Abstract
Background and purpose:
Experiments were performed to determine if capacitative Ca2+ entry (CCE) in canine pulmonary arterial smooth muscle cells (PASMCs) is dependent on InsP3 receptors or ryanodine receptors as induction of CCE is dependent on simultaneous depletion of the functionally separate InsP3- and ryanodine-sensitive sarcoplasmic reticulum (SR) Ca2+ stores in these cells.
Experimental approach:
Myocytes were isolated from canine pulmonary arteries using enzymatic procedures and were used within 8 h of preparation. Measurements of cytosolic Ca2+ were made by imaging fura-2 loaded individual myocytes that were perfused with physiological buffered saline solution with or without Ca2+.
Key results:
Treating myocytes with 10 μM cyclopiazonic acid (CPA), removing extracellular Ca2+, and briefly applying 10 mM caffeine and 10 μM 5-hydroxytryptamine (5-HT) depleted SR Ca2+ stores. Extracellular Ca2+ reintroduction caused cytosolic [Ca2+] to elevate above baseline signifying CCE. The InsP3 receptor inhibitors 2-aminobiphenylborate (50-75 μM; 2-APB) and xestospongin-C (20 μM; XeC) abolished CCE. Yet, CCE was unaffected by 10 μM or 300 μM ryanodine or 10 μM dantrolene, which modify ryanodine receptor activity. Higher dantrolene concentrations (50 μM), however, can inhibit both ryanodine receptors and InsP3 receptors, did reduce CCE. In contrast, CCE activated by hypoxia was unaffected by XeC (20 μM).
Conclusions and implications:
The results provide evidence that CCE activated by depletion of both InsP3 and ryanodine SR Ca2+ stores in canine PASMCs is dependent on functional InsP3 receptors, whereas the activation of CCE by hypoxia appears to be independent of functional InsP3 receptors.
Keywords: fura-2, sarcoplasmic reticulum, xestospongin-C, 5-hydroxytryptamine, 2-aminobiphenylborate, dantrolene, cyclopiazonic acid, hypoxia
Introduction
Two types of Ca2+ release channels are located on the sarcoplasmic reticulum (SR) of smooth muscle cells: ryanodine-sensitive channels (ryanodine receptors that are activated by rises in Ca2+), and inositol 1,4,5-triphosphate (InsP3)-sensitive Ca2+ channels that are activated by InsP3, which is produced downstream of neural or humoral stimulation of G-protein or tyrosine-coupled membrane bound receptors (Bootman and Berridge, 1995). Recent contractile and Ca2+ imaging studies from our laboratory demonstrated that functional differences exist in the SR Ca2+ stores of acutely isolated canine pulmonary arterial smooth muscle cells (PASMCs) (Jabr et al., 1997; Janiak et al., 2001). The contractile data showed that in canine pulmonary arterial rings phenylephrine caused contraction through release of Ca2+ from InsP3-sensitive stores (Somlyo and Somlyo, 1994). The α1 adrenoceptor-mediated contraction could be inhibited when the InsP3-sensitive Ca2+ stores were depleted of their Ca2+ in the presence of the sarcoplasmic–endoplasmic reticulum Ca2+ ATPase (SERCA) blocker cyclopiazonic acid (CPA) (Goeger et al., 1988) without affecting subsequent contraction due to release of ryanodine-sensitive Ca2+ stores with caffeine (Jabr et al., 1997). Similarly, depletion of ryanodine-sensitive Ca2+ stores by exposing cells to caffeine in the presence of ryanodine did not affect subsequent contraction due to release of the InsP3-sensitive Ca2+ stores. These experiments along with Ca2+ imaging experiments on canine PASMCs (Janiak et al., 2001) provided good evidence that in these cells, the InsP3- and ryanodine-sensitive Ca2+ stores were independent.
5-Hydroxytryptamine (5-HT) is a potent mediator of pulmonary hypertension by stimulating contraction and proliferation of PASMCs (McGoon and Vlietstra, 1984; MacLean et al., 2000) and its effects on intracellular Ca2+ and contractility are similar to those of phenylephrine. We have recently shown that Ca2+ release and contractility elicited by 5-HT is dependent on activation of InsP3 receptors, L-type Ca2+ channels as well as tonic Ca2+ entry pathways (Wilson et al., 2005). 5-HT, however, does not promote pulmonary arterial contraction through activation of ryanodine receptors (Wilson et al., 2005).
Depletion of SR Ca2+ stores in many cell types activates Ca2+ permeable store-operated currents (ISOC) on the plasma membrane, which replenish the empty stores through a process known as ‘capacitative Ca2+ entry' (CCE) (Putney, 1986). Recently, we reported that CCE (Wilson et al., 2002) as well as ISOC (Wilson et al., 2005) in canine PASMCs are activated in parallel with the organization of the SR Ca2+ stores; CCE and ISOC can be activated only with simultaneous InsP3 and ryanodine SR Ca2+ store depletion. 5-HT exposure alone was also not sufficient to activate CCE or ISOC in canine PASMCs (Wilson et al., 2005).
The mechanisms linking SR Ca2+ store depletion to CCE activation are diverse. CCE can be activated through a coupling of SR-bound InsP3 receptors (Ma et al., 2000) or possibly ryanodine receptors (Bennett et al., 1998; Kiselyov et al., 2001; Cherednichenko et al., 2004; Hurne et al., 2005) to store-operated channels. Decreases in the luminal Ca2+ content can also activate CCE independent of direct InsP3 receptor stimulation (Hofer et al., 1998). Alternatively, depletion of the SR Ca2+ stores may lead to the production and release of a calcium influx factor (CIF) (Trepakova et al., 2000) or STIM proteins (Roos et al., 2005; Zhang et al., 2005; Spassova et al., 2006) that activates CCE. A better understanding of the process of CCE activation in PASMCs is particularly important, since release of SR Ca2+ stores and induction of CCE pathways in canine (Jabr et al., 1997; Ng et al., 2005) and rat (Robertson et al., 2000; Wang et al., 2005; Weigand et al., 2005) have been implicated in the unique vasoconstrictor response of pulmonary arteries to hypoxia (HPV). Because CCE in canine PASMCs can be activated by simultaneous depletion (Wilson et al., 2002) of the functionally independent InsP3- and ryanodine-sensitive SR Ca2+ stores (Janiak et al., 2001), the present study was designed to examine whether store depletion per se is required for CCE activation in canine PASMCs or whether there is an additional involvement of InsP3 receptors or ryanodine receptors. We also tested whether functional InsP3 receptors are required for activation of CCE by hypoxia. An involvement of one or more of these receptors may provide evidence in support of a conformational coupling model of CCE. The findings indicate that functional InsP3 receptors, but not ryanodine receptors, are required for store depletion-induced CCE activation in canine PASMCs, whereas activation of CCE by hypoxia is independent of functional InsP3 receptors.
