Abstract
Gene therapy is emerging as a potential treatment option in patients suffering from a wide spectrum of cardiovascular diseases including coronary artery disease, peripheral vascular disease, vein graft failure and in-stent restenosis. Thus far preclinical studies have shown promise for a wide variety of genes, in particular the delivery of genes encoding growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) to treat ischaemic vascular disease both peripherally and in coronary artery disease. VEGF as well as other genes such as TIMPs have been used to target the development of neointimal hyperplasia to successfully prevent vein graft failure and in-stent restenosis in animal models. Subsequent phase I trials to examine safety of these therapies have been successful with low levels of serious adverse effects, and albeit in the absence of a placebo group some suggestion of efficacy. Phase 2 studies, which have incorporated a placebo group, have not confirmed this early promise of efficacy. In the next generation of clinical gene therapy trials for cardiovascular disease, many parameters will need to be adjusted in the search for an effective therapy, including the identification of a suitable vector, appropriate gene or genes and an effective vector delivery system for a specific disease target. Here we review the current status of cardiovascular gene therapy and the potential for this approach to become a viable treatment option.
Keywords: gene therapy, cardiovascular disease, therapeutic angiogenesis, peripheral vascular disease, coronary artery disease, neointimal hyperplasia, vein graft, in-stent restenosis
Introduction
Cardiovascular disease has been estimated to cause 17 million deaths per year globally and is one of the most common causes of death in the developed world (WHO, 2006). An epidemic of cardiovascular disease is now taking place and it has been estimated that it will be the leading cause of mortality in the developing world within the next decade (WHO, 2006). The emerging field of gene therapy is recognised as having the potential to add to the therapeutic armamentarium for cardiovascular disease by treating patients with disease, which is resistant to current approaches (Isner, 2002).
The introduction of transgenes into the vasculature has the advantage of providing long-term expression where required and also allows the therapy to specifically target the disease process involved. Ease of access to the circulatory system is another advantage for this type of therapy. Gene therapy in addition to helping control symptomatic presentation of cardiovascular disease may also be able to reverse the pathological processes involved. However, to achieve these objectives three goals must be met: suitable vectors must be generated, a suitable gene or group of genes must be identified and an appropriate delivery system needs to be developed (Figure 1). Optimal characteristics of these features may vary depending on the disease being targeted.
Figure 1.
The factors required for successful cardiovascular gene therapy.
This review will focus on four potential therapeutic targets for cardiovascular gene therapy using two therapeutic approaches. The first approach uses therapeutic angiogenesis to treat peripheral vascular disease and ischaemic heart disease. The second approach attempts to use gene therapy to prevent neointimal hyperplasia, which targets vein graft bypass failure and in-stent restenosis. Pharmacotherapy often requires long-term administration while rarely addressing the underlying pathophysiology. Interventional techniques are subject to failure in a significant proportion of cases. The formation of neointimal hyperplasia in vein grafts or stented vessels may cause failure of these therapeutic approaches. Even with optimal pharmacotherapy some forms of cardiovascular disease may be refractory to treatment and prognosis remains poor for many patients.
Vectors
Choosing the correct vector for gene therapy applications is probably the biggest challenge faced by gene therapists (Baker, 2004). The ideal vector would be noncytotoxic, highly efficient, have a large capacity, possess an appropriate tissue tropism preventing distal spread and be non-immunogenic. Recombinant viruses represent an efficient system for delivering genes to human tissues. Viruses commonly employed include retroviruses such as lentivirus, adenovirus and adeno-associated virus (AAV). However, there are many hazards associated with using viral gene therapy in humans. These include the induction of a host immune response, random insertional mutagenesis, the presence of wild-type vector in the administered preparation and unsuitable tissue tropism.
Viral vectors
Retroviruses were among the first vectors used for delivery of specific transgenes to the vasculature (Nabel et al., 1990). Retroviruses are RNA viruses, which insert their viral genome into the host chromosome. Although this results in prolonged tissue expression, it opens the putative possibility of random insertional mutagenesis and other forms of carcinogenesis. Despite this, retroviral vectors have been the most widely used vector in gene therapy clinical trials to date. The use of this vector to treat X-linked severe combined immunodeficiency syndrome, resulted in three patients developing a T-cell proliferation (Hacein-Bey-Abina et al., 2003) probably due to an altered IL-2 receptor with subsequent development of lymphoma (Woods et al., 2006). Moreover, retroviral vectors have poor uptake into nonmitotic cells, which are commonly found in vascular tissues. Developments to improve the safety and efficacy of these vectors include pseudotyping of retroviruses which changes the tropism of a virus due to alterations in its capsid proteins (Burns et al., 1993).
Lentiviruses are a subgroup of retroviruses which unlike the other members of the group are capable of transducing nondividing cells such as those found in the blood vessel wall (Totsugawa et al., 2002). The low titre achievable and safety concerns regarding random insertional events are drawbacks of this vector for use in gene therapy. However, initial work by Dishart et al. (2003) suggests that lentiviruses pseudotyped with the vesicular stomatis virus glycoprotein efficiently transduce vascular cells in vitro. Similarly, an in vivo gene therapy strategy using such vectors to deliver vascular endothelial growth factor (VEGF) and angiopoietin 2 to hindlimb ischaemia in a rabbit model has been effective (Conklin et al., 2005). Although to date, lentivirus remains little used in vascular gene therapy, its lower immunogenicity may offer advantages over other vector types.
Adenoviruses are double-stranded DNA viruses and unlike retroviruses do not integrate into the host genome and therefore do not have the associated risk of insertional mutagenesis. Adenoviral vectors are becoming increasingly popular as vectors in gene therapy with high titres achievable and a broad tissue tropism (OhNo et al., 1994; Varenne et al., 1998). They have been shown to be particularly effective in vascular cell types and can transduce quiescent cells efficiently. However, they may result in the induction of an immune response, which can decrease their effectiveness. The advent of the so-called ‘gutless' or helper dependent adenoviral vectors lacking any viral genetic elements may improve longer-term delivery by reducing the host immune response. They also have larger capacity than earlier generation adenoviruses and may allow multiple gene delivery in a single vector (Jozkowicz and Dulak, 2005).
AAVs are nonpathogenic parvoviruses that require cells to be doubly infected by a helper virus, such as adenovirus, to replicate in nature. They have recently emerged as potential gene therapy vectors. They have a small genome (<5 kb) and carry single-stranded DNA. Like adenoviral vectors, they can infect nondividing cells and can be concentrated to relatively high titres. In contrast to adenoviruses and similar to lentiviruses they can integrate into the host genome in nature but this property is lost in recombinant AAV, eliminating the risk of insertional mutagenesis (Young et al., 2000). AAVs have lower immunogenic potential than adenoviral vectors coupled with a prolonged expression. Although the most widely used serotype, AAV2, initially demonstrated some promise in the vasculature (Nicklin et al., 2001), further in vivo studies demonstrated AAV1 and 5 to be superior in vivo (Chen et al., 2005), whereas AAV7 and -8 have been shown to transduce endothelial cells poorly (Denby et al., 2005). The various serotypes identified have differing tissue tropisms, which are currently the source of active investigation. These serotypes may, in addition, be enhanced through tissue tropism modifications to their capsid proteins. Using in vivo biopanning, a study by Work et al. (2006) significantly improved delivery of AAV2-modified vectors to the vasculature.
Nonviral vectors
Naked plasmid DNA has been used to deliver genes effectively to the vasculature and although the efficiency is low this limitation can be overcome by the use of a potent secretory transgene such as VEGF (Majesky, 1996). Attempts to use artificially created liposome's to carry naked DNA as a method of gene delivery to the vasculature initially met with low efficiency and transient gene expression (Laitinen et al., 1997), although this is improving and has shown potential in therapeutic applications (Hedman et al., 2003; Deiner et al., 2006). Various polymers have also been proposed for use to improve nonviral delivery of genes. Nonviral delivery of DNA may be augmented at various levels including systemic delivery using polysine derivatives or at the organ level using hydrogels (Putnam, 2006). The biophysical properties of these compounds may enhance the stability, concentration as well as uptake of DNA by cells (Putnam, 2006) and require a systematic examination of their properties in this regard.
Other physical methods of delivering genes such as electroporation useful in many organs such as skeletal muscle and liver may also have a role in ensuring uptake of transgenes in the vasculature after either adventitial or luminal delivery (Dean, 2003). Direct injection followed by electroporation of the heart may also improve plasmid or liposome-based gene delivery (Harrison et al., 1998; Wang et al., 2001). A secretory product may prove useful in providing a paracrine effect from the genetically modified cells.
