Abstract
Background and purpose.
Somatostatin (SRIF-14) exerts broad spectrum antisecretory effects by activating the somatostatin 2 (sst2) receptor. The rat (r) sst2 receptor exists in ‘long' (sst2a) and ‘short' (sst2b) forms that differ in their C termini, while a single human (h) sst2a exists. This study compares the characteristics of recombinant rsst2a, rsst2b and hsst2a activation in human epithelia, and with native sst2 responses in rat colon.
Experimental approach.
Epithelial layers of each clone or rat colon were placed in Ussing chambers and short-circuit current (I SC) measured in response to SRIF-14 and chosen analogues. The relative potencies and ability to cause desensitization to SRIF-14 were assessed, and the affinities of the sst2 antagonist, D-Tyr8 CYN154806 for hsst2a, rsst2a and native rat colon sst2 receptors were established.
Key results.
Basolateral SRIF-14 responses were transient in hsst2a and rsst2a epithelia, but prolonged in rsst2b-expressing cells. Activation of rsst2a resulted in significant desensitization to SRIF-14 and receptor phosphorylation, whereas the rsst2b receptor did neither. Sst2-preferred agonists (BIM23190C and BIM23027) reduced I sc with similar potency and both caused complete desensitization to SRIF-14. CYN154806 antagonized hsst2a and rsst2a receptors with pK B values of 7.9 and 7.8, respectively. In rat colon mucosa, CYN154806 blocked SRIF-14 responses with a pA 2 value of 8.2, and BIM23190C responses with a pK B of 8.4.
Conclusions and implications.
SRIF-14 caused rapid rsst2a receptor phosphorylation and desensitization of epithelial antisecretory responses, neither of which occurred with the rsst2b receptor. These mechanisms are most likely to be a prerequisite for sensitivity to sst2-analogues with radiotherapeutic potential.
Keywords: somatostatin 2 receptor variants, sst2a, sst2b, epithelial ion transport
Introduction
SRIF-14 (somatotrophin release inhibiting factor, or somatostatin-14) is an enteric neuropeptide (Schultzberg et al., 1980; Ekblad et al., 1988) and endocrine cell product (Alumets et al., 1977) with broad spectrum antisecretory effects that include inhibition of gastric acid (Lloyd et al., 1995), intestinal electrolytes (Eklund et al., 1988; Knobloch et al., 1989; Ferrar et al., 1990) and endocrine secretions, for example, growth hormone, glucagon and insulin (for a review, see Weckbecker et al., 2003). These inhibitory actions are mediated by somatostatin 2 (sst2) receptors, one of five cloned sst receptor types that couple to pertussis toxin-sensitive Gi/o proteins and reduce adenylate cyclase activity (Siehler and Hoyer, 1999b) and can also modulate ion channels, for example, opening neuronal K+ channels (Hicks et al., 1998) or closing voltage-dependent Ca2+ channels (Kleuss et al., 1991) thereby reducing neuron excitability.
Two sst2 receptor spice variants are produced in mouse (Vanetti et al., 1993) and rat (r) tissues, alternative splicing occurring via a cryptic splice site within the coding sequence of the sst2 receptor gene (Schindler et al., 1998). Both rodent species express an sst2a receptor variant that is 23 amino acids longer than sst2b, but with different C termini (for a review, see Cole and Schindler, 2000). Activation of the murine (m) short isoform, msst2b, inhibited adenylate cyclase with greater efficacy and resulted in significantly less agonist-induced desensitization than the msst2a receptor (Vanetti et al., 1993). In contrast, no differences were observed between rsst2a and rsst2b signalling or desensitization after exposure to SRIF-14 (Schindler et al., 1998). The clearest discrimination between these two receptors' signalling capacities was described for the opposing actions of SRIF-14 on CHO cell proliferation; rsst2a-expressing cells exhibiting an inhibition, and rsst2b cells, a stimulation of peptide-induced proliferation (Alderton et al., 1998). In contrast with the proven production of two different sst2 isoforms in rat and mouse, only the human (h) sst2a has been identified as a functional protein, the hsst2b orthologue remaining putative (see Cole and Schindler, 2000).
The distribution patterns of the two sst2 variants differ in mouse and rat nervous systems, sst2a predominating in the central and peripheral nervous systems (Sarret et al., 1998; Cole and Schindler, 2000; Schulz et al., 2000). In the gastrointestinal tract, differences are also observed. In rat stomach and small intestine, for example, the sst2a receptor protein is expressed by neuroendocrine cells and enteric nerves (implicating indirect, as well as direct mechanisms of SRIF-14 action), while sst2b receptor labelling is found in a discrete subpopulation of rat parietal cells (Schindler and Humphrey, 1999). Warhurst et al. (1996) observed both sst2 receptor transcripts in rat colonic crypt extracts and with equal abundance. In the mouse stomach, parietal and endocrine cells express sst2 receptors, as do subpopulations of myenteric and submucous neurons, immunostaining for sst2a being colocalized with nitric oxide synthase immunoreactivity in inhibitory myenteric motor neurons (Allen et al., 2002). In the human gastrointestinal tract, sst2a is also expressed by endocrine cells (particularly, gastrin-containing cells in the small intestine), as well as myenteric and submucous neurons along the length of the intestine (Gugger et al., 2004) and this pattern is similar to that reported in the rat gastrointestinal tract (Schindler and Humphrey, 1999).
The extensive inhibitory nature of SRIF-14 actions stimulated early interest in the peptide for therapeutic benefit, for example, as a novel treatment for diabetes, of hormone-secreting tumours and hypersecretory diarrhoea, but the relative instability of plasma SRIF-14 was limiting. One of the first longer-acting cyclic SRIF-14 fragment analogues, octreotide (SMS 201-995, Sandostatin) is used, for example, to treat acromegaly, to prevent complications following pancreatic surgery and relieve symptoms such as chemotherapy-induced diarrhoea (Lamberts et al., 1996). Many other more stable cyclo-octapeptide analogues have since been produced with therapeutic, as well as diagnostic potential (Weckbecker et al., 2003) and some of these have been used in the present study, specifically, BIM23027 (amino acid sequence is as listed in McKeen et al. (1995)), BIM23190C, BIM23014C (Lanreotide) and BIM23268, together with the linear analogues BIM23052 and BIM23056 (amino acid sequences are as listed in Shimon et al. (1997)). Sst receptor-mediated endocytosis has also been utilized clinically to deliver stable radiolabelled SRIF-14 analogues into tumour cells that express sst2 receptors, thereby allowing metastases to be imaged (Breeman et al., 2001) and treated by receptor-targeted radiotherapy (Weckbecker et al., 2003).
