Abstract
Background and purpose:
Many compounds liberating NO (NO donors) have been used as therapeutic agents. Here we test two ruthenium nitrosyls, which release NO when activated by biological reducing agents, for their effects in vitro and in vivo against Trypanasoma cruzi, the agent responsible for the American trypanosomiasis (Chagas' disease).
Experimental approach:
Ruthenium NO donors were incubated with a partially drug-resistant strain of T. cruzi and the anti-proliferative and trypanocidal activities evaluated. In a mouse model of acute Chagas' disease, trypanocidal activity was evaluated by measuring parasitemia, survival rate of infected mice and elimination of amastigotes in myocardial tissue.
Key results:
In vitro, the observed anti-proliferative and trypanocidal activities of trans-[Ru(NO)(NH3)4isn](BF4)3 and trans-[Ru(NO)(NH3)4imN](BF4)3 were due to NO liberated upon reduction of these nitrosyls. Ru(NO)isn had a lower IC50epi (67 μM) than the NO donor, sodium nitroprusside (IC50epi=244 μM) and Ru(NO)imN (IC50try=52 μM) was more potent than gentian violet (IC50try=536 μM), currently used in the treatment of blood. Both ruthenium nitrosyls eliminated, in vivo, extracellular as well as intracellular forms of T. cruzi in the bloodstream and myocardial tissue and allowed survival of up to 80% of infected mice at a dose (100 nmol kg−1 day−1) much lower than the optimal dose for benznidazole (385 μmol kg−1 day−1).
Conclusions and implications:
Our data strongly suggest that NO liberated is responsible for the anti-proliferative and trypanocidal activities of the ruthenium NO donors and that these compounds are promising leads for novel and effective anti-parasitic drugs.
Keywords: ruthenium nitrosyl, NO donors, nitric oxide, trypanosomiasis, Trypanosoma cruzi, Chagas' disease, inorganic medicinal chemistry, benznidazole, cytotoxicity, Y strain
Introduction
Tropical diseases affect approximately a billion people and many do not yet have any adequate treatment. These diseases are mostly neglected by the pharmaceutical industry and affect the poorer and marginalized populations of the tropics and subtropics (Sachs, 2007). A major example is the American trypanosomiasis or Chagas' disease caused by the protozoan parasite Trypanosoma cruzi (T. cruzi), which affects approximately 16–18 million people in Latin America, with an additional 100 million people exposed to the risk of infection and a predicted annual death rate of 50 000 (Gelb and Hol, 2002).
The available chemotherapeutics for trypanosomiasis are still unsatisfactory. Benznidazole, the drug currently used in the treatment of Chagas' disease, is known to exhibit significant toxicity and it must be given under close medical supervision, due to its numerous side effects (Coura and Castro, 2002). Also, several strains of T. cruzi do not respond well to presently available antiparasitic drugs and thus a new and effective trypanocidal agent is urgently needed (Cerecetto and González, 2002). Against this background, nitric oxide (NO)-based therapies against T. cruzi, especially those involving the use of NO donor compounds, have provided an interesting and important alternative to existing trypanocidal treatments (Napoli and Ignarro, 2003). Pathophysiological concentrations of NO produced during the initial phase of acute infection might participate in the killing of the parasites by macrophages through NO-dependent mechanisms (Vespa et al., 1994). Classical NO donors such as SNAP (S-nitroso-acetyl-penicillamine) and SNP (sodium nitroprusside) are known to lyse the parasite, probably by inactivating the cysteine proteases of the parasite, due to the NO generated (Bocedi et al., 2004). In fact, gamma interferon (IFN-γ) is able to activate the inducible NO synthase (iNOS) and has trypanocidal activity (Gazzinelli et al., 1992; Cardillo et al., 1996). Inhibition of iNOS stops the trypanocidal effect of activated macrophages, suggesting that NO inhibits the growth of the parasite (Silva et al., 1995; Cardillo et al., 1996).
However, at higher levels, NO modifies normal cellular metabolism, causing a variety of still not well-characterized damage to the host cell (Martins et al., 1998; Bonavida et al., 2006).
In this context, ruthenium NO donors, trans-[RuII(NO+)(NH3)4L]3+ and [RuII(NO+)(Hedta)], are good models for assessing trypanocidal activity in vitro and in vivo as, apart from their low toxicity, water solubility and stability in aqueous media in the presence of oxygen, the NO released by these compounds at the site of action can be controlled through the judicious selection of the trans ligand (L) (Toledo et al., 2005). Additionally, these compounds are activated to release of NO by reducing agents present in biological media (Zanichelli et al., 2006). Hence, the features presented by these types of compounds are quite promising for designing metallopharmaceuticals, especially to combat infectious diseases where the NO concentration has to be high enough to prevent the development of parasites but not so high as to cause immunosuppression, inhibition of respiratory complexes and acotinase, DNA modifications or apoptosis in the host cells (Bogdan, 2001).
Here, we report the trypanocidal activity in vitro and in vivo of a series of ruthenium nitrosyls, trans-[RuII(NO+)(NH3)4L]X3, L=imidazole (imidazole coordinated by nitrogen (imN) or imidazole coordinated by carbon (imC)), pyridine (py), L-histidine (L-hist), sulphite (SO32−), pyrazine (pz), nicotinamide (nic), 4-picoline (4-pic), triethylphosphite ([P(OEt)3]), isonicotinamide (isn), isonicotinic acid (ina), X=BF4−, Cl− or PF6−, and [RuII(NO+)(Hedta)] against the Y strain of T. cruzi. The results are explained on the basis of the chemical properties of these compounds. The potential utility of these NO donors as drugs is also discussed.
Methods
Parasites
The Y strain of T. cruzi, a partially drug-resistant and highly virulent strain (Martínez-Díaz et al., 2001), was used for all experiments. Swiss mice were infected by intraperitoneal (i.p.) administration with 1.0 × 103 bloodstream trypomastigote (BT) forms of T. cruzi obtained from an intermediary strain-matched infected mouse. Before infection of intermediary mice, parasites were grown in Schneider's medium and purified from a monkey kidney fibroblast cell line, LLC-MK2.
Evaluation of the trypanocidal and antiproliferative activities in vitro
BT (Y strain) were obtained from the mice at the peak of parasitemia and resuspended to 1.0 × 106 parasites ml−1. Epimastigote forms were grown in Schneider's medium, supplemented with 20% fetal calf serum, harvested during the exponential phase of growth, washed in phosphate-buffered saline (PBS) and resuspended to 1.0 × 106 parasites ml−1. A volume of 200 μl of parasites was plated onto 96-well plates (in triplicate) and treated with the NO donors diluted in PBS (0.1, 0.5 and 1.0 mM) and incubated at 37°C, 5% CO2. Benznidazole and SNP from Aldrich Chemical Company (ACC) were used as the reference trypanocidal drug (positive control) and the reference NO donor, respectively, both diluted directly in PBS at 1.0 mM. Parasite viability was subsequently tested by determining the number of motile forms in a haemocytometer (Brener, 1962) and the percentage of trypanocidal activity (% TA) and the percentage of antiproliferative activity (growth inhibition, % GI) were calculated as follows: % TA=[1–(LDt/LCt)] × 100 and % GI={1–[(LDt−LDto)/(LCt−LCto)]} × 100, where LDt is the average of the number of motile forms in wells containing the drug at time t, LDto is the average of the number of motile forms in wells containing the drug at time t=zero, LCt is the average of the number of motile forms in wells in the absence of any compound at time t (negative control) and LCt is the average of the number of motile forms in wells in the absence of any compound at time t=zero (Saraiva et al., 2007). The concentration of compound corresponding to 50% antiproliferative or trypanocidal activities after 24 h of incubation was expressed as IC50epi (inhibitory concentration on epimastigotes forms) and IC50try (inhibitory concentration on trypomastigotes forms), respectively (Silva et al., 2006).
