Abstract
Background and purpose:
Myosin light chain kinase (MLCK) plays a pivotal role in regulation of cellular functions, the evidence often relying on the effects of extracelluarly administered drugs such as ML-9. Here we report that this compound exerts non-specific inhibitory actions on the TRPC6 channel, a transient receptor potential (TRP) protein.
Experimental approach:
Macroscopic and single channel currents were recorded from transfected HEK293 cells by patch-clamp techniques.
Key results:
Cationic currents elicited by carbachol (CCh; 100 μM) in HEK293 cells overexpressing murine TRPC6 (ITRPC6) were dose-dependently inhibited by externally applied ML-9 (IC50=7.8 μM). This inhibition was voltage-dependent and occurred as fast as external Na+ removal. Another MLCK inhibitor, wortmannin (3 μM), and MLCK inhibitory peptides MLCK-IP11-19 (10 μM) and -IP480-501 (1 μM) showed little effects on ITRPC6 density and the inhibitory efficacy of ML-9. The extent of the inhibition also unchanged with co-expression of wild-type or a dominant negative mutant of MLCK. Inhibitory effects of ML-9 on ITRPC6 remained unaffected whether TRPC6 was activated constitutively or by a diacylglycerol analogue OAG (100 μM). Similar rapid inhibition was also observed with a ML-9 relative, ML-7. Intracellular perfusion of ML-9 via patch pipette, dose-dependently suppressed ITRPC6. In inside-out patch configuration, bath application of ML-9 (and ML-7) rapidly diminished ∼35pS single TRPC6 channel activities. Contrarily, currents due to TRPC7 expression were rapidly enhanced by externally applied ML-9 and ML-7, which was not prevented by MLCK inhibitory peptides.
Conclusion and implications:
These results strongly suggest that ML compounds inhibit TRPC6 channels via a mechanism independent of inhibition of MLCK activity.
Keywords: transient receptor potential, receptor-operated Ca2+-permeable cation channel, TRPC6, TRPC7, myosin light chain kinase, naphthalene sulphonamide derivative, ML-9, dominant-negative MLCK
Introduction
The transient receptor potential (TRP) superfamily is a newly emerging channel gene family consisting of approximately 30 mammalian homologues, which encode, except for two isoforms, a Ca2+-permeable non-selective cation channel (NSCC). Among this superfamily, members of the TRPC family (TRPC1–7) show the highest sequence similarity to the archetypal Drosophila dTRP or dTRPL, and because of their activation via phospholipase Cβ or -γ (PLCβ or PLCγ)-linked receptors, are proposed to be the most promising candidates for store-operated and receptor-operated (and store-independent) Ca2+ channels (hereafter abbreviated as SOCCs and ROCCs, respectively) identified in various types of native cells (for review see Minke and Cook, 2002; Clapham, 2003; Montell, 2005).
TRPC6 belongs to the TRPC3/6/7 subfamily of the TRPC family. Heterologously expressed TRPC6 channels are activated by PLC-linked receptors via generation of a lipid mediator diacylglycerol, although a different mode of activation by activated inositol 1,4,5-triphosphate receptor or modulatory role of phosphatidylinositol (PI) and 4,5-bisphosphate (PIP2)/PI 3,4,5-trisphosphate (PIP3) have been suggested (Hardie, 2007; Kwon et al., 2007). The mRNA or protein of TRPC6 is ubiquitously expressed in vascular smooth muscle tissues (Beech et al., 2004; Dietrich et al., 2005; Inoue et al., 2006). Recent studies have suggested that this protein is an essential component of vascular α1-adrenoceptor NSCC activated during increased sympathetic activity (Inoue et al., 2001), and may also be activated by vasopressor hormones such as vasopressin, endothelin and angiotensin II (Jung et al., 2002; Soboloff et al., 2005; Maruyama et al., 2006). In cerebral resistance arteries, the generation of myogenic tone has been ascribed to TRPC6 (Welsh et al., 2002) as a mechanosensitive NSCC (Spassova et al., 2006), and in pulmonary artery, TRPC6 has been implicated as a SOCC in platelet-derived growth factor-stimulated or pathologically enhanced smooth muscle cell proliferation in idiopathic pulmonary arterial hypertension (Yu et al., 2003, 2004). Thus, TRPC6 is likely to play a pivotal role in both acute and long-term controls of vascular smooth muscle functions (Inoue et al., 2006).
It has been shown that heterologously expressed TRPC6 channels are subject to complex regulation by Ca2+ from both extracellular and intracellular sides and, most notably, its receptor-mediated activation depends critically on phosphorylation by calmodulin (CaM)-dependent kinase II, although sole activation of this enzyme by elevated Ca2+ concentration does not suffice to activate the channel (Shi et al., 2004). An analogous dependence on Ca2+/CaM-dependent kinase has been reported for the native vascular counterpart of TRPC6, α1-adrenoceptor NSCC, whose activation appears to involve Ca2+/CaM-mediated activation of myosin light chain kinase (MLCK) as an indispensable step (Aromolaran et al., 2000). A similar crucial role of MLCK in the regulatory process has also been reported for another TRPC member, TRPC5, based on siRNA silencing of endogenous MLCK (Kim et al., 2006) or co-expression of a dominant-negative mutant of MLCK (Shimizu et al., 2006). It is well accepted that MLCK has central importance in the generation of muscle contraction as well as regulation of other cellular activities tightly associated with cytoskeletal organization in non-muscle cells via phosphorylation of myosin light chain (MLC), including cytokinesis, integrin clustering at focal adhesions, stress fibre formation and regulation/trafficking of ion channels, transporters and exchangers (Kamm and Stull, 2001; Pfitzer, 2001).