The present study set out to determine whether SRIF-14 activation of recombinant hsst2a, rsst2a and rsst2b expressed in epithelial cells, differed (i) in their response time courses and pharmacology, (ii) in their agonist-induced phosphorylation and desensitization and (iii) whether their pharmacologies differed from that of native sst2 antisecretory responses in rat colon mucosa. Two SRIF-14 analogues were chosen for their sst2 affinity, namely, BIM23190C and BIM23027 (McKeen et al., 1995; Shimon et al., 1997; Siehler et al., 1999; Weckbecker et al., 2003). Also included were other SRIF-14 analogues known to stimulate sst5 receptors (BIM23268 and BIM23052, Shimon et al., 1997; Weckbecker et al., 2003) and the nonselective sst5/sst2/sst3 analogue, BIM23056 (Shimon et al., 1997; Siehler and Hoyer, 1999a, 1999b; Weckbecker et al., 2003) shown also to be an sst5 antagonist (Wilkinson et al., 1996). The affinity of the selective sst2 antagonist, D-Tyr8-CYN154806 (Ac-(4-NO2-Phe)-cyc(DCys-Tyr-DTrp-Lys-Thr-Cys)-DTyr-NH2) (Feniuk et al., 2000; Nunn et al., 2003) for each recombinant sst2 receptor was also determined and the antagonist used to confirm the predominant involvement of this receptor type in SRIF-14 responses in rat colon mucosa.
Methods
Cell culture and transfection
Colony 1 adenocarcinoma cells (from Dr S Kirkland; Marsh et al., 1993) were incubated in DMEM supplemented with 10% foetal calf serum, 100 μg ml−1 kanamycin and 1.2 μg ml−1 amphotericin B. Cell lines were grown at 37°C in a humidified atmosphere of 95% O2/5% CO2 and passaged when confluent by trypsinization (0.25% in versene). Stably transfected clones were generated by calcium phosphate co-precipitation followed by glycerol shock, using cDNA sequences encoding human sst2a (in pTEJ8, provided by Professor T. Schwartz, Panum Institute, Copenhagen, Denmark), and the rat sst2a or sst2b splice variants (N-terminal haemagglutinin (HA) epitope-tagged constructs in pcDNA3.1, from Dr M Schindler; Schindler et al., 1998). Colonies resistant to the antibiotic G418 (1.0 mg ml−1) were isolated directly, expanded and screened for sst2 receptor expression in short-circuit current (ISC) studies.
Animals
Male Sprague–Dawley rats (200–250 g, from Banton and Kingman, Hull, UK) were maintained in a 12 h light–dark cycle, with access to standard chow and water ad libitum. The descending colon was removed from rats killed by cervical dislocation, and placed in oxygenated Kreb's Henseleit (KH) buffer (constituents in mM: NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, glucose 11.1; pH 7.4) until dissection.
Short-circuit current studies
Colony 1 epithelial layers were grown to confluence (area 0.2 cm2) on collagen-coated Millipore filters, bathed at 37°C in oxygenated KH and voltage-clamped at 0 mV in Ussing chambers (DVC1000, WPI, Stevenage, UK) as described previously (Holliday et al., 2005). The resulting ISC was elevated by a maximal concentration of the secretagogue, vasoactive intestinal polypeptide (VIP) (30 nM, 20 min) before basolateral addition of sst2 ligands. Unless otherwise stated, agonist concentration–response relationships were constructed from single peptide additions. For the determination of CYN154806 IC50 and pKB values, epithelial layers were pretreated for 10 min with the antagonist before SRIF-14 application. At the end of each experiment, UK14,304 (1 μM) and piretanide (200 μM) were included as inhibitory controls.
Mucosal sheets from rat-descending colon (0.6 cm2 area) were voltage-clamped at 0 mV in oxygenated KH, as described previously (Cox et al., 1988). Concentration–response curves to SRIF-14 and other analogues were constructed by cumulative peptide additions to the basolateral reservoir. Changes to ISC levels were recorded continuously. Tetrodotoxin (TTX; 100 nM) was used to inhibit neuronal activity (as the submucous neuron innervation is intact in these preparations) before addition of the sst2 antagonist, CYN154806 10 min later. For the determination of CYN154806 pA2 value in mucosal preparations, the antagonist was added 10 min before the first agonist addition.
Phosphorylation measurements
Colony 1 clones were grown to 80% confluence in six-well plates, loaded with 50 μCi H3PO4 in phosphate-free KH buffer for 1 h at 37°C and treated with vehicle or 10 μM SRIF-14 for 5 min. HA-tagged sst2 receptors were then immunoprecipitated as described previously (Holliday et al., 2005). Briefly, cells were dissolved (2 h at 4°C) in RIPA buffer (50 mM Tris, 100 mM NaCl, 10 mM NaF, 10 mM Na4P2O7, 5 mM EDTA, 1.5% Nonidet P40, 0.5% sodium deoxycholate, 0.2% sodium dodecyl sulphate (SDS), 0.5 mM phenylmethylsulphonyl fluoride, 200 μM activated Na3VO4, 100 nM okadaic acid, 10 μg ml−1 leupeptin and aprotinin; pH 8.0) and samples were equalized for protein content (BCA protein assay, Pierce, Cheshire, UK). Immunoprecipitations were carried out overnight at 4°C by addition of anti-HA antibody (rat clone 3f10, Roche Molecular Biochemicals, Lewes, UK) directly conjugated to agarose, and then the washed precipitates were denatured in Laemmli loading buffer (80°C, 3 min). Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE; 10% Tris-HCl Ready Gels; Biorad, Hemel Hempstead, UK) and the dried gels were exposed to pre-flashed Amersham Hyperfilm MP for 72 h at –70°C to detect 32P labelling. To ensure equivalent receptor loading, immunoprecipitates resolved by SDS-PAGE were also transferred to polyvinyldifluoride membrane and probed overnight (18°C) with the 3f10 anti-HA antibody (100 ng ml−1 in TBST (50 mM Tris, 150 mM NaCl, 0.1% Tween 20; pH 7.5) containing 1% BSA and 0.02% NaN3). Western blots were developed using a goat anti-rat horseradish peroxidase-conjugated secondary antibody (1:5000 in TBST for 60 min; GE Biosciences, Little Chalfont, UK) and enhanced chemiluminescence detection (ECL plus; GE Biosciences).
Data analysis
Pooled data from ISC studies present the maximal changes in ISC as μA cm−2, mean±1 standard error of the mean (s.e.m.). Agonist pEC50 values and antagonist pIC50 values were obtained by nonlinear iterative fits to the combined data using GraphPad Prism (version 3.03, GraphPad Software Inc., San Diego, CA, USA). Affinity estimates for CYN154806 were obtained from the Gaddum equation (pKB, for epithelial layers) and Schild analysis (pA2, for rat colon studies). Statistical comparisons of two data sets were performed by use of Student's t-test, while multiple comparisons were obtained by one-way analysis of variance with Dunnett's post-test.