Delivery systems
Local gene delivery to focal lesions in the coronary or peripheral vasculature is ideally suited to the use of catheter-based vector delivery. Percutaneous delivery of gene vector-containing solutions to the vasculature has been attempted using various balloon catheters in animal models and in human trials (Hedman et al., 2003; Sharif et al., 2004). Double-balloon catheters were the first used and these form a space between them that allows infusion of vector-containing solutions (Goldman et al., 1987; Rome et al., 1994). Although prolonged, efficient delivery of adenoviral vectors to the arterial wall has been demonstrated using this technique it is possible that the total occlusion of vessels for significant periods of time may cause problems due to downstream ischaemia. Other catheters have also been used which have porous balloons through which vector-containing solutions infuse under high pressure (Hedman et al., 2003). However, these catheters are associated with low efficiency of delivery in animal models (Wolinsky and Thung, 1990). Other variations of catheter type which have been used for gene therapy applications include hydrogel catheters where the vector is incorporated into a gel on the surface of the catheter and dispatch catheters which maintain vascular flow during vector delivery but require a long dispatch time of 60 min and summarised by Sharif et al. (2004). In contrast to the other catheters mentioned thus far, infiltrator catheters allow injection of vector into the vessel wall via micro injection needles which decreases the chances of systemic spread of the vector and may improve medial delivery of the transgene (Wang et al., 2003).
Stents are an ideal platform for localised delivery of vectors to the vascular wall due to their widespread use, safety and permanent scaffold structure. Currently available drug eluting stents have already demonstrated the ability of a stent to act as a reservoir for therapeutic agents. Gene delivery from stents has been achieved using cellular-based techniques (Koren et al., 2006) as well as various formulations on the stent surface to alter the vector binding/release kinetics such as polyurethane (Takahashi et al., 2003), phosphorylcholine (Wu et al., 2003; Sharif et al., 2006), polylactic-polyglycolic acid (Klugherz et al., 2000) and bisphosphonate (Fishbein et al., 2006). Alternatively specific antibody tethering may also be used to attach the vector to the stent and this has been shown using anti-adenoviral antibodies (Klugherz et al., 2002). Localised delivery of adenoviral/plasmid vectors to the vasculature may be confined to the intimal or neointimal layers due to anatomical barriers (Rome et al., 1994). However this may be sufficient because overexpression of angiogenic factors or inhibitors of neointimal proliferation in the endothelial layer are only transiently required for the applications discussed in this review. However, medial delivery can also be possible through an intact elastic lamina using other vector types or may also be achieved incidentally by rupturing of the elastic lamina due to angioplasty.
Gene therapy strategies
Therapeutic angiogenesis
Introduction
A significant proportion of patients with ischaemic vascular disease remain refractory to pharmacological therapies and are unsuitable candidates for percutaneous or surgical interventions. This has led to the development of therapies to stimulate neovascularisation, a strategy known as therapeutic angiogenesis. This approach attempts to stimulate the development of collateral blood vessels, which result in the formation of bypass vessels around occluded arteries. Studies by Folkman et al. (1971) suggested that the process of vasculogenesis (de novo vessel formation) and angiogenesis (branches budding from existing vessels) are both essential for the establishment and maintenance of a vascular supply, and that angiogenic growth factors are required for neovascularisation (Folkman et al., 1971). Angiogenesis occurs in response to stimuli including hypoxia, ischaemia, mechanical stretch and inflammation (Folkman and Shing, 1992) and is mediated by the proliferation and migration of preexisting, fully differentiated endothelial cells, which line the parent vessel walls.
A thorough understanding of the biology of angiogenesis is required to predict the most appropriate genes for use in therapeutic angiogenesis. Under normal physiological conditions endothelial cells undergo a low level of turnover. However, under pathological conditions this can be significantly increased with a shift in balance of proangiogenic and angiostatic factors. For example, the development of collateral circulation in the myocardium occurs naturally in tissue that is exposed to chronic mild ischaemia. These conditions induce a revascularisation process, for example, through the activity of VEGF (Lee and Feldman, 1998; Lee et al., 2003).
VEGF acts on endothelial cells to increase proliferation and vascular permeability (Josko et al., 2000). VEGF has four isoforms 121, 159, 206 and 165 which act on tyrosine kinase receptors, FLK-1 and FT1. It can be induced in endothelial cells in response to a number of stimuli including hypoxia, interleukin (IL)-1, endothelin-1, cAMP, Ca2+, steroids and cytokines including tissue growth factor, platelet-derived growth factor and fibroblast growth factor (FGF). Alternatively cGMP decreases VEGF levels as does nitric oxide (NO) (Josko et al., 2000). In addition to VEGF a number of other candidate genes have been suggested for use in therapeutic angiogenesis including FGF, hepatocyte growth factor (HGF) as well as developmentally regulated endothelial locus 1 (Del-1) (Giordano et al., 1996; Aoki et al., 2000; Zhong et al., 2003).
FGFs are a group of growth factors that are released into the extracellular matrix by cell death or damage. Their potential use in therapeutic angiogenesis has been comprehensively reviewed by Khurana and Simons (2003). FGF consists of 22 related proteins, which affect cellular growth, proliferation and migration (Ornitz and Itoh, 2001). These proteins also act via tyrosine kinase receptors in this case four high affinity receptors. FGF3, -4 and -5 are known to have significant regulatory effects on angiogenesis. FGF2 has been shown to play a role in the generation of larger vessels not just capillary buds, which may be useful in relieving chronic, stable angina. FGFs are potent endothelial cell mitogens and also serve as ligands for other cell types including vascular smooth muscle cells and fibroblasts (Ornitz and Itoh, 2001). The FGFs, such as VEGF, also stimulate endothelial cell synthesis of proteases including plasminogen activator and metalloproteinases, important for extracellular matrix digestion in the process of angiogenesis.
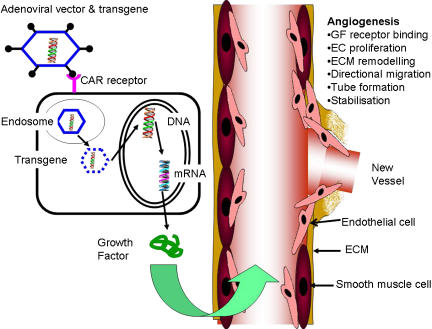
The strategy of therapeutic angiogenesis (Figure 2) has been used in peripheral vascular disease and coronary artery disease both in preclinical models (Table 1) and subsequently in clinical trials (Table 2). At critical points in the disease process a relatively brief expression of transgene may be sufficient which would not require efficient gene delivery, particularly if the transgene product is potent and secreted.
Figure 2.
Therapeutic angiogenesis. An example of adenoviral delivery of growth factor transgenes to the vasculature.
Table 1.
Summary of selected preclinical gene therapy studies
| Target | Model | Transgene | Study | End point |
|---|---|---|---|---|
| Peripheral vascular disease | HLI, rabbit | VEGF and bFGF | Kondoh et al. (2004) | Combination effective for collaterals |
| HLI, rabbit | bFGF | Ishii et al. (2004) | Significant collateralisation | |
| HLI, rabbit | VEGF165 and Ang-1 | Ohara et al. (2001) | Significant neovascularisation | |
| HLI, mouse | VEGF121, -165, -189 | Whitlock et al. (2004) | Combination effective | |
| Myocardial ischaemia | Ameroid LCCA, pig | VEGF165 | Laguens et al. (2002) | Increased cariomyocyte mitosis |
| Ameroid LCCA, mini-swine | VEGF165 | Zhang et al. (2002) | Decreased ischaemia area and increased collaterals | |
| Stress, pig | FGF4 | Gao et al. (2004) | No toxic effects | |
| Ameroid LCCA pig | HIFα | Heinl-Green et al. (2005) | Increased perfusion | |
| Neointimal hyperplasia | Vascular graft, pig | TIMP-3 | Akowuah et al. (2005) | Increased luminal area |
| Vascular graft, rat | Antisense TGF | Wolff et al. (2005) | Reduced neointimal hyperplasia | |
| Vascular graft, rabbit | NOS | West et al. (2001) | Reduced neointimal hyperplasia | |
| Vascular graft, pig | p53 | Wan et al. (2004) | Reduced neointimal hyperplasia | |
| PTA and stent, rat | iNOS | Fishbein et al. (2006) | Reduced ISR | |
| PTA and stent, pig | TIMP-3 | Johnson et al. (2005) | Reduced ISR | |
| PTCA and stent, rabbit | VEGF-2 | Walter et al. (2004) | Reduced ISR and increased RE |
Abbreviations: bFGF, basic fibroblast growth factor; HIFα, hypoxia-inducible factor α; HLI, hindlimb ischaemia; iNOS, inducible nitric oxide synthase; ISR, in-stent restenosis; LCCA, ameroid constrictor placed on the left circumflex artery; NOS, nitric oxide synthase; PTA, percutaneous transluminal angioplasty; RE, re-endothelialisation; TGF, tissue growth factor; TIMP-3, tissue inhibitor of matrix metalloproteinase-3; VEGF, vascular endothelial growth factor.
Table 2.
