Abstract
Background and purpose:
Rat stomach ECL cells secrete histamine and pancreastatin in response to gastrin and pituitary adenylate cyclase-activating peptide-27 (PACAP). This study applies microdialysis to explore how ECL cells in situ respond to PACAP and gastrin.
Experimental approach:
Both peptides were administered by microinfusion into the gastric submucosa. The microdialysate was analysed for histamine and pancreastatin (ECL-cell markers) and for somatostatin (D-cell marker).
Key results:
Microinfusion of PACAP (0.01–0.3 nmol μl−1) raised microdialysate histamine and pancreastatin dose-dependently. The response was powerful but short-lived. The response to gastrin was sustained at all doses tested. It is unlikely that the transient nature of the histamine response to PACAP reflects inadequate histamine synthesis, since the pancreastatin response to PACAP was short-lived too, and both gastrin and PACAP activated ECL-cell histidine decarboxylase. Unlike gastrin, PACAP mobilized somatostatin. Co-infusion of somatostatin abolished the histamine-mobilizing effect of PACAP. However, pretreatment with the somatostatin receptor type-2 antagonist (PRL-2903) did not prolong the histamine response to PACAP, suggesting that mobilization of somatostatin does not explain the transient nature of the response. Repeated administration of 0.1 nmol μl−1 of PACAP (1 h infusions, 1 h intervals) failed to induce a second histamine response. Pretreatment with a low dose of PACAP (0.03 nmol μl−1) abolished the response to a subsequent near-maximal PACAP challenge (0.3 nmol μl−1).
Conclusion:
The transient nature of the histamine response to PACAP reflects desensitization of the PACAP receptor and/or exhaustion of a specific storage compartment that responds to PACAP but not to gastrin.
Keywords: ECL cells, PACAP, histamine, pancreastatin, histidine decarboxylase, microdialysis, gastrin, somatostatin, rat stomach
Introduction
Histamine- and pancreastatin (PST)-producing enterochromaffin-like (ECL) cells are numerous in the oxyntic mucosa (Håkanson et al., 1994). They secrete histamine (and PST) in response to gastrin, and the histamine that is mobilized causes adjacent parietal cells to produce acid. While the existence and functional significance of a gastrin–ECL cell–parietal cell axis seems to be widely accepted (Lindström et al., 2001a), there is no consensus as to how the nervous system controls ECL cells and parietal cells. Most nerve fibres in the oxyntic mucosa derive from the enteric nervous system, which operates under vagal and sympathetic control. Candidate neurotransmitters in the enteric neurons comprise not only acetylcholine and noradrenaline, but also neuropeptides such as pituitary adenylate cyclase-activating peptide (PACAP) and vasoactive intestinal peptide (VIP; Ekblad et al., 1985, 1991; Green and Dockray, 1988). PACAP belongs to the glucagon/VIP/secretin peptide family and inhibits both basal and stimulated acid secretion (Mungan et al., 1992, 1995; Li et al., 2000; Piqueras et al., 2004; for a different point of view see Sandvik et al., 2001). Since histamine is a major stimulus of acid secretion, it is paradoxical that although PACAP stimulates ECL cell histamine secretion (Lindström et al., 1997, 2001b; Zeng et al., 1998, 1999; Lindström and Håkanson, 2001; Norlén et al., 2001), it does not stimulate acid secretion (Mungan et al., 1995). This apparent paradox may be resolved by assuming that PACAP (unlike gastrin) mobilizes not only histamine (from the ECL cells) but also somatostatin (SST; from the D cells; Hummelt et al., 1977; Schubert, 1991; Piqueras et al., 2004), SST being a well-known suppressor of parietal cell activity (Schubert and Makhlouf, 1991). Indeed, PACAP has been found to stimulate acid secretion in the anaesthetized rat following immunoneutralization of SST (Zeng et al., 1999), and Sandvik et al. (2001), working with the isolated, vascularly perfused stomach of the rat, found that PACAP at low doses stimulates acid secretion.
Interestingly, microdialysate experiments conducted in conscious rats revealed that the release of ECL cell histamine in response to local infusion of PACAP was powerful but short-lived, in contrast to the moderate and sustained response seen upon microinfusion of gastrin. The short duration of the histamine response to PACAP may reflect desensitization of the PACAP receptor (see, for example, Shintani et al., 2000), exhaustion of the releasable histamine pool, and/or mobilization of local agents, such as SST, which inhibit the activity of the ECL cells (Prinz et al., 1994a; Lindström et al., 1997; Norlén et al., 2001; Björkqvist et al., 2005).
The aim of the present study was to determine the characteristic features of the PACAP-evoked histamine response of ECL cells in situ. The mobilization of histamine and PST was monitored by sampling extracellular fluid from the submucosa of the oxyntic mucosa of conscious rats using a microdialysis technique (Kitano et al., 2000; Ericsson et al., 2003). PACAP and gastrin were compared with respect to their ability to mobilize histamine and PST, activate histidine decarboxylase (HDC) in the stomach wall, and release SST.
Methods
Animals
Female Sprague–Dawley rats (250–300 g) were kept at a 12-h light and 12-h dark cycle in plastic cages (4–6 in each cage) with free access to standard rat food pellets (Lactamin, Vadstena, Sweden) and tap water. When the rats were to be fasted, they were housed in individual cages with wire mesh bottoms for 24 h. During perfusion of the microdialysis probes, they were kept in Bollman-type restraining cages. One week before the experiments, all rats were thoroughly familiarized with such cages by training daily for 1–2 h. The studies were approved by the Local Animal Welfare Committee, Lund.
Drug treatments
Omeprazole was dissolved in 0.25% Methocel (Dow Corning, Midland, MI, USA) and administered once daily in the morning for 4 days, by oral gavage (400 mmol kg−1 day−1; Larsson et al., 1986; Konagaya et al., 2001). Control rats received the vehicle. Experiments were conducted 2–3 h after the last dose of omeprazole (or vehicle).
PRL-2903 was dissolved in 0.9% saline and given systemically (1.5 mg kg−1 as an i.v. bolus at time 0 followed by continuous i.v. infusion of 1.5 mg kg−1 h−1). Treatment with PRL-2903 was initiated concomitantly with infusion of PACAP. The dose used for i.v. administration was similar to that used in the studies of Piqueras et al. (2003), (2004).