Methods
Cell isolation
Smooth muscle cells were isolated from high-resistance canine pulmonary arteries as previously described (Janiak et al., 2001; Wilson et al., 2002). Mongrel dogs of either sex were killed with pentobarbital sodium (45 mg kg−1 intravenously) and ketamine (15 mg kg−1 intravenously), as approved by the University of Nevada, Reno Institutional Animal Care and Use Committee. The heart and lungs were excised en bloc. The third and fourth branches of pulmonary arteries were dissected at 5°C to decrease cellular metabolic activity. Pulmonary artery isolations and smooth muscle cell dispersions were made in a low-Ca2+ physiological saline solution (PSS) containing in mM: 125 NaCl; 5.36 KCl; 0.336 Na2HPO4; 0.44 K2HPO4; 11 N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES); 1.2 MgCl2; 0.05 CaCl2; 10 glucose; pH 7.4 (adjusted with Tris), osmolarity 300 mosM. Arteries were cleaned of connective tissue, cut into small pieces and placed in a tube containing fresh PSS. Tissue was immediately digested or cold stored in the refrigerator (5°C) up to 24 h. To disperse cells, tissue was placed in low-Ca2+ PSS enzymes containing (in mg ml−1): 0.5 collagenase type XI; 0.03 elastase type IV and 0.5 bovine serum albumin (fat-free) for 14–16 h at 5°C. The tissue was then washed several times with 5°C low-Ca2+ PSS solution and triturated with a fire-polished Pasteur pipette. The resulting dispersed PASMCs were cold stored at 5°C up to 8 h until experiments were performed. Approximately, 30% of the cells used in this study were dispersed from overnight storage of tissues and the remainder of the cells were dispersed from tissues isolated on the same day.
Fluorescence imaging
Cytosolic [Ca2+] was measured in canine PASMCs loaded with the ratiometric Ca2+-sensitive dye fura-2 AM (Molecular Probes, Eugene, OR, USA) using a dual excitation digital Ca2+ imaging system (IonOptix Inc., Milton, MA, USA) equipped with an intensified charge-coupled device (CCD). The imaging system was mounted on an inverted microscope (Nikon Inc., Melville, NY, USA) outfitted with a magnification of × 40 (NA 1.3, Nikon Inc.) oil immersion objective. Fura-2 AM was dissolved in dimethyl sulphoxide (DMSO) and added from a 1 mM stock to the cell suspension at a final concentration of 10 μM. Cells were loaded with fura-2 AM for 20–30 min in a perfusion chamber (Warner Instruments, Hamden, CT, USA) at room temperature in the dark. Cells were then washed for 30 min to allow for dye esterification at 2 ml min−1 with a balanced salt solution of the following composition (mM): 126 NaCl; 5 KCl; 0.3 NaH2PO4; 10 HEPES; 1 MgCl2; 2 CaCl2; 10 glucose; pH 7.4 (adjusted with NaOH) 285–305 mosM. Measurements of cytosolic [Ca2+] before and during CCE and pharmacological manipulation were made once the fura-2 fluorescence ratio stabilized. The Ca2+-free balanced salt solution was prepared by substituting MgCl2 for CaCl2 and adding 1 mM ethylene glycol tetraacetic acid (EGTA). Cells were illuminated with a xenon arc lamp at 340±15 and 380±12 nm (Omega Optical, Brattleboro, VT, USA), and emitted light was collected from regions that encompassed single cells with a CCD at 510 nm (Nikon Inc.). In most experiments, images were acquired at 1 Hz and stored on either compact disk or magnetic media for later analysis. Although it is difficult to precisely measure the intracellular calcium concentration ([Ca2+]i) (Baylor and Hollingworth, 2000), estimates were made from the relation [Ca2+]i=Kd(Sf2/Sb2)(R−Rmin)/(Rmax−R), where Rmin and Rmax are the F380/F340 ratios of Ca2+-free and Ca2+-saturated fura-2, respectively. Sf2 is the F380 of Ca2+-free fura-2 and Sb2 is F380 of Ca2+-bound fura-2. The values of Sf2 and Rmin were determined by bathing cells in a balanced salt solution that did not have any added Ca2+ and contained 10 mM EGTA and 1 μM ionomycin. The values of Sb2 and Rmax were determined by bathing cells in a balanced salt solution contained 10 mM Ca2+ and 1 μM ionomycin. The Kd for fura-2 was assumed to be 224 nM (Grynkiewicz et al., 1985). During the Ca2+ calibration, 5 mM 2,3-butanedione monoxime was added to the bathing solution to inhibit smooth muscle contraction (Waurick et al., 1999). Experimental temperature was 22–25°C.
In experiments where the effect of hypoxia was investigated, hypoxia was induced by switching normoxic balanced salt solution to hypoxic balanced salt solution, which continuously superfused the cells in the recording chamber as previously described (Ng et al., 2005). Hypoxic solution was prepared by continuous gassing with uncertified gas mixtures containing 95% N2 and 5% CO2 (Sierra Welding, Sparks, NV, USA). The uncertified gas mixture contained minimal amounts of oxygen, which equilibrated with the solution to avoid exposure of cells to anoxic condition. All solutions were saturated with either normoxic or hypoxic gas mixtures for at least 30 min before the start of perfusion, and maintained at pH 7.4. The PO2, measured in preliminary experiments with an O2-sensitive electrode (MI-730; Microelectrodes Inc., Bedford, NH, USA), was 145±1 mm Hg during normoxic PSS perfusion and fell to 15±1 mm Hg within 79±2 s of hypoxic exposure. The PO2 of hypoxic solutions was measured at the end of each experiment and was found to be 15–18 mm Hg, ensuring that the PO2 did not approach anoxia during recording of each experiment.
Statistical analysis
All data are presented as mean±s.e.m. Statistical difference within groups was determined with a two-tailed paired Student's t-test. Statistical difference between groups was determined with an unpaired Student's t-test. In cases where the data were not normally distributed, a Wilcoxon signed-rank sum test was used to test for differences within groups. A one-way analysis of variance with a Neuman–Keuls multiple comparison procedure was used to test for differences between groups. The specific test used for each data set is noted in the legend for each figure. A P-value <0.05 was accepted as statistically significant. The n values reported reflect the total number of cells tested. Multiple trials were performed and cells isolated from several animals for most experimental paradigms.
Chemicals and drugs
Ionomycin free acid and Xestospongin-C (XeC) were purchased from Calbiochem (San Diego, CA, USA), Fura-2 AM from Molecular Probes, ryanodine from Calbiochem LC Laboratories (Woburn, MA, USA) or Alomone Laboratories (Tel Aviv, Israel), and all other chemicals were purchased from Sigma (St Louis, MO, USA).