Summary of cardiovascular gene therapy and genetic decoy-based clinical trials
| DiseaseTarget | Trans gene | Study | Vector | Delivery system | Placebo | No | Primary end points | Result |
|---|---|---|---|---|---|---|---|---|
| CLI | HGF | Morishita et al. (2004) | Plasmid | IM | No | 6 | Pain, ABI, Ulcer | Improved |
| VEGF | Baumgartner et al. (1998) | Plasmid | Catheter | No | 9 | ABI, limb salvage | Improved | |
| VEGF | Isner et al. (1996) | Plasmid | Catheter | No | 1 | Angiography, Doppler | Improved | |
| PVD | VEGF | Kim et al. (2004) | Plasmid | IM | No | 9 | Angiography, ABI, pain | Improved |
| VEGF | Rajagopalan et al. (2001) | Adenovirus | IM | No | 5 | Endothelial activity | Improved | |
| Intermittent claudification | VEGF | Rajagopalan et al. (2003) (RAVE) | Adenovirus | IM | Yes | 105 | Peak walking time | NSD |
| VEGF | ||||||||
| Kusumanto et al. (2006) | Plasmid | IM | Yes | 27 | Amputation rate at 100 days | NSD | ||
| Angina | VEGF | Losordo et al. (2002) | Plasmid | Catheter | Yes | 19 | CCS angina class | Improved |
| Fortuin et al. (2003) | Plasmid | Thoracotomy | No | 5 | CCS angina class | Improved | ||
| FGF | Grines et al. (2002) (AGENT I) | Adenovirus | Catheter | Yes | 79 | ETT | Trend | |
| Grines et al. (2003) (AGENT II) | Adenovirus | IC injection | Yes | 52 | Reversible perfusion defect size | Trend | ||
| VBG | E2F decoy | Mann et al. (1999) (PREVENT I) | DNA | Instilled | Yes | 41 | Safety and occlusions | Trend |
| Grube et al. (2001) (PREVENT II) | DNA | Instilled | Yes | 200 | Occlusion, vessel wall thickness | Improved (30%) | ||
| Conte et al. (2005) (PREVENT III) | DNA | Instilled | Yes | 1404 | Reintervention | NSD | ||
| Alexander et al. (2005) (PREVENT IV) | DNA | Instilled | Yes | 3014 | Angiographic failure | NSD | ||
| ISR | VEGF | Laitinen et al. (2000) | PL/Lipo | I/P catheter | No | 15 | Safety, angiography | NSD |
| Hedman et al. (2003) | PL/Lipo/Ad | I/P catheter | No | 103 | Angiography, restenosis | NSD | ||
| (KAT I) | Myocardial perfusion |
Abbreviations: ABI, ankle-brachial index; Ad, adenovirus; CCS, Canadian Cardiovascular Society; CLI, chronic limb ischaemia; ETT, excercise treadmill test; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; I/P, infusion-perfusion; IC, intracoronary; IM, intramuscular; ISR, in-stent restenosis; Lipo, liposome; NSD, no significant difference; PL, plasmid; PVD, peripheral vascular disease; VBG, vascular bypass graft; VEGF, vascular endothelial growth factor.
Peripheral vascular disease
Atherosclerosis may develop in the peripheral circulation leading to critical limb ischaemia. It is estimated to develop in 500–1000 people per million per year and with age as a risk factor has an increasing prevalence in our aging society (Hooi et al., 2001; Morishita, 2004). The prognosis for patients with chronic critical leg ischaemia is often poor. These patients continue to suffer from the debilitating symptoms of their disease and remain at risk for limb amputation.
Preclinical studies
The favoured model for studying peripheral vascular disease is the hindlimb ischaemia model usually in mouse, rat or rabbit models (Takeshita et al., 1994; Taniyama et al., 2001; Dai et al., 2002; Xie et al., 2006) although the pig model has also been examined (Pipp et al., 2004). Both viral and nonviral methods have been used to deliver transgenes to the muscle of the ischaemic limb in the various models mentioned above. Nonviral methods include direct injection of plasmid DNA as well as utilising formulations of lipids or polymers to aid DNA uptake by cells (Ledley, 1994). Various growth factors have been studied and shown to induce angiogenesis in these models including FGF, HGF, IGF and in particular VEGF which has been shown to be successful in inducing collateralisation in both rat and rabbit hind-limb ischaemia models (Tsurumi et al., 1996; Rasmussen et al., 2002; Brar et al., 2004). Delivery has been shown to be effective whether by direct injection of naked DNA or by adenoviral delivery. An adenoviral vector carrying the VEGF 121 isoform and driven by a CMV promoter has been termed the BIOBYPASS and has shown promise in preclinical studies (Basara, 2001). Other methods of delivery of VEGF and FGF include a combined gene and cell therapy method, which packages these genes ex vivo into autologous fibroblasts. Interestingly, when fibroblasts transduced with both genes were introduced into the rabbit ischaemic limb there was a synergistic effect observed for neovascularisation (Kondoh et al., 2004). Other potential angiogens have also been identified as being potentially useful for treating peripheral ischaemia including the telomerase (Zaccagnini et al., 2005) or the extracellular matrix protein, del-1, a potent angiogenic factor during embryogenesis (Penta et al., 1999; Rezaee et al., 2002; Zhong et al., 2003).
Thus far, the majority of studies, which have examined the use of gene therapy in treating ischaemic disease, have used delivery of single genes. These single genes are either at critical control points in the underlying pathology or represent the start of a cascade of effects. Other potential methods, which have been examined in preclinical models, include the use of more than one active gene or the use of transcription factor overexpression, which would result in induction of a repertoire of genes. Elegant methods of ameliorating vasculogenesis have been put forward in preclinical models using bicistronic vectors carrying VEGF as well as angiopoeitin or using multiple isoforms of VEGF in various ratios (Niagara et al., 2004; Whitlock et al., 2004; Yu et al., 2006). Collectively, these data suggest that revascularisation may be more efficiently achieved through complex simultaneous activation of various pathways. Potential has also been shown using ex vivo gene therapy of fibroblasts using adenoviral vectors (Ohara et al., 2001; Ishii et al., 2004).
In summary, a large volume of preclinical efficacy and toxicology data paved the way for human studies, which will next be reviewed.
Clinical trials
Trials involving gene therapy to stimulate angiogenesis have been undertaken in patients with peripheral vascular disease. The efficacy of therapeutic angiogenesis using VEGF gene transfer has been reported in human patients with critical limb ischaemia (Baumgartner et al., 1998). Initially, the use of a hydrogel catheter with naked VEGF165 plasmid was proposed based on ischaemic hindlimb models (Isner et al., 1996). This procedure can effectively stimulate collateral blood vessel development in animal models; however, many patients do not have appropriate target lesions amenable to catheter-based gene delivery, so this was not considered an ideal treatment. Thus, intramuscular injection of naked plasmid encoding VEGF165 was used (Baumgartner et al., 1998). This trial involved small numbers of patients and did not enrol placebo controls but did demonstrate tolerability and promising clinical efficacy for treatment of peripheral arterial disease Kim et al. (2004). also reported the results of a phase I clinical trial using naked plasmid DNA encoding for VEGF165 in patients with severe debilitating peripheral vascular disease. Again the injections were well tolerated and results showed increased collateral vessel formation around the injection sites. Ischaemic pain and ischaemic ulcers in the affected limb were relieved or markedly improved in a significant number of patients but again a placebo control group was not included.
The above trials used naked plasmid DNA encoding for VEGF, however adenoviral VEGF121 has also been investigated with similar results. Initially, Rajagopalan et al. (2001) reported that administration of intramuscular AdVEGF121.10 to skeletal muscle of five patients with severe peripheral vascular disease improved endothelial function and lower extremity flow reserve.
HGF has also been examined in the context of treating peripheral ischaemia. A prospective, open-labelled clinical trial was carried out, which examined the safety and efficacy of intramuscular injections of HGF plasmid DNA in patients with chronic limb ischaemia (Morishita et al., 2004). This limited trial had promising initial results with the treatment reducing the pain index in 5/6 patients, increasing the ankle pressure index by >0.1 in all patients, and improving the size of ischaemic ulcers by >25% in four patients. However, as acknowledged by the authors, the small numbers and lack of a control group prevented meaningful conclusions being made regarding efficacy although short-term tolerability of the treatment was observed.
In contrast to the promising data emanating from preclinical and phase I studies, the Regional Angiogenesis with Vascular Endothelial Growth Factor trial (RAVE Trial), which was reported in 2003, demonstrated a less optimistic outcome using intramuscular AdVEGF121 (Rajagopalan et al., 2003). This trial was a phase II, randomised, double-blind, placebo-controlled trial examining the safety and efficacy of administration of intramuscular VEGF 121 isoform to the lower extremities of patients with disabling unilateral intermittent claudication. The outcome of this study demonstrated that administration of AdVEGF121 was not associated with improved exercise performance or quality of life. A similar lack of efficacy for a primary endpoint in preventing amputations has been observed in placebo-controlled trials where plasmid VEGF165 was delivered intramuscularly to diabetic patients with critical limb ischaemia. However, ulceration rates and other clinical end points were reduced (Kusumanto et al., 2006).
Coronary artery disease
At present the accepted method of treatment for coronary artery disease is by pharmacotherapy, risk factor modification or through invasive interventions. These interventions include percutaneous transcoronary angioplasty (PTCA) with or without stenting or coronary artery bypass grafting (CABG). Patients may fail to respond to risk factor modification or pharmacotherapy. In addition, recurrent coronary ischaemia may occur, subsequent to either intervention, due to graft failure or in-stent restenosis. These patients, refractory to current therapies, would be the main targets for gene therapy approaches to treatment initially. However, it is evident that once such therapies become more accepted that a broader patient population may then be amenable to treatment using these strategies.
