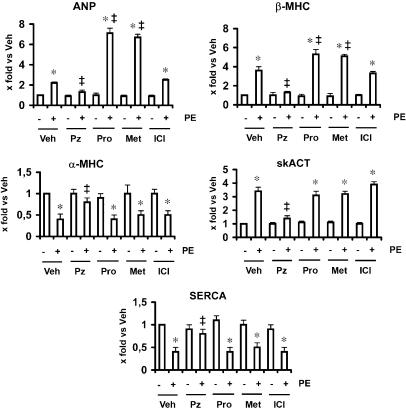
We next investigated the effects of propranolol on the foetal gene response induced by phenylephrine in cultured neonatal cardiomyocytes. Treatment of cardiomyocytes with phenylephrine induced the expression of foetal genes such as ANP, β-MHC and skACT, associated with the decrease in the expression of adult genes such as α-MHC and SERCA (Figure 3). These responses were completely inhibited by prazosin (Figure 3). Conversely, treatment with propranolol enhanced phenylephrine-stimulated ANP and β-MHC gene expression (Figure 3).
Figure 3.
Expression of phenylephrine-induced foetal genes in cultured neonatal cardiomyocytes. Cardiomyocytes were stimulated with phenylephrine (PE) (25 μM) for 24 h in the presence or absence of propranolol (Pro) (1 μM), metoprolol (Met) (1 μM), ICI 118551 (ICI) (1 μM) or prazosin (Pz) (2 μM). At the end of treatments, total RNA was isolated and real-time TaqMan quantitative PCR was used to assess the expression of the foetal genes. Summary data for ANP, β-MHC, skACT, α-MHC and SERCA from three separate experiments performed in triplicate are shown. Data are presented as 2−ΔΔCt. Expression of ANP, β-MHC and skACT genes significantly increased in response to PE (2.2-, 3.6- and 3.4-fold, respectively). In propranolol-treated cardiomyocytes, induction of the foetal genes by PE was enhanced (ANP, 7.1-fold; and β-MHC, 5.4-fold). Similar results were obtained with metoprolol, but not with ICI 118551. Veh, vehicle. *P<0.05, vs corresponding control group; ‡P<0.05, vs PE group. ANP, atrial natriuretic peptide; β-MHC, β-myosin heavy chain; skACT, skeletal α-actin; α-MHC, α-myosin heavy chain; SERCA, sarcoplasmic reticulum Ca2+-ATPase.
To evaluate the relative contribution of the β-adrenoceptor subtypes to the effects of propranolol on phenylephrine-induced foetal gene expression, a selective β1-adrenoceptor antagonist, metoprolol, and a selective β2-adrenoceptor antagonist, ICI 118551, were also tested. Metoprolol, similarly to propranolol, enhanced ANP and β-MHC gene expression, while ICI 118551 failed to affect phenylephrine-stimulated foetal gene expression (Figure 3).
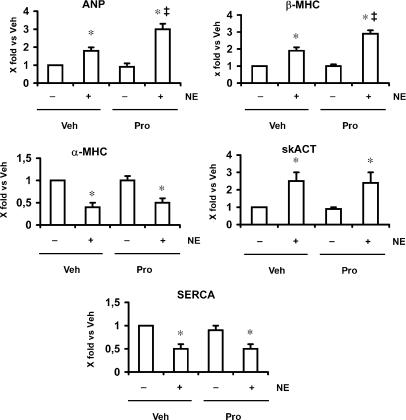
Effect of propranolol on the noradrenaline-induced foetal gene response
The results described above suggested that β-adrenoceptor stimulation was responsible for negatively modulating foetal gene expression. To further confirm that the effects of propranolol on the foetal gene expression are dependent on β-adrenoceptor blockade, cells were treated with noradrenaline, which is an agonist at both α- and β-adrenoceptors. Treatment of cardiomyocytes with noradrenaline induced the expression of foetal genes such as ANP, β-MHC and skACT, associated with the decrease in the expression of adult genes such as α-MHC and SERCA (Figure 4). Again, treatment with propranolol enhanced noradrenaline-stimulated ANP and β-MHC gene expression (Figure 4).
Figure 4.