Evaluation of the trypanocidal activity in vivo (acute model)
Female Swiss mice (6–8 weeks old) were infected by injecting 1.0 × 102 or 1.0 × 103 BT per mouse. The animals were housed in temperature-controlled rooms (22–25°C) and received water and food ad libitum in the animal facilities of the Departamento de Bioquímica e Imunologia, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Brazil. All NO donors were injected i.p. in 100 μl of PBS. All procedures performed during the study described herein were approved by the Ethics Committee on Animal Research of the Universidade de São Paulo. The course of infection was monitored by counting the number of motile trypomastigotes in blood samples (5 μl) drawn from the tail veins, as described previously (Brener, 1969). The histological analyses were carried out on heart tissues of groups of six infected and non-infected mice at 15th day after the infection. The hearts were fixed in a solution of formaldehyde (10%) in PBS embedded in paraffin, sectioned, stained with hematoxylin-eosin (H&E), and examined by light microscopy.
Statistical analysis
The results presented here are expressed as mean±s.e.m. The Mann–Whitney and Kruskal–Wallis procedures were used to determine the statistical significance of the inter-group comparison. Results were considered statistically significant when P<0.05.
Chemicals and reagents
Ruthenium trichloride from ACC was the starting material for the synthesis of all ruthenium complexes described herein. All solvents were purified following known procedures (Perrin et al., 1980) and doubly distilled water was used throughout. All the syntheses and manipulations were carried out under argon atmosphere (Shriver, 1969).
Synthesis of the ruthenium compounds
The [Ru(NH3)5Cl]Cl2, trans-[Ru(NH3)4(HSO3)2], trans-[Ru(NH3)4(SO2)Cl]Cl (Vogt et al., 1965), trans-[Ru(NO)(NH3)4L]X3, L=imN, imC, py, pz, 4-pic, L-hist, nic, isn, P(OEt)3, SO32−, and X=BF4−, Cl− or PF6− (Borges et al., 1998; Lopes et al., 2001, 2004) and [Ru(NO)(Hedta)] (Zanichelli et al., 2004) complexes were synthesized and characterized following published procedures. The trans-[Ru(NO)(NH3)4ina](BF4)3 was also prepared by adapting the procedures published by Borges et al. (1998). Yield=60%. For trans-[Ru(NO)(NH3)4ina](BF4)3: Theoretical: H, 3.40; N, 13.58; C, 11.64; Ru, 16.31. Found: H, 3.46; N, 13.61; C, 11.85; Ru, 16.57. Relevant infrared absorption bands, cm−1: 3240 br s [νNH, νOH], 1934 s [νNO+], 1639 m [δHOH, δdNH], 1326 m [δsNH], 843 m [ρ(NH3), δ(NH)out of plane], 618 w [νM−NO], 570 w [δRu−NO] and 481 w[νM−NH3], where br=broad, s=strong, m=medium and w=weak. Electrochemical data: E(NO+/NO0)=0.061 V vs normal hydrogen electrode (NHE). Ultraviolet (UV)-visible data: 228 nm (ɛ=3.3±0.7 × 103 M−1 cm−1); 270 nm (ɛ=1.0±0.4 × 103 M−1 cm−1), pH=3.1±0.2, μ=0.1 M.
Instrumentation
Microanalyses of hydrogen, carbon and nitrogen were carried out by using an EA 1110 CHNS-O CE Instrument. Analysis of ruthenium was performed according to the method proposed by Clarke (1978), using a polarized Zeeman atomic absorption spectrophotometer, Hitachi (model Z-8100), with a Hitachi hollow cathode lamp, 12 mA, and λ=349.9 nm.
UV-visible measurements were performed in a 1.0 cm quartz cell on a Hewlett–Packard diode array model 8452A spectrophotometer. IR spectra were recorded on a Bomem FTIR, model MB-102, spectrophotometer in the 400–4000 cm−1 range, in potassium bromide pellets.
A polarographic analyzer/stripping voltammeter model 264A from Princeton Applied Research attached to a microcomputer and employing Microquímica Eletrochemical Software was used for the electrochemical measurements. The electrochemical cell used was a conventional three-electrode type with an aqueous saturated calomel electrode as a reference electrode and a glassy-carbon and platinum wire with a small platinum plate at the end as working and auxiliary electrodes, respectively. However, for convenience the final electrochemical data were expressed against NHE.
Results
In vitro antiproliferative activity
Preliminary experiments carried out to determine the in vitro antiproliferative activity of trans-[Ru(NO)(NH3)4L]X3, X=BF4− or PF6− and [Ru(NO)(Hedta)] compounds were set up using cultures of epimastigotes forms. Table 1 summarizes the data of the antiproliferative activity of these NO donors in the exponential phase of T. cruzi growth expressed as the % GI. As shown in this table, the compounds where L=pz, isn, L-Hist, imN, py, and nic all exhibited greater antiproliferative activity than SNP, under practically all conditions tested. These data also indicate that the trans-[Ru(NO)(NH3)4pz](BF4)3 compound was the most effective NO donor tested. However, according to previous studies (Rodriguez et al., 1997), this complex also exhibited higher nonspecific cytotoxicity on V-79 cells (inhibitory concentration on V-79 cells (IC50V79)=120 μM) than the others nitrosyls (IC50V79 varying from 410 μM for L=L-hist to 2260 μM for L=P(OEt)3), and, therefore, we decided not use the trans-[Ru(NO)(NH3)4pz](BF4)3 complex for in vivo experiments in this present study.
Table 1.
Antiproliferative activity of the NO-donors on epimastigote forms at different concentrations and time of incubation
|
Antiproliferative activity (% GI) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T=1 h |
T=4 h |
T=24 h |
||||||||
| NO donors |
Concentrations (mM) |
|||||||||
| 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | IC50epi (μM) | |
| t-[Ru(NO)(NH3)4pz]3+ | 30±4 | 66±5 | 87±3 | 73±4 | 85±3 | 90±9 | 89±4 | 92±5 | 100 | 56 |
| t-[Ru(NO)(NH3)4isn]3+ | 15±4 | 19±3 | 70±6 | 27±5 | 55±4 | 72±2 | 75±2 | 86±4 | 91±7 | 67 |
| t-[Ru(NO)(NH3)4L-Hist]3+ | 31±3 | 49±3 | 57±7 | 36±2 | 60±8 | 62±4 | 68±5 | 71±4 | 77±5 | 78 |
| t-[Ru(NO)(NH3)4imN]3+ | 16±6 | 37±4 | 54±3 | 35±5 | 39±2 | 65±4 | 58±4 | 65±4 | 78±4 | 86 |
| t-[Ru(NO)(NH3)4py]3+ | 12±9 | 55±5 | 62±6 | 26±4 | 47±3 | 65±4 | 56±6 | 78±4 | 85±4 | 90 |
| t-[Ru(NO)(NH3)4nic]3+ | 25±5 | 63±3 | 72±4 | 39±2 | 61±6 | 89±8 | 47±5 | 81±4 | 89±6 | 136 |
| SNP | 19±5 | 35±2 | 51±4 | 22±5 | 56±4 | 65±3 | 41±4 | 66±6 | 74±4 | 244 |
| [Ru(NO)(Hedta)] | 28±4 | 50±4 | 56±8 | 20±3 | 50±4 | 56±5 | 33±2 | 72±7 | 89±4 | 275 |
| t-[Ru(NO)(NH3)4P(OEt)3]3+ | 57±4 | 68±9 | 51±3 | 12±3 | 35±4 | 58±4 | 25±4 | 69±6 | 74±5 | 328 |
Abbreviation: NO, nitric oxide.