Preclinical studies
Gene therapy treatment of myocardial ischaemia is a more difficult proposition than peripheral ischaemia due to issues of access and delivery of the vector. To investigate putative gene therapies, it is common to use larger animal models (Hughes et al., 2003) including the pig (Laguens et al., 2002; Zhang et al., 2002; Grines et al., 2003a; Gao et al., 2004; Heinl-Green et al., 2005; Horvath et al., 2005). However, there are limitations to the animal data generated because it is difficult to mimic the human condition of chronic stable ischaemia and easier to induce an acute injury through a myocardial infarction.
Similarly to peripheral ischaemia models both VEGF (Laguens et al., 2002; Zhang et al., 2002) and FGF (Grines et al., 2003a; Gao et al., 2004; Horvath et al., 2005) and in particular FGF-4 are popular targets for gene therapy to treat chronic and acute myocardial ischaemia. Results using these genes have been promising in animal models as have other molecular targets including transcription factors and the master-switch HIF-1α (Shyu et al., 2002; Heinl-Green et al., 2005).
Studies have also examined methods to improve the efficiency of gene delivery to the diseased myocardium. An elegant method has been identified to express transgenes only under appropriately hypoxic conditions to avoid pathological angiogenesis (Tang et al., 2002, 2005). This is achieved using a hypoxia-inducible VEGF gene therapy system using the erythropoietin enhancer and a water-soluble lipopolymer or alternatively using a double plasmid system. Cardiomyocytes may also be intrinsically protected from hypoxic damage by the induction of Akt, which may aid myocyte survival, through upstream overexpression of insulin-like growth factor-1 (Chao et al., 2003). An exciting area of advancement in the field of gene therapy is the combined use of both gene therapy with stem cell therapy in particular the use of bone marrow-derived mesenchymal stem cell (MSC) lines. These pluripotent cells can differentiate into myocytes, but have only demonstrated minor improvement in the function of damaged myocardium when transplanted in a pig model (Shake et al., 2002). However, the work of Mangi et al. (2003), and Noiseux et al. (2006) has demonstrated that overexpression of Akt in MSCs ex vivo, significantly improves the effects of this approach in restoring myocardial function in vivo after ischaemic damage. It is probable that such effects are most likely paracrine in nature rather than due to an enhanced survival of MSCs in situ conferred by Akt.
Clinical trials
The system for vector delivery assumes considerable importance in the heart. The various studies reviewed below have examined methods of direct injection to the myocardium through a mini-thoracotomy or in conjunction with CABG (Rosengart et al., 1999b) or through infusion from an intracoronary catheter or cardiac navigation system allows system which is direct intramyocardial delivery to viable areas of myocardium (Kastrup et al., 2005).
A study by Losordo et al. (2002) examined the use of naked plasmid DNA encoding VEGF delivered directly to the myocardium by injection through a mini-thoracotomy. The patients had stable chronic angina refractory to current treatments with Canadian Class 3–4 angina. Symptomatic relief in all five patients was observed between 10 and 30 days later suggesting that gene expression had reached significant levels. All patients had improved anginal status and were able to decrease medication use. In addition, harder end points such as myocardial perfusion assessed using SPECT-sestambi and angiography, were improved in all patients. However, only a small number of patients were used (five) in this study and it was not placebo-controlled. A similar phase I study was carried out by direct myocardial injection in combination with CABG and demonstrated promising trends regarding excercise treadmill test (ETT) and anginal class at 6-month follow-up (Rosengart et al., 1999a). A subsequent study by Fortuin et al. (2003) delivered naked plasmid DNA via a thoracotomy. At 1-year follow-up similarly encouraging results were seen in 30 patients with refractory angina. Improved ETT times, anginal class and decreased frequency of medication use were observed in comparison to baseline and this peaked at 6 months. Follow-up persisted to 12 months and as reported by Reilly et al. (2005), out to 24 months. Dose escalation was used in the study but the improvements were not dose-dependent. However, the low numbers and lack of a placebo control group limit the usefulness of the data generated.
Based on the promising results using recombinant FGF in a phase I trial (Schumacher et al., 1998a, 1998b), the first study to use adenovirus to deliver FGF was carried out (AGENT 1) (Grines et al., 2002). This study used catheters to deliver Ad5-FGF4 to the myocardium of 79 angina patients. The trial was a multicentre, double-blind, randomised, placebo-controlled study. It aimed again to establish safety in the patient population and also to assess some pharmacokinetic data such as vector distribution. In the latter case, the vector was distributed to the pulmonary circulation but was not excreted in the urine nor was any evidence of it found in germ cells using PCR. It should be noted that FGF4 binds to the proteoglycans of the cells it is secreted by and therefore may have less systemic effects. No retinopathy, angioma or myocarditis was observed. Notable again in two cases a malignancy was unmasked during the trial. The first was a colorectal cancer, which metastasised but was more likely due to hereditary nonpolyposis colorectal cancer owing to family history. Ad5-FGF4 could not be detected in the neoplasm. Another patient developed a glioblastoma. An improved ETT was noted in treated groups compared with baseline values but when compared with placebo controls this was not significant. The study was discontinued as enough safety data had been generated to design AGENT2 (Grines et al., 2003b). This subsequent study had 52 patients and examined dose escalation and looked at reversible perfusion defect size as an end point. Again the active treatment group had no significant benefit when compared to controls.
As comprehensively reviewed by Isner et al. (2001) the relative safety of angiogenic gene therapy and in particular delivery of the VEGF gene to the myocardium has been repeatedly demonstrated. Studies using this approach have a low number of significant adverse events compared with the general population. Of concern are the theoretical risks concerning the effect angiogenesis may have on accelerating disease states already present in the patient. It has been suggested that angiogenesis could contribute to an acceleration of atherosclerotic disease due to increased perfusion of fibrofatty plaques and it has been shown experimentally that inhibition of plaque angiogenesis can reduce the growth of atherosclerotic lesions. However, gene delivery of angiogenic agents such as VEGF has not been shown to have an adverse effect on atherosclerotic lesions in current phase I trials. This is most likely due to the relatively localised and low levels of angiogenic agents involved. Other potential adverse outcomes for angiogenic-based gene therapy include the development of vascular malformations, diabetic retinopathy and oedema. Again the occurrence of these adverse effects has been rare in patients undergoing gene therapy. It has also been suggested that angiogenic gene therapy could potentially contribute to the progression of neoplasia by contributing to the vascularisation of an existing primary mass aiding growth and metastatic spread. However, in the present short-term studies, which have been conducted an increased risk of neoplasia has not been observed. However, some adverse effects may not become apparent until larger and longer-term studies have been conducted with effective monitoring and reporting of any such outcomes.
Summary
Thus far, the promise shown by preclinical studies of therapeutic angiogenesis using gene therapy has not been matched by clinical trial results. Even in cases where efficacy has been noted (Losordo et al., 2002; Fortuin et al., 2003), the inclusion of a placebo control group invariably eliminates this difference. This may be due to the fact that multiple genes may be required to establish a suitably angiogenic local environment. Single genes have been used to date and the use of vectors with a larger capacity such as herpes simplex virus type-1 may allow multiple genes to be expressed to provoke an angiogenic environment (Epstein, 2005). Alternatively, the most useful gene may not yet have been discovered. The time course of expression may also be very important and prolonged low-level expression may be more suitable to establish efficacy. Another issue may be the route of vector delivery. For example, in the AGENT trial, the vector was delivered by an intracoronary route, whereas a more direct delivery to the myocardium using the minimally invasive NOGA system may be more effective method of delivering genes to the heart (Kastrup et al., 2005). Finally, the extent of angiogenesis observed in the placebo group in phase II studies is a significant issue, which should be considered in the design of future trials in this area.
Several positive features have been elucidated from the clinical data to date in the area of biosafety and tolerability (Rosengart et al., 1999a, 1999b). Therapeutic angiogenesis is a theoretically perilous strategy considering the potential risks associated with unrestrained blood vessel formation in the eye and in particular in the area of malignancies. The studies to date, however, have suggested that the strategy is safe without an increased rate of adverse outcomes in the active treatment groups.
Prevention of neointimal hyperplasia
Introduction
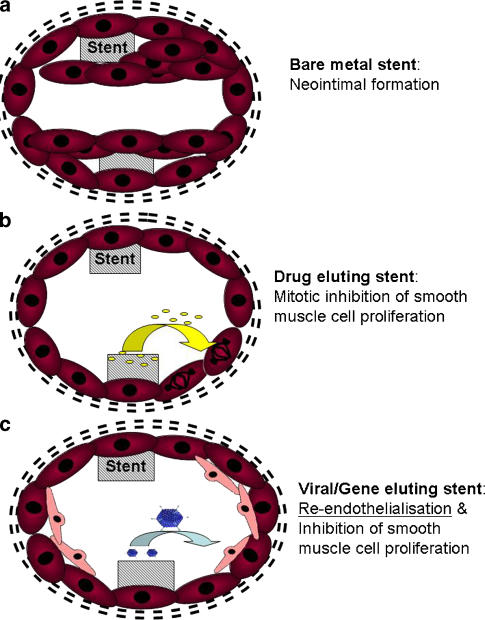
The development of neointimal hyperplasia contributes to the pathogenesis of both vein graft failure and in-stent restenosis (Figure 3). Neointimal graft wall thickening is an adaptive reaction that provides haemodynamic stability to the graft. However, this leads to the upregulated expression of proinflammatory growth factors, cytokines and adhesion molecules by the neointimal vascular smooth muscle cells. The resulting inflammation results in dysfunction of the overlying endothelium.