Expression of noradrenaline-induced foetal genes in cultured neonatal cardiomyocytes. Cardiomyocytes were stimulated with noradrenaline (NE) (10 μM) for 24 h in the presence or absence of propranolol (Pro) (1 μM). Summary data for ANP, β-MHC, skACT, α-MHC and SERCA from three separate experiments performed in triplicate are shown. Data are presented as 2−ΔΔCt. Expression of ANP, β-MHC and skACT genes significantly increased in response to NE (1.8-, 1.9- and 2.5-fold, respectively). In propranolol-treated cardiomyocytes, induction of the foetal genes by NE was enhanced (ANP, 3.0-fold; and β-MHC, 2.9-fold). Veh, vehicle. *P<0.05, vs corresponding control group; ‡P<0.05, vs NE group. ANP, atrial natriuretic peptide; β-MHC, β-myosin heavy chain; skACT, skeletal α-actin; α-MHC, α-myosin heavy chain; SERCA, sarcoplasmic reticulum Ca2+-ATPase.
Discussion
In the present study, we provide evidence that propranolol, a non-selective β-adrenoceptor antagonist endowed with anti-hypertrophic activity, enhances cardiomyocyte foetal gene expression in response to hypertrophic stimuli via β1-adrenoceptor blockade. Additionally, given the lack of proportionality between changes of cardiac mass and mRNA levels of foetal genes, it is suggested that induction of the foetal genes and cardiomyocyte growth occurred as independent processes under pressure-overload conditions.
Foetal gene programme and β-blockers
To our knowledge, this is the first time that propranolol is shown to upregulate ANP, β-MHC and skACT gene expression in response to hypertrophic stimuli in cardiomyocytes. Considering that pressure overload triggers an enhanced sympathetic drive to the heart (Siri, 1988; Ganguly and Sherwood, 1991), our results could be interpreted as indicating that β-adrenoceptor stimulation has a suppressive role in regulating foetal gene expression. However, we also found that both propranolol and metoprolol were able to enhance ANP and β-MHC gene expression in response to phenylephrine, an α1-adrenoceptor agonist. This is an apparent discrepancy because phenylephrine, at high concentrations, causes cross-stimulation of β-adrenoceptors in cultured rat cardiomyocytes. Indeed, Markou et al. (2004) reported that pretreatment of cardiomyocytes with prazosin or propranolol alone partially inhibited phosphorylation of cAMP responsive element binding protein by phenylephrine, whereas addition of both antagonists completely prevented it. Furthermore, we have observed that phenylephrine at concentrations of 25 μM, which is that used in the present study, causes a significant increase in the intracellular cAMP amount (data unpublished).
The results of the present study are in contradiction with those of other studies designed to evaluate the effects of β-blockers on the foetal gene profile in failing hearts. In a rat model of heart failure induced by chronic pressure overload (Takeo et al., 2000), administration of propranol for 8 weeks caused an increase in SERCA and α-MHC gene expression along with a decrease in β-MHC gene expression. Changes in myocardial foetal gene expression were associated with a significant improvement both in diastolic and systolic cardiac function. Moreover, in a clinical study performed in patients with idiopathic dilated cardiomyopathy (Lowes et al., 2002), long-term treatment with carvedilol or metoprolol induced an improvement in left ventricular ejection fraction along with a more ‘physiological' myocardial gene expression. It was also shown that the increase in SERCA and α-MHC gene expression, coupled with a significant reduction in β-MHC, was only observed in β-blocker-treated patients who had an increase in the left ventricular ejection fraction. We cannot, so far, reconcile the differences between these results and ours. One possibility, which remains to be investigated, is that β-adrenoceptor blockade causes a paradoxical enhancement of the foetal gene expression at a very early stage of the mouse model of TAC-induced cardiac hypertrophy, when cardiac function is still unchanged. It is also possible that the discordant responses are due to differences in dose and duration of treatment.