Notably, such biological significance of MLCK has been demonstrated based on the effectiveness of agents that are thought to specifically inhibit the MLCK activity. A naphthalene sulphonamide derivative, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl] (ML-9), is a membrane permeable, organic MLCK inhibitor frequently used in various functional studies. Especially, in experiments where intracellular administration of MLCK-specific peptides is not feasible, the effectiveness of this compound has often been regarded as an important indication of the involvement of MLCK activity (Saitoh et al., 1987). ML-9 and its congener [1-(5-iodonaphthalene-1-sulphonyl)homopiperazine, HCl] (ML-7) have been reported to inhibit ROCC and SOCC activities or Ca2+ influx evoked by receptor stimulation (Kim et al., 1997; Wingard and Murphy, 1999; Aromolaran et al., 2000; Kuroiwa-Matsumoto et al., 2000), and several other membrane ion-transporting proteins including a TRPC member TRPC5 (Kim et al, 2006; Shimizu et al., 2006), voltage-dependent K+ channels, an Na+, K+, Cl− co-transporter and Na+/H+ exchanger (Wu et al., 1998; Szaszi et al., 2000; Akar et al., 2001). In addition, in our preliminary experiments, we have found that other TRPC channels, such as TRPC6 and -7, are also subject to modulation by this compound. In considering such a broad target spectrum, a question inevitably arises as to whether the observed inhibitory effects of ML-9 genuinely reflect the specific inhibition of MLCK activity or may involve some non-specific actions. If the latter is true, the interpretation of studies based solely on the effects of ML-9 and its relative compounds could be misleading and thus may require re-evaluation. To clarify this point, in this study, we examined in detail the effects of ML-9 on heterologously expressed TRPC6 channels, in comparison with its closest relative TRPC7 (Okada et al., 1999). We have found that ML-9 (and ML-7 as well) exerted rapid inhibition of the TRPC6 channel and enhancement of the TRPC7 channel, neither of which seemed to involve the inhibition of MLCK activity.
Methods
Cell culture and transfection
Human embryonic kidney 293 (HEK293) cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and maintained in 60 mm culture dishes. For transfection, HEK293 cells were re-seeded in a 35 mm culture dish and were allowed to grow to about 50% confluency. Cells were then transfected with a mixture of 2 μg plasmid vector (pCI-neo) incorporated with the whole cDNA sequence of murine TRPC6 (Inoue et al., 2001; Shi et al., 2004) or TRPC7 (Okada et al., 1999) and 0.4 μg pCI-neo-πH3-CD8 (cDNA of the T-cell antigen CD8) with the aid of 20 μl of a transfection reagent SuperFect (Qiagen, Germany). Polystyrene bead-conjugated anti-CD8 antibody (Dynabeads M-450 CD8, Dynal Biotech, Oslo, Norway) was used to select cells successfully expressing TRPC proteins, as performed previously (Shi et al., 2004). With this method, more than 90% of selected cells showed large inward currents in response to muscarinic receptor stimulation. After incubating for about 24 h, the cells were trypsinized and re-seeded onto coverslips pre-coated with 100 μg ml−1 poly-L-lysine. Electrophysiological measurements were performed within 24–48 h thereafter.
In some experiments where wild-type MLCK (wt-MLCK) cloned from HEK293 cells or its dominant-negative mutant lacking Lys561 (mut-MLCK) (Shimizu et al., 2006) was co-expressed with TRPC6 (Figure 6), we used a fivefold higher amount of plasmid DNA for MLCK (10 μg) than TRPC6 (2 μg) to carry out the transfection. Although it was not possible to exactly evaluate the rate of successful co-expression of both gene products in the same cell, we anticipated that this would be high enough, based on the results of CD8 co-expression (>90%; see above).
Electrophysiology
Two modes of the patch-clamp technique were employed in the present study, that is nystatin-perforated and conventional whole-cell recordings. For both recordings, microglass pipettes with a resistance of 2.5–4 MΩ (when filled with internal solution) were used. Voltage generation and current signal acquisition were implemented with a high-impedance, low-noise, patch-clamp amplifier (EPC9; HEKA Electronics, Germany). Sampled data were low-passed filtered at 1 kHz and digitized at 5 kHz (for example, Figure 2a, current–voltage relationship: I–V curve). Longer time-frame recordings (for example, whole-cell current traces in Figure 1) were performed in conjunction with an A/D, D/A converter, PowerLab/400 (AD Instruments, Australia; sampling rate: 100 Hz), and data evaluation was made by using the accessory software, Chart v3.6. The extent of inhibition or enhancement of TRPC6 and -7 currents were calculated as the ratio of their amplitudes after, to just before, application of the ML compounds. Because of progressive desensitization, which was most evident with carbachol (CCh) as an agonist, it was difficult to specify the time point at which the effects of drugs reached the maximum. We therefore empirically chose the time point of 5 s after the onset of drug application. Although this would introduce some errors, with the same method, we could get similar results with 1-oleoyl-2-acetyl-sn-glycerol (OAG) to activate these currents, which showed much slower desensitization. Concentration–response relationships were constructed by fitting data points with a non-linear least-square routine using the Hill equation:
where I, C, IC50 and nH denote the normalized current amplitude (the ratio of current amplitudes after, to before, drug application), drug concentration, the half-maximal inhibitory concentration and a cooperative factor, respectively.
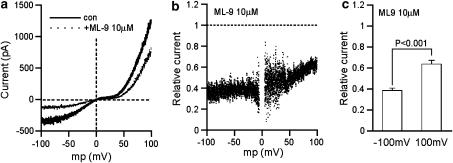
Figure 2.
Voltage-dependency of ML-9's effects on ITRPC6. (a) Current–voltage (I–V) relationships of ITRPC6 before (bold curve) and during (dotted curve) application of 10 μM ML-9. These curves were constructed by applying a 2 s rising ramp voltage, which can be seen as short vertical deflections in Figure 1a. (b) Voltage-dependent effects of ML-9. The fraction of ITRPC6 remaining in the presence of 10 μM ML-9 is calculated from the I–V curves in (a), and plotted against the membrane potential. Values near the reversal potential are omitted because of large scattering due to ‘division by zero'. (c) The fractions of ITRPC6 remaining after ML-9 inhibition at −100 and 100 mV averaged from seven different experiments. Paired t-test (n=7). ITRPC6, TRPC6 current; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl].
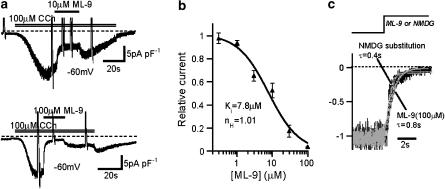
Figure 1.