Results
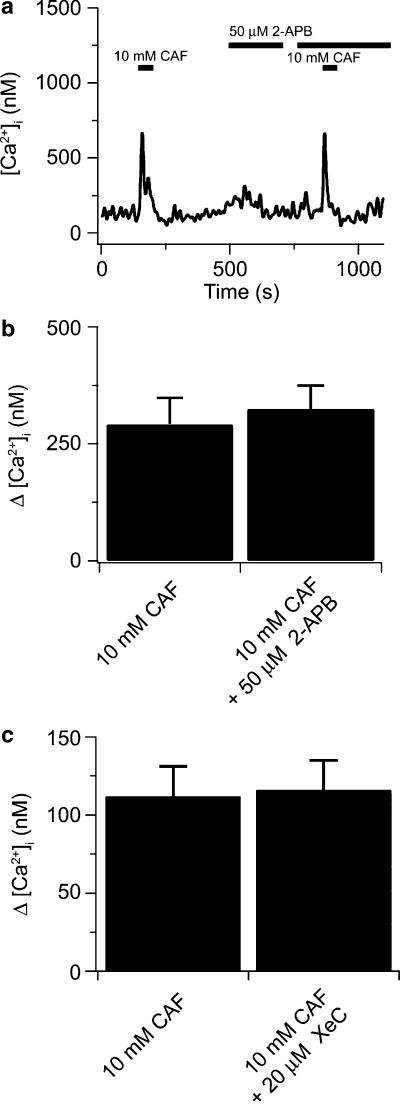
Recently, we have shown that 50–75 μM 2-APB as well as 20 μM XeC significantly attenuated the cytosolic Ca2+ increases elicited by 5-HT (Wilson et al., 2005). To test the specificity of these agents, we now initially examined whether 2-APB or XeC might also inhibit CAF-induced ryanodine receptor-mediated cytosolic [Ca2+] increases as well. Figure 1a shows that a 30-s 10 mM caffeine exposure caused a rapid, transient increase in cytosolic [Ca2+] of 537 nM and this was not affected by exposure to 50 μM 2-APB, where 10 mM caffeine caused a 557 nM cytosolic [Ca2+] increase. Figure 1b summarizes the results from nine cells and shows clearly that 50 μM 2-APB did not appear to inhibit ryanodine receptor activity. Very similar results were observed with XeC (Figure 1c). The cytosolic Ca2+ increase induced by caffeine (10 mM) was not affected by the addition of XeC (20 μM).
Figure 1.
2-APB and XeC does not inhibit caffeine-elicited cytosolic Ca2+ increases in canine PASMCs. (a) 10 mM caffeine (CAF) induced Ca2+ transients in the absence then the presence of 50 μM 2-APB. (b) Bars show the magnitude of the peak cytosolic Ca2+ increase elicited by 10 mM caffeine before and during 50 μM 2-APB. There was no significant difference between the two groups using a two-tailed paired t-test (n=9). (c) Bars show the magnitude of the peak cytosolic Ca2+ increase elicited by 10 mM caffeine before and during 20 μM XeC. There was no significant difference between the two groups using a two-tailed paired t-test (n=23). Bars represent means±s.e.m. 2-APB, 2-aminobiphenylborate; PASMCs, canine pulmonary arterial smooth muscle cells; XeC, xestospongin-C.
Role of InsP3 receptors and ryanodine receptors in store depletion-induced CCE
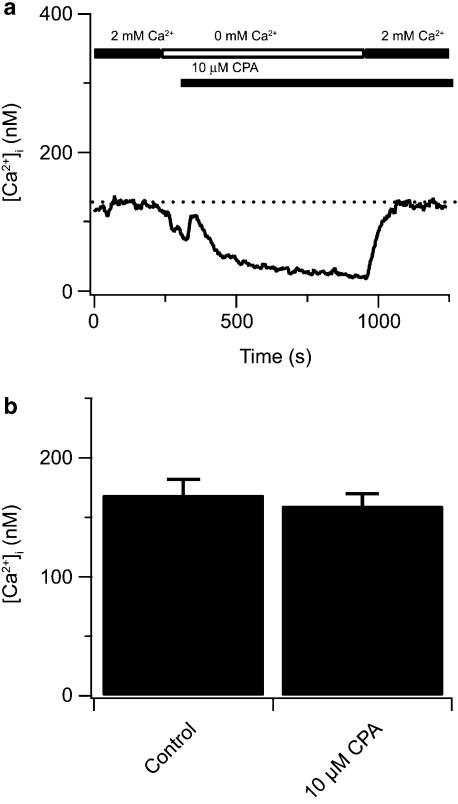
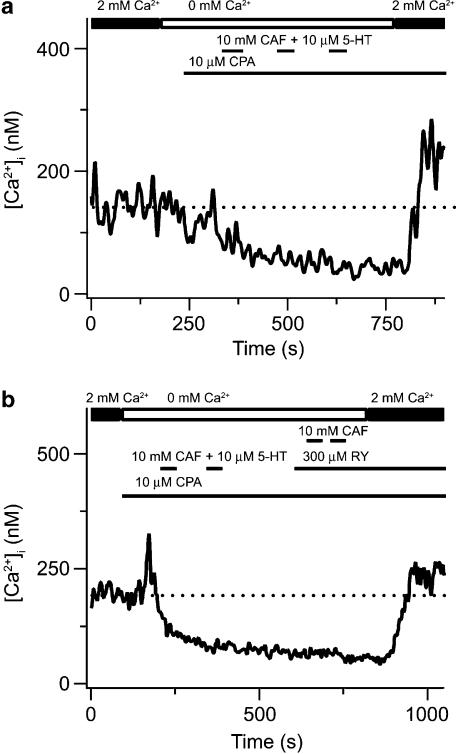
Experiments were carried out to address the possibility that InsP3 receptors are functionally coupled to CCE in canine PASMCs, as previously demonstrated in HEK 293 cells expressing transfected canonical transient receptor potential (TRPC) TRPC3 channels (Ma et al., 2000). Figure 2 shows the effects of the SERCA inhibitor CPA on the cytosolic [Ca2+]. CPA, at the concentration used in the present studies, has been used to deplete intracellular Ca2+ stores in many smooth muscle cells including rabbit portal vein myocytes (Albert and Large, 2002) and rat uterine myocytes (Shmigol et al., 1999) as well as canine pulmonary arteries (Jabr et al., 1997) and smooth muscle cells (Janiak et al., 2001; Wilson et al., 2002). Specifically, CPA selectively depletes the InsP3-sensitive Ca2+ stores in canine pulmonary arteries, as caffeine can still elicit ryanodine-sensitive Ca2+ release and contractility in the presence of CPA (Jabr et al., 1997; Janiak et al., 2001). As demonstrated previously, CPA exposure is only expected to activate CCE in canine PASMCs when combined with ryanodine-sensitive Ca2+ store depletion. Figure 2a shows that Ca2+ re-addition following 10 μM CPA for ∼5 min in a Ca2+-free bathing did not cause any rise in cytosolic [Ca2+] above basal values in a single PASMC, with cytosolic [Ca2+] remaining at 123 nM. Figure 2b summarizes data illustrating that CPA exposure in a Ca2+-free bathing solution does not elicit any increase in Ca2+ above basal values with Ca2+ re-addition.
