Abstract
While the expression patterns of cardiac hypertrophy-related genes have been well documented and widely used as markers for hypertrophy, recent research has revealed uncoupling of hypertrophy-related gene profiles and hypertrophic growth. The role of β-adrenergic signalling in the development of hypertrophy is incompletely understood. The finding of an upregulated expression of hypertrophy-related genes but a suppressed hypertrophy following β-blockade reveals previously unrecognized sympatho-adrenergic mechanisms of hypertrophic growth.
Keywords: hypertrophy, β-blocker, β-adrenoceptors, gene expression, natriuretic peptides
Activation of the sympathetic nervous system and myocardial hypertrophy occur in the setting of cardiovascular disease and precipitate progression of cardiac remodelling, dysfunction and heart failure. Although there has been no convincing evidence for a direct antihypertrophic effect of β-adrenoceptor antagonists (β-blockers), a prohypertrophic action of β-adrenergic signalling has been shown by experimental and clinical studies (Zahabi et al., 2003; Burns et al., 2007).
Pathological hypertrophy is associated with a well-documented pattern of gene expression, including reactivation of a set of fetal genes like atrial or B-type natriuretic peptides (ANP, BNP), β-myosin heavy chain (β-MHC) and α-skeletal actin (α-SKA), and downregulation of adult cardiac genes, most notably sarcoendoplastic reticulum Ca2+ ATPase (SERCA) and α-MHC. Such a transcriptional profile, particularly ANP upregulation, has been used as measure of hypertrophy in vivo and in vitro. Although poorly defined, there also exist intrinsic signal networks that counter-regulate hypertrophic growth.
In the current issue of the BJP, Patrizio et al. (2007) report an interesting finding; treatment with β-blockers in models of cardiac hypertrophy in vivo (transverse aortic constriction (TAC)) and in vitro (cardiomyocytes treated with phenylephrine or noradrenaline) suppressed hypertrophic growth even though expression of fetal genes was further upregulated. In the TAC model, sympatho-adrenergic signalling contributes to hypertrophic growth, as shown by a suppressed left ventricle hypertrophy in dopamine-β-hydroxylase-null mice, depleted of catecholamines (Esposito et al., 2002). Patrizio et al. (2007) took a good approach by investigating the effect of β-blockers both in vivo and in vitro. They tested propranolol, metoprolol (β1-selective) and ICI-118551 (β2-selective) with findings showing a class effect mediated by β1-adrenoceptors.
This study (Patrizio et al., 2007) is the first to show such paradoxical combinations using β-blockers commonly prescribed to patients with heart disease. Actually, uncoupling of hypertrophy-related gene profile and hypertrophic growth has been noticed in recent years by studies using genetically engineered models or gene targeting. For instance, lack of fetal gene expression was reported in α1A- and α1B-adrenoceptor dual-knockout mice with severe pressure-overload hypertrophy (O'Connell et al., 2006). Conversely, α1A-adrenoceptor transgenic mice had increased expression of ANP but did not develop hypertrophy nor exacerbated pathological hypertrophy (Lin et al., 2001; Du et al., 2006a). In cultured cardiomyocytes, inactivation of activating protein 1 function reversed hypertrophy-related gene profile evoked by phenylephrine, but hypertrophy remained unaltered (Jeong et al., 2005). Uncoupling of expression of individual fetal genes has also been reported. Cardiac overexpression of glycogen synthase kinase-3β (GSK3β) inhibited hypertrophy due to either calcineurin overexpression, isoproterenol administration or TAC, phenotypes associated with further elevation of ANP expression but downregulation of both BNP and β-MHC (Antos et al., 2002). Similarly, concomitant expression of modulatory calcineurin-interacting protein 1 markedly inhibited calcineurin-mediated hypertrophy, but expression of ANP was further activated and that of α-SKA inhibited (Hill et al., 2002). All these findings suggest that expression of individual fetal and adult genes in the hypertrophic myocardium is regulated by distinct signal mechanisms.
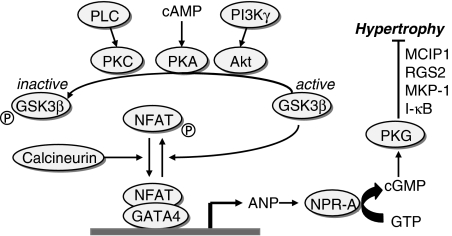
Signalling mechanisms responsible for the findings by Patrizio et al. (2007) remain unexplored. Studies using genetically engineered models targeting ANP or the natriuretic peptide receptor-A (NPR-A) have provided strong evidence for an antihypertrophic property of the ANP/NPR-A/PKG signalling pathway under basal or pathological conditions, as summarized in Table 1. This signal pathway counteracts multiple hypertrophic signal pathways including those involving nuclear factor-κB (NF-κB), p-38-mitogen-activated protein kinase (p38-MAPK), calcineurin/nuclear factor of activated T cell (NFAT) and protein kinase C (Figure 1). Inhibition of TAC-hypertrophy with a further elevation of ANP expression was observed in mice treated with 17β-estradiol (van Eickels et al., 2001), the effect mediated through the NPR-A/cGMP-dependent protein kinase (PKG) pathway (van Eickels et al., 2001; Du et al., 2006b).