Results are mean±s.e.m., n=4–6, P<0.05. Antiproliferative activity expressed as the percentage of growth inhibition at the concentrations shown. IC50epi corresponds to the concentration with 50% antiproliferative activity, after 24 h of incubation.
In similar experiments, the corresponding trans-[Ru(NH3)4L(SO4)]Cl, L=isn, imN, nic, ina, py, L-hist, and 4-pic, trans-[Ru(H2O)(NH3)4P(OEt)3](PF6)2 and [Ru(Hedta)Cl]Cl, all compounds that do not have the NO molecule coordinated were found not to exhibit any antiproliferative or trypanocidal activities (data not shown).
In vitro trypanocidal activity on BT
Table 2 shows the results of the time- and concentration-dependent activity of these NO donors on BT. The nitrosyls, trans-[Ru(NO)(NH3)4pz](BF4)3, trans-[Ru(NO)(NH3)4L-hist](BF4)3, trans-[Ru(NO)(NH3)4imN](BF4)3, trans-[Ru(NO)(NH3)4SO3]Cl, trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3 and trans-[Ru(NO)(NH3)4ina](BF4)3, were as effective as SNP in inducing lysis of trypomastigotes when incubated under the same conditions. However, in previous studies, SNP shows marked cytotoxicity on macrophages (IC50V79=60 μM) (Torsoni et al., 2002) and, therefore, its use as a chemoprophylaxis agent is not practical, as the IC50V79 was almost the same as the IC50try value.
Table 2.
Trypanocidal activity of the NO-donors on BT forms at different concentrations and time of incubation
|
Trypanocidal activity (% TA) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T=1 h |
T=4 h |
T=24 h |
||||||||
| NO donors |
Concentrations (mM) |
|||||||||
| 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | IC50try (μM) | |
| t-[Ru(NO)(NH3)4pz]3+ | 30±4 | 66±5 | 100 | 85±4 | 100 | 100 | 100 | 100 | 100 | 50 |
| t-[Ru(NO)(NH3)4L-hist]3+ | 36±4 | 86±4 | 88±4 | 58±4 | 79±4 | 83±5 | 98±4 | 100 | 100 | 51 |
| t-[Ru(NO)(NH3)4imN]3+ | 87±6 | 91±5 | 92±4 | 68±4 | 97±3 | 100 | 97±4 | 100 | 100 | 52 |
| SNP | 56±7 | 78±3 | 90±4 | 75±4 | 89±7 | 92±4 | 97±4 | 98±5 | 100 | 52 |
| Benznidazole | 0 | 7±6 | 12±4 | 15±5 | 21±5 | 37±7 | 89±8 | 92±5 | 100 | 53 |
| t-[Ru(NO)(NH3)4SO3]+ | 40±5 | 80±2 | 88±4 | 47±7 | 74±6 | 86±4 | 85±4 | 88±4 | 100 | 59 |
| t-[Ru(NO)(NH3)4P(OEt)3]3+ | 57±3 | 68±2 | 86±4 | 62±4 | 90±4 | 92±4 | 84±4 | 92±8 | 100 | 60 |
| t-[Ru(NO)(NH3)4ina]3+ | 55±4 | 83±4 | 87±5 | 56±4 | 64±2 | 77±5 | 79±4 | 100 | 100 | 63 |
| t-[Ru(NO)(NH3)4py]3+ | 60±2 | 79±4 | 83±3 | 49±6 | 76±4 | 80±4 | 67±4 | 91±6 | 92±5 | 75 |
| t-[Ru(NO)(NH3)4isn]3+ | 90±4 | 81±5 | 81±7 | 59±4 | 55±5 | 52±7 | 65±7 | 83±4 | 97±4 | 77 |
| t-[Ru(NO)(NH3)4nic]3+ | 36±6 | 60±4 | 67±5 | 38±2 | 63±4 | 93±4 | 44±5 | 86±4 | 100 | 158 |
| t-[Ru(NO)(NH3)44-pic]3+ | 48±4 | 77±3 | 90±5 | 35±3 | 50±4 | 78±4 | 44±5 | 76±4 | 95±3 | 177 |
| [Ru(NO)(Hedta)] | 12±7 | 15±6 | 82±4 | 20±3 | 50±4 | 56±3 | 33±3 | 72±2 | 89±2 | 275 |
| t-[Ru(NO)(NH3)4imC]3+ | 5±4 | 8±3 | 14±8 | 11±7 | 12±7 | 18±4 | 15±5 | 21±5 | 26±4 | ≈3400 |
Abbreviations: BT, bloodstream trypomastigote; NO, nitric oxide.
Results are mean±s.e.m., n=4–8, P<0.05. Trypanocidal activity expressed as the percentage of the number of lysed trypomastigotes compared to the negative control. IC50try corresponds to the concentration with 50% trypanocidal activity after 24 h of incubation.
These same compounds exhibited, on average 10-fold greater trypanocidal activity than the gentian violet (IC50try=536 μM), a phenylmethane dye currently recommended by the World Heath Organization in the treatment of blood banks in endemic areas to prevent the transmission of Chagas' disease by blood transfusion (Silva et al., 2006). In contrast to the reference anti-parasitic drug, benznidazole, that showed a very low activity in the first 4 h of incubation, the number of lysed parasites compared to the negative control, for the ruthenium complexes after 1 h of incubation (37°C, 5% CO2; 1 mM) was found to increase in the following sequence: trans-[Ru(NO)(NH3)4isn]3+ <trans-[Ru(NO)(NH3)4nic]3+ <[Ru(NO)(Hedta)] ∼ trans-[Ru(NO)(NH3)4py]3+ <trans-[Ru(NO)(NH3)4P(OEt)3]3+ ∼ trans-[Ru(NO)(NH3)4ina]3+ ∼ trans-[Ru(NO)(NH3)4SO3]+ ∼ trans-[Ru(NO)(NH3)4L-hist]3+ <SNP=trans-[Ru(NO)(NH3)44-pic]3+ <trans-[Ru(NO)(NH3)4imN]3+ <trans-[Ru(NO)(NH3)4pz]3+. The trans-[Ru(NO)(NH3)4L]3+ species, where L=imN, SO32−, ina, pz, L-hist, nic, and P(OEt)3, and SNP, all at 1 mM, induced 100% lysis of the BT forms after 24 h incubation.