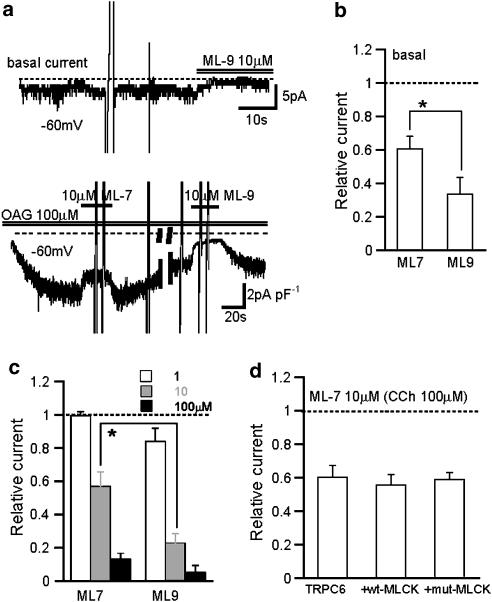
Inhibitory effects of ML-9 on TRPC6 current (ITRPC6). Nystatin-perforated recording in normal external solution with a holding potential: −60 mV. (a) Typical traces for the inhibitory effects of ML-9 of 10 μM (upper) and 100 μM (lower) on CCh (100 μM)-evoked ITRPC6. (b) Relationship between ML-9 concentration and relative ITRPC6 amplitude after inhibition. Relative amplitude is calculated as the ratio of current amplitudes 5 s after, to just before, application of ML-9. Data points are fitted by the Hill equation with non-linear least-square routine (IC50=7.8 μM, nH=1.0, n=4–6; see the Methods). (c) Expanded time courses of the effects of Na+ removal (NMDG substitution) and 100 μM ML-9 on ITRPC6. Representative of separate six experiments. Each current trace is normalized to its amplitude just before drug application and then superimposed. The time courses of the currents are fitted by an exponential function: I=I0 exp(−(t−t0)/τ)+C (smooth curves), where I0, t0, τ and C denote the amplitude of drug-inhibited component, the onset of drug application, time constant of drug's effect and the steady level reached after drug application, respectively, and I0+C is set to be −1. τ Values (in seconds) in the figure indicate the results of best fit by non-linear least-square routine. CCh, carbachol; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl]; NMDG, N-methyl D-glucamine.
For single-channel recordings, electrodes having a resistance of 4–5 MΩ (with the pipette solution described below) were used for the inside-out (I/O) configuration. Sampled data were low-pass filtered at 1 kHz and stored on a computer hard disc after digitization at 10 kHz. Single channel analysis was made using the software ‘Clampfit' v.9.2 (Axon Instruments, Foster City, CA, USA). To evaluate the unitary current amplitude, all-points-in-open-state amplitude histograms were constructed. The extent of single-channel current inhibition by ML compounds was calculated as the ratio of mean currents (averaged for 2 s) 5 s after, to just before, application of the drugs. Determination of the levels of baseline and channel openings and construction of all-points-in-open-state amplitude histograms were performed by using the ‘event detection' analysis of Clampfit v.9.2.
Drugs were rapidly applied onto cells or excised patch membrane by using a fast solution change device called ‘Y-tube' as performed previously (Inoue et al., 2001; Shi et al., 2004).
All experiments were performed at room temperature (22–26°C).
Solutions
Solutions with the following compositions were used. Pipette solution for nystatin-perforated recording (in mM): 140 CsCl, 2 MgCl2, 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 10 glucose (adjusted to pH 7.2 with Tris base); pipette solution for conventional whole-cell recording (in mM): 120 CsOH, 120 aspartate, 20 CsCl, 2 MgCl2, 1 EGTA (ethylene glycol bis(β-aminoethylether)-N,N,N',N'-tetraacetic acid), 0.4 CaCl2, 10 HEPES, 2 ATP, 0.1 GTP, 10 glucose (adjusted to pH 7.2 with Tris base). Various concentrations of ML-9 or MLCK inhibitory peptides (see below) were included in the latter internal solution where necessary. The normal external solution contained (in mM): 140 NaCl, 5 KCl, 1 CaCl2, 1.2 MgCl2, 1 EGTA, 10 HEPES, 10 glucose (pH 7.4, adjusted with Tris base). For Ca2+-free external solution, Ca2+ was omitted from normal external solution. Pipette solution for I/O recording contained (in mM): 140 NaCl, 5 tetraethylammonium-Cl, 0.1 DIDS [4,4-diisothiocyanostilbene-2,2-disulphonic acid, 2Na], 1.2 MgCl2, 1 CaCl2, 10 HEPES, 10 glucose, 0.1 CCh (pH 7.4, adjusted with Tris base). Bathing solution for I/O recording (in mM): 120 CsOH, 120 aspartate, 20 CsCl, 2 MgSO4, 2 EGTA, 0.4 Ca, 10 HEPES, 2 ATP, 0.1 GTP (pH 7.2, adjusted with Tris base).
Statistics
All data are expressed as means±s.e.m. Paired and unpaired Student's t-test was used to evaluate statistical significance with a criterion of P<0.05. For multiple comparisons, statistical significance was evaluated by analysis of variance followed by Bonferroni's t-test.
Chemicals
ML-9, ML-7, wortmannin, DIDS, OAG and MLCK inhibitory peptide MLCK-IP480−501 were purchased from Calbiochem (La Jolla, CA, USA), MLCK-IP11−19 from Alexis (Nottingham, UK); Carbachol (CCh) and HEPES from Sigma (St Louis, MO, USA); ATP, GTP, and EGTA from Dojindo (Kumamoto, Japan). Stock solutions for ML-9, ML-7, DIDS and OAG were made by dissolving them in dimethylsulphoxide (DMSO) and then diluting more than 1000 times for use. We confirmed that, up to 0.1%, DMSO produced no detectable changes in basal currents or those activated by CCh or OAG.