Materials
Cell culture materials were from the following origin: DMEM and G418 sulphate (Invitrogen, Paisley, UK); foetal calf serum, kanamycin and amphotericin B (ICN Biomedicals, Oxford, UK); trypsin (Lorne Laboratories, Reading, UK). H332PO4 (10 mCi ml−1) was from Amersham Biosciences (Little Chalfont, Bucks, UK). Agonists BIM23104C, BIM23190C, BIM23052, BIM23056 and BIM23268 (see Shimon et al., 1997 for sequences) were provided by Biomeasure Inc. (Milford, MA 01757, USA); while D-Tyr8-CYN154806 and BIM23027 (McKeen et al., 1995) were kind gifts of Dr W Feniuk (Glaxo Institute of Applied Pharmacology, Cambridge, UK). Other peptides were purchased from Bachem (Merseyside, UK); all were stored as single use frozen aliquots of aqueous solution. Piretanide was obtained from Hoechst Marion Roussel (Swindon, UK), and other reagents were from Sigma-Aldrich (Poole, UK) or VWR International (Poole, UK). UK14,304 (5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine) was prepared as a 10 mM solution in dimethylsulphoxide; other chemicals were made up as aqueous stock solutions.
Results
Epithelial responses to recombinant sst2 receptors
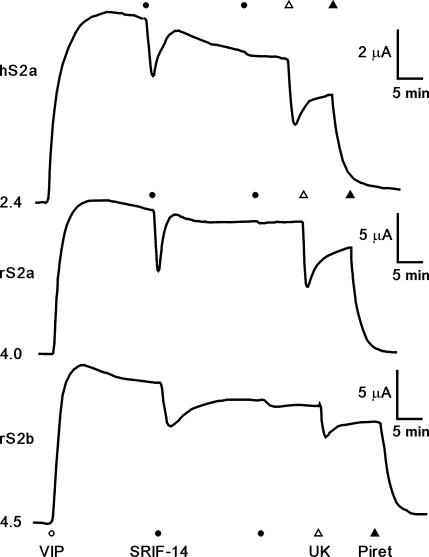
We isolated stably transfected Colony 1 cell lines expressing the hsst2a (hS2a), rsst2a (rS2a) or rsst2b (rS2b). These clones exhibited higher basal resistances than non-transfected Colony 1 cells in ISC studies, but in each case VIP-stimulated sustained elevations in ISC with similar potencies (pEC50 range: 8.08–8.48, data not shown), reflecting electrogenic chloride secretion. Moreover, VIP responses in Colony 1, hS2a, rS2a and rS2b epithelial layers were all inhibited by subsequent UK14,304 activation of endogenous α2 receptors (Figure1 and Table 1), or by blockade of basolateral Na+/K+/2Cl− co-transport using piretanide (Figure 1). However, in contrast to non-transfected host cells, basolateral SRIF-14 decreased VIP-stimulated ISC in hS2a, rS2a and rS2b clones with equivalent potency (pEC50 range: 7.64–7.86) and similar levels of inhibition, that is, 29–43% of the VIP response after 100 nM SRIF-14 (Figure1 and Table 1). SRIF-14 (100 nM) also inhibited basal ISC levels, by −13.0±2.8 μA cm−2 (n=4) in rS2a cells and by −13.0±3.8 μA cm−2 (n=4) in the rS2b epithelial clone. Apical SRIF-14 addition (100 nM) had little effect on VIP-elevated ISC in rS2a (−1.1±0.4 μA cm−2, n=4) or rS2b epithelial layers (−3.5±0.5 μA cm−2, n=3), indicating preferential targeting of both sst2 receptor variants to the epithelial basolateral membrane.
Figure 1.
Representative ISC recordings from Colony 1 hS2a, rS2a and rS2b clones. Confluent epithelial layers were stimulated with 30 nM vasoactive intestinal polypeptide (which produced a sustained rise in ISC) then by 100 nM SRIF-14 (two additions 20 min apart), 1 μM UK14,304 (UK) and 200 μM piretanide (Piret) as indicated. Initial ISC levels (in μA) are given to the left of each trace. Note the smaller μA scale for the hS2a trace. SRIF-14, somatotrophin release inhibiting factor; UK14,304, 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine; VIP, vasoactive intestinal polypeptide.
Table 1.
Electrophysiological parameters and responses to basolateral stimuli in untransfected Colony 1 cells and sst2 receptor clones
| Basal R | Basal ISC | VIP 30 nM ΔISC |
SRIF-14 |
UK14,304 | ||
|---|---|---|---|---|---|---|
| Cells | (Ω cm2) | (μA cm−2) | (μA cm−2) | pEC50 | 100 nM ΔISC (μA cm−2) | 1 μM ΔISC (μA cm−2) |
| Colony 1 | 39.3±0.6 (531) | 11.0±0.4 (531) | +29.5±1.2 (200) | — | 0.0±0.0 (14) | −10.4±0.9 (4) |
| hS2a | 131.2±4.1 (125) | 9.9±4.9 (125) | +22.1±1.0 (103) | 7.64±0.07 | −9.6±1.1 (8) | −5.8±0.5 (6) |
| rS2a | 100.1±2.5 (237) | 50.6±1.7 (237) | +73.2±1.8 (189) | 7.67±0.07 | −22.4±2.2 (6) | −25.3±3.4 (6) |
| rS2b | 63.4±2.8 (126) | 25.2±1.1 (126) | +83.0±3.3 (105) | 7.86±0.07 | −24.3±4.4 (6) | −19.5±3.6 (6) |
Abbreviations: SRIF-14, somatotrophin release inhibiting factor; UK14,304, 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine; VIP, vasoactive intestinal polypeptide.
pEC50 values for SRIF-14 were calculated from pooled single addition concentration–response relationships for the inhibition of 30 nM VIP-stimulated ISC (n=3–8).
Values in parentheses indicate the number of observations for basal resistances (R) and ISC, and for the change in ISC (ΔISC) after each agonist addition at the optimal concentrations shown.
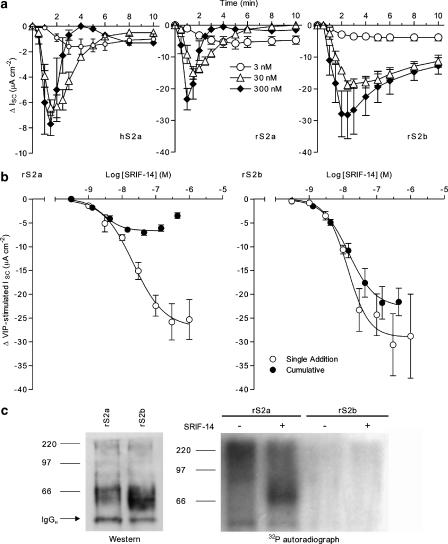
Sst2a and sst2b receptor desensitization
Figure 2a illustrates the time-profiles of sst2a and sst2b-mediated ISC responses following basolateral SRIF-14 addition. In both hS2a and rS2a cells, SRIF-14 responses were transient, with increased agonist concentrations leading to both a reduction in the time-to-peak (1–1.5 min after 300 nM SRIF-14), and a more rapid return to ISC levels before sst2a stimulation (3–4 min after 300 nM). In contrast to the short-lived nature of rat and human sst2a responses, the inhibition of VIP-elevated ISC by SRIF-14 in rS2b epithelial layers was more sustained (Figure 2a). For example, responses at 10 min after 300 nM SRIF-14 had decayed to 47.2±3.3% (n=4) of the peak levels at 2.5–3 min, compared to only 6.4±1.8% (n=5; P<0.001) in rS2a cells.