Table 1.
Summary of findings from genetically engineered mice indicating antihypertrophic action of natriuretic peptide/GC signal pathway
| Model | Cardiac phenotypes |
|---|---|
| ANP KO (Wang et al., 2003) | Hypertrophy at baseline and exacerbated hypertrophy and fibrosis under pressure-overload |
| Corin KO (Chan et al., 2005) | Hypertension and cardiac hypertrophy |
| NPR-A KO (Oliver et al., 1997; Knowles et al., 2001; Franco et al., 2004; Tokudome et al., 2005) | Cardiac hypertrophy and sudden death at baseline; exacerbated hypertrophy by calcineurin activation or by pressure-overload |
| Cardiac NPR-A KO (Holtwick et al., 2003) | Mild hypertrophy, hypotension at baseline; exaggerated pressure-overload hypertrophy |
| TG-DN-NPR-A (Patel et al., 2005) | Increased severity of pressure-overload hypertrophy and fibrosis |
| NPR-A TG (Kishimoto et al., 2001) | Reduced heart size |
| TG-CA-GC (Zahabi et al., 2003) | Inhibited hypertrophy by isoproterenol or pressure-overload |
Abbreviations: ANP, atrial natriuretic peptide; CA, constitutively active; DN, dominant negative; KO, knockout; NPR-A, natriuretic peptide receptor-A; TG, transgenic.
Figure 1.
Signal pathways that promote ANP expression while inhibiting myocardial hypertrophy. ANP, atrial natriuretic peptide; I-κB, NF-κB inhibitor; MKP-1, MAPK phosphatase-1; RGS2, regulator of G-protein signalling 2.
How does β-blockade upregulate ANP expression in hearts of sham-operated and TAC animals? Recent studies have shown that ANP expression is controlled by signal pathways involving calcineurin, phosphoinositide 3-kinase (PI3Kγ) and protein kinase B (Akt)/GSK3β. Activation of nuclear Akt by viral or transgenic means, selectively increased ANP expression (Tsujita et al., 2006). Upon β-adrenoceptor activation, ANP expression is promoted via Ca2+/calcineurin signalling but suppressed by inactivation of GSK3β following its phosphorylation by Akt or cAMP-dependent protein kinase (Figure 1) (Morisco et al., 2000). Thus, GSK3β suppresses hypertrophy while it activates ANP expression (Antos et al., 2002) (Figure 1). In addition, following β-adrenoceptor activation, PI3Kγ and β-adrenoceptor kinase-1 are recruited by β-arrestins to the ligand-activated β-adrenoceptors, a process necessary to free Gβγ and to induce β-adrenoceptor desensitization (Esposito et al., 2002; Nienaber et al., 2003). If this β-adrenoceptor/PI3Kγ colocalization is associated with a reduced nuclear PI3Kγ/Akt activity, one would expect a disinhibition of GSK3β by β-adrenoceptor blockade, as tested by Patrizio et al. (2007), thereby promoting ANP expression via calcineurin/NFAT signalling (Figure 1). This and other possibilities remain to be tested.
The ‘contradictory' findings by Patrizio et al. (2007) reveal our incomplete understanding on the role of β-adrenoceptor in hypertrophic development and hence the effect of β-blockers. If β-blockade increases ANP expression, one would expect a suppressed expression of at least some hypertrophy-related genes by β-adrenoceptor activation. Clinical studies on patients with dilated cardiomyopathy showed that treatment with β-blockers inhibited the expression of ANP and β-MHC and restored that of α-MHC and SERCA (Lowes et al., 2002). Thus, caution is required when extrapolating the findings from the mouse TAC model to clinical situations.
The findings by Patrizio et al. (2007) would have been strengthened by providing measures of cardiomyocyte hypertrophy (such as cell size, protein synthesis), exploring potential signalling mechanisms and validating the results from pharmacological approaches by using genetically engineered models, such as β-adrenoceptor knockout mice. Actually, a recent paper from the same group found no difference between the β1- and β2-adrenoceptor dual-knockout and wild-type mice in the extent of TAC-induced hypertrophy, fetal gene expression and fibrosis (Palazzesi et al., 2006), findings contradictory to the current report (Patrizio etal., 2007). Furthermore, although hypertrophy was inhibited, β-blockade had no effect on the suppressed SERCA expression (Patrizio etal., 2007). It would be interesting to know the chronic impact of this phenomenon. Thus, further research with extended study periods or using different heart disease models would be worthwhile.