In vivo experiments (acute model)
At light of the above findings, the compounds trans-[Ru(NO)(NH3)4imN](BF4)3 and trans-[Ru(NO)(NH3)4isn](BF4)3, now referred to as Ru(NO)imN and Ru(NO)isn, respectively, were selected for assessment in an in vivo model as, apart from their high trypanocidal and antiproliferative activities observed in the in vitro experiments, these two compounds exhibited lower cytotoxicity than the trans-[Ru(NO)(NH3)4pz]3+ and SNP (Rodriguez et al., 1997). Furthermore, the trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3 (LD50=257.5 μmol kg−1) is 17-fold less toxic in vivo than SNP (LD50=15 μmol kg−1) (Torsoni et al., 2002). Similarly, in the toxicity up-and-down tests performed with Swiss mice for [Ru(NO)(Hedta)], no death was observed in doses up to 90 μmol kg−1 (Zanichelli et al., 2004). At present, LD50 data for all the other compounds are not available. However, taking in account, the similarity of the compounds of this series, it is reasonable to suppose that the LD50 for these compounds will be similar. Therefore, the doses given in this study of Ru(NO)imN and Ru(NO)isn would be, at least, a 100-fold lower than the expected LD50.
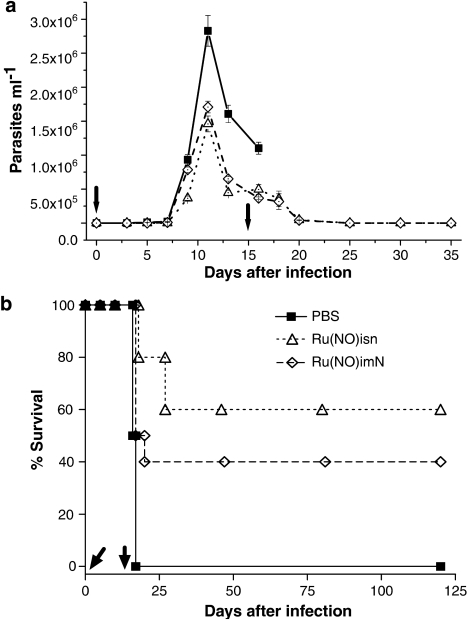
Animals infected with 1.0 × 103 BT per mouse and treated with a single daily dose of 100 nmol Ru(NO)isn or Ru(NO)imN per kg of body weight (58.2 μg kg−1 day−1 and 52.8 μg kg−1 day−1, respectively) during 15 consecutive days, exhibited a lower parasitemia than the group treated with only PBS (Figure 1a). This dose is 10 times smaller than the highest that can be given i.p. without observing any overt toxicity in T. cruzi-infected mice. Furthermore, 60% of the mice treated with the Ru(NO)isn and 40% of the mice treated with Ru(NO)imN in these experiments survived for more of 120 days, whereas all those treated with only the saline solution died before the 18th day (Figure 1b).
Figure 1.
Parasitemia and survival of Swiss mice infected with T. cruzi and treated with Ru(NO)isn or Ru(NO)imN. The mice were infected with T. cruzi (Y strain, 1.0 × 103 BT per mouse) and treated with Ru(NO)isn or Ru(NO)imN (100 nmol kg−1) daily for 15 consecutive days. Another group of mice received only PBS. Parasitemia levels are shown in (a) and survival curves in (b). Data are representative of three independent experiments with similar results, n=6. Arrows indicate treatment period. Another group of infected mice received benznidazole at 100 nmol kg−1 day−1 (26 μg kg−1 day−1), but no increased survival was observed (data not shown). PBS, phosphate-buffered saline.
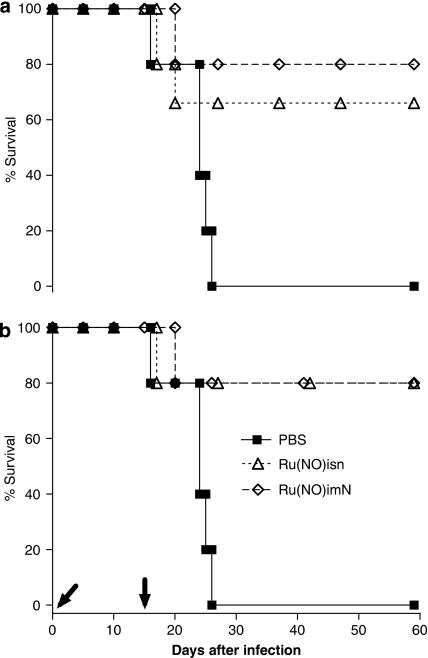
For the purpose of comparison and with the aim of increasing the survival time of the control group, the mice were inoculated with a 10-fold lower inoculum, 1.0 × 102 BT per mouse and treated with the same doses of Ru(NO)isn and Ru(NO)imN. The protocols for these tests are summarized in Table 3. According to Figures 2a and b, the survival of the group of mice that received daily doses of 100 nmol kg−1 of Ru(NO)imN during 15 consecutive days (group 1) was similar to that treated with the same dose only on the 5th, 6th and 7th days (group 2). This survival was found to be 80% for a period of up to 60 days. However, when the treatment was carried out with the Ru(NO)isn, the survival was 66% for group 1 and 80% for group 2.
Table 3.
Chronogram of treatment, parasitemia monitored and infection in Swiss mice with 5 at 8 weeks old
| Experiment | Infection | Treatment | Parasitemia monitored |
|---|---|---|---|
| Control group | 1.0 × 102 BT mouse−1 | 15 consecutive days with PBS | 5th, 10th and 15th day |
| Group 1 | 1.0 × 102 BT mouse−1 | 15 consecutive days with Ru(NO)imN and Ru(NO)isn | 5th, 10th and 15th day |
| Group 2 | 1.0 × 102 BT mouse−1 | 5th, 6th and 7th daysa with Ru(NO)imN and Ru(NO)isn | 10th day |
| Group 3 | 1.0 × 102 BT mouse−1 | –1th, 3th, 6th and 9th days with Ru(NO)imN and Ru(NO)isn | 10th day |
Critical days which precede the parasitemia peak. Infection is shown as number of BT per mouse injected as the initial inoculum.
Figure 2.
Survival curves of Swiss mice infected with T. cruzi and treated with Ru(NO)isn or Ru(NO)imN. The mice were infected with T. cruzi (Y strain, 1.0 × 102 BT per mouse) and treated with Ru(NO)isn or Ru(NO)imN (100 nmol kg−1) according to the protocol described in Table 3, (a) group 1 and (b) group 2. Another group of mice received only PBS. Data are representative of three independent experiments with similar results, n=6. Arrows indicate treatment period. PBS, phosphate-buffered saline.
In other experiments, Swiss mice were similarly infected and treated with benznidazole at 100 nmol kg−1 day−1 (26 μg kg−1 day−1) during 15 consecutive days but no increased survival was observed with this dose (data no shown).
Histological analysis
Swiss mice were i.p. infected with 1.0 × 103 BT per mouse and treated with the Ru(NO)imN or Ru(NO)isn during 15 consecutive days with a dose of 100 nmol kg−1 day−1. On the 15th day after infection, the survivor mice of the control group (treated only with PBS) and that of the group treated with the nitrosyls were killed and the hearts processed for H&E staining. Hearts from a group of uninfected mice were processed similarly, for comparison.