Figure 2.
Desensitization of sst2a and sst2b receptors. Time courses from hS2a, rS2a and rS2b cells (a) show the reductions in vasoactive intestinal polypeptide-stimulated ISC following SRIF-14 addition (t=0), at 3 nM (n=3–4), 30 nM (n=3–4) or 300 nM (n=3–5). SRIF-14 concentration–response relationships in rS2a and rS2b epithelial layers (b) were constructed from responses to single agonist concentrations (n=3–8), or to cumulative agonist additions (n=3–4). Sigmoidal fits to the pooled data (excluding the 444 nM data point for rS2a-cumulative responses) yielded the pEC50 values and maximal SRIF-14 responses given in Table 1 and the text. In (c), immunoprecipitated HA-tagged rat sst2a and sst2b receptors were resolved by SDS-PAGE and transferred to polyvinyl difluoride membrane for western blotting with anti-HA (left hand photograph). Sst2 proteins were identified as broad bands of 58–83 kDa (sst2a) and 53–83 kDa (sst2b), in addition to bands corresponding to the light (25 kDa, data not shown) and heavy (IgGH) chains of the immunoprecipitating antibody. To measure phosphorylation, HA-tagged receptors were immunoprecipitated under the same conditions, from rS2a and rS2b cells labelled with 32Pi and treated with vehicle or 10 μM SRIF-14 for 5 min. The autoradiograph (right, 72 h) of the dried SDS-PAGE gel indicates agonist-induced phosphorylation of rat sst2a, but not sst2b receptors. SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; SRIF, somatotrophin release inhibiting factor
We next compared rS2a and rS2b concentration–response curves constructed from single agonist additions, with cumulative relationships in which sequential applications of agonist were made at the peak of the previous SRIF-14 response (Figure 2b). The cumulative data for rS2b clones yielded a SRIF-14 pEC50 of 7.83±0.04 and a maximal ISC response of −21.8±3.4 μA cm−2 (n=4, 144 nM), similar to values obtained from single peptide additions (Table 1). However, rS2a cumulative SRIF-14 responses were bell-shaped, with a 10-fold higher pEC50 for the inhibitory portion of the curve (8.55±0.08), and a maximal response at 44 nM (−7.0±0.6 μA cm−2, n=4) that was only 31% of the peak ISC decrease to 100 nM SRIF-14 (Table 1).
To measure phosphorylation, the HA-tagged rat sst2a and sst2b receptors were immunoprecipitated from epithelial clones loaded with 32Pi, and incorporation of radiolabelled phosphate was determined by gel autoradiography. These experiments were technically challenging, involving the use of a high agonist concentration (10 μM SRIF-14 for 5 min) to overcome the diffusion barrier to the basolateral epithelial domain, and limited by variable background in the autoradiographs from the presence of genomic DNA contamination in the samples. Western blots probed with anti-HA revealed specific broad bands in immunoprecipitates from rS2a (58–83 kDa) and rS2b (53–83 kDa) epithelia (Figure 2c), which were absent in samples from non-transfected Colony 1 cells. Despite the similar loading of receptor proteins, 32Pi labelling of an equivalent band was observed only in rS2a immunoprecipitates (autoradiograph, Figure 2c), following stimulation by SRIF-14. In contrast, no phosphorylation of sst2b receptors could be detected.
Effects of sst agonists and antagonist CYN154806 on recombinant sst2 receptor-expressing epithelia
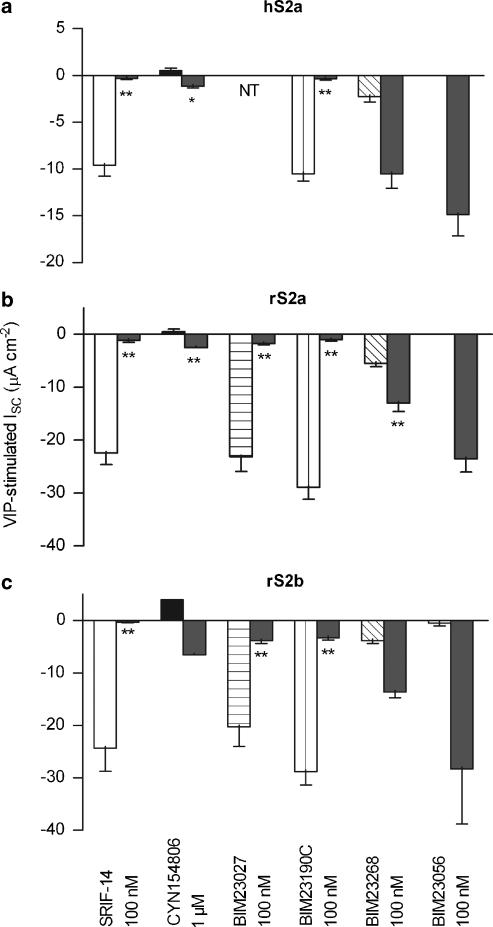
The sst2-preferred peptides BIM23027 and BIM23190C were both full agonists in the transfected clones (Figure 3), displaying equivalent potencies and response time-profiles to SRIF-14 in rS2a and rS2b epithelial layers. BIM23027 inhibited VIP-stimulated ISC with pEC50 values of 7.41±0.26 (rS2a; n=4) and 7.50±0.18 (rS2b; n=3–5), BIM23190C pEC50 values were 7.58±0.14 in rS2a cells (n=3–5) and 7.66±0.18 in the rS2b clone (n=3–5). The sst5-preferred agonist BIM23268 (100 nM) elicited only small ISC decreases in each clone, with slight reductions in the subsequent sensitivity to 100 nM SRIF-14 (Figure 3). BIM23056 (a partial agonist at sst5, sst3 and sst2 receptors, 100 nM) did not affect VIP-elevated ISC, nor did it alter the responses to SRIF-14 added 20 min later (Figure 3).
Figure 3.