Implantation of the microdialysis probe and sampling of microdialysate
A flexible microdialysis probe (MAB3.8.4, AgnTho's AB, Stockholm, Sweden; length of membrane, 4 mm; outer diameter, 0.57 mm, 35 kDa cutoff) was implanted in the acid-producing part of the stomach as described previously (Kitano et al., 2000; Ericsson et al., 2003). Surgery was performed under chloral hydrate anaesthesia (300 mg kg−1 i.p.). All rats were fasted for 24 h before the start of the microdialysis, which was performed 3 days after implantation of the probe. All rats were awake during the experiments since anaesthesia has been shown to suppress histamine mobilization from the ECL cells (Norlén et al., 2000). The inlet tube to the microdialysis probe was connected to a microinfusion pump (Model 361, Sage instrument; ATI Orion, Boston, MA, USA) and the outlet was allowed to drain into 300 μl polyethylene vials. Microdialysis (that is, perfusion with saline 1.2 μl min−1) started at 07 h. After a 2-h-equilibration period, collection of microdialysate samples commenced. The time taken for the solution to be transported from membrane to the outlet of the probe (3 min) was corrected. Each rat and each probe was used once only. After completion of the experiments, the rats were killed by an overdose of chloral hydrate and the position of the probes in the submucosa of the stomach wall was verified by visual inspection.
Local administration of agents: study design
Microdialysate samples for measurement of basal histamine, PST and/or SST were collected for 2 h in fasted rats. At this time point (time 0), saline was replaced by saline containing the different challenging agents to be tested: PACAP was infused at different concentrations and gastrin 0.1 nmol μl−1 (1.2 μl min−1) for 3 h. Microdialysate samples were collected every 20 min (for histamine) or every 30 min (for PST) during the first hour of stimulation, then every hour for 3 or 4 h. In some of these experiments, specimens of the stomach wall surrounding the probe were collected for determination of the HDC activity.
Rats were pretreated with omeprazole or vehicle for 4 days and received PACAP via the microdialysis probe for 3 h.
Rats received local microinfusion of α-fluoromethylhistidine (α-FMH; 0.1 nmol μl−1)+gastrin (0.1 nmol μl−1) for 3 h, after basal microdialysate samples had been collected for 1 h.
PACAP (0.1 nmol μl−1) was microinfused locally for 3 h followed by microinfusion of PACAP+i.v. infusion of gastrin (5 nmol kg−1 h−1) for another 2 h.
PACAP (0.1 nmol μl−1) and/or gastrin (0.1 nmol μl−1) were microinfused locally for 3 h after basal microdialysate samples had been collected for 1 h.
PACAP (0.1 nmol μl−1) was microinfused twice for 1 h with 1 h interval between the infusions.
PACAP (0.1 nmol μl−1) was microinfused and the concentration of SST was measured in the microdialysate.
Rats received SST (0.1 nmol μl−1; Norlén et al., 2001) via the microdialysis probe for 1 h followed by coadministration of PACAP (0.1 nmol μl−1)+SST or gastrin (0.1 nmol μl−1)+SST for 3 h.
Microinfusion of PACAP (0.1 nmol μl−1) and of PACAP+SST (0.1 nmol μl−1) was combined with the i.v. infusion of the SST receptor type 2 (SSTR2) antagonist PRL-2903 (1.5 mg kg−1 bolus followed by 1.5 mg kg−1 h−1 for 3 h).
Analysis of microdialysate
Histamine, PST and SST were measured by the use of commercially available radioimmunoassay (RIA) kits: a histamine RIA kit from Immunotech (Marseille, France) and PST and SST RIA kits from Euro-Diagnostika (Malmö, Sweden). We used 5–10 μl of microdialysate for measurement of histamine, 25–50 μl for PST and 50 μl for SST. The histamine and SST concentrations were expressed in nanomoles and picomoles, respectively, per litre microdialysate. The concentration of PST-like immunoreactivity was expressed as picomole equivalents of rat PST per litre.
Determination of serum gastrin
The serum gastrin concentration was determined by RIA (Stadil and Rehfeld, 1973), using antiserum no. 2604 (a kind gift from Dr JF Rehfeld, Copenhagen, Denmark), and expressed as picomole equivalents of rat gastrin-17 per litre.
Determination of HDC activity in the oxyntic mucosa
Minute specimens (2 × 12 mm) of the stomach wall embedding the microdialysis probes were collected from along the probes by means of a razor blade. The specimens were weighed and homogenized in ice-cold 0.1 M sodium phosphate buffer (pH 7.4) to a concentration of 100 mg ml−1. Aliquots (80 μl) of the homogenates were incubated with L-[1-14C]histidine (specific activity 50 mCi mmol−1), 0.5 mM L-histidine, and 0.01 mM pyridoxal-5-phosphate in a total volume of 160 μl at 37°C for 1 h as described previously (Larsson et al., 1986). The HDC activity was expressed as picomole 14CO2 mg−1 h−1.
Statistical analysis
Data are presented as mean values±s.e.mean. Differences between groups were analysed with analysis of variance followed by Dunnet's or Bonferroni's multiple comparison tests.
Chemicals
Human Leu15-gastrin-17 was purchased from Research Plus (South Plainfield, NJ, USA). Rat PACAP-27 was from Bachem (Bubendorf, Switzerland) and from the Yanaihara Institute (Shizuoka, Japan). The proton pump inhibitor omeprazole was from AstraZeneca (Mölndal, Sweden). α-FMH, an irreversible inhibitor of HDC (see Andersson et al., 1992), was obtained from Sigma (St Louis, MO, USA). SST was from Bachem. The selective SSTR2 antagonist PRL-2903 (Kawakubo et al., 1999) was generously provided by Dr DH Coy (Tulane University, New Orleans, LA, USA).