Foetal gene programme and cardiac hypertrophy
With few exceptions, pathological cardiac hypertrophy has been associated with the upregulation of foetal genes such as ANP, β-MHC and skACT. The significance of this association, however, is still not clear. To date, there are two opposing views. One position interprets these changes as an integral part of a more complex adaptive and cardioprotective process aimed at reducing energy demand. Specifically, β-MHC would allow the heart to work more efficiently when chronically overloaded (Izumo et al., 1988), and ANP would exert local anti-hypertrophic actions (Holtwick et al., 2003). The other view holds that the induction of the foetal gene programme can compromise cardiac contractility and therefore contribute to the progression to heart failure. This view is consistent with the poor tolerance of transgenic hearts expressing β-MHC to mechanical or pharmacological cardiovascular stress (Krenz and Robbins, 2004). Our data show that the increased expression of the foetal genes is not associated with early deterioration of cardiac function and contractility, at least over the short term, which supports the adaptive hypothesis.
Because of the strong association between cardiomyocyte hypertrophy and induction of foetal genes, expression of some of these genes has often been used as a marker of hypertrophy. ANP, β-MHC and skACT have each been observed to be regulated, individually or in combination, in various mouse models of cardiac hypertrophy and measurements of ANP, β-MHC and skACT gene expression are an important tool to sub-classify hypertrophy phenotypes. Given that propranolol causes an enhancement in cardiac mRNA levels of ANP, β-MHC and skACT genes that is unrelated to enlargement of cardiomyocytes, caution must be exerted when hypertrophic markers are selected for evaluating the extent of hypertrophic response in the presence of β-adrenoceptor blockade. Our observations also suggest that the signalling pathways leading to changes in gene expression and cell size in response to the pressure overload are divergent. Further, blockade of β-adrenoceptors positively modulates induction of the foetal gene programme. This view is consistent with the results of other studies indicating that the induction of the foetal gene programme and hypertrophic growth of cardiomyocytes can be disassociated (Boluyt et al., 1997; Jeong et al., 2005).
At this time, the mechanism responsible for the anti-hypertrophic effect of propranolol remains to be elucidated. In an earlier report (Marano et al., 2002), we hypothesized that propranolol attenuates LVH via a β-adrenoceptor-independent mechanism, perhaps related to the local anaesthetic/membrane stabilizing action of the drug which seems to be due to sodium channel blockade. However, studies performed on heterozygous knockout mice for the SCN5A sodium channel gene do not support that hypothesis (data unpublished). Another possibility, that remains to be explored in future studies, is that propranolol, under pressure-overload conditions, promotes β-arrestin recruitment to β-adrenoceptors, confining activated extracellular signal-regulated kinases (ERKs) to the cytoplasm and thus attenuating the effect of activated ERKs on the phosphorylation of early growth response factor-1, an inducible trascriptional factor involved in cardiac hypertrophy induced by pressure overload (Buitrago et al., 2005). Indeed, recent evidence indicate that β-arrestins segregate activated ERK to specific subcellular locations preventing its translocation in the nucleus (Shenoy and Lefkowitz, 2005).
In summary, our results indicate that, in the presence of hypertrophic stimuli, propranolol promotes expression of the foetal gene programme via β1-adrenoceptor blockade. Additionally, it is suggested that foetal gene expression and cardiomyocyte growth are not tightly linked processes under pressure-overload conditions.
Acknowledgments
We are grateful to Mr Valerio Vago for technical support. This work was supported in part by grants from the Italian Minister of Health to GM (2004/N01).
Abbreviations
- TAC
thoracic aortic coarctation
Conflict of interest
The authors state no conflict of interest.