Figure 2.
Cyclopiazonic acid (CPA) alone does not effectively activate CCE in canine PASMCs. (a) Effect of 10 μM CPA and extracellular Ca2+ removal on cytosolic [Ca2+] during extracellular Ca2+ re-addition. (b) Bars indicate the cytosolic [Ca2+]. Dashed line shows resting cytosolic [Ca2+]. There was no significant difference between the basal [Ca2+] measurements and those during CPA using a two-tailed paired t-test (n=27). Bars represent means±s.e.m. CCE, capacitative Ca2+ entry; PASMCs, canine pulmonary arterial smooth muscle cells.
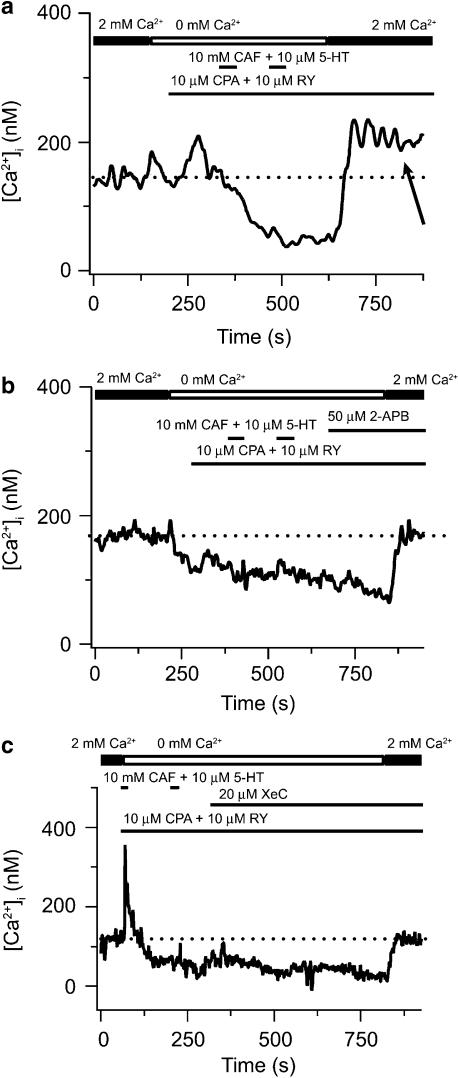
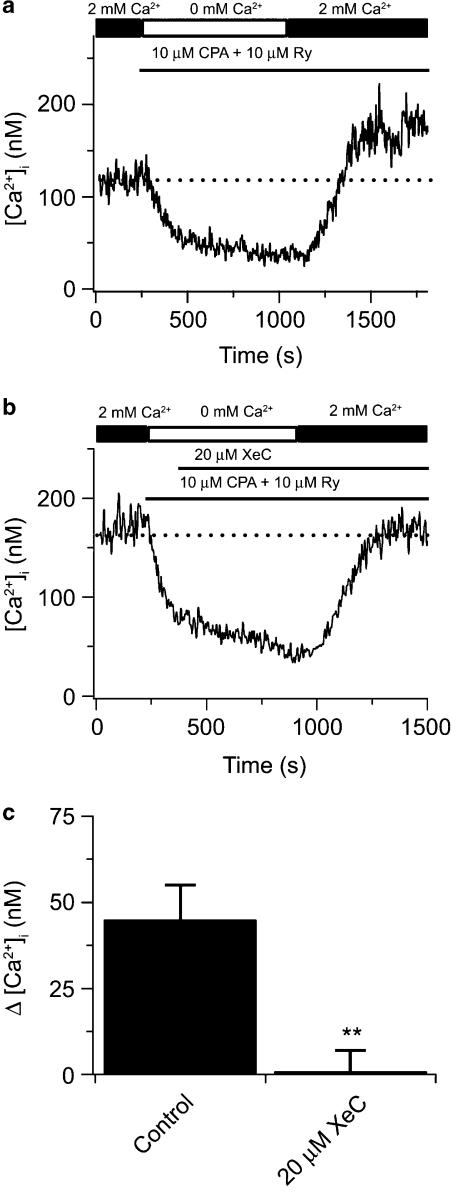
Figure 3 shows the effects of fully depleting both SR Ca2+ stores on the cytosolic [Ca2+] and the actions of 2-APB and XeC on CCE in canine PASMCs. We have recently shown that 2-APB as well as XeC inhibit 5-HT-elicited Ca2+ increases (Wilson et al., 2005), consistent with inhibition of InsP3 receptors (Gafni et al., 1997; Wu et al., 2000; Ta et al., 2005). Figure 3a shows that in a single PASMC the SR Ca2+ stores could be fully depleted by perfusing with a Ca2+-free bathing solution in the continuous presence of 10 μM CPA and 10 μM ryanodine, and exposing the cell twice for 30 s to 10 mM caffeine and 10 μM 5-HT. We have used protocols similar to this one to simultaneously deplete InsP3- and ryanodine-sensitive intracellular Ca2+ stores (Janiak et al., 2001) and activate CCE in canine PASMCs (Wilson et al., 2002; Ng et al., 2005). Subsequent addition of 2 mM extracellular Ca2+ while in the continued presence of 10 μM CPA and 10 μM ryanodine caused cytosolic [Ca2+] to rise 66 nM above basal values (arrow), indicating the activation of CCE. Figure 3b shows results from a similar experiment, except following complete store depletion where 50 μM 2-APB was added to the bathing solution in the absence and then the presence of 2 mM extracellular Ca2+. Subsequent addition of 2 mM extracellular Ca2+ in the continued presence of 50 μM 2-APB, 10 μM CPA and 10 μM ryanodine did not elicit any rise in cytosolic [Ca2+] above basal levels. Figure 3c shows results from an experiment where 20 μM XeC was added to the bathing solution following depletion of the InsP3- and ryanodine-sensitive Ca2+ stores in the absence and then presence of 2 mM extracellular Ca2+. Addition of 2 mM extracellular Ca2+ in the continued presence of 20 μM XeC, 10 μM CPA and 10 μM ryanodine did not cause any rise in cytosolic Ca2+ above basal levels. The mean data for these experiments from a number of cells are shown in Figure 5a.
Figure 3.
2-APB and XeC inhibit store depletion-induced CCE in canine PASMCs. (a) Effect of extracellular Ca2+ removal, 10 μM CPA, 10 μM ryanodine (RY), and sequential exposure to 10 mM caffeine (CAF) and 10 μM 5-HT on cytosolic [Ca2+] during extracellular Ca2+ re-addition. (b) Effect of 50 μM 2-APB on cytosolic [Ca2+] during extracellular Ca2+ re-addition following depletion of the SR Ca2+ stores. (c) Effect of 20 μM XeC on cytosolic [Ca2+] during extracellular Ca2+ re-addition following depletion of the SR Ca2+ stores. Dashed line shows resting cytosolic [Ca2+]. 2-APB, 2-aminobiphenylborate; 5-HT, 5-hydroxytryptamine; CCE, capacitative Ca2+ entry; CPA, cyclopiazonic acid; PASMCs, canine pulmonary arterial smooth muscle cells; SR, sarcoplasmic reticulum; XeC, xestospongin-C.