Figure 3.
Inhibition of in-stent restenosis (a) using drug-eluting stents (b) and viral/gene-eluting stents (c).
Gene therapy offers a potential role in this setting by targeting:
The modulation of smooth muscle cell migration
The modulation of smooth muscle cell proliferation
Smooth muscle cell apoptosis
The acceleration of re-endothelialisation and improvement of endothelial function.
A number of potentially therapeutic genes, which are key to neointimal formation, have been identified (Baker, 2002). These include tissue inhibitors of matrix metalloproteinases (TIMP-3) (Akowuah et al., 2005), nitric oxide synthase (NOS) (West et al., 2001) and p53 (George et al., 2001; Wan et al., 2004). The use of E2F decoy oligodeoxynucleotides to modulate gene expression is not strictly gene therapy but this related approach will be also be discussed here. A summary of preclinical and clinical trials using these therapeutic approaches can be seen in Tables 1 and 2, respectively.
Vein graft failure
Direct access to the vein during surgery offers a major potential benefit as a target for gene therapy. The vein graft can be manipulated and therapeutic genes, which may prevent subsequent graft failure can be applied ex vivo.
Preclinical studies
Overexpression of TIMPs prevents the digestion of the extracellular matrix by matrix metalloproteinases, which is required for smooth muscle cell migration. Studies involving the overexpression of TIMP 1–3 in human saphenous vein cell cultures ex vivo, demonstrated inhibition of migration of smooth muscle cells thus reducing neointimal formation. TIMP-3 blocks neointimal formation in vivo in pig grafts at 28 days post-surgery (Akowuah et al., 2005). TIMP-3 is thought to work best because of its ability to bind to the extracellular matrix, induce smooth muscle cell apoptosis and block migration of smooth muscle cells.
Other approaches, which have proven successful in various animal models of neointimal hyperplasia in vein grafts (Petrofski et al., 2004), include successful attempts to downregulate the expression of chemotactic and mitogenic factors in the graft or to inhibit the signalling cascade responsible for neointimal hyperplasia (Wolff et al., 2005).
Studies by West et al. (2001) have shown that Adenoviral mediated gene transfer of neuronal NOS (nNOS) can improve endothelial cell function as well as blocking smooth muscle cell proliferation following vein bypass grafting . They demonstrated that NOS gene transfer intraoperatively in vein grafts can result in reduced endothelial activation and inflammation. In mature vein grafts this leads to decreased smooth muscle cell intimal hyperplasia. Furthermore, overexpression of NOS during the early phase of vein graft remodelling results in intimal smooth muscle cells which are modulated toward a more differentiated phenotype with reduced smooth muscle cell-related superoxide production. This suggests that both reduced smooth muscle cell proliferation and intimal hyperplasia may be achieved through increased production of NO. Furthermore, such an increase in NO can also result in a reduction in superoxide production thus protecting the vein graft from damage by reactive oxygen species. It may be that early and transient increases in NO can result in long-term benefits, which do not require persistent overexpression of NOS making vein grafts a potential target for gene delivery methods intraoperatively to prevent vein graft failure. This approach to preventing neointimal formation has been further confirmed by in vivo experiments comparing other NOS isoforms (Cooney et al., 2006).
It has been shown that wild-type p53 gene transfer inhibits neointimal formation in human saphenous vein segments by modulation of smooth muscle cell migration and induction of apoptosis (George et al., 2001). The same group also studied adenoviral-mediated gene transfer of p53 in porcine interposition vein grafts and found that it resulted in increased lumen size and inhibition of neointimal formation (Wan et al., 2004).
Antisense oligonucleotides that block cell-cycle gene expression can inhibit experimental neointimal hyperplasia, stabilise vein graft endothelial function and prevent graft atherosclerosis (Morishita et al., 1995). The more cell-cycle regulatory genes blocked by an agent, the more effective is the therapy. Thus, an ideal target for cell-cycle blockade is E2F, a transcription factor that leads to upregulation of up to 12 cell-cycle genes. A strategy to block E2F using a double-stranded decoy oligodeoxynucleotide that bears the consensus E2F-binding site (E2F decoy oligodeoxynucleotides) is a good single agent strategy for prevention of vein graft disease. Ex vivo studies in rabbits have shown that vein grafts treated intraoperatively with E2F decoy oligodeoxynucleotides resulted in inhibition of neointimal hyperplasia and graft atherosclerosis for up to 6 months.
Clinical trials
On the basis of potential shown of this approach to block cellular proliferation using E2F decoy oligodeoxynucleotides, the PREVENT Trial entered a phase 1 trial. This was a single-centre, prospective, randomised controlled trial of ex vivo therapy for human vascular bypass grafts which manipulated gene expression using an E2F decoy oligodeoxynucleotide (Mann et al., 1999). There was a 74% reduction in vascular cell proliferation in the E2F decoy oligodeoxynucleotide treated group. This group also displayed fewer graft occlusions at 30 days compared with the untreated group (11.8 and 25%, respectively) as well as fewer nontechnical primary failures among high-risk patients. Similar safety and tolerability results were observed for the PREVENT II trial, which used this gene manipulation strategy for coronary bypass grafts (Grube, 2001). Although the phase 1 trial (PREVENT Trial) showed promising results, subsequent studies were less positive including recently published results of the phase III, multicentre, randomised double-blinded, placebo-controlled trial of 3014 patients undergoing primary CABG surgery with at least two planned saphenous grafts and without concomitant valve surgery (Alexander et al., 2005; Conte et al., 2005, 2006). The vein grafts were treated ex vivo with either edifoligide (E2F decoy oligodeoxynucleotide) or placebo. The trial showed that edifoligide had no effect on vein graft failure compared to the placebo-treated group (45.2 vs 46.3%, respectively).
In-stent restenosis
As previously mentioned, stented vessels may undergo restenosis in up to 30% of patients, 6 months post-PTCA with stenting (Rome et al., 1994). To prevent this complication, drug-eluting stents have been used to prevent vascular smooth muscle proliferation, which is the major contributor to in-stent restenosis. These stents have a polymer coating capable of stably eluting anticoagulants or antimitotic drugs. However, drug-eluting stents also inhibit re-endothelialisation of the stented segment making it liable to thrombosis. Furthermore, although this method tackles the physical blockade of the stent it does not address the underlying pathology of the disease process. Therefore, it may become attractive to combine stenting with gene delivery to both prevent in-stent restenosis and to allow a stable platform for gene delivery over time which may ameliorate the disease process.
Preclinical studies
The initial concept of using genetically modified organisms to help prevent in-stent restenosis was published by Dichek et al. (1989), who established that genetically altered endothelial cells overexpressing, human-type tissue plasminogen activator or reporter transgenes could be seeded and grown onto intravascular stents (Koren et al., 2006). To examine in-stent restenosis larger animal models are generally used including the rabbit, pig and monkey (Sharif et al., 2004). A rodent model is also in use (Fishbein et al., 2006). Studies have focused on establishing proof of principle for various genes as well as the technical aspects of when and how to deliver the genes from the stent or via a catheter. Numerous genes have shown promise in reducing in-stent restenosis in animal models. These include VEGF, inducible NOS (iNOS) and TIMPs, which have been effective in animal models (Sharif et al., 2004). The development of stents, which elute genes directly via plasmids or adenoviral vectors, has proven to be an effective means of delivering transgenes (Walter et al., 2004; Johnson et al., 2005; Sharif et al., 2006). However, such direct gene-eluting stents have yet to be examined in human trials with catheter-based infusion techniques being favoured thus far (Hedman et al., 2003).
Clinical trials
Once again VEGF was the initial anti-restenotic gene chosen for use due to its known effect in inducing NO and prostacyclin, which both inhibit VSMC migration and proliferation. Laitinen et al. (2000) examined a small number of patients (15) undergoing PTCA or PTCA with stenting. VEGF was delivered using plasmid/liposome DNA subsequent to and at the site of the PTCA procedure but before any stenting procedure. The gene transfer involved infusion of vector for 10 min through an infusion–perfusion catheter. This study used reporter genes in a control group and found the procedure to be well tolerated with little or not adverse effects but with no benefit on restenosis rates (Laitinen et al., 2000). Although long term (up to 180 days) and localised gene delivery was observed, the lack of beneficial or adverse effects may be due to the low efficiency gene delivery in this trial.
A subsequent study based on the results of Laitinen et al. (2000) was termed the Kuopio Angiogenesis Trial (KAT) and this also used an infusion–perfusion catheter. This examined the safety and feasibility of catheter-based gene delivery in 103 patients (Hedman et al., 2003). The study was a double-blind, randomised, placebo-controlled study. In addition to controls and plasmid/liposome-mediated delivery of VEGF, a third group had VEGF delivered using an adenovirus. The treatment was again well tolerated with serious adverse effects similar to those in the general population. However, at 6 months follow-up no difference was noted in lumen diameter within the stent using angiography, although a low overall rate of restenosis was observed in the study (6%). Also of note, an increased myocardial perfusion was observed at 6 months follow-up in the AdVEGF-treated group. This may be due to the prolonged expression of VEGF from adenoviral delivery and its more potent effect on angiogenesis than on NO induction.