Results
Mobilization of histamine and PST in response to microinfusion of gastrin and/or PACAP
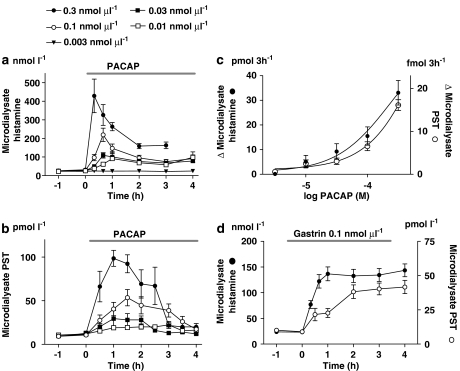
Local microinfusion of PACAP mobilized histamine and PST in a dose-dependent manner. With low doses of PACAP (0.01–0.03 nmol μl−1), the histamine response was modest and long-lasting. At doses of 0.1 and 0.3 nmol μl−1, the microdialysate histamine concentration increased 8 and 15-fold, respectively, after 20–40 min of PACAP infusion and then declined gradually. The levels measured 3–4 h later were still higher than the basal level at the start of the infusion (Figure 1a). Microdialysate PST concentrations increased 5 and 10 times respectively, reaching maximum after 1–1.5 h and then declining slowly to the basal level after 3–4 h of infusion (Figure 1b). Integrated dose–response curves are shown in Figure 1c. A near-maximally effective dose of gastrin (0.1 nmol μl−1; Norlén et al., 2001) evoked a sustained fourfold increase in the microdialysate concentrations of both histamine and PST (Figure 1d).
Figure 1.
Time course of enterochromaffin-like (ECL)-cell histamine (a) and pancreastatin (PST) (b) mobilization in response to increasing doses of pituitary adenylate cyclase-activating peptide (PACAP) administered via the microdialysis probes (local microinfusion; horizontal line). (c) Dose–response curves for the histamine- and PST-releasing effects of PACAP (integrated 3-h increments). (d) Histamine and PST mobilization in response to local microinfusion of a near-maximal dose of gastrin (0.1 nmol μl−1) is shown for comparison. Means±s.e.means (vertical lines) are shown; n=4–9.
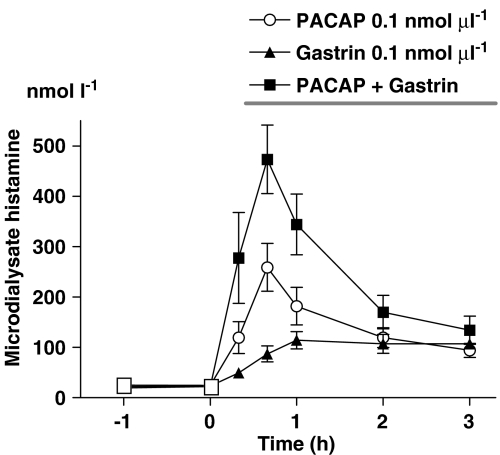
The concomitant microinfusion of PACAP (0.1 nmol μl−1) and gastrin (0.1 nmol μl−1) seemed to induce an additive histamine response in comparison to the responses to PACAP and gastrin, given separately (Figure 2). In fact, the calculated integrated 3-h-histamine response to PACAP+gastrin was quite similar to the sum of the responses to PACAP and to gastrin (42.6±6.05 versus 43.3±7.04 pmol 3 h−1).
Figure 2.
Histamine mobilization in response to microinfusion of pituitary adenylate cyclase-activating peptide (PACAP) (0.1 nmol μl−1), or gastrin (0.1 nmol μl−1) and of PACAP+gastrin. The combination of the two peptides seemed to induce an additive effect. Means±s.e.means (vertical lines) are shown; n=5–9.
HDC activation by gastrin and PACAP microinfusion
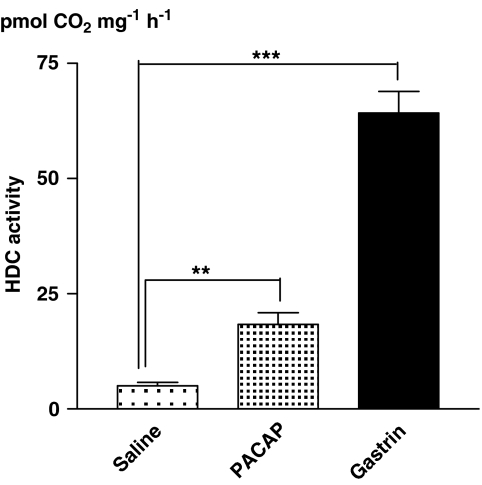
Local microinfusion of gastrin (0.1 nmol μl−1) induced a 13-fold increase (compared to saline-treated fasted rats) of the HDC activity (3 h after start of microinfusion) in stomach wall specimens collected from along the probe. The corresponding effect of PACAP (0.1 nmol μl−1) was a 3-fold HDC increase (Figure 3).
Figure 3.
Histidine decarboxylase (HDC) activity in homogenates of small specimens collected from the stomach wall along the microdialysis probe after local microinfusion of saline, pituitary adenylate cyclase-activating peptide (PACAP; 0.1 nmol μl−1) or gastrin (0.1 nmol μl−1) for 3 h. Means±s.e.means (vertical lines) are shown; n=5–6. **P<0.01, ***P<0.001.
Effect of PACAP microinfusion on mobilization of histamine and PST in hypergastrinaemic rats
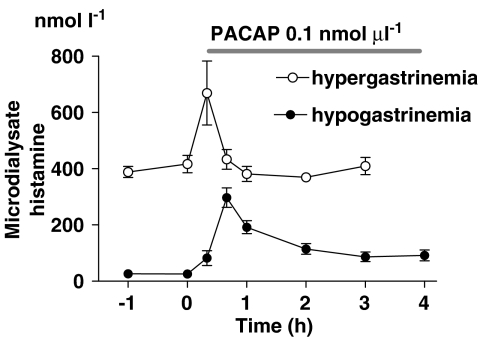
To assess whether the comparatively low HDC-activating effect of PACAP might explain the short duration of the histamine response, we compared the PACAP-evoked histamine response in fasted rats (low HDC activity) to that in hypergastrinaemic rats (high HDC activity). Acid blockade induced by treatment with the proton pump inhibitor omeprazole for 4 days raised the serum gastrin concentration (415±26 pmol l−1 in freely fed omeprazole-treated rats versus 11±0.7 pmol l−1 in vehicle-treated fasted rats), increased the oxyntic mucosal HDC activity 30-fold (179±24 pmol 14CO2 mg−1 h−1 in omeprazole-treated rats versus 5.8±0.6 pmol 14CO2 mg−1 h−1 in fasted rats), the basal microdialysate histamine concentration (402±25 nmol l−1 in omeprazole-treated rats versus 28±2.6 nmol l−1 in fasted rats; Figure 4) and the basal PST concentration (47±11 pmol l−1 in omeprazole-treated rats versus 10±1.6 pmol l−1 in fasted rats). However, the magnitude of the histamine and PST responses to PACAP (rise over basal level, that is the increment in absolute values) in omeprazole-treated rats was similar to that seen in vehicle-treated fasted rats (Figure 4). The microdialysate histamine concentration in the omeprazole-treated rats was back to prestimulation levels after 1 h, and the microdialysate PST concentration was back after 2 h (not shown), despite continued infusion of PACAP. Clearly, the transient nature of the histamine response to PACAP is not due to inadequate HDC activation.