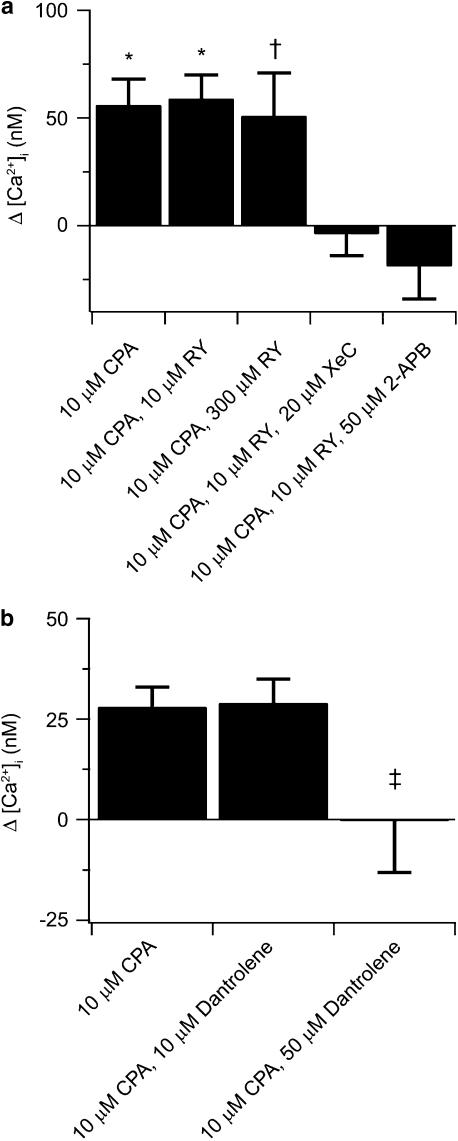
Figure 5.
Effects of pharmacological manipulation of InsP3 or ryanodine receptor activity on store depletion-induced CCE in canine PASMCs. (a) Summary of the cytosolic [Ca2+] during extracellular Ca2+ re-addition following depletion of the SR Ca2+ stores under control conditions (n=38) and in the presence of 10 μM (n=28) or 300 μM (n=8) ryanodine, 20 μM XeC (n=10) or 50 μM 2-APB (n=23). (b) Summary of the cytosolic [Ca2+] during extracellular Ca2+ re-addition following depletion of the SR Ca2+ stores under control conditions (n=192) and in the presence of 10 μM (n=102) or 50 μM (n=81) dantrolene. Bars indicate the change in cytosolic [Ca2+] compared to the resting cytosolic [Ca2+] and represent means±s.e.m. Means significantly different from their controls by * two-tailed paired t-test (P<0.001), or † a signed-rank test, ‡ significantly different from other conditions (one-way ANOVA with Neuman–Keuls multiple comparison procedure; P<0.05). 2-APB, 2-aminobiphenylborate; ANOVA, analysis of variance; CCE, capacitative Ca2+ entry; InsP3, inositol 1,4,5-triphosphate; PASMCs, canine pulmonary arterial smooth muscle cells; SR, sarcoplasmic reticulum; XeC, xestospongin-C.
Experiments were then performed to determine the involvement of ryanodine receptors in store depletion-induced CCE activation. Figure 4a shows that in a single canine PASMC, the SR Ca2+ stores could be fully depleted by perfusing with a Ca2+-free bathing solution in the continuous presence of 10 μM CPA and exposing the cell three times for 30 s to 10 mM caffeine and 10 μM 5-HT. Subsequent addition of 2 mM extracellular Ca2+ while in the continued presence of 10 μM CPA activated CCE. In this cell, cytosolic [Ca2+] increased to 65 nM above basal values. Figure 4b shows results from a similar experiment, except the protocol was designed to alter the conformation of the ryanodine receptors. The efficacy of ryanodine binding to ryanodine receptors is dependent on activation and opening of ryanodine receptors as well as the ryanodine concentration and exposure time. Thus, following depletion of the InsP3- and ryanodine-sensitive Ca2+ stores, 300 μM ryanodine was added in the absence of extracellular Ca2+ and the cell was exposed twice for 30 s to 10 mM caffeine to promote ryanodine binding. Based on this ryanodine concentration and the duration of exposure, it is predicted that the ryanodine receptors would either be locked partially open, in a sub-conductance state, or would be in a closed inactivated state (Lattanzio et al., 1987; Humerickhouse et al., 1993). Subsequent addition of 2 mM extracellular Ca2+ in the continued presence of 300 μM ryanodine and 10 μM CPA still elicited a rise in cytosolic [Ca2+] of 45 nM above basal values.
Figure 4.
Ryanodine does not inhibit store depletion-induced CCE in canine PASMCs. (a) Effect of extracellular Ca2+ removal, 10 μM CPA, and sequential exposure to 10 mM caffeine (CAF) and 10 μM 5-HT on cytosolic [Ca2+] during extracellular Ca2+ re-addition. (b) Effect of 300 μM ryanodine (RY) on cytosolic [Ca2+] during extracellular Ca2+ re-addition following depletion of the SR Ca2+ stores. CCE, capacitative Ca2+ entry; CPA, cyclopiazonic acid; PASMCs, canine pulmonary arterial smooth muscle cells; SR, sarcoplasmic reticulum.
Figure 5a summarizes results illustrating that InsP3 receptor but not ryanodine receptor activity is important for store depletion-induced CCE activation in canine PASMCs. Depletion of the intracellular Ca2+ stores in the presence of CPA and in the absence or presence of 10 μM or 300 μM ryanodine all resulted in CCE, while InsP3 receptor inhibition abolished CCE. Subsequent to depletion of the InsP3- and ryanodine-sensitive Ca2+ stores addition of 2 mM Ca2+ to the bathing solution containing 10 μM CPA elicited a significant elevation in cytosolic [Ca2+] above basal value. Cells that were exposed to 10 μM ryanodine, as well as CPA, had similar significant cytosolic [Ca2+] increases. Raising the ryanodine concentration to 300 μM, ryanodine did not affect the cytosolic [Ca2+] increase (Figure 5a). These SR Ca2+ store depletion-induced rises in cytosolic Ca2+ were prevented by adding 20 μM XeC, or 50 μM 2-APB (Figure 5a).