Abbreviations
- Akt
protein kinase B
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- GC
guanylate cyclase
- GSK3β
glycogen synthase kinase-3β
- MHC
myosin heavy chain
- NFAT
nuclear factor of activated T cell
- NPR
natriuretic peptide receptor
- PI3K
phosphoinositide 3-kinase
- PKG
cGMP-dependent protein kinase
- SERCA
sarcoendoplasmic reticulum Ca2+ ATPase
- α-SKA
α-skeletal actin
- TAC
transverse aorta constriction
References
- Antos CL, McKinsey TA, Fey N, Kutschke W, McAnally J, Shelton JM, et al. Activated glycogen synthase-3β suppresses cardia hypertrophy in vivo. Proc Nat Acad Sci USA. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatiral natriuretic peptide convertase corin. Proc Nat Acad Sci USA. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Sivananthan MU, Ball SG, Mackintosh AF, Mary DA, Greenwood JP. Relationship between central sympathetic drive and magnetic resonance imaging-determined left ventricular mass in essential hypertension. Circulation. 2007;115:1999–2005. doi: 10.1161/CIRCULATIONAHA.106.668863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, Su Y, et al. Transgenic α1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res. 2006a;71:735–743. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Du XJ, Lu F, Kiriazis H. Sex dimorphism in cardiac pathophysiology: experimental findings, hormonal mechanisms, and molecular mechanisms. Pharmacol Ther. 2006b;111:434–475. doi: 10.1016/j.pharmthera.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- Franco V, Chen Y-F, Oparil S, Feng JA, Wang D, Hage F, et al. Atrial natriuretic peptide dose-dependently inhibits pressure overload-induced cardiac remodeling. Hypertension. 2004;44:746–750. doi: 10.1161/01.HYP.0000144801.09557.4c. [DOI] [PubMed] [Google Scholar]
- Hill JA, Rothermel B, Yoo KD, Cabuay B, Demetroulis E, Weiss RM, et al. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. J Biol Chem. 2002;277:10251–10255. doi: 10.1074/jbc.M110722200. [DOI] [PubMed] [Google Scholar]
- Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong MY, Kinugawa K, Vinson C, Long CS. AFos dissociates cardiac myocyte hypertrophy and expression of the pathological gene program. Circulation. 2005;111:1645–1651. doi: 10.1161/01.CIR.0000160367.99928.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci USA. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, et al. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, et al. Targeted α1A-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. 2001;89:343–350. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- Morisco C, Zebrowski D, Condorelli G, Tsichlis P, Vatner SF, Sadoshima J. The Akt-glycogen synthase kinase 3β pathway regulates transcription of atrial natriuretic factor induced by β-adrenergic receptor stimulation in cardiac myocytes. J Biol Chem. 2000;275:14466–14475. doi: 10.1074/jbc.275.19.14466. [DOI] [PubMed] [Google Scholar]
- Nienaber JJ, Tachibana H, Naga Prasad SV, Esposito G, Wu D, Mao L, et al. Inhibition of receptor-localized PI3K preserves cardiac β-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest. 2003;112:1067–1079. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, et al. α1-Adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzesi S, Musumeci M, Catalano L, Patrizio M, Stati T, Michienzi S, et al. Pressure overload causes cardiac hypertrophy in β1- and β2-adrenergic receptor double knockout mice. J Hypertens. 2006;24:563–571. doi: 10.1097/01.hjh.0000203843.41937.2a. [DOI] [PubMed] [Google Scholar]
- Patrizio M, Stati T, Musumeci M, Fasanaro P, Palazzesi S, Catalano L, et al. Propranolol causes a paradoxical enhancement of cardiomyocyte fetal gene response to hypertrophic stimuli Br J Pharmacol 2007152216–222.(this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JB, Valencik ML, Pritchett AM, Burnett JC, Jr, McDonald JA, Redfield MM. Cardiac-specific attenuation of natriuretic peptide A receptor activity accentuates adverse cardiac remodeling and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2005;289:H777–H784. doi: 10.1152/ajpheart.00117.2005. [DOI] [PubMed] [Google Scholar]
- Tokudome T, Horio T, Kishimoto I, Soeki T, Mori K, Kawano Y, et al. Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides. Circulation. 2005;111:3095–3104. doi: 10.1161/CIRCULATIONAHA.104.510594. [DOI] [PubMed] [Google Scholar]
- Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, et al. Nuclear targeting of Akt antagonizes aspects of cardiomocyte hypertrophy. Proc Natl Acad Sci USA. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eickels M, Grohé C, Cleutjens JPM, Janssen BJ, Wellens HJ, Doevendans PA. 17β-Estradil attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104:1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, et al. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- Zahabi A, Picard S, Fortin N, Reudelhuber TL, Deschepper CF. Expression of constitutively active guanylate cyclase in cardiomyocytes inhibits the hypertrophic effects of isoproterenol and aortic constriction on mouse hearts. J Biol Chem. 2003;278:47694–47699. doi: 10.1074/jbc.M309661200. [DOI] [PubMed] [Google Scholar]