Figure 4.
Histamine mobilization in response to local microinfusion of pituitary adenylate cyclase-activating peptide (PACAP) (horizontal line) in hypergastrinaemic rats (omeprazole pretreatment for 4 days) and hypogastrinaemic rats (food deprivation for 24 h). The high basal histamine concentrations in the microdialysate in the hypergastrinaemic rats can be ascribed to the high serum gastrin concentration (see text). Means±s.e.means (vertical lines) are shown; n=6.
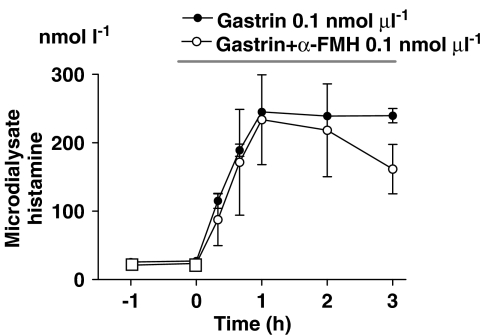
Histamine mobilization in response to microinfusion of gastrin after HDC blockade
If histamine resynthesis is essential for the duration of the histamine response to gastrin, then inhibition of HDC would shorten the response. To this end, gastrin and the HDC inhibitor α-FMH were microinfused simultaneously. However, co-infusion of α-FMH did not overtly affect either the magnitude or the duration of the histamine response to gastrin during the 3-h period of the study (Figure 5).
Figure 5.
Histamine mobilization in response to local microinfusion of gastrin or gastrin+α-fluoromethylhistidine (α-FMH), as indicated by the horizontal line. Means±s.e.means (vertical lines) are shown; n=4–6.
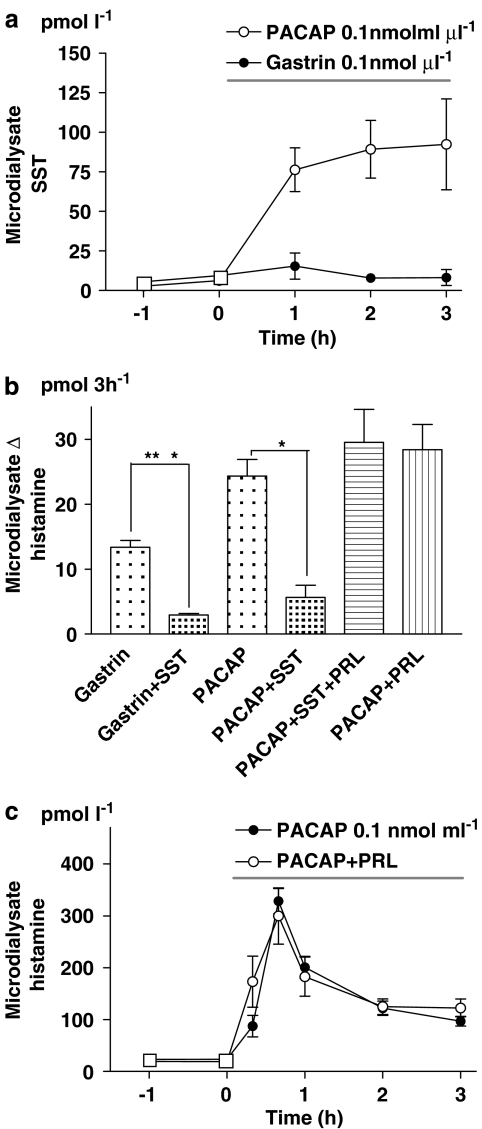
Somatostatin mobilization by gastrin and PACAP microinfusion
Microinfusion of PACAP raised the microdialysate SST level more than 10 times within 1 h. Gastrin in contrast did not mobilize SST (Figure 6a).
Figure 6.
(a) Somatostatin (SST) mobilization in response to local microinfusion of pituitary adenylate cyclase-activating peptide (PACAP; horizontal bar). Gastrin was without effect; n=5–8. (b) Histamine mobilization (integrated 3-h response) following microinfusion of gastrin or PACAP, PACAP with SST, PACAP with the SST receptor type 2 (SSTR2) antagonist PRL-2903 (PRL) i.v. (1.5 mg kg−1 bolus followed by 1.5 mg kg−1 h−1 for 3 h) or PACAP with SST and PRL; n=6–10. (c) Histamine mobilization in response to PACAP microinfusion (horizontal line) with or without the concomitant intravenous infusion of PRL; n=5, *P<0.05. In (a–c) means±s.e.means (vertical lines) are shown.
Effect of somatostatin and somatostatin receptor type 2 blockade on histamine mobilization in response to PACAP
The histamine response to gastrin and PACAP microinfusion was reduced by about 80 and 70%, respectively, upon coadministration of SST (Figure 6b). The SST receptor of the ECL cells has been identified as type 2 (SSTR2) (Prinz et al., 1994a). The SSTR2 antagonist PRL-2903 effectively blocked the inhibitory effect of SST but failed to affect either the magnitude or the duration of the histamine response to PACAP (Figures 6b and c).