Summary
It would seem likely that the effective long-term prevention of in-stent restenosis using vascular gene therapy is an achievable goal, although to date clinical results have not reflected this. It may be that the impetus in developing such strategies using gene therapy has not been great enough particularly in the era of drug-eluting stents (Burt and Hunter, 2006). However, it is clear from longer-term studies examining drug-eluting stents that late complications such as thrombosis are a significant problem with that therapy (Joner et al., 2006). Strategies to prevent neointimal hyperplasia while ensuring the integrity of the endothelium may be achievable using stent-based gene delivery and this is an area worthy of further study. As there is no effective means of preventing vein graft failure gene therapy approaches to this condition again focusing on genes, which may inhibit intimal hyperplasia while preserving the endothelium, would be attractive. The exciting, emerging technology of small interfering RNA which earned its discoverers, Professor Mello and Professor Fire the 2006 Nobel prize in Physiology and Medicine may also become a more attractive gene-based therapeutic strategy in the future for cardiovascular disease processes. The technology can down-regulate activated genes associated with pathological cascades and has been shown to successfully target genes associated with vein graft failure by preventing neointimal hyperplasia in an in vivo model (Banno et al., 2006).
Future perspectives
Gene-based therapy for patients with refractory or recurrent cardiovascular disease has been the subject of extensive investigation. Although efficacy has been demonstrated in animal models and safety in phase I human studies, unequivocal evidence of efficacy has not been demonstrated in placebo-controlled trials. (Simari and O'Brien, 2002; Yla-Herttuala et al., 2004). The reason for this is not clear but future approaches will require optimisation of vectors for gene delivery, use of alternative genes and/or families of genes and the use of optimal in vivo gene delivery systems, which will result in efficient gene delivery to the target tissues. These approaches will need careful evaluation in animal models followed by appropriate clinical translation. From experience to date, evidence of efficacy should be examined in properly designed placebo-controlled clinical studies. Gene therapy approaches are particularly relevant in conditions such as ‘the no-option revascularisation patient' or vein bypass graft failure where current alternative therapies are suboptimal. They may also be relevant in the prevention of in-stent restenosis if reduction of intimal hyperplasia can be prevented while maintaining endothelial integrity. In addition, gene therapy approaches to cardiovascular disease will have to demonstrate advantages over conventional therapies, which make the above-mentioned disease targets attractive.
Conflict of interest
The authors state no conflict of interest.
References
- Akowuah EF, Gray C, Lawrie A, Sheridan PJ, Su CH, Bettinger T, et al. Ultrasound-mediated delivery of TIMP-3 plasmidDNA into saphenous vein leads to increased lumen size in a porcine inteposition graft model. Gene Therapy. 2005;12:1154–1157. doi: 10.1038/sj.gt.3302498. [DOI] [PubMed] [Google Scholar]
- Alexander JH, Ferguson TB, Jr, Joseph DM, Mack MJ, Wolf RK, Gibson CM, et al. The PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) trial: study rationale, design, and baseline patient characteristics. Am Heart J. 2005;150:643–649. doi: 10.1016/j.ahj.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Aoki M, Morishita R, Taniyama Y, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by hepatocyte growth factor: potential gene therapy for ischemic diseases. J Atheroscler Thromb. 2000;7:71–76. doi: 10.5551/jat1994.7.71. [DOI] [PubMed] [Google Scholar]
- Baker AH. Gene therapy for bypass graft failure and restenosis. Pathophysiol Haemost Thromb. 2002;32:389–391. doi: 10.1159/000073606. [DOI] [PubMed] [Google Scholar]
- Baker AH. Designing gene delivery vectors for cardiovascular gene therapy. Prog Biophys Mol Biol. 2004;84:279–299. doi: 10.1016/j.pbiomolbio.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Banno H, Takei Y, Muramatsu T, Komori K, Kadomatsu K. Controlled release of small interfering RNA targeting midkine attenuates intimal hyperplasia in vein grafts. J Vasc Surg. 2006;44:633–641. doi: 10.1016/j.jvs.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Basara N. AdGVVEGF121.10 (GenVec) Curr Opin Investig Drugs. 2001;2:792–795. [PubMed] [Google Scholar]
- Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, et al. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart Endocrinology 200414524–35.discussion 21–23 [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W BUrrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt HM, Hunter WL. Drug-eluting stents: a multidisciplinary success story. Adv Drug Deliv Rev. 2006;58:350–357. doi: 10.1016/j.addr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Chao W, Matsui T, Novikov MS, Tao J, Li L, Liu H, et al. Strategic advantages of insulin-like growth factor-I expression for cardioprotection. J Gene Med. 2003;5:277–286. doi: 10.1002/jgm.347. [DOI] [PubMed] [Google Scholar]
- Chen S, Kapturczak M, Loiler SA, Zolotukhin S, Glushakova OY, Madsen KM, et al. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum Gene Ther. 2005;16:235–247. doi: 10.1089/hum.2005.16.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin LD, McAninch RE, Schulz D, Kaluza GL, LeMaire SA, Coselli JS, et al. HIV-based vectors and angiogenesis following rabbit hindlimb ischemia. J Surg Res. 2005;123:55–66. doi: 10.1016/j.jss.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery J Vasc Surg 200643742–751.discussion 751 [DOI] [PubMed] [Google Scholar]
- Conte MS, Lorenz TJ, Bandyk DF, Clowes AW, Moneta GL, Seely BL. Design and rationale of the PREVENT III clinical trial: edifoligide for the prevention of infrainguinal vein graft failure. Vasc Endovascular Surg. 2005;39:15–23. doi: 10.1177/153857440503900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney R, Hynes SO, Sharif F, Howard L, O'Brien T. Effect of gene delivery of NOS isoforms on intimal hyperplasia and endothelial regeneration after balloon injury. Gene Therapy. 2006;14:396–404. doi: 10.1038/sj.gt.3302882. [DOI] [PubMed] [Google Scholar]
- Dai Q, Thompson MA, Pippen AM, Cherwek H, Taylor DA, Annex BH. Alterations in endothelial cell proliferation and apoptosis contribute to vascular remodeling following hind-limb ischemia in rabbits. Vasc Med. 2002;7:87–91. doi: 10.1191/1358863x02vm430oa. [DOI] [PubMed] [Google Scholar]
- Dean DA. Electroporation of the vasculature and the lung. DNA Cell Biol. 2003;22:797–806. doi: 10.1089/104454903322625000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner C, Schwimmbeck PL, Koehler IS, Loddenkemper C, Noutsias M, Nikol S, et al. Adventitial VEGF165 gene transfer prevents lumen loss through induction of positive arterial remodeling after PTCA in porcine coronary arteries. Atherosclerosis. 2006;189:123–132. doi: 10.1016/j.atherosclerosis.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Denby L, Nicklin SA, Baker AH. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Therapy. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- Dichek DA, Neville RF, Zwiebel JA, Freeman SM, Leon MB, Anderson WF. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation. 1989;80:1347–1353. doi: 10.1161/01.cir.80.5.1347. [DOI] [PubMed] [Google Scholar]
- Dishart KL, Denby L, George SJ, Nicklin SA, Yendluri S, Tuerk MJ, et al. Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: implications for gene therapy. J Mol Cell Cardiol. 2003;35:739–748. doi: 10.1016/s0022-2828(03)00136-6. [DOI] [PubMed] [Google Scholar]
- Epstein AL. HSV-1-based amplicon vectors: design and applications. Gene Therapy. 2005;12 Suppl 1:S154–S158. doi: 10.1038/sj.gt.3302617. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Alferiev IS, Nyanguile O, Gaster R, Vohs JM, Wong GS, et al. Bisphosphonate-mediated gene vector delivery from the metal surfaces of stents. Proc Natl Acad Sci USA. 2006;103:159–164. doi: 10.1073/pnas.0502945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133:275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuin FD, Vale P, Losordo DW, Symes J, DeLaria GA, Tyner JJ, et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol. 2003;92:436–439. doi: 10.1016/s0002-9149(03)00661-1. [DOI] [PubMed] [Google Scholar]
- Gao MH, Lai NC, McKirnan MD, Roth DA, Rubanyi GM, Dalton N, et al. Increased regional function and perfusion after intracoronary delivery of adenovirus encoding fibroblast growth factor 4: report of preclinical data. Hum Gene Ther. 2004;15:574–587. doi: 10.1089/104303404323142024. [DOI] [PubMed] [Google Scholar]
- George SJ, Angelini GD, Capogrossi MC, Baker AH. Wild-type p53 gene transfer inhibits neointima formation in human saphenous vein by modulation of smooth muscle cell migration and induction of apoptosis. Gene Therapy. 2001;8:668–676. doi: 10.1038/sj.gt.3301431. [DOI] [PubMed] [Google Scholar]
- Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- Goldman B, Blanke H, Wolinsky H. Influence of pressure on permeability of normal and diseased muscular arteries to horseradish peroxidase. A new catheter approach. Atherosclerosis. 1987;65:215–225. doi: 10.1016/0021-9150(87)90037-2. [DOI] [PubMed] [Google Scholar]
- Grines C, Rubanyi GM, Kleiman NS, Marrott P, Watkins MW. Angiogenic gene therapy with adenovirus 5 fibroblast growth factor-4 (Ad5FGF-4): a new option for the treatment of coronary artery disease. Am J Cardiol. 2003a;92:24N–31N. doi: 10.1016/s0002-9149(03)00965-2. [DOI] [PubMed] [Google Scholar]
- Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003b;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- Grube E. PRoject of Ex-vivo Vein graft ENgineering via Transfection (PREVENT) II trial 2001Anaheim, California; Presented at the annual Amercian Heart Association Scientific Sessions, November 11–14, 2001 [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated cloncal T cell prlofieration in two patients after gene therapy fo SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Harrison RL, Byrne BJ, Tung L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998;435:1–5. doi: 10.1016/s0014-5793(98)00987-9. [DOI] [PubMed] [Google Scholar]
- Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- Heinl-Green A, Radke PW, Munkonge FM, Frass O, Zhu J, Vincent K, et al. The efficacy of a ‘master switch gene' HIF-1alpha in a porcine model of chronic myocardial ischaemia. Eur Heart J. 2005;26:1327–1332. doi: 10.1093/eurheartj/ehi223. [DOI] [PubMed] [Google Scholar]
- Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153:666–672. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- Horvath KA, Lu CY, Robert E, Pierce GF, Greene R, Sosnowski BA, et al. Improvement of myocardial contractility in a porcine model of chronic ischemia using a combined transmyocardial revascularization and gene therapy approach. J Thorac Cardiovasc Surg. 2005;129:1071–1077. doi: 10.1016/j.jtcvs.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Hughes GC, Post MJ, Simons M, Annex BH. Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol. 2003;94:1689–1701. doi: 10.1152/japplphysiol.00465.2002. [DOI] [PubMed] [Google Scholar]
- Ishii S, Koyama H, Miyata T, Nishikage S, Hamada H, Miyatake S, et al. Appropriate control of ex vivo gene therapy delivering basic fibroblast growth factor promotes successful and safe development of collateral vessels in rabbit model of hind limb ischemia. J Vasc Surg. 2004;39:629–638. doi: 10.1016/j.jvs.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Isner JM. Myocardial gene therapy. Nature. 2002;415:234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- Isner JM, Vale PR, Symes JF, Losordo DW. Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ Res. 2001;89:389–400. doi: 10.1161/hh1701.096259. [DOI] [PubMed] [Google Scholar]
- Isner JM, Walsh K, Symes J, Pieczek A, Takeshita S, Lowry J, et al. Arterial gene transfer for therapeutic angiogenesis in patients with peripheral artery disease. Hum Gene Ther. 1996;7:959–988. doi: 10.1089/hum.1996.7.8-959. [DOI] [PubMed] [Google Scholar]
- Johnson TW, Wu YX, Herdeg C, Baumbach A, Newby AC, Karsch KR, et al. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol. 2005;25:754–759. doi: 10.1161/01.ATV.0000157582.33180.a9. [DOI] [PubMed] [Google Scholar]
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Josko J, Gwozdz B, Jedrzejowska-Szypulka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6:1047–1052. [PubMed] [Google Scholar]
- Jozkowicz A, Dulak J. Helper-dependent adenoviral vectors in experimental gene therapy. Acta Biochim Pol. 2005;52:589–599. [PMC free article] [PubMed] [Google Scholar]
- Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Khurana R, Simons M. Insights from angiogenesis trials using fibroblast growth factor for advanced arteriosclerotic disease. Trends Cardiovasc Med. 2003;13:116–122. doi: 10.1016/s1050-1738(02)00259-1. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jang SY, Park JI, Byun J, Kim DI, Do YS, et al. Vascular endothelial growth factor-induced angiogenic gene therapy in patients with peripheral artery disease. Exp Mol Med. 2004;36:336–344. doi: 10.1038/emm.2004.44. [DOI] [PubMed] [Google Scholar]
- Klugherz BD, Jones PL, Cui X, Chen W, Meneveau NF, DeFelice S, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- Klugherz BD, Song C, DeFelice S, Cui X, Lu Z, Connolly J, et al. Gene delivery to pig coronary arteries from stents carrying antibody-tethered adenovirus. Hum Gene Ther. 2002;13:443–454. doi: 10.1089/10430340252792576. [DOI] [PubMed] [Google Scholar]
- Kondoh K, Koyama H, Miyata T, Takato T, Hamada H, Shigematsu H. Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. Cardiovasc Res. 2004;61:132–142. doi: 10.1016/j.cardiores.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Koren B, Weisz A, Fischer L, Gluzman Z, Preis M, Avramovitch N, et al. Efficient transduction and seeding of human endothelial cells onto metallic stents using bicistronic pseudo-typed retroviral vectors encoding vascular endothelial growth factor. Cardiovasc Revasc Med. 2006;7:173–178. doi: 10.1016/j.carrev.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006;17:683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- Laguens R, Cabeza Meckert P, Vera Janavel G, Del Valle H, Lascano E, Negroni J, et al. Entrance in mitosis of adult cardiomyocytes in ischemic pig hearts after plasmid-mediated rhVEGF165 gene transfer. Gene Therapy. 2002;9:1676–1681. doi: 10.1038/sj.gt.3301844. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Hartikainen J, Hiltunen MO, Eranen J, Kiviniemi M, Narvanen O, et al. Catheter-mediated vascular endothelial growth factor gene transfer to human coronary arteries after angioplasty. Hum Gene Ther. 2000;11:263–270. doi: 10.1089/10430340050016003. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Zachary I, Breier G, Pakkanen T, Hakkinen T, Luoma J, et al. VEGF gene transfer reduces intimal thickening via increased production of nitric oxide in carotid arteries. Hum Gene Ther. 1997;8:1737–1744. doi: 10.1089/hum.1997.8.15-1737. [DOI] [PubMed] [Google Scholar]
- Ledley FD. Non-viral gene therapy. Curr Opin Biotechnol. 1994;5:626–636. doi: 10.1016/0958-1669(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Lee JS, Feldman AM. Gene therapy for therapeutic myocardial angiogenesis: a promising synthesis of two emerging technologies. Nat Med. 1998;4:739–742. doi: 10.1038/nm0698-739. [DOI] [PubMed] [Google Scholar]
- Lee M, Rentz J, Bikram M, Han S, Bull DA, Kim SW. Hypoxia-inducible VEGF gene delivery to ischemic myocardium using water-soluble lipopolymer. Gene Therapy. 2003;10:1535–1542. doi: 10.1038/sj.gt.3302034. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- Majesky MW. A little VEGF goes a long way. Therapeutic angiogenesis by direct injection of vascular endothelial growth factor-encoding plasmid DNA. Circulation. 1996;94:3062–3064. doi: 10.1161/01.cir.94.12.3062. [DOI] [PubMed] [Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Mann MJ, Whittemore AD, Donaldson MC, Belkin M, Conte MS, Polak JF, et al. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- Morishita R. Perspective in progress of cardiovascular gene therapy. J Pharmacol Sci. 2004;95:1–8. doi: 10.1254/jphs.95.1. [DOI] [PubMed] [Google Scholar]
- Morishita R, Aoki M, Hashiya N, Makino H, Yamasaki K, Azuma J, et al. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44:203–209. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- Morishita R, Gibbons GH, Horiuchi M, Ellison KE, Nakama M, Zhang L, et al. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci USA. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EG, Plautz G, Nabel GJ. Site specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990;249:1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- Niagara MI, Haider H, Ye L, Koh VS, Lim YT, Poh KK, et al. Autologous skeletal myoblasts transduced with a new adenoviral bicistronic vector for treatment of hind limb ischemia. J Vasc Surg. 2004;40:774–785. doi: 10.1016/j.jvs.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Buening H, Dishart KL, de Alwis M, Girod A, Hacker U, et al. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol Ther. 2001;4:174–181. doi: 10.1006/mthe.2001.0424. [DOI] [PubMed] [Google Scholar]
- Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Ohara N, Koyama H, Miyata T, Hamada H, Miyatake SI, Akimoto M, et al. Adenovirus-mediated ex vivo gene transfer of basic fibroblast growth factor promotes collateral development in a rabbit model of hind limb ischemia. Gene Therapy. 2001;8:837–845. doi: 10.1038/sj.gt.3301475. [DOI] [PubMed] [Google Scholar]
- OhNo T, Gordon D, San H, Pompili VJ, Imperiale MJ, Nabel GJ, et al. Gene therapy for vascular smooth musle cell proliferaion after arterial injury. Science. 1994;265:781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:3005.1–3005.12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penta K, Varner JA, Liaw L, Hidai C, Schatzman R, Quertermous T. Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J Biol Chem. 1999;274:11101–11109. doi: 10.1074/jbc.274.16.11101. [DOI] [PubMed] [Google Scholar]