Results
Features of heterologously expressed TRPC6 current
Under the nystatin-perforated voltage-clamp (holding potential: −60 mV), in non-transfected HEK293 cells, little discernible current was induced by external application of 100 μM CCh (current density at −60 mV, 0.32±0.15 pA pF−1; n=12). In contrast, in nearly all HEK cells transfected with TRPC6 plasmids and positive to anti-CD8 beads selection, the density of basal current was increased to 1.0±0.5 pA pF−1 (n=25) and that of CCh-induced current at −60 mV was as large as 41.7±8.4 pA pF−1 (n=25). Replacement of external Na+ by a large impermeant cation, N-methyl D-glucamine (NMDG), reversibly abolished the CCh-induced current (Cl− substitution with benzene sulphonate was ineffective; data not shown). The reversal potential of CCh-induced current was near zero under normal ionic conditions (Figure 2a). These results together indicate the non-selective permeation of cations. The current–voltage (I–V) relationship of CCh-induced current showed an S-shape double rectification (Figure 2a), and application of La3+ (100 μM) or pretreatment with the phospholipase C inhibitor U73122 (10 μM) strongly inhibited the current (data not shown). These features match those reported previously (Inoue et al., 2001), indicating that CCh-induced current observed in this study reflect the activation of TRPC6 channels via stimulation of muscarinic receptors endogenous to HEK293 cells (hereafter designated as ITRPC6).
Acute actions of ML-9 on ITRPC6
External application of ML-9 caused a dose-dependent inhibition of ITRPC6 (Figure 1). The inhibition was detectable at 1 μM or lower concentrations and almost complete at 100 μM (lower trace in Figure 1a). The Hill analysis of ML-9 concentration−ITRPC6 amplitude relationship gives the IC50 of 7.8 μM and cooperative factor of 1.0 (Figure 1b). The inhibition of ITRPC6 by ML-9 occurred rapidly, the rate of onset being comparable to that of external Na+ substitution with NMDG (time constant τ; 0.61±0.09 s vs 0.46±0.06 s, for 100 μM ML-9 and NMDG, respectively, n=6, P<0.01; Figure 1c). The extent of the inhibition was relieved by membrane depolarization (Figures 2a and b). The ratio of ITRPC6 amplitude after, to before, application of 10 μM ML-9, which was calculated from the I–V relationship constructed by a 2 s rising ramp voltage, was different at −100 or 100 mV (P<0.001 with paired t-test, n=7; Figure 2c). These results indicate that there is a moderate voltage-dependency present in the inhibitory actions of ML-9 on ITRPC6.
Other MLCK inhibitors are ineffective on ITRPC6
The rapidity and voltage-dependence of ITRPC6 inhibition by ML-9 makes us suspect that the inhibition may arise from a mechanism not involving MLCK activity. To clarify this point, we employed other manoeuvres known to affect the MLCK activity.
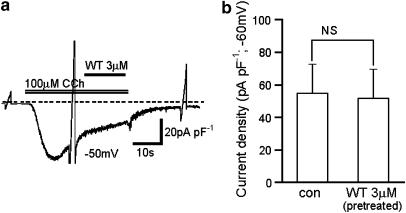
Wortmannin is another frequently used organic MLCK inhibitor structurally unrelated to ML-9 (IC50 in vitro, 200 nM; Yano et al., 1993), although non-specifically inhibiting the phophatidylinositol-3-, -4- and MAP-kinases. This compound was however, entirely ineffective on ITRPC6 at the concentration of 3 μM which has been reported to effectively inhibit MLCK activity in smooth muscle (Burdyga and Wray, 1998; Longbottom et al., 2000; Ito et al., 2004), whether applied acutely following full ITRPC6 activation by CCh or by pretreatment for 5–10 min before application of CCh (Figures 3a and b).
Figure 3.
Wortmannin was ineffective on ITRPC6. Nystatin-perforated recording at −60 mV. (a) Actual trace of ITRPC6. Wortmannin (WT) (3 μM) was introduced into the bath 5–10 min before application of CCh. In this experiment, WT was further applied after activation of ITRPC6 by CCh (100 μM), but no discernible change was observed. (b) Current density of ITRPC6 with and without pretreatment with 3 μM WT. P>0.05 with unpaired t-test (n=6). CCh, carbachol; ITRPC6, TRPC6 current.
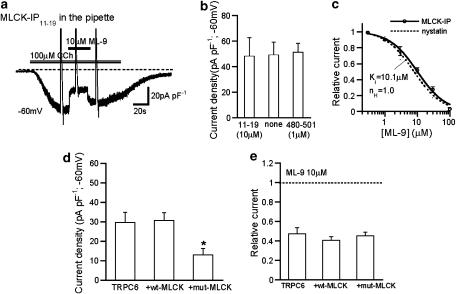
In the next step, we examined the effects of two MLCK-specific inhibitory peptides (MLCK-IP11−19 and MLCK-IP480−501). It has been reported that the IC50 value for MLCK-IP480−501 is about 0.25 μM (Smith et al., 1990) and 5 μM is high enough for MLCK-IP11−19 to inhibit the smooth muscle MLCK activity (Aromolaran et al., 2000). As demonstrated in Figure 4a, 10 μM MLCK-IP11−19 or 1 μM MLCK-IP480−501, which was included in the patch pipette, failed to reduce the density of ITRPC6 (Figure 4b). Moreover, the efficacy of ML-9 to inhibit ITRPC6 remained almost unaffected even after intracellular application of these MLCK inhibitory peptides (Figure 4c vs Figure 1b). The ML-9 concentration ITRPC6 amplitude curves obtained in the presence and absence of MLCK peptides nearly superimpose, giving similar IC50 values (10.1 vs 7.8 μM).
Figure 4.
Intracellular perfusion of MLCK-inhibitory peptides and dominant negative inhibition of MLCK did not affect the inhibitory efficacy of ML-9 on ITRPC6. Conventional whole-cell recording at −60 mV. To reduce the degree of desensitization, Ca2+-free rather than normal external solution was used as the bathing solution. (a) Representative trace for the extracellular ML-9 (10 μM) effects on ITRPC6 in the presence of 10 μM MLCK IP11–19 peptide in the pipette. (b) Summary of the effects of 10 μM MLCK IP11–19 and 1 μM MLCK IP480–501 on ITRPC6 density (n=7–11). (c) ML-9 concentration–inhibition curves for ITRPC6 with (solid) and without (nystatin-perforated recording; dotted; taken from Figure 1b) inclusion of MLCK inhibitory peptides (10 μM MLCK IP11–19 or 1 μM MLCK IP480–501). The solid curve is drawn according to the best fit of the data points with Hill equation (see Methods). IC50=10.1 μM; nH=1.0 (n=4–6). There was no statistically significant difference between two curves with ANOVA. (d and e) Influence of overexpression of wild type (wt-MLCK) or a dominant-negative mutant of MLCK (mut-MLCK) on current density of ITRPC6 (d; n=15–18) and inhibitory efficacy of ML-9 for ITRPC6 (e; n=8–11). (d) *P<0.05 from other columns with ANOVA and Bonferroni's t-test. ANOVA, analysis of variance; IP, inhibitory peptides; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl]; MLCK, myosin light chain kinase.