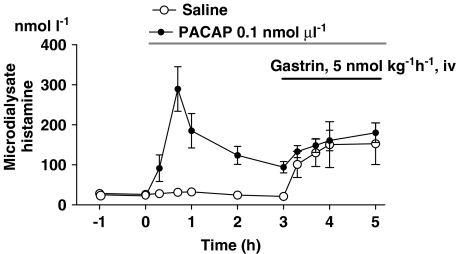
Histamine mobilization in response to i.v. infusion of gastrin superimposed on PACAP microinfusion
Microinfusion of PACAP (0.1 nmol μl−1) resulted in histamine mobilization that reached its peak after 40 min and then declined gradually. After 3 h of PACAP stimulation, the microdialysate histamine concentration remained approximately three times higher than basal (Figure 1, Figure 7). At this point, i.v. infusion of gastrin triggered a histamine response that was similar in magnitude (absolute values) and shape to the response to gastrin alone (Figure 7). This suggests that the transient nature of the PACAP-evoked histamine response does not reflect depletion of all releasable ECL-histamine (although it might reflect the depletion of a specific histamine pool that responds to PACAP but not to gastrin).
Figure 7.
Effect of intravenous infusion of a near-maximally effective dose of gastrin on histamine mobilization in rats administered either pituitary adenylate cyclase-activating peptide (PACAP) or saline via microdialysis probes. Means±s.e.means (vertical lines) are shown; n=5–7.
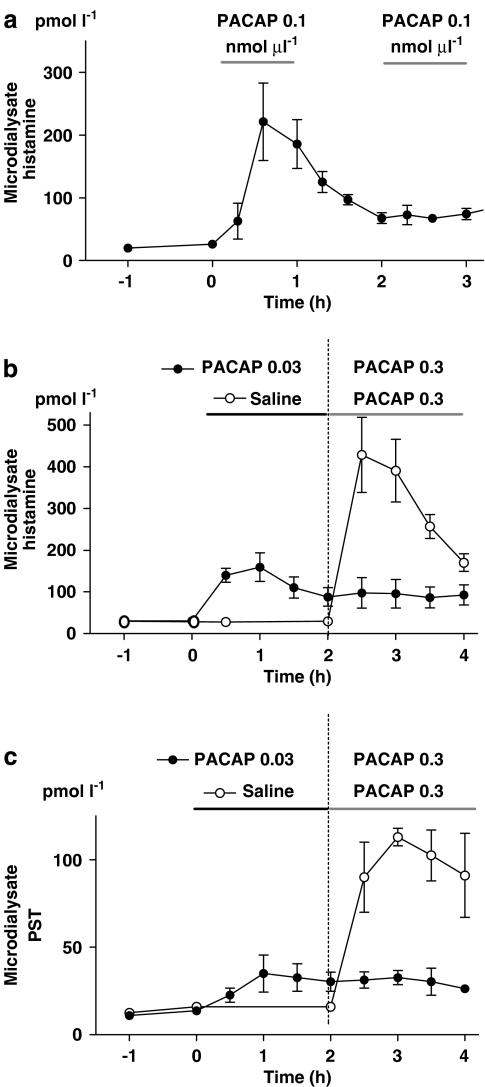
Histamine and PST mobilization in response to repeated microinfusion of PACAP
Repeated microinfusion of PACAP (0.1 nmol μl−1, 1 h interval) failed to induce renewed histamine mobilization after the initial spectacular response (Figure 8a). Moreover, a submaximally effective dose of PACAP (0.03 nmol μl−1), microinfused for 2 h, abolished both the histamine and the PST response to a subsequently given, near-maximally effective dose of PACAP (0.3 nmol μl−1; Figures 8b and c).
Figure 8.
(a) Histamine mobilization in response to repeated 1 h administrations of pituitary adenylate cyclase-activating peptide (PACAP; 0.1 nmol μl−1, local microinfusion) given at 1 h intervals. n=6. Histamine (b) and pancreastatin (PST) (c) mobilization in response to microinfusion of a near-maximally effective dose of PACAP in rats pretreated with a low dose of PACAP (indicated by horizontal lines); n=5. In (a–c) means±s.e.means (vertical lines) are shown.
This finding supports the view that the cessation of the response to PACAP reflects receptor desensitization rather than depletion of releasable histamine and PST.
Discussion and conclusion
Acid secretion is induced by vagal excitation and by a rise in circulating gastrin. The latter peptide hormone acts by stimulating the release of histamine (Kahlson et al., 1964) from the ECL cells (Håkanson et al., 1977; Sandvik et al., 1987; Chen et al., 1994; Prinz et al., 1994b; Lindström et al., 1997, 2001b), which in turn stimulates the parietal cell to produce HCl (Kahlson et al., 1964; Black and Shankley, 1987; Waldum et al., 1991). This pathway is referred to as the gastrin–ECL cell–parietal cell axis (Lindström et al., 2001a). The vagal input to the stomach is transmitted through command neurons in the stomach wall. Among candidate neurotransmitters in these command neurons are not only acetylcholine but also neuropeptides, such as PACAP and VIP (Ekblad et al., 1991; Sundler et al., 1992). Transmitters from the enteric neurons may stimulate the parietal cells directly and/or indirectly via the release of gastrin from the G cells or histamine from the ECL cells. While ECL cells do not seem to be capable of responding to acetylcholine (Lindström et al., 1997; Lindström and Håkanson, 2001; Norlén et al., 2001), it has been shown that they possess receptors that enable them to respond to PACAP and VIP, and secrete histamine (Sandor et al., 1996; Lindström et al., 1997, 2001b; Zeng et al., 1998, 1999; Norlén et al., 2001; Björkqvist et al., 2005). The PACAP neurons in the stomach wall probably operate under vagal control and, consequently, PACAP may be released from vagally stimulated, local nerve fibres to regulate the activity of ECL cells (and parietal cells) together with circulating gastrin. In fact, there is experimental evidence in favour of the view that vagal stimulation augments the response of the ECL cells to gastrin (Qvigstad et al., 1999; Norlén et al., 2005). Although direct evidence is lacking, it is not inconceivable that PACAP is involved in the vagal control of ECL cells (and parietal cells). In the present study, we confirm the finding that PACAP mobilizes histamine (and PST) from ECL cells in conscious rats and explore the mechanisms behind the release, in an attempt to explain the characteristic transient response pattern (see below).