Responses to SRIF-14 analogues in Colony 1 clones. Epithelial layers from hS2a (a), rS2a (b) or rS2b cells (c) were pre-stimulated with 30 nM vasoactive intestinal polypeptide for 20 min, followed by SRIF-14, BIM agonists, or D-Tyr8 CYN154806 (n=3–6). The pooled changes in ISC to these peptides (mean±1 s.e.m.) are indicated by the left hand of each pair of columns (open for SRIF-14, filled or hatched for analogues). A second addition of 100 nM SRIF-14 was added after 20 min (SRIF-14 or BIM agonists) or 10 min (CYN154806), giving the indicated reductions in ISC (right hand column of each pair). *P<0.05, **P<0.01 compared to control 100 nM SRIF-14 responses (open columns). BIM23027 was not tested (NT) in hS2a cells and CYN154806 data for rS2b was n=1. D-Tyr8 CYN154806, Ac-(4-NO2-Phe)-cyc(DCys-Tyr-DTrp-Lys-Thr-Cys)-DTyr-NH2; SRIF-14, somatotrophin release inhibiting factor.
The D-Tyr8 isomer of the peptide sst2 antagonist CYN154806 (1 μM) produced small increases in ISC, and attenuated peak SRIF-14 responses added 10 min later in hS2a, rS2a and rS2b clones (Figure 3). CYN154806 pIC50 values for the inhibition of 100 nM SRIF-14 responses were 7.13±0.17 (hS2a) and 7.48±0.04 (rS2a; both n=3–6). SRIF-14 concentration–response relationships were parallel-shifted to the right to the same degree in hS2a and rS2a epithelial layers by 30 nM CYN154806, with no change in the maximum (data not shown). The resulting pEC50 values in the presence of antagonist were 7.17±0.08 (hS2a) and 7.04±0.10 (rS2a; each n=3–8), yielding pKB values for the hsst2a and rsst2a receptor of 7.9 and 7.8, respectively.
Responses to SRIF-14 and analogues in rat descending colon mucosa
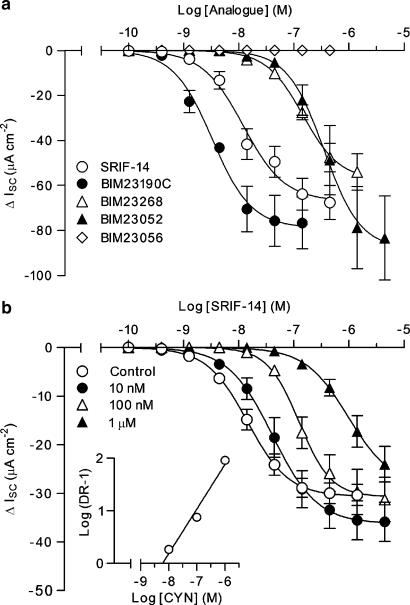
Isolated preparations of rat descending colon mucosa exhibited basal resistances of 93.2±4.0 Ω cm2 and ISC levels of 48.7±3.7 μA cm−2 (n=76). SRIF-14, BIM23190C, BIM23014C, BIM23268 and BIM23052 all inhibited the basal ISC, with maximal sustained responses of similar magnitude. Concentration–response relationships (Figure 4a and Table 2) revealed the following order of agonist potency: BIM23190C⩾BIM23014C>SRIF-14>BIM23052=BIM23268. BIM23056 was inactive up to the highest concentration tested (444.4 nM; as observed previously in colonic mucosa; McKeen et al., 1995) and a subsequent addition of SRIF-14 (100 nM) still reduced ISC by 77.0±8.0% (n=6; compared with controls of 71.2±5.2%, n=6).
Figure 4.
Responses to SRIF-14 and BIM agonists in rat descending colon mucosa. In (a), pooled cumulative concentration–response curves for SRIF-14 (n=18), BIM23190C (n=3–5), BIM23052 (n=4), BIM23268 (n=6) or BIM23056 (n=3) produced the pEC50 values presented in Table 2. (b) The effect of CYN154806 pretreatment (CYN; 10 nM–1 μM; n=5) on the SRIF-14 concentration–response curve (antagonist pEC50 values are quoted in the text) in tetrodotoxin (100 nM)-pretreated tissues. The resulting Schild plot (inset) had a slope of 0.85 and a pA2 value of 8.2. SRIF, somatotrophin release inhibiting factor.
Table 2.
The potencies and peak effect of sst receptor agonists in rat descending colon mucosa
| Agonist | Sst receptor preference and order | pEC50 | Peak cumulative ΔISC (μA cm−2) |
|---|---|---|---|
| SRIF-14 | Nonselective (2, 3, 5 ⩾1, 4) | 7.92±0.08 (18) | −67.6±7.5 (444 nM) |
| BIM23190C | Sst2-preferred(2≫5>3≫4, 1) | 8.48±0.06 (5) | −76.7±11.9 (144 nM) |
| BIM23014C | Sst2-preferred (2≫5>3≫4, 1) | 8.21±0.05 (2–5) | −107.8±14.3 (144 nM) |
| BIM23052 | Sst5-preferred (5>3, 2⩾4⩾1) | 6.46±0.03 (4) | −83.3±18.6 (4.44 μM) |
| BIM23268 | Sst5-preferred (5≫2, 4, 1>3) | 6.84±0.03 (6) | −54.0±8.1 (1.44 μM) |
| BIM23056 | Partial agonist at all (5>2, 3⩾4⩾1) | Inactive (3) | 0.0±0.0 (444 nM) |
Abbreviation: SRIF, somatotrophin release inhibiting factor.
The described sst receptor preferences of SRIF-14 and BIM analogues are based on binding IC50 values obtained at recombinant human sst1–5 receptors in stably transfected CHO-K1 cells (Shimon et al. (1997)).
pEC50 values (with n numbers) and peak agonist responses (including the highest cumulative peptide concentration used, in brackets) were calculated from the concentration–response relationships presented in Figure 4a.
To measure epithelial responses to these agonists in the absence of potentially confounding neuronal sst receptor-mediated effects, we added the neurotoxin, TTX (100 nM) to mucosal preparations. TTX reduced the basal ISC slightly (−5.6±0.9 μA cm−2; n=40, as shown previously, Ferrar et al., 1990), but had no significant effect on the subsequent pEC50 values for BIM23190C (8.61±0.33, n=5) or BIM23268 (6.84±0.30, n=4) compared with controls (Table 2). Pre-incubation of TTX-treated tissues with CYN154806 (10 min, produced no change in ISC) elicited rightward shifts in the SRIF-14 concentration–response curve without a change in the maximum inhibitory response (Figure 4b), yielding pEC50 values of 7.38±0.01 (10 nM antagonist), 6.90±0.02 (100 nM) and 6.01±0.03 (1 μM, each n=5). The linearity and slope (0.85±0.13) of the resulting Schild analysis was consistent with reversible, competitive antagonism, and gave a pA2 value of 8.2. The BIM23190C concentration–response curve was also right-shifted (pEC50: 7.20±0.05, compared to 8.61±0.05 in control tissues; n=5) in the presence of 100 nM CYN154806 and the resulting pKB value (8.4) was similar to the pA2 obtained with SRIF-14 as the agonist. The effect of CYN154806 on sst5 preferred agonist, BIM23268-stimulated responses was less pronounced, with a small decrease in agonist potency after 100 nM antagonist (pEC50: 7.21±0.07 compared to control value of 7.49±0.18, n=3–5), resulting in a 10-fold lower pKB estimate of 7.0.