References
- Boluyt M, Long X, Eschenhagen T, Mende U, Schmitz W, Crow MT, et al. Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. Am J Physiol. 1995;269:H638–H647. doi: 10.1152/ajpheart.1995.269.2.H638. [DOI] [PubMed] [Google Scholar]
- Boluyt MO, Zheng J-S, Younes A, Long X, O'Neill L, Silverman H, et al. Rapamycin inhibits α1-adrenergic receptor-stimulated cardiac myocyte hypertrophy but not activation of hypertrophy-associated genes. Evidence for involvement of p70 S6 kinase. Circ Res. 1997;81:176–186. doi: 10.1161/01.res.81.2.176. [DOI] [PubMed] [Google Scholar]
- Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- Buitrago M, Lorenz K, Maass AH, Oberdorf-Maass S, Keller U, Schmitteckert EM, et al. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nat Med. 2005;11:837–844. doi: 10.1038/nm1272. [DOI] [PubMed] [Google Scholar]
- Ganguly PK, Sherwood GR. Noradrenaline turnover and metabolism in myocardium following aortic constriction in rats. Cardiovasc Res. 1991;25:579–585. doi: 10.1093/cvr/25.7.579. [DOI] [PubMed] [Google Scholar]
- Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong MY, Kinugawa K, Vinson C, Long CS. AFos dissociates cardiac myocyte hypertrophy and expression of the pathological gene. Circulation. 2005;111:1645–1651. doi: 10.1161/01.CIR.0000160367.99928.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I, Kurabayashi M, Shibazaki Y, Takaku F, Yazaki Y. Molecular cloning and characterization of a Ca2++Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum: regulation of its expression by pressure overload and developmental stage. J Clin Invest. 1989;83:1102–1108. doi: 10.1172/JCI113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004;44:2390–2397. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- Marano G, Palazzesi S, Fadda A, Vergari A, Ferrari AU. Attenuation of aortic banding-induced cardiac hypertrophy by propranolol is independent of beta-adrenoceptor blockade. J Hypertens. 2002;20:763–769. doi: 10.1097/00004872-200204000-00036. [DOI] [PubMed] [Google Scholar]
- Marano G, Palazzesi S, Vergari A, Catalano L, Gaudi S, Testa C, et al. Inhibition of left ventricular remodelling preserves chamber systolic function in pressure-overloaded mice. Pflugers Arch. 2003;446:429–436. doi: 10.1007/s00424-003-1059-2. [DOI] [PubMed] [Google Scholar]
- Marano G, Vergari A, Catalano L, Gaudi S, Palazzesi S, Musumeci M, et al. Na+/H+ exchange inhibition attenuates left ventricular remodeling and preserves systolic function in pressure-overloaded hearts. Br J Pharmacol. 2004;141:526–532. doi: 10.1038/sj.bjp.0705631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou T, Hadzopoulou-Cladaras M, Lazou A. Phenylephrine induces activation of CREB in adult rat cardiac myocytes through MSK1 and PKA signaling pathways. J Mol Cell Cardiol. 2004;37:1001–1011. doi: 10.1016/j.yjmcc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Nishio R, Shioi T, Sasayama S, Matsumori A. Carvedilol increases the production of interleukin-12 and interferon-γ and improves the survival of mice infected with the encephalomyocarditis virus. J Am Coll Cardiol. 2003;41:340–345. doi: 10.1016/s0735-1097(02)02711-0. [DOI] [PubMed] [Google Scholar]
- Ostman-Smith I. Reduction by oral propranolol treatment of left ventricular hypertrophy secondary to pressure-overload in the rat. Br J Pharmacol. 1995;116:2703–2709. doi: 10.1111/j.1476-5381.1995.tb17230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992;267:10551–10560. [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Angiotensin II-stimulated signaling through G proteins and β-arrestin. Sci STKE. 2005;311:cm 14. doi: 10.1126/stke.3112005cm14. [DOI] [PubMed] [Google Scholar]
- Siri FM. Sympathetic changes during development of cardiac hypertrophy in aortic-constricted rats. Am J Physiol. 1988;255:H452–H457. doi: 10.1152/ajpheart.1988.255.3.H452. [DOI] [PubMed] [Google Scholar]
- Takeo S, Elmoselhi AB, Goel R, Sentex E, Wang J, Dhalla NS. Attenuation of changes in sarcoplasmic reticular gene expression in cardiac hypertrophy by propranolol and verapamil. Mol Cell Biochem. 2000;213:111–118. doi: 10.1023/a:1007120332587. [DOI] [PubMed] [Google Scholar]
- van den Bosch BJC, Lindsey PJ, van den Burg CMM, van der Vlies SA, Lips DJ, van der Vusse GJ, et al. Early and transient gene expression changes in pressure overload-induced cardiac hypertrophy in mice. Genomics. 2006;88:480–488. doi: 10.1016/j.ygeno.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen Fand Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]