The histamine response to PACAP differs from that to gastrin
At a near-maximally effective PACAP dose (0.3 nmol μl−1), the histamine response was strong (15-fold increase over basal) but short-lived as was the PST response (Figure 1). By comparison, the histamine and PST responses to a near-maximal dose of gastrin (0.1 nmol μl−1) were weaker but lasted longer. The histamine response to the combined microinfusion of PACAP and gastrin seemed to be additive, favouring the view that PACAP and gastrin draw histamine from intracellular stores that are at least partly different (Figure 2; see also Lindström et al., 2001a). The gradual decline in microdialysate histamine in response to PACAP (despite continued PACAP microinfusion) may be explained in various ways: depletion of histamine because of inadequate histamine resynthesis, release of SST and/or other local inhibitors of the ECL cells, downregulation of the PACAP receptor and/or depletion of histamine (and PST) from a restricted, PACAP-sensitive pool.
Is the histamine response to PACAP short-lasting because of slow histamine resynthesis?
PACAP was a less powerful activator of HDC than gastrin. It is possible therefore that the PACAP-evoked increase in HDC activity is too small to allow histamine resynthesis to compensate fully for the PACAP-induced secretion of histamine. The following observations argue against this hypothesis:
In omeprazole-treated (hypergastrinaemic) rats, which have much higher HDC activity than fasted vehicle-treated rats, the submucosal histamine and PST concentrations were high (a consequence of activation of the ECL cells; Konagaya et al., 2001) but the mobilization of ECL-cell histamine and PST in response to PACAP was quantitatively similar to that in hypogastrinaemic rats and remained short-lasting.
In view of the fact that the histamine response to gastrin seemed unchanged after acute HDC blockade (α-FMH), it seems unlikely that a low HDC activity could explain why the histamine response to PACAP was short-lived.
Intravenous infusion of gastrin during ongoing microinfusion of PACAP resulted in prompt histamine mobilization, indicating that the ECL cells also retain releasable histamine after exposure to PACAP.
Finally, the PST response to PACAP was also quite short-lived, indicating that mechanisms other than histamine depletion are of overriding importance.
Is the histamine response to PACAP short-lasting because of somatostatin mobilization?
PACAP (unlike gastrin) was found to raise the SST concentration in the microdialysate 10-fold, and coadministration of PACAP and SST abolished the histamine response to PACAP. SST is known to interact with SSTR2 on the ECL cells (Prinz et al., 1994a; Björkqvist et al., 2005). However, SSTR2 blockade, which abolished the effects of exogenous SST, did not enhance or prolong the histamine response to PACAP. Thus, although exogenous SST effectively inhibited the response to PACAP, it seems unlikely that endogenous SST is responsible for the short duration of the PACAP response.
Repeated administration of PACAP resulted in a dramatically reduced histamine response. It is unlikely that this can be explained by the mobilization of SST: a time interval of 60 min between PACAP challenges should be enough to clear SST (and other possible inhibitors mobilized by PACAP) from the mucosa/submucosa. Together, these findings argue against the view that endogenous SST acts to shorten PACAP-evoked histamine mobilization. Still, the possibility that other local inhibitors may be instrumental in bringing about a short-lasting response cannot be excluded.
Is the histamine response to PACAP short-lasting because of depletion of a specific PACAP-sensitive compartment in the ECL cell or because of desensitization of the PACAP receptor?
As would be expected, ‘unprovoked' ECL cells in omeprazole-treated (hypergastrinaemic) rats released more histamine than ECL cells in fasted (hypogastrinaemic) rats (Konagaya et al., 2001). Interestingly, PACAP provocation mobilized the same amount of histamine in omeprazole-treated rats as in fasted rats (the response was equally short-lived in both groups of rats). This finding seems to favour the view that PACAP is capable of exhausting a specific, PACAP-sensitive histamine compartment that does not seem to increase in size in response to long-standing hypergastrinaemia. In contrast, the histamine compartment that is being tapped by gastrin was greatly increased as a result of the hypergastrinaemia.
Our observation that repeated PACAP stimulation caused release of histamine in response to the first but not to subsequent challenges can be explained either by PACAP receptor desensitization or by depletion of a sequestered compartment that responds to PACAP but not to gastrin.
The fact that the histamine/PST response to PACAP is short-lived also at submaximally effective doses of PACAP (0.1 nmol μl−1) favours the desensitization hypothesis rather that the depletion hypothesis, because the low PACAP dose mobilized modest amounts of histamine only—not enough to exhaust the histamine store. Indeed, pretreatment with an even lower dose of PACAP (0.03 nmol μl−1) abolished the histamine/PST response to a subsequently given, near-maximal effective dose of PACAP (0.3 nmol μl−1; Figure 8), despite the fact that the histamine/PST stores were not depleted (as shown by the remaining histamine response to gastrin; Figure 7). The finding that the histamine response was more rapid and more short-lasting than the PST response seem to contradict both hypotheses, in that both histamine and PST should decrease in parallel if the receptor was downregulated, or if the stores were depleted. However, the delay in the PST response to PACAP may reflect simply a slow diffusion rate of peptides in general, including PST (there was also a delay in the PST response to gastrin).
It seems likely that receptor desensitization is at least partly responsible for the cessation of the ECL-cell response to PACAP, and that depletion of a PACAP-sensitive storage compartment may contribute to this effect.
In conclusion, microdialysis studies revealed that local administration of PACAP by microinfusion into the stomach wall mobilized histamine and PST from the ECL cells (transient response) and SST from the D cells (sustained response). Repeated stimulation with PACAP failed to induce a second histamine response. Although PACAP released SST from the D cells, SST does not seem to be responsible for the decrease in histamine mobilization, since SSTR2 blockade failed to affect the histamine response to PACAP. The characteristically short-lived histamine (and PST) response to PACAP and the large reduction in the amount of histamine that is released following a repeated PACAP challenge may reflect PACAP receptor desensitization. Alternatively, the short-lived PACAP-evoked response may reflect depletion of a specific sequestered intracellular histamine compartment that responds to PACAP but not to gastrin.