Discussion
SRIF-14 antisecretory responses, desensitization and phosphorylation of recombinant sst2-expressing epithelia.
We characterized the splice variants of the human and rat sst2 receptors in stably transfected epithelial cells, to assess how differences between their respective C termini altered functional responses. Sst2a and sst2b receptors were both targeted to the basolateral epithelial membrane, indicating that the shortened C-tail of the sst2b receptor did not cause its apical misdirection after synthesis (Beau et al., 2004). In each cell line (hS2a, rS2a and rS2b), basolateral SRIF-14 inhibited VIP-stimulated Isc levels (pEC50 7.64–7.86), as expected from the preference of the former receptors for Gi/o protein coupling (Schindler et al., 1998; Siehler and Hoyer, 1999b). However, SRIF time-courses were strikingly more transient in hS2a and rS2a cells than observed for rS2b responses. Together with the comparison between SRIF cumulative and single addition concentration–response relationships (Figure 2b), these data provide strong evidence that both sst2a receptors undergo much more rapid desensitization than the rsst2b variant.
As we also observed in rS2a epithelial cells, native and recombinant sst2a receptors are phosphorylated within minutes (Liu et al., 2003, 2005; Tulipano et al., 2004), either by a G-protein-coupled receptor kinase, GRK2, after homologous SRIF-14 stimulation (Elberg et al., 2002; Tulipano et al., 2004), or after heterologous activation of protein kinase C by other G-protein-coupled receptors (Hipkin et al., 2000; Elberg et al., 2002). Phosphorylated sst2a receptors bound to SRIF-14 recruit β-arrestin adaptor proteins, preventing G-protein coupling (desensitization) and also initiating clathrin-mediated internalization (Tulipano et al. 2004). SRIF-14 and its peptide analogues (but not small molecule agonists) stimulate similar patterns of sst2a β-arrestin2 recruitment and internalization (Liu et al., 2005), consistent with the identical time-course profiles of SRIF-14, BIM23027 and BIM23190C in hS2a and rS2a cells. The reduced desensitization observed here for the sst2b variant is supported by an earlier study on mouse sst2a and sst2b isoforms (Vanetti et al., 1993), but differs from the comparison of rat sst2a and sst2b receptors made by Schindler et al. (1998). However, these authors used a much longer conditioning SRIF-14 treatment (60 min) to establish sst2a and sst2b desensitization, which contrasts with our examination of the real-time effects of a single agonist concentration over a shorter period (20 min). Our demonstration for the first time that rsst2b receptors (containing 3 C-terminal Ser/Thr residues) are not phosphorylated as efficiently as the rsst2a isoform (10 C-tail sites) suggests the underlying mechanism. In particular, alanine mutation of a C-terminal Thr cluster surrounded by acidic residues (E352TTET) inhibits sst2a phosphorylation and β-arrestin2 recruitment (Tulipano et al., 2004), and the absence of this key sequence in the sst2b receptor may be sufficient to explain its resistance to phosphorylation and functional desensitization.
Comparison of hsst2a and rsst2a receptor pharmacology and desensitization
There were no apparent differences in the sensitivity of epithelial layers expressing either hsst2a or rsst2a to SRIF-14, the sst2 antagonist CYN154806 or BIM compounds; sst2 agonist BIM23190C or the sst5-preferred BIM23268. Both epithelial clones were insensitive to the nonselective sst5/sst2/sst3 agonist, BIM23056. CYN154806 was a competitive antagonist, blocking antisecretory responses with the same potency (pKB values of 7.9 and 7.8) for hsst2a and rsst2a and this was similar to the potencies obtained for the cyclic octapeptide in other functional assays (Feniuk et al., 2000; they also used the D-Tyr8 isomer of CYN154806).
The sst5-preferred agonists, BIM23268 (Figure 3) and BIM23052 (data not shown) were partial activators at the highest concentration tested (100 nM) and they only caused partial subsequent desensitization of SRIF-14 responses. BIM23056 had no effect alone, or on subsequent SRIF-14 responses (Figure 3) and we conclude it has a very low affinity for sst2 receptors in epithelia, or in rat mucosal preparations (see below). Our observations are in keeping with reports that BIM23056 has a higher affinity for sst5 (Shimon et al., 1997; Siehler et al., 1998) and is actually a potent sst5 antagonist (Wilkinson et al., 1996). As our epithelia only express sst2 receptors, we would not expect to see any sst5-mediated effects to this, or other less potent sst5 analogues. We have ascertained that BIM23190C and BIM23037 are full sst2 agonists, while BIM23268 and BIM23052 were less potent in all preparations. It is notable that in another colonic adenocarcinoma cell line (Col-24 cells, that constitutively express other Gi-coupled receptors; Cox et al., 2001) BIM23268 was a potent antisecretory agonist (exhibiting an EC50 value of 8.7 nM) and thus these cells appear to express sst5 receptors constitutively (Cox et al., unpublished observations).
Sst2 receptor pharmacology in rat-isolated colon mucosa
Reverse transcription-PCR analysis of crypt epithelia isolated from the rat descending colon, show expression of sst2a and sst2b receptors together with sst1 transcripts and low levels of sst5 RNA (but no sst3 or sst4 products, Warhurst et al., 1996). Given this combination of sst receptor expression, we might predict a mixed pharmacology. However, the sst2-preferring agonists BIM23190C, BIM23014C (Table 2) and SRIF-14 were potent antisecretory agonists with similar efficacy, producing long-lasting reductions in ISC (as shown previously for SRIF-14; Ferrar et al., 1990; McKeen et al., 1995). These inhibitory responses were unaffected by the neurotoxin, TTX and are therefore epithelial in origin. However, they were abolished by CYN154806, while BIM23056 (with purported sst5/sst2/sst3 activity, Weckbecker et al., 2003; and sst5 antagonism; Wilkinson et al., 1996) had no significant sst2 activity in our epithelial models. Colonic SRIF-14 and BIM23190C responses were both blocked by CYN154806 (with a pA2 value of 8.2 and pKB of 8.4, respectively) and the cyclic octapeptide was an order of magnitude less potent at inhibiting the antisecretory responses of sst5-preferred (but also sst2-activating) BIM23268 (pKB of 7.0). CYN154806 has in addition been found to have a low affinity for sst5 receptors (two orders of magnitude less than its reported sst2 affinity; Feniuk et al., 2000; Nunn et al., 2003), so inhibition, albeit at high nanomolar antagonist concentrations might be expected in colonic mucosa.