Using ryanodine as a tool to assess the role of ryanodine receptors in CCE could be problematic since it may be difficult to separate its ability to block ryanodine receptors from its ability to promote store depletion. We therefore performed a separate series of experiments using the ryanodine receptor inhibitor, dantrolene (Zucchi and Ronca-Testoni, 1997; Laporte et al., 2004; MacMillan et al., 2005; Zheng et al., 2005). Dantrolene was also chosen because at higher concentrations, it significantly reduces InsP3 receptor-induced Ca2+ responses (MacMillan et al., 2005). Thus, it can be used to confirm the finding that InsP3 receptors are important for store depletion-induced CCE in canine PASMCs. Control experiments were performed using the same protocol as in Figure 4a, whereas experiments involved dantrolene were performed using the same protocol as in Figure 4b and the results are summarized in Figure 5b. Depletion of the intracellular Ca2+ stores in the presence of 10 μM CPA and in the presence or absence of 10 μM dantrolene resulted in CCE, whereas 50 μM dantrolene abolished CCE. In control experiments, there was a significant elevation in cytosolic [Ca2+] above basal values, and cells that were exposed to 10 μM dantrolene had equivalent cytosolic [Ca2+] increases. However, those exposed to the higher concentration of dantrolene (50 μM) did not have any cytosolic [Ca2+] response. These results confirm that InsP3 receptor, but not ryanodine receptor, activity is important for store depletion-induced CCE in canine PASMCs.
Alternatively, the apparent requirement for functional InsP3 receptor activity for store depletion-induced CCE might be explained by the fact that in these experiments, an InsP3 agonist, 5-HT, was used with CPA to facilitate InsP3 store depletion, and the ability of 2-APB and XeC to inhibit CCE might rather reflect incomplete store depletion due to interference with the actions of 5-HT. To test this possibility, we repeated the experiments shown in Figure 3 in the absence of 5-HT, using CPA alone to deplete InsP3-sensitive Ca2+ stores. As shown in Figures 6a–c, 20 μM XeC, was still capable of inhibiting store depletion-induced CCE even in the absence of InsP3 receptor activation.
Figure 6.
XeC inhibits store depletion-induced CCE in the absence of 5-HT in canine PASMCs. (a) Effect of extracellular Ca2+ removal, 10 μM CPA, 10 μM ryanodine on cytosolic [Ca2+] during extracellular Ca2+ re-addition. (b) Effect of 20 μM XeC on cytosolic [Ca2+] during extracellular Ca2+ re-addition following depletion of the SR Ca2+ stores in the absence of 5-HT. (c) Bars indicate the cytosolic [Ca2+]. Dashed line shows resting cytosolic [Ca2+]. There was a significant difference between the increase in cytosolic [Ca2+] induced by store depletion, in the absence (n=38) and presence (n=47) of 20 μM XeC using an unpaired t-test (**P<0.001). Bars represent means±s.e.m. 5-HT, 5-hydroxytryptamine; CCE, capacitative Ca2+ entry; CPA, cyclopiazonic acid; PASMCs, canine pulmonary arterial smooth muscle cells; SR, sarcoplasmic reticulum; XeC, xestospongin-C.
Role of InsP3 receptors in hypoxia-induced CCE
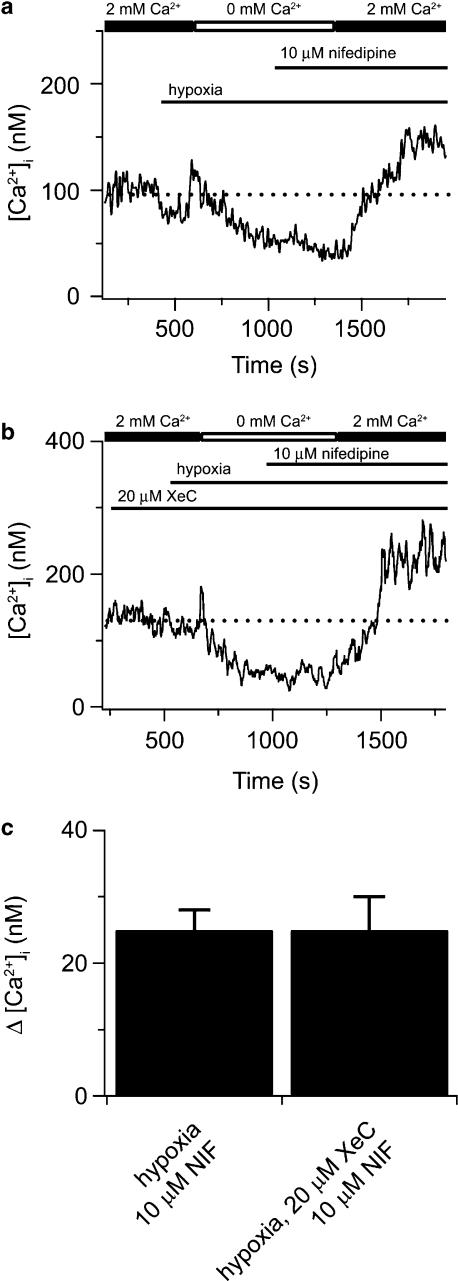
In our earlier study (Ng et al., 2005), we found that pharmacological pre-depletion of intracellular Ca2+ stores prevented further activation of CCE by hypoxia, suggesting that hypoxic activation of CCE may simply be due to depletion of intracellular Ca2+ stores. We, therefore, examined whether activation of CCE by acute exposure to hypoxia would exhibit the same dependence on functional InsP3 receptor activity as store depletion-induced CCE (cf Figure 5). Figure 7a illustrates an experiment in which hypoxia is shown to activate CCE in a canine PASMC. During exposure to a Ca2+-free bathing solution, the cell was exposed to hypoxia, which elicited an initial transient rise in cytosolic [Ca2+] due to Ca2+ release from intracellular stores (Ng et al., 2005). In the continued presence of hypoxia, subsequent addition of 2 mM extracellular Ca2+ caused cytosolic [Ca2+] to rise above basal values, indicative of CCE activation. Nifedipine was used in these experiments to eliminate Ca2+ entry through voltage-dependent Ca2+ channels. This nifedipine-insensitive rise in cytosolic [Ca2+] following addition of extracellular Ca2+ has been shown to be blocked by SKF 96365 and Ni2+, inhibitors of CCE channels (Ng et al., 2005). Figure 7b illustrates the effects of 20 μM XeC on the hypoxia-induced activation of CCE. Exposure to XeC failed to block the hypoxia-induced activation of CCE. Figure 7c summarizes similar results from cells in the absence and presence of XeC.
Figure 7.
XeC does not inhibit hypoxia-induced CCE in canine PASMCs. (a) Effect of extracellular Ca2+ removal and hypoxia on cytosolic [Ca2+] during extracellular Ca2+ re-addition in the presence of 10 μM nifedipine (NIF). (b) Effect of 20 μM XeC on cytosolic [Ca2+] during extracellular Ca2+ re-addition following hypoxic stimulation in the presence of 10 μM nifedipine. (c) Bars indicate the cytosolic [Ca2+]. Dashed line shows resting cytosolic [Ca2+]. There was no significant difference between the increase in cytosolic [Ca2+] induced by hypoxia, in the absence (n=28) and presence (n=34) of 20 μM XeC using an unpaired t-test. Bars represent means±s.e.m. CCE, capacitative Ca2+ entry; PASMCs, canine pulmonary arterial smooth muscle cells; XeC, xestospongin-C.