Taken together, these results strongly suggest that MLCK plays, if any, only a minor role in the regulation of TRPC6 channels overexpressed in HEK293 cells.
Overexpression of wild type or dominant-negative MLCK does not affect the inhibitory actions of ML-9
To validate further the above conclusion and also explore possible long-term consequences of MLCK inhibition, we carried out the dominant-negative suppression of MLCK by co-expressing a non-functional form of MLCK (mut-MLCK; see Methods; Shimizu et al., 2006) with TRPC6 protein. As graphically summarized in Figure 4, expression of mut-MLCK did not affect the extent of fast inhibition by ML-9 (10 μM; Figure 4e), although it moderately decreased the density of ITRPC6 (Figure 4d). On the other hand, overexpression of wt-MLCK induced no significant changes in either the density or extent of the inhibition of CCh-induced ITRPC6 (Figures 4d and e), or in basal current in the absence of receptor stimulation (data not shown). These results further reinforce the idea that MLCK is not a major target of the fast inhibitory effects of ML-9 on ITRPC6.
ITRPC6 inhibition by ML-9 and ML-7 does not depend on the mode of activation
The rapid and MLCK-independent actions of ML-9 tempt us to speculate that the site of actions of ML-9 may be close to or on the TRPC6 channel itself. To gain more insight into this possibility, we examined the effects of ML-9 on TRPC6 channels, which were activated in different ways. As demonstrated and summarized in Figure 5, even when ITRPC6 was induced by a membrane-permeable analog of diacylglycerol OAG (100 μM) by bypassing the receptor/G-protein/phospholiapse C pathway to activate TRPC6 channels more directly, ML-9 exerted a similar extent of inhibition to that seen with ITRPC6 activated with CCh (Figures 5a and c). Similarly, the extent of inhibition of small basal currents, which probably reflect constitutive activation of expressed TRPC6 channels, did not change from those obtained with CCh and OAG (Figures 5a and b). These results strongly support the site of action of ML-9 being in close proximity to the TRPC6 channel.
Figure 5.
Activation mode of TRPC6 did not affect the efficacy of ML-9 and ML-7. Conventional whole-cell recording at −60 mV with Ca2+-free external solution. (a) Representative traces showing the effects of ML-9 and ML-7 on basal (upper) and OAG (100 μM; lower)-induced ITRPC6. (b and c) Summary of the effects of ML-9 and ML-7 on basal ITRPC6 at 10 μM (b) and on OAG-induced ITRPC6 at three different concentrations (1, 10, 100 μM) (c). n=6–7. (d) Inhibitory efficacy of ML-7 for ITRPC6 with overexpression of wild type (wt-MLCK) or a dominant negative mutant of MLCK (mut-MLCK). n=8–11. *P<0.05. ML-7, [1-(5-iodonaphthalene-1-sulphonyl)homopiperazine, HCl]; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl]; MLCK, myosin light chain kinase.
In addition, we tested another ML compound, ML-7, and found that, except for its slightly weaker efficacy, the inhibitory effects of this compound did not essentially differ from those of ML-9 being independent of MLCK (Figures 5b–d). We also found that another naphthalene sulphonamide derivative W-7 (50 μM) caused an acute inhibition of ITRPC6, but the time course of this inhibition appeared much slower than ML-9 or ML-7 (data not shown) and was not further pursued in the present study.
ML-9 and ML-7 can rapidly act on TRPC6 channel from the inside of the membrane
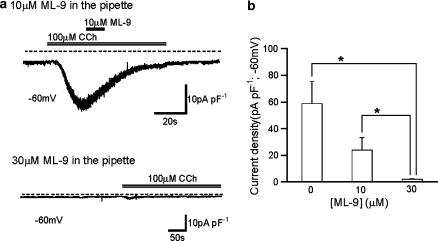
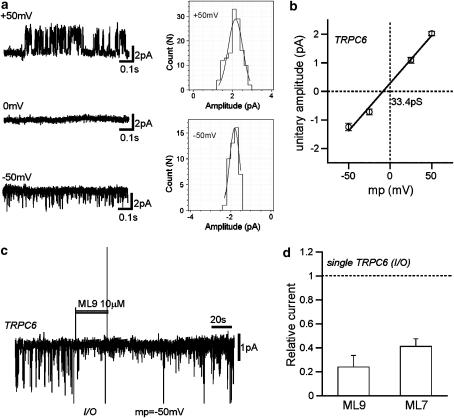
In the final stage of the present study, we questioned if there was ‘membrane sidedness' for the rapid action of ML-9. As shown in Figures 6a and b, after intracellular perfusion of ML-9, the density of ITRPC6 activated by CCh was reduced dose-dependently; more than half and almost complete inhibition occurred at 10 and 30 μM, respectively. Importantly, after equilibration of internal perfusion with ML-9, its external application at the same concentration failed to produce further inhibition (Figure 6a). These results suggest that ML-9 may access almost equally, from the outside or the inside of the cell membrane, a site(s) associated with TRPC6 channel inhibition. Consistent with this speculation, bath application of ML-9 as well as its congener ML-7, rapidly diminished ∼35 pS single TRPC6 channel activities in cell-free, inside-out patch configuration (Figure 7). It was, however, difficult to determine unequivocally whether the inhibition occurred due to reduced unitary amplitude, reduced open probability or both, because of infrequent openings of the channel after application of these drugs. In any case, this finding further corroborates the conclusion above that the inhibitory effects of ML-9 (and ML-7) were not mediated by the inhibition of MLCK activity but were, rather, direct actions on the TRPC6 channel.