In conclusion, the rapid agonist-induced sst2a receptor phosphorylation and coincident desensitization that we have observed is consistent with the pronounced sst2a receptor internalization that Cole and Schindler (2000) and others (Hipkin et al., 2000) described. This acute process has already been harnessed to deliver stable radiolabelled SRIF-14 analogues into sst2-expressing neuroendocrine tumours to allow either imaging (Breeman et al., 2001) or targeted chemotherapy and radiotherapy treatment (for a review, see Hofland and Lamberts, 2003; Weckbecker et al., 2003). However, certain patients with islet or carcinoid tumours can become insensitive to treatment with long-term SRIF-agonists such as lanreotide or octreotide (Hofland and Lamberts, 2003; Zomerhuis et al., 2005). It remains to be seen whether sst2a receptor desensitization contributes in any way to this chronic reduction in efficacy.
Acknowledgments
This work was initially funded by the Special Trustees for St Thomas Hospital and subsequently the Kimmel Cancer Foundation. We thank Biomeasure Inc. (Milford, MA 01757, USA) for the BIM analogues, and Dr W. Feniuk (then at Glaxo Institute of Applied Pharmacology, University of Cambridge, UK) for BIM23027 and CYN154806. We also thank Professor T Schwartz (Panum Institute, Copenhagen, Denmark) and Dr M Schindler (now at Boehringer Ingelheim Pharma KG, Biberach an der Riss, Germany) for generously providing hsst2a, rsst2a and rsst2b cDNA's respectively.
Abbreviations
- D-Tyr8 CYN154806
Ac-(4-NO2-Phe)-cyc(DCys-Tyr-DTrp-Lys-Thr-Cys)-DTyr-NH2
- DMEM
Dulbecco's modified Eagle's medium
- HA
haemagglutinin
- hS2a
human sst2a clone
- rS2a
rat sst2a clone
- rS2b
rat sst2b clone
- ISC
short-circuit current
- KH
Kreb's Henseleit
- SRIF-14
somatotrophin release inhibiting factor (somatostatin)14–28
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
- UK14,304
5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine
Conflict of interest
The authors state no conflict of interest.
References
- Alderton F, Fan T-PD, Schindler M, Humphrey PPA. Rat somatostatin sst2(a) and sst2(b) receptor isoforms mediate opposite effects on cell proliferation. Br J Pharmacol. 1998;125:1630–1633. doi: 10.1038/sj.bjp.0702282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Canty AJ, Schulz S, Humphrey PPA, Emson PC, Young HM. Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of sstr2 knockout/lacZ knockin mice. J Comp Neurol. 2002;454:329–340. doi: 10.1002/cne.10466. [DOI] [PubMed] [Google Scholar]
- Alumets J, Sundler F, Håkanson R. Distribution, ontogeny and ultra-structure of somatostatin-immunoreactive cells in the pancreas and the gut. Cell Tissue Res. 1977;185:465–479. doi: 10.1007/BF00220651. [DOI] [PubMed] [Google Scholar]
- Beau I, Groyer-Picard MT, Desroches A, Condamine E, Leprince J, Tome JP, et al. The basolateral sorting signals of the thyrotropin and luteinizing hormone receptors: an unusual family of signals sharing an unusual distal intracellular localization, but unrelated in their structures. Mol Endocrinol. 2004;18:733–746. doi: 10.1210/me.2003-0130. [DOI] [PubMed] [Google Scholar]
- Breeman WA, de Jong M, Kwekkeboom DJ, Valkema R, Bakker WH, Koojj PP, et al. Somatostatin receptor-mediated imaging and therapy: basic science, current knowledge, limitations and future perspectives. Eur J Nucl Med. 2001;28:1421–1429. doi: 10.1007/s002590100502. [DOI] [PubMed] [Google Scholar]
- Cole SL, Schindler M. Characterisation of somatostatin sst2 receptor splice variants. J Physiol. 2000;94:217–237. doi: 10.1016/s0928-4257(00)00207-2. [DOI] [PubMed] [Google Scholar]
- Cox HM, Cuthbert AW, Håkanson R, Wahlestedt C. The effect of neuropeptide Y and peptide YY on electrogenic ion transport in rat intestinal epithelia. J Physiol. 1988;398:65–80. doi: 10.1113/jphysiol.1988.sp017029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox HM, Tough IR, Zandvliet DWJ, Holliday ND. Constitutive neuropeptide Y Y4 receptor expression in human colonic adenocarcinoma cell lines. Br J Pharmacol. 2001;132:345–353. doi: 10.1038/sj.bjp.0703815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E, Ekman R, Håkanson R, Sundler F. Projections of peptide-containing neurons in rat colon. Neuroscience. 1988;27:655–674. doi: 10.1016/0306-4522(88)90296-5. [DOI] [PubMed] [Google Scholar]
- Eklund S, Sjöqvist A, Fahrenkrug J, Jodal M, Lundgren O. Somatostatin and met-enkephalin inhibit cholera toxin-induced jejunal net fluid secretion and release VIP in the cat in vivo. Acta Physiol Scand. 1988;133:551–557. doi: 10.1111/j.1748-1716.1988.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Elberg G, Hipkin RW, Schonbrunn A. Homologous and heterologous regulation of somatostatin receptor 2. Mol Endocinol. 2002;16:2502–2514. doi: 10.1210/me.2002-0207. [DOI] [PubMed] [Google Scholar]
- Feniuk W, Jarvie E, Luo J, Humphrey PPA. Selective somatostatin sst2 receptor blockade with the novel cyclic octapeptide, CYN-154806. Neuropharmacology. 2000;39:1443–1450. doi: 10.1016/s0028-3908(00)00035-6. [DOI] [PubMed] [Google Scholar]
- Ferrar JA, Cuthbert AW, Cox HM. The antisecretory effects of somatostatin and analogues in rat descending colon mucosa. Eur J Pharmacol. 1990;184:295–303. doi: 10.1016/0014-2999(90)90621-c. [DOI] [PubMed] [Google Scholar]
- Gugger M, Waser B, Kappeler A, Schonbrunn A, Reubi JC. Cellular detection of sst2A receptors in human gastrointestinal tissue. Gut. 2004;53:1431–1436. doi: 10.1136/gut.2004.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GA, Feniuk W, Humphrey PPA. Outward current produced by somatostatin (SRIF) in rat anterior cingulated pyramidal cells in vitro. Br J Pharmacol. 1998;124:252–258. doi: 10.1038/sj.bjp.0701824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkin RW, Wang Y, Schonbrunn A. Protein kinase C activation stimulates the phosphorylation and internalization of the sst2A somatostatin receptor. J Biol Chem. 2000;275:5591–5599. doi: 10.1074/jbc.275.8.5591. [DOI] [PubMed] [Google Scholar]