Discussion and conclusion
The results of this study provide evidence that store depletion-induced CCE in canine PASMCs is dependent on functional InsP3 receptor activity. Store depletion-induced CCE, however, does not appear to be critically dependent on ryanodine receptor activity. This work complements findings of other groups (Kiselyov et al., 1998, 1999; Ma et al., 2000), which illustrate a requirement of InsP3 receptor activity as an important component of CCE and store-operated channel activity.
The mechanism of CCE activation in canine PASMCs is complex, requiring an integration of separate signals. CCE is dependent on InsP3 receptors, as shown by the inhibition of CCE by 2-APB, XeC and high concentrations of dantrolene. While each of these pharmacological agents may not be entirely selective, we have capitalized on their common antagonism of the InsP3 receptor. 2-APB is known to inhibit InsP3 receptors as well as the activity of some store-operated channels (Prakriya and Lewis, 2001, 2002; Lievremont et al., 2005) and SERCA-dependent Ca2+ uptake (Missiaen et al., 2001). The dose–response relationship for 2-APB inhibition of 5-HT-mediated Ca2+ increases that we have recently reported (Wilson et al., 2005) match that shown for inhibition of CCE-mediated Ca2+ increases. However, since 2-APB may also block the non-selective cation channels responsible for CCE at concentrations similar to those that block InsP3 receptors, one must be cautious of reaching more than a speculative conclusion. We therefore chose to also examine the effects of the InsP3 receptor antagonist XeC (Gafni et al., 1997; Kiselyov et al., 1998; Ta et al., 2005) on 5-HT-elicited Ca2+ responses (Wilson et al., 2005) as well as CCE. Our previously reported data illustrate that XeC blocked 5-HT-induced Ca2+ increases with similar efficacy to its inhibition of muscarinic receptor-mediated Ca2+ increases in murine colonic smooth muscle (Bayguinov et al., 2001). This leads to the presumption that InsP3 receptor activation mediates the 5-HT-induced Ca2+ responses. Moreover, XeC at these same concentrations inhibited store depletion-induced CCE in the present experiments. It therefore appears that the effects of XeC in our experiments can be attributed to inhibition of InsP3 receptors and not due to direct interaction with non-selective cation channels responsible for CCE, since XeC had no effect on hypoxia-induced CCE. Our findings with dantrolene are comparable to those of MacMillan et al. (2005), who showed that 50 μM, but not 10 μM, dantrolene inhibit InsP3-induced Ca2+ responses in colonic myocytes.
Our experimental evidence demonstrates that altering the ryanodine receptor open state by promoting ryanodine binding or through inhibition by 10 μM dantrolene does not affect store depletion-induced CCE. The expectation was that if ryanodine receptors were as critically involved in the process of CCE activation in smooth muscle as they are in excitation-coupled Ca2+ entry pathways described in skeletal myocytes (Cherednichenko et al., 2004; Hurne et al., 2005), there should have been gross changes in CCE magnitude. Our data therefore do not support a model of tight coupling between the ryanodine receptor and the store-operated channels underlying store depletion-induced CCE in canine pulmonary arterial smooth muscle. Instead, the data lend support to the theory that this form of CCE activation requires coupling of InsP3 receptors to the store-operated channels that are responsible for CCE and concomitant depletion of the functionally separate InsP3- and ryanodine-sensitive Ca2+ stores in these canine PASMCs. Thus, there appears to be regulatory feedback from the SR Ca2+ stores to the store-operated channels through both passive depletion of the SR Ca2+ stores and the activity of InsP3 receptors. Our data, however, does not rule out a role of coupling of ryanodine receptors and the store-operated channels responsible for hypoxia-induced CCE, since hypoxic-mediated increases in contractile tension and cytosolic [Ca2+] in canine pulmonary arterial smooth muscle are significantly attenuated by pretreatment with ryanodine and caffeine (Jabr et al., 1997).
There are a number of possible explanations for why depletion of the ryanodine-sensitive Ca2+ stores is necessary to induce CCE even though ryanodine receptor activation is not. An obvious possibility is that functional ryanodine-sensitive SR Ca2+ stores buffer enhanced Ca2+ influx. This is highly unlikely, since our data shows that CCE (Wilson et al., 2002) and ISOC (Wilson et al., 2005) are activated only when the functionally independent InsP3- and ryanodine-sensitive SR Ca2+ stores are depleted. Specifically, neither CCE (Wilson et al., 2002) nor ISOC (Wilson et al., 2005) are active when the ryanodine-sensitive SR Ca2+ stores are filled, and thus they cannot operate as a Ca2+ sink. More generally, functional Ca2+ stores should alter the time to reach the steady-state cytosolic [Ca2+], but not the final steady-state cytosolic [Ca2+], which is dependent on the balance of the inward and outward Ca2+ fluxes across the plasma membrane (Smith et al., 1996). A more likely possibility is that, in canine PASMCs, a passive Ca2+ leak pathway from the ryanodine-sensitive SR Ca2+ store coordinates with the function of InsP3 receptors to regulate the activity of the store-operated channels responsible for CCE.
Depletion of the SR Ca2+ stores may regulate the activity of intracellular signaling pathways in canine PASMCs. The decreased luminal Ca2+ concentration could induce activation of Ca2+ independent phospholipase A2 (Smani et al., 2003), which may be involved with CIF generation and store-operated channel and CCE activation in vascular smooth muscle cells (Trepakova et al., 2000). CCE could also be due to translocation of STIM1 from the SR to the plasma membrane, which activates calcium release-activated channels (CRAC) in T lymphocytes (Roos et al., 2005; Zhang et al., 2005; Spassova et al., 2006) and TRPC1 (Huang et al., 2006; Lopez et al., 2006).
Capacitative calcium entry has been linked to various ion channels. Orai1 are novel transmembrane proteins that may be the molecular correlate for CRAC in T lymphocytes and other cells (Peinelt et al., 2006). Yet, Orai1 works in concert with STIM proteins through an unresolved mechanism to regulate store depletion Ca2+ entry and ICRAC (Mercer et al., 2006; Peinelt et al., 2006; Soboloff et al., 2006). Other candidate channel proteins responsible for CCE in canine PASMCs include TRPC channels, which are a class of non-selective cation channels that have also been proposed as molecular correlates of CCE in some cells, including smooth muscle (Beech, 2005). The sensitivity of CCE in canine PASMCs to pharmacological inhibitors and the biophysical characteristics of ISOC differs from that of CRAC channels in lymphocytes and is more typical of some TRPC channels. TRPC channels are expressed in canine PASMCs (Walker et al., 2001). Depletion of intracellular Ca2+ stores activates TRPC4 (McKay et al., 2000), whereas stimulation of G-protein-coupled receptors and diacylglycerols activates TRPC6 and TRPC7, independent of Ca2+ store depletion (Hofmann et al., 1999; Okada et al., 1999; McKay et al., 2000; Beck et al., 2006; Maruyama et al., 2006; Vazquez et al., 2006). TRPC4 has an InsP3 receptor binding site (Boulay et al., 1999) and activation of TRPC3 is coupled to InsP3 receptor function (Ma et al., 2000). Most relevant to pulmonary arterial function, it has been suggested that TRPC1 and TRPC6 may mediate store-operated Ca2+ entry and receptor-operated Ca2+ entry in rat PASMCs, respectively, and expression of both were significantly elevated in chronic hypoxia (Lin et al., 2004).