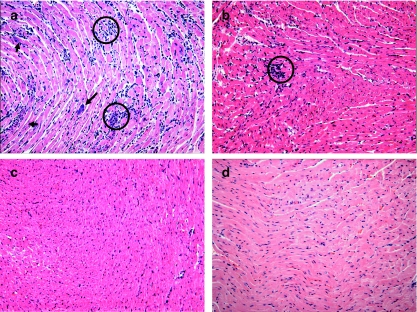
Microscopy revealed that the control-infected mice showed several nests of amastigotes (intracellular forms of T. cruzi) in the myocardial tissue, whereas no nests were observed in the hearts of the mice treated with Ru(NO)imN and Ru(NO)isn. Furthermore, the results obtained from these experiments showed that chemotherapy with these complexes decreases the occurrence of myocarditis (less inflammatory infiltrates), principally for the Ru(NO)imN compound (Figure 3).
Figure 3.
Histological patterns of heart sections of mice infected with T. cruzi 1.0 × 103 BT mouse−1 and treated with (a) PBS, (b) Ru(NO)isn or (c) Ru(NO)imN during 15 consecutive days or (d) non-infected mice. On the 15th day after infection, the mice were killed and their hearts processed for H&E staining. Note the intensity of the inflammatory process with mononuclear cell infiltrates and necrosis in (a) but not in (c) or (d). Arrows indicate the nests of amastigotes and circles indicate inflammatory infiltrate. Photomicrographs are representative of three independent experiments with similar results. Final magnification: × 200. BT, bloodstream trypomastigote; H&E, hematoxylin and eosin; PBS, phosphate-buffered saline.
Discussion and conclusions
Endogenous or exogenous NO exhibit antiparasitic effects on both protozoan and metazoan (Ascenzi et al., 2003). However, NO production needs to be tightly controlled to limit cytotoxic damage to the host cells. The protective and toxic effects of NO are frequently observed during infection with parasites such as T. cruzi and T. brucei (Ascenzi et al., 2003; Silva et al., 2003). During acute infection with T. cruzi, the cytokines, IFN-γ and tumour necrosis factor-α (TNF-α), and several chemoattractant molecules are produced (Gazzinelli et al., 1992; Cardillo et al., 1996). These cytokines play essential roles in the induction of iNOS and in the NO-dependent lysis of T. cruzi by murine macrophages. On the other hand, NO can also suppress the immune response to T. cruzi through the induction of apoptosis in T cells (Martins et al., 1998). Furthermore, expression of cardiac iNOS has been associated with myocardial dysfunction (Silva et al., 2003). Therefore, NO donors that can release this molecule judiciously are promising leads to new antiparasitic drugs, especially in environments where the NO concentration has to be tightly controlled.
trans-[RuII(NO+)(NH3)4L]3+ and [RuII(NO+)(Hedta)] undergo one-electron reduction centred in the coordinated NO+ group (see below, 1a) followed by dissociation of NO (1b), which can be controlled through the judicious selection of the L (Toledo et al., 2005).
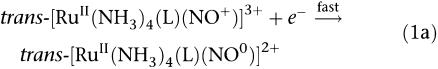 |
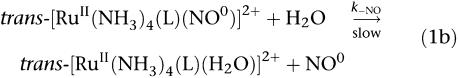 |
The in vitro or in vivo reduction step can be attributed to biological reducing agents such as nicotinamide adenine dinucleotide phosphate (Toledo et al., 2004), sulphydryl groups (Roncaroli and Olabe, 2005) or single electron transfer proteins (Allardyce and Dyson, 2001). As a consequence, NO is liberated from trans-[RuII(NO0)(NH3)4L]2+ species, with a different specific rate constants (k−NO), which for these complexes varies from 0.043 s−1 (L=isn) to 0.987 s−1 (L=P(OEt)3) at 25°C (Tfouni et al., 2003). For [RuII(NO0)(Hedta)]−, k−NO is equal to 2.1±0.4 × 10−3 s−1 (Zanichelli et al., 2004). Thus, all these NO donors differ from one another with respect to the reduction potential of the RuIINO+/RuIINO0 couple (E(NO+/NO0)) and the specific rate constant for NO release, k−NO.
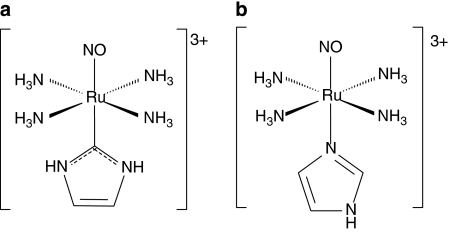
The data of Tables 1 and 2 strongly suggest that NO molecules released by nitrosyls upon reduction play essential roles in the antiproliferative and trypanocidal activities as in similar experiments, the related trans-[Ru(NH3)4L(SO4)]Cl and [Ru(Hedta)Cl]Cl species, which are not able to act as a NO donors, did not exhibit any activity. A correlation trend between the antiproliferative activity (% GI on epimastigote forms after 1 h of incubation) and the reduction potential of the fragment RuNO+/RuNO0 was also observed. As illustrated by Table 4, as the reduction potential of the NO+/NO0 couple becomes more positive (thus being more easily reduced), the % GI observed increases. This tendency should be compatible with the minor effect observed for the [Ru(NO)(Hedta)], trans-[Ru(NO)(NH3)4L-hist](BF4)3 and trans-[Ru(NO)(NH3)4imN](BF4)3 compounds as for these compounds, the (E(NO+/NO0)) fragment is more negative than that of the other NO donors (Tfouni et al., 2003). In agreement with the arguments above, the trans-[Ru(NO)(NH3)4imC]3+, which differs structurally from the trans-[Ru(NO)(NH3)4imN]3+ only through being bound to the carbon atom (C2) of the imidazole, rather than the nitrogen (Figure 4), despite its quite favourable k−NO=4 s−1, shows a very low trypanocidal activity up to 1 mM (Table 2), as its reduction potential is partially inaccessible to the biological reducing agents (−0.32 V vs NHE) (Lopes et al., 2001).
Table 4.
Trend of correlation between the antiproliferative activity, the redox potential E(NO+/NO0) and rate constant of NO release in a range of donors
| Compounds | % GI1h | E(NO+/NO0) vs NHE (V) | k-NO (s−1) |
|---|---|---|---|
| t-[Ru(NO)(NH3)4pz]3+ | 87±3 | 0.112 | 0.070 |
| t-[Ru(NO)(NH3)4nic]3+ | 72±4 | 0.072 | 0.025 |
| t-[Ru(NO)(NH3)4isn]3+ | 70±6 | 0.052 | 0.043 |
| t-[Ru(NO)(NH3)4py]3+ | 62±6 | 0.012 | 0.060 |
| t-[Ru(NO)(NH3)4L-Hist]3+ | 57±7 | −0.108 | 0.140 |
| [Ru(NO)(Hedta)] | 56±8 | −0.098 | 0.002 |
| t-[Ru(NO)(NH3)4imN]3+ | 54±3 | −0.118 | 0.160 |
| t-[Ru(NO)(NH3)4P(OEt)3]3+ | 51±3 | 0.132 | 0.987 |
| SNP | 51±4 | −0.195 | ND |
| t-[Ru(NO)(NH3)4(H2O)]3+ | ND | −0.148 | 0.040 |
Abbreviation: ND, not determined; NHE, normal hydrogen electrode; NO, nitric oxide.
% GI1h, percentage of growth inhibition on epimastigote forms after 1 h of incubation at a concentration of 1 mM. Results are mean±s.e.m. of the percentage number of lysed epimastigotes forms compared to the control.
Figure 4.
Structures of (a) trans-[Ru(NO)(NH3)4imC]3+ (E(NO+/NO0)=−0.32 V vs NHE and k−NO=4 s−1 at 25°C) and (b) trans-[Ru(NO)(NH3)4imN]3+ (E(NO+/NO0)=−0.118 V vs NHE and k−NO=0.16 s−1 at 25°C).