Figure 6.
Concentration-dependent inhibition of ITRPC6 by intracellularly applied ML-9. ML-9 was included in the pipette under conventional whole-cell clamp (−60 mV), with bath containing Ca2+-free external solution. (a) Typical traces of ITRPC6 with intrapipette inclusion of 10 μM ML-9, which abolished the inhibitory effect of extracellularly applied ML-9 at the same concentration (upper), and of 30 μM ML-9, which strongly inhibited ITRPC6 (lower). (b) Summary of the effects of intracellularly applied ML-9 on ITRPC6 density. *P<0.05 with ANOVA and Bonferroni's t-test. n=5–8. ANOVA, analysis of variance; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl].
Figure 7.
Effects of ML-9 and ML-7 on single TRPC6 channels. Inside-out (I/O) patch recording with CCh (100 μM) in the pipette. To suppress K+ and Cl− channels, 5 mM tetraethylammonium and 100 μM DIDS were also added in the pipette. (a) Actual traces at three different potentials (−50, 0 and 50 mV). Boxes on the right indicate all-points-in-open-state amplitude histograms with the results of Gaussian fit (smooth curves). (b) I–V relationship for single TRPC6 channel. Linear regression of data points between −50 and 50 mV gives a unitary conductance of 33.4 pS (n=5–8), which is in good accordance with the value obtained previously (Shi et al., 2004). (c) Inhibition of single TRPC6 channel activity by 10 μM ML-9, which was applied rapidly into the bath at the bar. Two large vertical deflections indicate the electrical artefacts of a solenoid valve. (d) Summary of the inhibitory effects of 10 μM ML-9 and ML-7 on single TRPC6 channels. The extent of single-channel current inhibition by ML compounds was calculated as the ratio of mean currents (averaged for 2 s) 5 s after, to just before, application of the drugs. n=8–11. CCh, carbachol; DIDS, [4,4-diisothiocyanostilbene-2,2-disulphonic acid, 2Na]; ML-7, [1-(5-iodonaphthalene-1-sulphonyl)homopiperazine, HCl]; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl].
TRPC7 channels show the opposite sensitivity to ML compounds
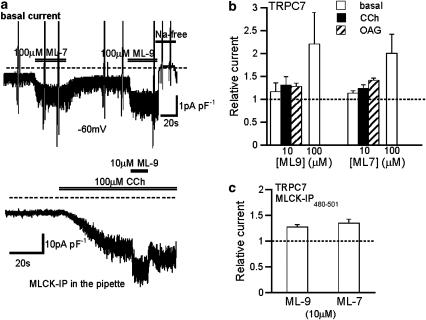
TRPC7 possesses a high degree of similarity to TRPC6 but shows distinct properties with respect to the sensitivity to Ca2+ and some organic compounds (Inoue et al., 2001; Shi et al., 2004). We were thus intrigued to see how ML compounds affect this TRPC isoform. Somewhat surprisingly, both ML-9 and ML-7 caused a rapid and reversible enhancement rather than inhibition of currents arising from TRPC7 expression, whether they were constitutively activated (basal) or activated via muscarinic receptor stimulation (CCh) or more directly by OAG (Figures 8a and b). As in the case of TRPC6, these actions do not seem to involve the inhibition of MLCK activity, since prior intracellular application of MLCK-IP480−501 could not prevent this enhancement (Figure 8c).
Figure 8.
Enhancing actions of ML-compounds on macroscopic TRPC7 current (ITRPC7). Conventional whole-cell recording at −60 mV with Ca2+-free external solution. (a) Representative records of basal (upper) and CCh-induced (lower) ITRPC7 demonstrating the enhancing effect of ML-9 and ML-7. (b) Fractional change in ITRPC7 amplitude after rapid application of ML-9 and ML-7. n=6–8. (c) Ineffectiveness of MLCK inhibitory peptide (MLCK-IP480–501=1 μM) on the effects of extracellular ML-9 and ML-7 on CCh (100 μM)-induced ITRPC7. n=5–6. CCh, carbachol; IP, inhibitory peptides; ML-7, [1-(5-iodonaphthalene-1-sulphonyl)homopiperazine, HCl]; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl]; MLCK, myosin light chain kinase.
Discussion
The unexpected new finding of the present study is that ML-9 (and its congener ML-7 as well), which has frequently been used to suppress MLCK activities, exerts rapid reversible inhibitory actions on TRPC6 channels via a mechanism independent of MLCK. Although enhancing rather than inhibitory, similarly rapid effects of ML-9 were also observed on TRPC7, a closest homologue of TRPC6. This conclusion is supported by several different lines of evidence.
Firstly, neither wortmannin, a structurally different MLCK inhibitor (Davies et al., 2000; Bain et al., 2003) nor MLCK-specific inhibitory peptides (MLCK-IP11−19 and MLCK-IP480−501) targeted to the catalytic and ATP binding domains of MLCK (Ikebe, 1990; Kemp et al., 1991), affected the extent of channel activation (that is, current density) or of inhibition by ML-9. Ineffectiveness of these peptide inhibitors is unlikely due to their insufficiently high concentrations (cf Smith et al., 1990; Aromolaran et al., 2000) or incomplete intracellular perfusion, since we employed patch pipettes whose input and resultant series resistances had been low enough to attain almost complete inhibition of calmodulin-dependent kinase II activity by its inhibitory peptides (2.5–4 and <10 MΩ, respectively; see Shi et al., 2004). Secondly, the fast inhibitory actions of ML-9 on TRPC6 current were not appreciably affected by overexpression of wild-type or a dominant-negative mutant MLCK, despite that this procedure was anticipated to alter the MLCK activity and, in turn, the phosphorylated status of target proteins. Thirdly, a similar extent of inhibitory actions by ML-9 was still observed on single TRPC6 channel activities (∼35 pS) recorded in cell-free, inside-out patch configuration, where a complete set of intracellular constituents/enzymes requisite for phosphorylation/dephosphorylation is unlikely to be retained. Finally, the onset of ML-9 action was extremely fast (less than 1 s), being comparable to those of Na+ substitution by NMDG (Figure 1b), and the extent of TRPC6 inhibition was significantly relieved by membrane depolarization even during a short ramp potential (2 s; Figure 2b). Such rapid actions cannot be accounted for by a series of biochemical reactions, exemplified by facilitated internalization of channel protein from the cell surface following MLCK inhibition, which has recently been demonstrated for a different TRPC subfamily member TRPC5 (Shimizu et al., 2006). This internalization process has been shown to result from reduced MLC phosphorylation and consequent cytoskeletal rearrangements, the whole process requiring minutes to become overt.