Acknowledgments
The study was supported by grants from the Royal Physiographic Society, Lund, A Påhlsson's Foundation, the Å Wiberg Foundation, the Crafoord Foundation and the Medical Faculty of Lund University, Lund, Sweden. The generous gift of PACAP-27 from Professor Chizuko Yanaihara at the Yanaihara Institute, Shizuoka, Japan, is gratefully acknowledged.
Abbreviations
- HDC
histidine decarboxylase
- PACAP
pituitary adenylate cyclase-activating peptide
- SSTR2
somatostatin receptor type 2
- VIP
vasoactive intestinal peptide
Conflict of interest
The authors state no conflict of interest.
References
- Andersson K, Chen D, Håkanson R, Mattsson H, Sundler F. Enterochromaffin-like cells in the rat stomach: effect of α-FMH-evoked histamine depletion. A chemical, histochemical and electron microscopic study. Cell Tissue Res. 1992;270:7–13. doi: 10.1007/BF00381874. [DOI] [PubMed] [Google Scholar]
- Björkqvist M, Bernsand M, Eliasson L, Håkanson R, Lindström E. Somatostatin, misoprostol and galanin inhibit gastrin- and PACAP-stimulated secretion of histamine and pancreastatin from ECL cells by blocking specific Ca2+ channels. Regul Pept. 2005;130:81–90. doi: 10.1016/j.regpep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Black JW, Shankley NP. How does gastrin act to stimulate oxyntic cell secretion. Trends Pharmacol Sci. 1987;8:486–490. [Google Scholar]
- Chen D, Monstein H-J, Nylander A-G, Zhao C-M, Sundler F, Håkanson R. Acute response of rat stomach enterochromaffin-like cells to gastrin. Secretory activation and adaptation. Gastroenterology. 1994;107:18–27. doi: 10.1016/0016-5085(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Ekelund M, Graffner H, Håkanson R, Sundler F. Peptide-containing nerve fibers in the stomach wall of rat and mouse. Gastroenterology. 1985;89:73–85. doi: 10.1016/0016-5085(85)90747-4. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Håkanson R, Sundler F.Innervation of the stomach of rat and man with special reference to the endocrine cells The Stomach as an Endocrine Organ 1991Elsevier: Amsterdam; 79–95.In: Håkanson R, Sundler F (eds) [Google Scholar]
- Ericsson P, Norlén P, Bernsand M, Alm P, Höglund P, Håkanson R. ECL cell histamine mobilization studied by gastric submucosal microdialysis in awake rats: methodological considerations. Pharmacol Toxicol. 2003;93:57–65. doi: 10.1034/j.1600-0773.2003.930201.x. [DOI] [PubMed] [Google Scholar]
- Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea pig. Neuroscience. 1988;25:181–193. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Håkanson R, Chen D, Sundler F.The ECL cells Physiology of the Gastrointestinal Tract, 3rd edn. Vol 2 1994Raven Press: New York; 1171–1184.In: Johnson LR (ed) [Google Scholar]
- Håkanson R, Hedenbro J, Liedberg G, El Munshid HA, Rehfeld JF. Gastrin: obligatory intermediate in the postprandial mobilization of gastric histamine in the rat. Experientia. 1977;33:1541–1542. doi: 10.1007/BF01918860. [DOI] [PubMed] [Google Scholar]
- Hummelt H, Jennewein HM, Treichel R, Waldeck F. Somatostatin: mode of action on gastric acid secretion in dogs. Digestion. 1977;15:151–155. doi: 10.1159/000197996. [DOI] [PubMed] [Google Scholar]
- Kahlson G, Rosengren E, Svahn D, Thunberg R. Mobilization and formation of histamine in the gastric mucosa as related to acid secretion. J Physiol. 1964;174:400–416. doi: 10.1113/jphysiol.1964.sp007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo K, Coy DH, Walsh JH, Taché Y. Urethane-induced somatostatin mediated inhibition of gastric acid: reversal by the somatostatin 2 receptor antagonist, PRL-2903. Life Sci. 1999;10:115–120. doi: 10.1016/s0024-3205(99)00340-9. [DOI] [PubMed] [Google Scholar]
- Kitano M, Norlén P, Håkanson R. Gastric submucosal microdialysis: a method to study gastrin- and food-evoked mobilization of ECL-cell histamine in conscious rats. Regul Pept. 2000;86:113–123. doi: 10.1016/s0167-0115(99)00096-8. [DOI] [PubMed] [Google Scholar]
- Konagaya T, Bernsand M, Norlén P, Håkanson R. Mobilization of rat stomach ECL-cell histamine in response to short- or long-term treatment with omeprazole and/or YF 476 studied by gastric submucosal microdialysis in conscious rats. Br J Pharmacol. 2001;133:37–42. doi: 10.1038/sj.bjp.0704037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Carlsson E, Mattson H, Lundell L, Sundler F, Sundell G, et al. Plasma gastrin and gastric enterochromaffin-like cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986;90:391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- Li P, Chang T-M, Coy D, Chey WY. Inhibition of gastric acid secretion in rat stomach by PACAP is mediated by secretin, somatostatin and PGE2. Am J Physiol. 2000;278:G121–G127. doi: 10.1152/ajpgi.2000.278.1.G121. [DOI] [PubMed] [Google Scholar]
- Lindström E, Björkqvist M, Boketoft Å, Chen D, Zhao CM, Kimura K, et al. Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells. Regul Pept. 1997;71:73–86. doi: 10.1016/s0167-0115(97)01018-5. [DOI] [PubMed] [Google Scholar]
- Lindström E, Chen D, Norlén P, Andersson K, Håkanson R. Control of gastric acid secretion: the gastrin–ECL cell–parietal cell axis. Comp Biochem Physiol A Mol Integr Physiol. 2001a;128:505–514. doi: 10.1016/s1095-6433(00)00331-7. [DOI] [PubMed] [Google Scholar]