The compound trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3, despite its well-known ability to liberate NO (Torsoni et al., 2002; Zanichelli et al., 2006), exhibits a smaller effect compared to other NO donors dealt with in this study. Despite its quite favourable ENO+/NO0 and k−NO values, trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3 is unstable under physiological conditions yielding as products trans-[Ru(H2O)(NH3)4P(OEt)3]2+ and trans-[Ru(NO)(H2O)(NH3)4]3+. The first product does not exhibit any trypanocidal activity, while the reduction potential of the second is sufficiently negative (E(NO+/NO0)=−0.148 V vs NHE) to hamper its reduction under the experimental conditions investigated.
Transmission of T. cruzi by blood transfusion of immigrants originating from endemic areas of South America is becoming a source of concern in areas free from vectorial transmission such as the USA, Canada and European countries (Castro et al., 2006). Therefore, there is the need to develop new compounds for chemoprophylaxis in place of gentian violet, which, despite its efficacy, has restrictions to its use (Chiari et al., 1996). The trans-[Ru(NO)(NH3)4pz](BF4)3 (IC50try=50 μM), trans-[Ru(NO)(NH3)4L-hist](BF4)3 (IC50try=51 μM), trans-[Ru(NO)(NH3)4imN](BF4)3 (IC50try=52 μM), trans-[Ru(NO)(NH3)4SO3]Cl (IC50try=59 μM), trans-[Ru(NO)(NH3)4P(OEt)3](PF6) (IC50try=60 μM) and trans-[Ru(NO)(NH3)4ina](BF4) (IC50try=63 μM) compounds exhibited on average, 10-fold greater trypanocidal activity than gentian violet (IC50try=536 μM) after 24 h of incubation (Silva et al., 2006) and thus these compounds could be promising chemoprophylaxis agents in the treatment of infected blood. Furthermore, the IC50try values for these NO donors are up to 37 times lower than their respective nonspecific cytotoxicity values, whereas benznidazole, the drug available for Chagas' disease in humans, has a narrower therapeutic window (Coura and Castro, 2002).
We found that Ru(NO)isn markedly increased survival (to 60%) in animals inoculated with1.0 × 103 BT per mouse and treated daily with 100 nmol kg−1 day−1 (58.2 μg kg−1 day−1) of this compound. However, when the inoculation was carried out with an inoculum 10-fold lower (1.0 × 102 BT per mouse), the group treated daily and that treated only on critical days preceding the parasitemia peak were found to exhibit the same survival (80%). This suggested that administering daily doses on the 3 days preceding the parasitemia peak was enough to achieve good survival of the infected mice. These results are compatible with the in vivo experiments reported by Silva et al. (1992) showing that treatment with the anti IFN-γ antibody was more effective in the increased parasitemia and mortality, when given on days closest to the infection, while treatment on the 11th day post-infection, or later, was ineffective. Groups of mice inoculated with 1.0 × 102 BT per mouse and treated on alternate days (data not shown) were found to exhibit similar survival (50–60%) to those inoculated with the higher dose, 1.0 × 103 BT per mouse, and treated during 15 consecutive days, thus supporting the above arguments.
In a similar study in an acute model of Chagas' disease, benznidazole produced a survival rate of 90–100% when infected mice were treated for 20 consecutive days (Molina et al., 2000). However, the daily dose used in that study was 3850-fold higher (385 μmol kg−1 day−1=100 mg kg−1 day−1) than that presented here for Ru(NO)isn and Ru(NO)imN. Furthermore, infected mice treated with benznidazole at the lower dose, (100 nmol kg−1 day−1/26 μg kg−1 day−1) did not show increased survival, whereas the survival of mice treated with Ru(NO)imN or Ru(NO)isn at the same molar dose was 40 and 60%, respectively, for a period of up to 120 days (Figure 1).
The cellular diffusion and half-life of the NO molecule in red blood cells are important factors in a better understanding of the trypanocidal activity of NO. The calculated diffusion constant for the NO molecule employing Stokes' law resulted in D=3.360 μm2 s−1 at 37°C (Lancaster Jr, 2000), and is in good agreement with the value measured in water; D=3.300 μm2 s−1 (Malinsk et al., 1993) and in brain; D=3.810 μm2 s−1 (Meulemans, 1994). Therefore, a value of 3.5±0.3 × 103 μm2 s−1 can be taken as a good estimate for the diffusion constant of NO in the bloodstream. Although the observed half-life of NO in vivo was less than 1 s, nevertheless, it is reasonable to suggest that 50% of the NO molecules do survive for long enough to cover a volume of up to 65 mm3 within their estimated first half-lives (Pacher et al., 2007). Thus, it is likely that the NO molecules released by our NO donors do not only act at the site of liberation but also at a considerable distance from the site. This NO property is an important factor to be considered, as charged, inorganic, water-soluble NO-scavengers should remain preferentially in the bloodstream instead of crossing lipophilic host cell membranes (Fricker, 1995). It is likely that the NO donors studied must also have faced some resistance to cross these membranes but the extracellularly released NO could still diffuse into the cell (Zanichelli et al., 2006). This explains the results obtained from analyses of the heart photomicrographs. These micrographs showed that Ru(NO)imN and Ru(NO)isn were able to eliminate extracellular as well as intracellular forms of the Y strain of T. cruzi, thus reducing the inflammatory infiltrates in the myocardial tissue (Figure 3).
In the acute phase of T. cruzi infection, an intense myocarditis is frequently found (Rossi and Bestelli, 1995; Machado et al., 2000). The observation that cardiomyocytes produce NO in response to TNF-α, interleukin-1β and iNOS expression (Machado et al., 2000) added to the fact that these cytokines are detected in hearts of T. cruzi infected rats (Chandrasekar et al., 1998) is indicative of the fact that the local NO production is essential in controlling development of the parasite in myocardial tissue and the consequent typical cardiomyopathy. Therefore, the fact that Ru(NO)isn and Ru(NO)imN are efficient in eliminating amastigotes in myocardium tissue is further strong evidence of their antichagassic properties.
These nitrosyl compounds were shown to exhibit a hypotensive effect in different normotensive and hypertensive mouse models (Barros et al., 2002; Torsoni et al., 2002) and in aortic rings (Zanichelli et al., 2004, 2007) and this effect was therefore considered when planning the in vivo experiments in the present study. Previous results showed that a 100 nmol kg−1 dose of trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3, given intravenous, caused a 26% drop in blood pressure within the first 15 min (Torsoni et al., 2002). This compound also exhibited hypotensive effects when given i.p. and relaxed aortic rings, without endothelium and pre-contracted with noradrenaline. However, the dilator effect of Ru(NO)imN in similarly prepared aortic rings was almost negligible, 0% in the first 15 min and only 12% after an hour (Zanichelli et al., 2007). Thus, it is reasonable to suppose that the hypotensive effects of Ru(NO)isn and Ru(NO)imN would be much lower than that of trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3 in vivo, reflecting their much lower rate constant for NO release (Table 4).