- Hofland LJ, Lamberts SWJ. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocrinol Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- Holliday ND, Lam C-W, Tough IR, Cox HM. Role of the C-terminus in neuropeptide Y Y1 receptor desensitisation and internalisation. Mol Pharmacol. 2005;67:655–664. doi: 10.1124/mol.104.006114. [DOI] [PubMed] [Google Scholar]
- Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- Knobloch SF, Diener M, Rummel W. Antisecretory effects of somatostatin and vasopressin in the rat colon descendens in vitro. Reg Peptides. 1989;25:75–85. doi: 10.1016/0167-0115(89)90250-4. [DOI] [PubMed] [Google Scholar]
- Lamberts SWJ, van der Lely A, de Herder WW, Hofland LJ. Octreotide. Drug therapy. New Engl J Med. 1996;334:246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- Liu Q, Cescato R, Dewi DA, Rivier J, Reubi J-C, Schonbrunn A. Receptor signaling and endocytosis are differentially regulated by somatostatin analogs. Mol Pharmacol. 2005;68:90–101. doi: 10.1124/mol.105.011767. [DOI] [PubMed] [Google Scholar]
- Liu Q, Reubi J-C, Wang Y, Knoll BJ, Schonbrunn A. In vivo phosphorylation of the somatostatin 2A receptor in human tumors. J Clin Endocrinol Metabol. 2003;88:6073–6079. doi: 10.1210/jc.2003-030986. [DOI] [PubMed] [Google Scholar]
- Lloyd KCK, Wang J, Aurang K, Gronhed P, Coy DH, Walsh JH. Activation of somatostatin receptor subtype 2 inhibits acid secretion in rats. Am J Physiol. 1995;268:G102–G108. doi: 10.1152/ajpgi.1995.268.1.G102. [DOI] [PubMed] [Google Scholar]
- Marsh KA, Stamp GW, Kirkland SC. Isolation and characterisation of multiple cell types from a single human colonic carcinoma. J Pathol. 1993;170:441–450. doi: 10.1002/path.1711700407. [DOI] [PubMed] [Google Scholar]
- McKeen ES, Feniuk W, Humphrey PPA. Somatostatin receptors mediating inhibition of basal and stimulated electrogenic ion transport in rat isolated distal colonic mucosa. Naunyn-Schmied Arch Pharmacol. 1995;352:402–411. doi: 10.1007/BF00172777. [DOI] [PubMed] [Google Scholar]
- Nunn C, Schoeffter P, Langenegger D, Hoyer D. Functional characterization of the putative somatostatin sst2 receptor antagonist CYN 154806. Naunyn-Schmied Arch Pharmacol. 2003;367:1–9. doi: 10.1007/s00210-002-0656-5. [DOI] [PubMed] [Google Scholar]
- Sarret P, Botto JM, Vincent JP, Mazella J, Beaudet A. Preferential expression of sst2A over sst2B somatostatin receptor splice variant in rat brain and pituitary. Neuroendocrinology. 1998;68:37–43. doi: 10.1159/000054348. [DOI] [PubMed] [Google Scholar]
- Schindler M, Humphrey PPA. Differential distribution of somatostatin sst2 receptor splice variants in rat gastric mucosa. Cell Tissue Res. 1999;297:163–168. doi: 10.1007/s004410051344. [DOI] [PubMed] [Google Scholar]
- Schindler M, Kidd EJ, Carruthers AM, Wyatt MA, Jarvie EM, Sellers LA, et al. Molecular cloning and functional characterization of a rat somatostatin sst2(b) receptor splice variant. Br J Pharmacol. 1998;125:209–217. doi: 10.1038/sj.bjp.0702064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzberg M, Hökfelt T, Nilsson G, Terenius L, Rehfeld JF, Brown M, et al. Distribution of peptide- and catecholamine-containing neurons in the gastrointestinal tract of rat and guinea-pig. Neuroscience. 1980;5:689–744. doi: 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Schulz S, Handel M, Schreff M, Schmidt H, Höllt V. Localisation of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J Physiol. 2000;94:259–264. doi: 10.1016/s0928-4257(00)00212-6. [DOI] [PubMed] [Google Scholar]
- Shimon I, Yan X, Taylor JE, Weiss MH, Culler MD, Melmed S. Somatostatin receptor subtype specificity in human fetal pituitary cultures, differential role of SSTR2 and SSTR5 for growth hormone, thyroid-stimulating hormone, and prolactin regulation. J Clin Invest. 1997;99:789–798. doi: 10.1172/JCI119225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler S, Hoyer D. Characterisation of human recombinant somatostatin receptors. 2. Modulation of GTPγS binding. Naunyn-Schmied Arch Pharmacol. 1999a;360:500–509. doi: 10.1007/s002109900142. [DOI] [PubMed] [Google Scholar]
- Siehler S, Hoyer D. Characterisation of human recombinant somatostatin receptors. 3. Modulation of adenylate cyclase activity. Naunyn-Schmied Arch Pharmacol. 1999b;360:510–521. doi: 10.1007/s002109900143. [DOI] [PubMed] [Google Scholar]
- Siehler S, Seuwen K, Hoyer D. [125I][Tyr3]octreotide labels human somatostatin sst2 and sst5 receptors. Eur J Pharmacol. 1998;348:311–320. doi: 10.1016/s0014-2999(98)00159-9. [DOI] [PubMed] [Google Scholar]
- Siehler S, Seuwen K, Hoyer D. Characterisation of recombinant somatostatin receptors. 1. Radioligand binding studies. Naunyn-Schmied Arch Pharmacol. 1999;360:488–499. doi: 10.1007/s002109900141. [DOI] [PubMed] [Google Scholar]
- Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Höllt V, Schulz S. Differential beta-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–21382. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]
- Vanetti M, Vogt G, Höllt V. The two isoforms of the mouse somatostatin receptor (mSSTR2A and mSSTR2B) differ in coupling efficiency to adenylate cyclase and in agonist-induced receptor desensitisation. FEBS Lett. 1993;331:260–266. doi: 10.1016/0014-5793(93)80349-y. [DOI] [PubMed] [Google Scholar]
- Warhurst G, Higgs NB, Fakhoury H, Warhurst AC, Garde J, Coy DH. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology. 1996;111:325–333. doi: 10.1053/gast.1996.v111.pm8690197. [DOI] [PubMed] [Google Scholar]
- Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Disc. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- Wilkinson GF, Thurlow RJ, Sellers LA, Coote JE, Feniuk W, Humphrey PPA. Potent antagonism by BIM-23056 at the human recombinant somatostatin sst5 receptor. Br J Pharmacol. 1996;118:445–447. doi: 10.1111/j.1476-5381.1996.tb15423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomerhuis MT, Hussain SM, Feelders RA, van der Lely AJ, de Herder WW. Octreotide exerts only acute, but not sustained, effects on MRI enhancement of liver metastases in carcinoid syndrome. Neuroendocrinology. 2005;82:41–48. doi: 10.1159/000090636. [DOI] [PubMed] [Google Scholar]