Thus, the most straightforward interpretation of the above observations is that ML-9 acts on a site directly or closely associated with the gating or ion permeation of TRPC6 channels to exert its inhibition, although its longer application might also reduce the cell membrane expression of the channel protein via a MLCK-dependent mechanism (see also the following paragraph). Interestingly, it has been reported that flufenamate, a general NSCC blocker, and external Ca2+, can also exert similar rapid but contrary effects on TRPC6 and -7 channels (potentiation and inhibition, respectively; Inoue et al., 2001; Shi et al., 2004). In contrast, no such reciprocal effects were observed on the two TRPC isoforms by a widely used NSCC channel blocker SK&F96365, which caused only inhibition (Shi and Inoue, unpublished observation). Furthermore, W7, another naphthalene sulphonamide derivative capable of blocking MLCK, also inhibited TRPC6 current but only with a much slower time course than the ML compounds (see above). At present, structural basis for these pharmacological differences remain entirely unclear. Future mutagenic scanning of amino-acid residues near the putative pore region (and if available, those responsible for gating) of TRPC6 and -7 channels may help clarify this point.
Despite that distinct Ca2+/CaM-dependent kinases are involved in the activation process, both overexpressed TRPC6 channel and its likely native counterpart α1-adrenoceptor NSCC in rabbit portal vein myocytes (Aromolaran et al., 2000) are susceptible to micromolar concentrations of ML-9. However, careful comparison of the results of Hill analysis reveals that the IC50 value is significantly lower for α1-adrenoceptor NSCCs (2.0 μM) than that for expressed TRPC6 channels (7.8 μM), and the former appears even lower than that required to inhibit MLCK activity in vitro (3.8 μM; Saitoh et al., 1987). More importantly, the cooperativity factor (nH) for α1-adrenoceptor NSCC (2.5) is more than twice that of overexpressed TRPC6 channel which is equal to unity (1.0) (Aromolaran et al., 2000; present study). These stoichiometric data suggest that ML-9 may exert at least two distinct effects, that is inhibition of MLCK activity and more direct inhibition of NSCC. Both effects seem to be present in the α1-adrenoceptor NSCC, whereas only the latter may be operative in TRPC6 channels expressed in HEK293 cells where, as compared with calmodulin-dependent kinase II, the physiological impact of MLCK activity on NSCC activation would be much weaker than in muscle tissues. Indeed, this may have partly been manifested as reduced availability of TRPC6 channel with overexpresion of mutant MLCK (Figure 4d), but we did not further pursue this issue in the present study. On the other hand, ML-7, another naphthalene sulphonamide derivative, is more than 30-fold more potent in inhibiting MLCK (IC50=300 nM; Saitoh et al., 1987) than the TRPC6 channel (IC50 appears slightly larger than 10 μM; see Figure 5c). This means that even though this compound's MLCK-independent effect on TRPC6 channel would be operative in the micromolar range, this would already be masked by its submicromolar inhibitory effect on MLCK, thus the former effect would not be clearly observable. This actually seems to occur in rabbit portal vein α1-adrenoceptor NSCC, which ML-7 (IC50=0.8 μM) inhibits more effectively than ML-9 but the value of nH is nearly equal to unity (Aromolaran et al., 2000). In this regard, to separate the effects on MLCK and TRPC6 channel, ML-7 may be more advantageous than ML-9, when it is used at low micromolar concentrations.
The non-specific inhibitory effects of ML-9 on Ca2+ transporting activities have been documented in the literature. For instance, in porcine aortic endothelial cells, one study reported that bradykinin or thapsigargin-induced Ca2+ entry was suppressed by both ML-9 and wortmannin with a parallel inhibition of MLC phosphorylation (Watanabe et al., 1998). However, in the same preparation, another study provided a conflicting evidence that only ML-9 but not wortmannin-inhibited thapsigargin-induced Ca2+ entry (Kuroiwa-Matsumoto et al., 2000). In guinea pig tracheal smooth muscle, while ML-9 was found to be able to reduce Ca2+ entry and concomitant contraction induced by high K+, methacholine or thapsigargin, wortmannin only inhibited the contraction without affecting [Ca2+]i (Ito et al., 2004). All these findings appear to be compatible with the idea that ML-9 may act as a direct inhibitor of several different types of Ca2+ entry channels rather than via its effects on MLCK activity. TRPC6 is known to be a major TRPC isoform expressed in vascular smooth muscle cells (Inoue et al., 2006), but its relatively abundant expression has also been detected in airway smooth muscle cells and endothelial cells (Ong et al., 2003; Yip et al., 2004). It is thus possible that part of the inhibitory actions of ML-9 observed in the above studies may also reflect the non-specific effects of ML-9 on Ca2+ entry channels per se, as observed in the present study.
In summary, the present results clearly show that ML-9 effectively inhibited TRPC6 channel in a manner independent of MLCK inhibition. This inhibition is likely to occur through an interaction with a site(s) closely associated with the TRPC6 channel protein, and may rely on subtle structural differences between different TRPC isoforms, since ML-9 exhibits the opposite, enhancing, effects on TRPC7 channels which shows a high degree of amino-acid sequence identity over the six transmembrane domains. This non-specificity could mislead the conclusion of studies investigating the functional significance of MLCK activity on Ca2+ transporting activity, if evaluation is made based solely on ML-9's effects. This potential problem would become particularly serious when smooth muscle tissues are of interest, where MLCK functions as the dominant effector of Ca2+/CaM-dependent pathway and expression of TRPC6 protein may also be ubiquitous.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant nos. 30400154 and 30400329) and grants-in-aid from the JSPS to RI (Grant nos.14570079 and 17590221).