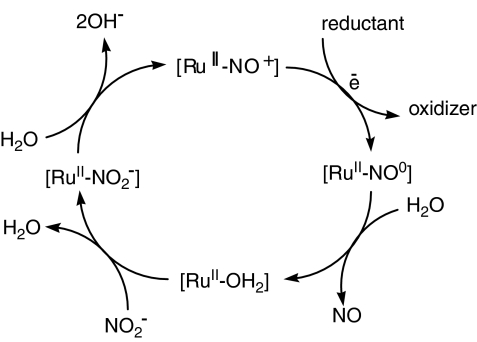
It is important to point out that these compounds are able to catalyse the conversion of nitrite into nitrosyl. In the presence of a suitable reducing agent, [RuIINO+]3+ species generate [RuIINO0]2+. The NO ligand is then hydrated and the [RuIIH2O]2+ species is formed. Since NO2− present in plasma can reach a concentration of up to 0.5 μmol l−1 h−1 (Himeno et al., 2004) and the reaction between NO2− and ruthenium species is fast (Zanichelli et al., 2006; Osti and Franco, 2007), [RuIINO2−]+ species can be formed in vivo. When the hydrogen ion concentration is higher than 10 nM (as it is in the bloodstream), the conversion of nitrite to NO can occur following the cycle proposed in the Figure 5. This potential catalytic ability of this series of compounds is supposed to provide an additional source of nitrosyl compounds in the body and is therefore an incentive for us to pursue this line of study.
Figure 5.
Scheme of the potential catalytic cycle for conversion of nitrite to nitric oxide by nitrosyl compounds.
In summary, the data obtained strongly suggest that Ru(NO)isn and Ru(NO)imN are effective against T. cruzi, and that administering the compounds on days preceding the parasitemia peak can be envisaged as a preliminary protocol for the chemotherapy of Chagas' disease. Studies to determine the optimal dose for these compounds in a chronic model for Chagas' disease in mice and to better understand the hypotensive effects in T. cruzi infected are now underway.
Acknowledgments
We are thankful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Millennium Institute for Vaccine Development and Technology (MIVDT/CNPq), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Ref. Proc.: 06/53266-4) for their financial support. The gift of a sample of trans-[Ru(NO)(NH3)4imC](PF6)3 by Professor Luiz GF Lopes is very much acknowledge.
Abbreviations
- BT
bloodstream trypomastigote forms of Trypanosoma cruzi
- E(NO+/NO0)
reduction potential of the RuNO+/RuNO0 couple
- % GI
percentage of growth inhibition on epimastigote forms
- IC50try
inhibitory concentration on trypomastigotes forms
- IC50V79
inhibitory concentration on V-79 cells
- imC
imidazole coordinated by carbon
- imN
imidazole coordinated by nitrogen
- ina
isonicotinic acid
- isn
isonicotinamide
- k−NO
specific rate constant for NO release
- L
trans ligand
- L-hist
L-histidine
- NHE
normal hydrogen electrode
- nic
nicotinamide
- PBS
phosphate-buffered saline
- 4-pic
4-picoline
- [P(OEt)3]
triethylphosphite
- py
pyridine
- pz
pyrazine
- Ru(NO)imN
trans-[RuII(NO+)(NH3)4imN](BF4)3
- Ru(NO)isn
trans-[RuII(NO+)(NH3)4isn](BF4)3
- SNAP
S-nitroso-acetyl-penicillamine
- SNP
sodium nitroprusside
- % TA
percentage of trypanocidal activity
- T. cruzi
Trypanosoma cruzi
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Allardyce CS, Dyson PJ. Ruthenium in medicine: current clinical uses and future prospects. Platinum Metal Rev. 2001;45:62–69. [Google Scholar]
- Ascenzi P, Bocedi A, Gradoni L. The anti-parasitic effects of nitric oxide. IUBMB Life. 2003;55:573–578. doi: 10.1080/15216540310001639265. [DOI] [PubMed] [Google Scholar]
- Barros BF, Toledo JC, Jr, Franco DW, Tfouni E, Krieger MH. A new inorganic vasodilator, trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3: hypotensive effect of endothelium-dependent and – independent vasodilators in different hypertensive animals models. Nitric Oxide. 2002;7:50–56. doi: 10.1016/s1089-8603(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Bocedi A, Gradoni L, Menegatti E, Ascenzi P. Kinetics of parasite cysteine proteinase inactivation by NO-donors. Biochem Biophys Res Commun. 2004;315:710–718. doi: 10.1016/j.bbrc.2004.01.113. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immun. 2001;10:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Bonavida B, Khineche S, Huerta-Yepez S, Garbán H. Therapeutic potential of nitric oxide in cancer. Drug Resist Updat. 2006;9:157–173. doi: 10.1016/j.drup.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Borges SSS, Davanzo CU, Castellano EE, Z-Zchpector J, Silva SC, Franco DW. Ruthenium nitrosyl complexes with N-heterocyclic ligands. Inorg Chem. 1998;37:2670–2677. doi: 10.1021/ic951563s. [DOI] [PubMed] [Google Scholar]
- Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop. 1962;4:389–396. [PubMed] [Google Scholar]
- Brener Z. The behavior of slender and stout forms of Trypanosoma cruzi in the blood-stream of normal and immune mice. Ann Trop Med Parasitol. 1969;63:215–220. doi: 10.1080/00034983.1969.11686622. [DOI] [PubMed] [Google Scholar]
- Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin-10: the role of NK cell. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro JA, Meca MM, Bartel LC. Toxic side effect of drugs used to treat Chagas's disease (American trypanosomiasis) Hum Exp Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- Cerecetto H, González M. Chemotherapy of Chagas' disease: status and new development. Curr Top Med Chem. 2002;2:1187–1213. doi: 10.2174/1568026023393066. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Melby PC, Troyer DA, Colston JT, Freeman GL. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am J Pathol. 1998;152:925–934. [PMC free article] [PubMed] [Google Scholar]
- Chiari E, Oliveira AB, Prado MA, Alves RJ, Galvao L, Araujo FG. Potential use of WR6026 as prophylaxis against transfusion-transmitted American trypanosomiasis. Antimicrob Agents Chemother. 1996;40:613–615. doi: 10.1128/aac.40.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MJ. Electrochemistry, synthesis, and spectra of pentaammineruthenium(III) complexes of cytidine, adenosine, and related ligands. J Am Chem Soc. 1978;100:5068–5075. [Google Scholar]
- Coura JR, Castro SL. A critical review on Chagas' disease chemotherapy. Mem Instit Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- Fricker SP. Ruthenium, nitric oxide and disease. Platinum Metal Rev. 1995;59:150–159. [Google Scholar]
- Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immun. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- Gelb MH, Hol WGJ. Drugs to combat tropical protozoan parasites. Science. 2002;297:343–344. doi: 10.1126/science.1073126. [DOI] [PubMed] [Google Scholar]
- Himeno M, Ishibashi T, Nakano S, Furuya K, Yoshida J, Kigashi T, et al. Implication of steady state concentrations of nitrite and nitrate metabolites of nitric oxide in plasma and whole blood in healthy human subjects. Clin Exp Pharmacol Physiol. 2004;31:591–596. doi: 10.1111/j.1440-1681.2004.04060.x. [DOI] [PubMed] [Google Scholar]