- Lindström E, Eliasson L, Bjorkqvist M, Håkanson R. Gastrin and the neuropeptide PACAP evoke secretion from rat stomach histamine-containing (ECL) cells by stimulating influx of Ca2+ through different Ca2+ channels. J Physiol. 2001b;535:663–677. doi: 10.1111/j.1469-7793.2001.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström E, Håkanson R. Neurohormonal regulation of secretion from isolated rat stomach ECL cells: a critical reappraisal. Regul Pept. 2001;2:169–180. doi: 10.1016/s0167-0115(00)00217-2. [DOI] [PubMed] [Google Scholar]
- Mungan Z, Hammer RA, Akarca US, Komaki G, Ertan A, Arimura A. Effect of PACAP on gastric acid secretion in rats. Peptides. 1995;16:1051–1056. doi: 10.1016/0196-9781(95)00083-v. [DOI] [PubMed] [Google Scholar]
- Mungan Z, Ozman V, Ertan A, Arimura A. Pituitary adenylate cyclase activating polypeptide-27 (PACAP-27) inhibits pentagastrin-stimulated gastric acid secretion in conscious rats. Regul Pept. 1992;38:199–206. doi: 10.1016/0167-0115(92)90102-z. [DOI] [PubMed] [Google Scholar]
- Norlén P, Bernsand M, Konagaya T, Håkanson R. ECL-cell histamine mobilization in conscious rats: effects of locally applied regulatory peptides, candidate neurotransmitters and inflammatory mediators. Br J Pharmacol. 2001;134:1767–1777. doi: 10.1038/sj.bjp.0704419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlén P, Ericsson P, Kitano M, Ekelund M, Håkanson R. The vagus regulates histamine mobilization from rat stomach ECL cells by controlling their sensitivity to gastrin. J Physiol. 2005;564:895–905. doi: 10.1113/jphysiol.2005.082677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlén P, Kitano M, Lindström E, Håkanson R. Anaesthetic agents inhibit gastrin-stimulated but not basal histamine release from rat stomach ECL cells. Br J Pharmacol. 2000;130:725–730. doi: 10.1038/sj.bjp.0703347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras L, Taché Y, Martinez V. Somatostatin receptor type 2 mediates bombesin-induced inhibition of gastric acid secretion in mice. J Physiol. 2003;549:889–901. doi: 10.1113/jphysiol.2003.039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras L, Taché Y, Martinez V. Peripheral PACAP inhibits gastric acid secretion through somatostatin release in mice. Br J Pharmacol. 2004;142:67–78. doi: 10.1038/sj.bjp.0705739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz C, Sachs G, Walsh JH, Coy DH, Wu S. The somatostatin receptor subtype on rat enterochromaffin-like cells. Gastroenterology. 1994a;107:1067–1074. doi: 10.1016/0016-5085(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Prinz C, Scott DR, Hurwitz D, Helander HF, Sachs G. Gastrin effects on isolated rat enterochromaffin-like cells in primary culture. Am J Physiol. 1994b;267:G663–G675. doi: 10.1152/ajpgi.1994.267.4.G663. [DOI] [PubMed] [Google Scholar]
- Qvigstad G, Björgaas M, Eide I, Sandvik AK, Waldum HL. Vagal stimulation augments maximal (penta)gastrin-stimulated acid secretion in humans. Acta Physiol Scand. 1999;165:277–281. doi: 10.1046/j.1365-201x.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- Sandor A, Kidd M, Lawton GP, Miu K, Tang LH, Modlin IM. Neurohormonal modulation of rat enterochromaffin-like cell histamine secretion. Gastroenterology. 1996;100:1084–1092. doi: 10.1053/gast.1996.v110.pm8612997. [DOI] [PubMed] [Google Scholar]
- Sandvik AK, Cui G, Bakke I, Munkvold B, Waldum HL. PACAP stimulates gastric acid secretion in the rat by inducing histamine release. Am J Physiol. 2001;281:G997–G1003. doi: 10.1152/ajpgi.2001.281.4.G997. [DOI] [PubMed] [Google Scholar]
- Sandvik AK, Waldum HL, Kleveland PM, Schutze-Søgnen B. Gastrin produces an immediate and dose-dependent histamine release preceding acid secretion in the totally isolated, vascularly perfused rat stomach. Scand J Gastroenterol. 1987;22:803–808. doi: 10.3109/00365528708991918. [DOI] [PubMed] [Google Scholar]
- Schubert ML. The effect of vasoactive intestinal polypeptide on gastric acid secretion is predominately mediated by somatostatin. Gastroenterology. 1991;100:1195–1200. [PubMed] [Google Scholar]
- Schubert ML, Makhlouf GM.The somatostatin cells as a local modulator of gastric function The Stomach as an Endocrine Organ 1991Elsevier: Amsterdam; 99–119.In: Håkanson R, Sundler F (eds). [Google Scholar]
- Shintani N, Hashimoto H, Kunugi A, Koyama Y, Yamamoto K, Tomimoto S, et al. Desensitization, surface expression, and glycosylation of a functional, epitope-tagged type I PACAP (PAC1) receptor. Biochim Biophys Acta. 2000;1509:195–202. doi: 10.1016/s0005-2736(00)00295-9. [DOI] [PubMed] [Google Scholar]
- Stadil F, Rehfeld JF. Determination of gastrin in serum. An evaluation of the reliability of a radioimmunoassay. Scand J Gastroenterol. 1973;8:101–112. [PubMed] [Google Scholar]
- Sundler F, Ekblad E, Absood A, Håkanson R, Köves K, Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46:439–454. doi: 10.1016/0306-4522(92)90064-9. [DOI] [PubMed] [Google Scholar]
- Waldum HL, Sandvik AK, Brenna E, Petersen H. The gastrin–histamine sequence in the regulation of gastric acid secretion. Gut. 1991;32:698–700. doi: 10.1136/gut.32.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng N, Athmann C, Kang T, Lyu R-M, Walsh JH, Ohning GV, et al. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest. 1999;104:1383–1391. doi: 10.1172/JCI7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng N, Kang T, Lyu R-M, Wong H, Wen Y, Walsh JH, et al. The pituitary adenylate cyclase activating polypeptide type 1 receptor (PAC1-R) is expressed on gastric ECL cells: evidence by immunocytochemistry and RT-PCR. Ann NY Acad Sci. 1998;865:147–156. doi: 10.1111/j.1749-6632.1998.tb11173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]