- Petrofski JA, Hata JA, Gehrig TR, Hanish SI, Williams ML, Thompson RB, et al. Gene delivery to aortocoronary saphenous vein grafts in a large animal model of intimal hyperplasia. J Thorac Cardiovasc Surg. 2004;127:27–33. doi: 10.1016/j.jtcvs.2003.07.032. [DOI] [PubMed] [Google Scholar]
- Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Mohler ER, III, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Shah M, Luciano A, Crystal R, Nabel EG. Adenovirus-mediated gene transfer of VEGF(121) improves lower-extremity endothelial function and flow reserve. Circulation. 2001;104:753–755. doi: 10.1161/hc3201.095192. [DOI] [PubMed] [Google Scholar]
- Rasmussen HS, Rasmussen CS, Macko J, Yonehiro G. Angiogenic gene therapy strategies for the treatment of cardiovascular disease. Curr Opin Mol Ther. 2002;4:476–481. [PubMed] [Google Scholar]
- Reilly JP, Grise MA, Fortuin FD, Vale PR, Schaer GL, Lopez J, et al. Long-term (2-year) clinical events following transthoracic intramyocardial gene transfer of VEGF-2 in no-option patients. J Interv Cardiol. 2005;18:27–31. doi: 10.1111/j.1540-8183.2005.04026.x. [DOI] [PubMed] [Google Scholar]
- Rezaee M, Penta K, Quertermous T. Del1 mediates VSMC adhesion, migration, and proliferation through interaction with integrin alpha(v)beta(3) Am J Physiol Heart Circ Physiol. 2002;282:H1924–H1932. doi: 10.1152/ajpheart.00921.2001. [DOI] [PubMed] [Google Scholar]
- Rome JJ, Shayani V, Newman KD, Farrell S, Lee SW, Virmani R, et al. Adenoviral vector-mediated gene transfer into sheep arteries using a double-balloon catheter. Hum Gene Ther. 1994;5:1249–1258. doi: 10.1089/hum.1994.5.10-1249. [DOI] [PubMed] [Google Scholar]
- Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR, et al. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA Ann Surg 1999a230466–470.discussion 470–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999b;100:468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998b;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- Schumacher B, von Specht BU, Haberstroh J, Pecher P. The stimulation of neo-angiogenesis in the ischemic heart by the human growth factor FGF. J Cardiovasc Surg (Torino) 1998a;39:445–453. [PubMed] [Google Scholar]
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects Ann Thorac Surg 2002731919–1925.discussion 1926 [DOI] [PubMed] [Google Scholar]
- Sharif F, Daly K, Crowley J, O'Brien T. Current status of catheter- and stent-based gene therapy. Cardiovasc Res. 2004;64:208–216. doi: 10.1016/j.cardiores.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Sharif F, Hynes SO, McMahon J, Cooney R, Conroy S, Dockery P, et al. Gene-eluting stents: comparison of adenoviral and adeno- associated viral gene delivery to the blood vessel wall in vivo. Hum Gene Ther. 2006;17:741–750. doi: 10.1089/hum.2006.17.741. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Wang MT, Wang BW, Chang CC, Leu JG, Kuan P, et al. Intramyocardial injection of naked DNA encoding HIF-1alpha/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc Res. 2002;54:576–583. doi: 10.1016/s0008-6363(02)00259-6. [DOI] [PubMed] [Google Scholar]
- Simari RD, O'Brien T. Clinical trials in vascular gene therapy: the missing piece of the puzzle. Arch Pathol Lab Med. 2002;126:317–319. doi: 10.5858/2002-126-0317-CTIVGT. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Palmer-Opolski M, Smith RC, Walsh K. Transgene delivery of plasmid DNA to smooth muscle cells and macrophages from a biostable polymer-coated stent. Gene Therapy. 2003;10:1471–1478. doi: 10.1038/sj.gt.3302010. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Jackson M, Qian K, Phillips MI. Hypoxia inducible double plasmid system for myocardial ischemia gene therapy. Hypertension. 2002;39:695–698. doi: 10.1161/hy0202.103784. [DOI] [PubMed] [Google Scholar]
- Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, et al. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: molecular mechanisms of delayed angiogenesis in diabetes. Circulation. 2001;104:2344–2350. doi: 10.1161/hc4401.098470. [DOI] [PubMed] [Google Scholar]
- Totsugawa T, Kobayashi N, Okitsu T, Noguchi H, Watanabe T, Matsumura T, et al. Lentiviral transfer of the LacZ gene into human endothelial cells and human bone marrow mesenchymal stem cells. Cell Transplant. 2002;11:481–488. [PubMed] [Google Scholar]
- Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, et al. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94:3281–3290. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- Varenne O, Pislaru S, Gillijns H, Van Pelt N, Gerard RD, Zoldhelyi P, et al. Local adenovirus-mediated transfer of human endothelial nitric oxide synthase reduces luminal narrowing after coronary angioplasty in pigs. Circulation. 1998;98:919–926. doi: 10.1161/01.cir.98.9.919. [DOI] [PubMed] [Google Scholar]
- Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004;110:36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- Wan S, George SJ, Nicklin SA, Yim AP, Baker AH. Overexpression of p53 increases lumen size and blocks neointima formation in porcine interposition vein grafts. Mol Ther. 2004;9:689–698. doi: 10.1016/j.ymthe.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Wang K, Kessler PD, Zhou Z, Penn MS, Forudi F, Zhou X, et al. Local adenoviral-mediated inducible nitric oxide synthase gene transfer inhibits neointimal formation in the porcine coronary stented model. Mol Ther. 2003;7:597–603. doi: 10.1016/s1525-0016(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bai Y, Price C, Boros P, Qin L, Bielinska AU, et al. Combination of electroporation and DNA/dendrimer complexes enhances gene transfer into murine cardiac transplants. Am J Transplant. 2001;1:334–338. doi: 10.1034/j.1600-6143.2001.10408.x. [DOI] [PubMed] [Google Scholar]
- West NE, Qian H, Guzik TJ, Black E, Cai S, George SE, et al. Nitric oxide synthase (nNOS) gene transfer modifies venous bypass graft remodeling: effects on vascular smooth muscle cell differentiation and superoxide production. Circulation. 2001;104:1526–1532. doi: 10.1161/hc3801.095693. [DOI] [PubMed] [Google Scholar]
- Whitlock PR, Hackett NR, Leopold PL, Rosengart TK, Crystal RG. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol Ther. 2004;9:67–75. doi: 10.1016/j.ymthe.2003.09.014. [DOI] [PubMed] [Google Scholar]
- World Health Organisation Cardiovascular Diseases 2006. Viewed 1 September 2006
- Wolff RA, Ryomoto M, Stark VE, Malinowski R, Tomas JJ, Stinauer MA, et al. Antisense to transforming growth factor-beta1 messenger RNA reduces vein graft intimal hyperplasia and monocyte chemotactic protein 1. J Vasc Surg. 2005;41:498–508. doi: 10.1016/j.jvs.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Thung SN. Use of a perforated balloon catheter to deliver concentrated heparin into the wall of the normal canine artery. J Am Coll Cardiol. 1990;15:475–481. doi: 10.1016/s0735-1097(10)80079-8. [DOI] [PubMed] [Google Scholar]
- Woods NB, Bottero V, Schmidt M, Von Kalle C, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- Work LM, Buning H, Hunt E, Nicklin SA, Denby L, Britton N, et al. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther. 2006;13:683–693. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Wu YX, Johnson T, Herdeg C, Baumbach A, Newby AC, Karsch KK, et al. Stent-based local delivery of therapeutic adenovirus effectively reduces neointimal proliferation in porcine coronaries. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:1263–1265. [PubMed] [Google Scholar]
- Xie D, Li Y, Reed EA, Odronic SI, Kontos CD, Annex BH. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J Vasc Surg. 2006;44:166–175. doi: 10.1016/j.jvs.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Markkanen JE, Rissanen TT. Gene therapy for ischemic cardiovascular diseases: some lessons learned from the first clinical trials. Trends Cardiovasc Med. 2004;14:295–300. doi: 10.1016/j.tcm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Young SM, Jr, McCarty DM, Degtyareva N, Samulski RJ. Roles of adeno-associated virus Rep protein and human chromosome 19 in site-specific recombination. J Virol. 2000;74:3953–3966. doi: 10.1128/jvi.74.9.3953-3966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lei L, Liang Y, Hinh L, Hickey RP, Huang Y, et al. An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice. FASEB J. 2006;20:479–481. doi: 10.1096/fj.04-3670fje. [DOI] [PubMed] [Google Scholar]
- Zaccagnini G, Gaetano C, Della Pietra L, Nanni S, Grasselli A, Mangoni A, et al. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J Biol Chem. 2005;280:14790–14798. doi: 10.1074/jbc.M414644200. [DOI] [PubMed] [Google Scholar]
- Zhang D, Gai L, Fan R, Dong W, Wen Y. Efficacy and safety of therapeutic angiogenesis from direct myocardial administration of an adenoviral vector expressing vascular endothelial growth factor 165. Chin Med J (Engl) 2002;115:643–648. [PubMed] [Google Scholar]
- Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, et al. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112:30–41. doi: 10.1172/JCI17034. [DOI] [PMC free article] [PubMed] [Google Scholar]