- Lancaster JR., JrThe physiological properties of nitric oxide. Determinants of the dynamics of NO in tissue Nitric Oxide 2000Academic Press: NovaYork/Londres; 209–223.In: Ignarro L (eds). [Google Scholar]
- Lopes LGF, Castellano EE, Ferreira AG, Davanzo CU, Clarke MJ, Franco DW. Reactivity of trans-[Ru(NH3)4P(OEt)3NO]X3, (X=PF6−, CF3COO−): modulation of the release of NO by the trans-effect. Inorg Chim Acta. 2004;358:2883–2890. [Google Scholar]
- Lopes LGF, Wieraszko A, El-Sherif Y, Clarke M. The trans-labilization of nitric oxide in Ru-II- complexes by C-bound imidazoles. Inorg Chim Acta. 2001;312:15–22. [Google Scholar]
- Machado FS, Martins GA, Aliberti JCS, Mestriner FLAC, Cunha FQ, Silva JS. Trypanosoma cruzi-infected cardiomuocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102:3003–3008. doi: 10.1161/01.cir.102.24.3003. [DOI] [PubMed] [Google Scholar]
- Malinsk T, Taha Z, Grunfeld S, Patton S, Kapturezak M, Tmbolian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrine microsensors. Biochem Biophys Res Commun. 1993;193:1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- Martínez-Díaz RA, Escario JA, Nogal-Ruiz JJ, Gómez-Barrio A. Biological characterization of Trypanosoma cruzi strains. Mem Inst Oswaldo Cruz. 2001;96:53–59. doi: 10.1590/s0074-02762001000100006. [DOI] [PubMed] [Google Scholar]
- Martins GA, Cardoso AG, Aliberti JCS, Silva JS. Nitric oxide-induced apoptotic cell death in the acute phase of Trypanosoma cruzi infection in mice. Immun Lett. 1998;63:113–120. doi: 10.1016/s0165-2478(98)00066-2. [DOI] [PubMed] [Google Scholar]
- Meulemans A. Diffusion coefficients and half-lives of nitric oxide and N-nitroso-L-arginine in rat cortex. Neurosci Lett. 1994;171:89–93. doi: 10.1016/0304-3940(94)90612-2. [DOI] [PubMed] [Google Scholar]
- Molina J, Martins-Filho O, Brener Z, Romanha AJ, Loebenberg D, Urbina JA. Activity of the triazole derivative SCH 56592 (Posaconazole) against drug-resitent strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob Agents Chemother. 2000;44:150–155. doi: 10.1128/aac.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Ignarro LJ. Nitric oxide-releasing drugs. Annual Rev Pharmacol Toxicol. 2003;43:97–123. doi: 10.1146/annurev.pharmtox.43.100901.140226. [DOI] [PubMed] [Google Scholar]
- Osti RZ, Franco DW.Aspects of nitrite association with trans-[Ru(NH3)4P(OEt)3(H2O)]2+ Polyhidron 2007 10.1016/j.poly.2007.05.017doi [DOI]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin DD, Armarego WLF, Perrin DR. Purification of Laboratory Chemicals. Pergamon Press: Elmsford, USA; 1980. [Google Scholar]
- Rodriguez JA, Souza-Torsoni A, Franco DW, Haun M. XXVIa renião anual da sociedade brasileira de bioquímica e biologia molecular-SBBQ, Caxambu, 3rd–6th May, S-32. 1997.
- Roncaroli F, Olabe JA. The reactions of nitrosyl complexes with cysteine. Inorg Chem. 2005;44:4719–4727. doi: 10.1021/ic048156d. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Bestelli RB. The challenge of Chagas' disease cardiomyophaty: the pathogenic roles of autonomic abnormalites, autoimmune mechanisms an microvascular changes, and therapeutic implications. Cardiology. 1995;86:1–7. doi: 10.1159/000176822. [DOI] [PubMed] [Google Scholar]
- Sachs JD. The neglected tropical diseases. Sci Am. 2007;296:33A. doi: 10.1038/scientificamerican0107-33a. [DOI] [PubMed] [Google Scholar]
- Saraiva J, Vega C, Rolon M, Silva R, Silva MLA, Donate PM, et al. In vitro and in vivo activity of lignan lactones derivatives agaisnt Trypanossoma cruzi. Parasitol Res. 2007;100:791–795. doi: 10.1007/s00436-006-0327-4. [DOI] [PubMed] [Google Scholar]
- Shriver DF. The manipulation of Air-sensitive Compound. McGraw-Hill: New York; 1969. [Google Scholar]
- Silva JS, Machado FS, Martins GA. The role of nitric oxide in the pathogenesis of Chagas' disease. Front Biosci. 2003;1:s314–s325. doi: 10.2741/1012. [DOI] [PubMed] [Google Scholar]
- Silva JS, Morrissey PJ, Grabstein KH, Mohler KM, Anderson D, Reed SG. IL-10 and IFN-γ regulation of experimental Trypanosoma cruzi infection. J Exper Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JS, Vespa GNR, Cardoso MA, Aliberti JC, Cunha FQ. TNF-α mediates resistance to Trypanosoma cruzy infection in mice by inducing nitric oxide production in infected IFN-γ-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RSF, Costa EM, Trindade ULT, Teixeira DV, Pinto MCFR, Santos GL, et al. Synthesis of naphthofuranquinones with activity against Trypanosoma cruzi. Eur J Med Chem. 2006;41:526–530. doi: 10.1016/j.ejmech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Tfouni E, Krieger M, McGarvey B, Franco DW. Structure, chemical and photochemical reactivity and biological activity of some ruthenium nitrosyl complexes. Coord Chem Rev. 2003;236:57–69. [Google Scholar]
- Toledo JC, Lima Neto BS, Franco DW. Mutual effects in the chemical properties of the ruthenium metal center and ancillary ligands upon coordination. Coord Chem Rev. 2005;249:419–431. [Google Scholar]
- Toledo JC, Silva HAS, Scarpellini M, Mori V, Camargo AJ, Bertoti M, et al. Ruthenium tetraamine as a model of nitric oxide donor compounds. Eur J Inorg Chem. 2004;9:1879–1885. [Google Scholar]
- Torsoni AS, Barros BF, Toledo JC, Haun M, Krieger MH, Tfouni E, et al. Hypotensive properties and acute toxicity of trans-[Ru(NH3)4P(OEt)3(NO)](PF6)3, a new nitric oxide donor. Nitric Oxide. 2002;6:247–354. doi: 10.1006/niox.2001.0409. [DOI] [PubMed] [Google Scholar]
- Vespa GNR, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and direct kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt LH, Jr, Katz JL, Wiberly S. The crystal and molecular structure of ruthenium-sulfur dioxide coordination compounds. I. Chlorotetraammine(sulfur dioxide)ruthenium (II) chloride. Inorg Chem. 1965;4:1157–1160. [Google Scholar]
- Zanichelli PG, Estrela HFG, Spadari-Bratfisc RC, Grasse-Kassisse DM, Franco DW. The effects of ruthenium compounds on vascular smooth muscle. Nitric Oxide. 2007;16:189–196. doi: 10.1016/j.niox.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Zanichelli PG, Miotto AM, Estrela HFG, Soares FR, Grassi-Kassisse DM, Spadari-Bratfisch RC, et al. The [Ru(Hedta)NO]0,1− system: structure, chemical and biological assays. J Inorg Biochem. 2004;98:1921–1932. doi: 10.1016/j.jinorgbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Zanichelli PG, Sernaglia RL, Franco DW. Immobilization of the [RuII(edta)NO+] íon on surface of functionalized sílica gel. Langmuir. 2006;22:203–208. doi: 10.1021/la051852l. [DOI] [PubMed] [Google Scholar]