Abbreviations
- CaM
calmodulin
- CaMKII
calmodulin-dependent kinase II
- ML-9
[1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl]
- ML-7
[1-(5-iodonaphthalene-1-sulphonyl)homopiperazine, HCl]
- MLCK
myosin light chain kinase
- MLCK-IP
myosin light chain kinase inhibitory peptide
- mut-MLCK
mutant myosin light chain kinase
- NMDG
N-methyl D-glucamine
- OAG
1-oleoyl-2-acetyl-sn-glycerol
- TRP
transient receptor potential protein
- TRPC6
TRP canonical subfamily isoform 6
- TRPC7
TRP canonical subfamily isoform 7
- wt-MLCK
wild-type myosin light chain kinase
Conflict of interest
The authors state no conflict of interest.
References
- Akar F, Jiang G, Paul RJ, O'Neill WC. Contractile regulation of the Na+-K+-2Cl− cotransporter in vascular smooth muscle. Am J Physiol Cell Physiol. 2001;281:C579–C584. doi: 10.1152/ajpcell.2001.281.2.C579. [DOI] [PubMed] [Google Scholar]
- Aromolaran AS, Albert AP, Large WA. Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes. J Physiol. 2000;524:853–863. doi: 10.1111/j.1469-7793.2000.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Mclauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga TV, Wray S. The effect of inhibition myosin light chain kinase by wortmannin on intracellular Ca2+, electrical activity and force in phasic smooth muscle. Pflugers Arch. 1998;436:801–803. doi: 10.1007/s004240050705. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Rost BR, Gudermann T. The diacylglycerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflugers Arch. 2005;451:72–80. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M. Mode of inhibition of smooth muscle myosin light chain kinase by synthetic peptide analogs of the regulatory site. Biochem Biophys Res Commun. 1990;168:714–720. doi: 10.1016/0006-291x(90)92380-i. [DOI] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, et al. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Ito S, Kume H, Honjo H, Kodama I, Katoh H, Hayashi H, et al. ML-9, a myosin light chain kinase inhibitor, reduces intracellular Ca2+ concentration in guinea pig trachealis. Er J Pharmacol. 2004;486:325–333. doi: 10.1016/j.ejphar.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Pearson RB, House CM. Pseudosubstrate-based peptide inhibitors. Methods Enzymol. 1991;201:287–304. doi: 10.1016/0076-6879(91)01026-x. [DOI] [PubMed] [Google Scholar]
- Kim MT, Kim BJ, Lee JH, Kwon SC, Yeon DS, Yang DK, et al. Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am J Physiol Cell Physiol. 2006;290:C1031–C1040. doi: 10.1152/ajpcell.00602.2004. [DOI] [PubMed] [Google Scholar]
- Kim YC, Kim SJ, Kang TM, Suh SH, So I, Kim KW. Effects of myosin light chain kinase inhibitors on carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Pflugers Arch. 1997;434:346–353. doi: 10.1007/s004240050407. [DOI] [PubMed] [Google Scholar]
- Kuroiwa-Matsumoto M, Hirano K, Ahmed A, Kawasaki J, Nishimura J, Kanaide H. Mechanisms of the thapsigargin-induced Ca2+ entry in situ endothelial cells of the porcine aortic valve and the endothelium-dependent relaxation in the porcine coronary artery. Br J Pharmacol. 2000;131:115–123. doi: 10.1038/sj.bjp.0703548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbottom ER, Luckas MJ, Kupittayanant S, Badrick E, Shmigol T, Wray S. The effects of inhibiting myosin light chain kinase on contraction and calcium signaling in human and rat myometrium. Pflugers Arch. 2000;440:315–321. doi: 10.1007/s004240000305. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Nakanishi Y, Walsh EJ, Wilson DP, Welsh DG, Cole WC. Heteromultimeric TRPC6–TRPC7 channels contribute to arginine vasopressin-induced cation current of A7r5 vascular smooth muscle cells. Circ Res. 2006;98:1520–1527. doi: 10.1161/01.RES.0000226495.34949.28. [DOI] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;272:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Ong HL, Brereton HM, Harland ML, Barritt GJ. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology. 2003;8:23–32. doi: 10.1046/j.1440-1843.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, et al. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Wakamori M, Ishii M, Okada T, Takahashi M, Seto M, et al. Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J Physiol. 2006;570:219–235. doi: 10.1113/jphysiol.2005.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MK, Colbran RJ, Soderling TR. Specificity of autoinhibitory domain peptides for four protein kinases. Implications for intact cell studies of protein kinase function. J Biol Chem. 1990;265:1837–1840. [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem. 2005;280:39786–39794. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaszi K, Kurashima K, Kapus A, Paulsen A, Kaibuchi K, Grinstein S, et al. RhoA and rho kinase regulate the epithelial Na+/H+ exchanger NHE3. Role of myosin light chain phosphorylation. J Biol Chem. 2000;275:28599–28606. doi: 10.1074/jbc.M001193200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Takahashi R, Zhang XX, Goto Y, Hayashi H, Ando J, et al. An essential role of myosin light-chain kinase in the regulation of agonist- and fluid flow-stimulated Ca2+ influx in endothelial cells. FASEB J. 1998;12:341–348. doi: 10.1096/fasebj.12.3.341. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Wingard CJ, Murphy RA. Inhibition of Ca2+-dependent contraction in swine carotid artery by myosin kinase inhibitors. Gen Pharmacol. 1999;32:483–494. doi: 10.1016/s0306-3623(98)00289-4. [DOI] [PubMed] [Google Scholar]
- Wu RL, Butler DM, Barish ME. Potassium current development and its linkage to membrane expansion during growth of cultured embryonic mouse hippocampal neurons: sensitivity to inhibitors of phosphatidylinositol 3-kinase and other protein kinases. J Neurosci. 1998;18:6261–6278. doi: 10.1523/JNEUROSCI.18-16-06261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, et al. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- Yip H, Chan WY, Leung PC, Kwan HY, Liu C, Huang Y, et al. Expression of TRPC homologs in endothelial cells and smooth muscle layers of human arteries. Histochem Cell Biol. 2004;122:553–561. doi: 10.1007/s00418-004-0720-y. [DOI] [PubMed] [Google Scholar]
- Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, et al. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]