It is interesting that the functionally independent InsP3- and ryanodine-sensitive SR Ca2+ stores in canine PASMCs must be simultaneously depleted to activate CCE (Wilson et al., 2002) and ISOC (Wilson et al., 2005). Our original prediction was that CCE and ISOC activation would be graded and dependent on the size of the InsP3- and ryanodine-sensitive SR Ca2+ stores and the extent of store depletion. Possibly, a constitutive Ca2+ entry pathway provides sufficient Ca2+ entry to refill an empty Ca2+ store when the other store is full, which then inhibits CCE activity. This is supported by our previously reported Mn2+ quench data (Wilson et al., 2002, 2005), which illustrates there is a tonic Ca2+ influx pathway that contributes to pulmonary arterial contractility elicited by 5-HT (Wilson et al., 2005).
Several previous studies (Jabr et al., 1997; Robertson et al., 2000; Ng et al., 2005; Wang et al., 2005; Weigand et al., 2005) have provided evidence that hypoxic increases in intracellular Ca2+ in PASMCs may well involve the activation of CCE. The underlying mechanism(s) involved is unknown, but it may be due to a direct effect of hypoxia on CCE Ca2+ entry pathways, the ability of hypoxia to directly deplete intracellular Ca2+ stores or due to hypoxic facilitation of signal transduction pathways linking store depletion to CCE activation. The observation that store depletion-induced CCE in canine PASMCs is dependent on functional InsP3 receptor activity, whereas hypoxia-induced CCE is not, suggests that while hypoxia-induced CCE may involve store depletion, other mechanisms as well are likely to be involved.
Acknowledgments
We thank Phillip Keller and Shen Xiao-Ming for technical assistance and Normand Leblanc for insightful discussions. This work was supported by NIH grants HL49254 and P20RR15518 from NCRR (JRH) and HL10476 and AI055642 (SMW). Sean M Wilson is a faculty research fellow at the University of Mississippi.
Abbreviations
- 2-APB
2-aminobiphenylborate
- [Ca2+]i
intracellular Ca2+ concentration
- CCE
capacitative Ca2+ entry
- CIF
calcium influx factor
- CPA
cyclopiazonic acid
- CRAC
calcium release-activated channels
- DMSO
dimethyl sulphoxide
- HPV
hypoxic pulmonary vasoconstriction
- InsP3
inositol 1,4,5-triphosphate
- ISOC
store-operated currents
- PASMCs
pulmonary arterial smooth muscle cells
- PSS
physiological saline solution
- SERCA
sarcoplasmic–endoplasmic reticulum Ca2+ ATPase
- SR
sarcoplasmic reticulum
- TRPC
canonical transient receptor potential
- XeC
xestospongin-C
Conflict of interest
The authors state no conflict of interest.
References
- Albert AP, Large WA. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol. 2002;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayguinov O, Hagen B, Sanders KM. Muscarinic stimulation increases basal Ca2+ and inhibits spontaneous Ca2+ transients in murine colonic myocytes. Am J Physiol (Cell Physiol) 2001;280:C689–C700. doi: 10.1152/ajpcell.2001.280.3.C689. [DOI] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Measurement and interpretation of cytoplasmic [Ca2+] signals from calcium-indicator dyes. News Physiol Sci. 2000;15:19–26. [PubMed] [Google Scholar]
- Beck B, Zholos A, Sydorenko V, Roudbaraki M, Lehen'kyi V, Bordat P, et al. TRPC7 is a receptor-operated DAG-activated channel in human keratinocytes. J Invest Dermatol. 2006;126:1982–1993. doi: 10.1038/sj.jid.5700352. [DOI] [PubMed] [Google Scholar]
- Beech DJ. Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2005;32:597–603. doi: 10.1111/j.1440-1681.2005.04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Bootman MD, Berridge MJ, Cheek TR. Ca2+ entry into PC12 cells initiated by ryanodine receptors or inositol 1,4,5-trisphosphate receptors. Biochem J. 1998;329:349–357. doi: 10.1042/bj3290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, et al. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, et al. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci USA. 2004;101:15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Goeger DE, Riley RT, Dorner JW, Cole RJ. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988;37:978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hofer AM, Fasolato C, Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+] J Cell Biol. 1998;140:325–334. doi: 10.1083/jcb.140.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Humerickhouse RA, Besch HR, Jr, Gerzon K, Ruest L, Sutko JL, Emmick JT. Differential activating and deactivating effects of natural ryanodine congeners on the calcium release channel of sarcoplasmic reticulum: evidence for separation of effects at functionally distinct sites. Mol Pharmacol. 1993;44:412–421. [PubMed] [Google Scholar]
- Hurne AM, O'Brien JJ, Wingrove D, Cherednichenko G, Allen PD, Beam KG, et al. Ryanodine receptor type 1 (RyR1) mutations C4958S and C4961S reveal excitation-coupled calcium entry (ECCE) is independent of sarcoplasmic reticulum store depletion. J Biol Chem. 2005;280:36994–37004. doi: 10.1074/jbc.M506441200. [DOI] [PubMed] [Google Scholar]
- Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol. 1997;122:21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol (Cell Physiol) 2001;280:C22–C33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Mignery GA, Zhu MX, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell. 1999;4:423–429. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Shin DM, Shcheynikov N, Kurosaki T, Muallem S. Regulation of Ca2+-release-activated Ca2+ current (Icrac) by ryanodine receptors in inositol 1,4,5-trisphosphate-receptor-deficient DT40 cells. Biochem J. 2001;360:17–22. doi: 10.1042/0264-6021:3600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, et al. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56:439–513. doi: 10.1124/pr.56.4.1. [DOI] [PubMed] [Google Scholar]
- Lattanzio FA, Jr, Schlatterer RG, Nicar M, Campbell KP, Sutko JL. The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem. 1987;262:2711–2718. [PubMed] [Google Scholar]
- Lievremont JP, Bird GS, Putney JW., Jr Mechanism of inhibition of TRPC cation channels by 2-aminoethoxydiphenylborane. Mol Pharmacol. 2005;68:758–762. doi: 10.1124/mol.105.012856. [DOI] [PubMed] [Google Scholar]
- Lin M-J, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, et al. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed hTRPC1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- Ma HT, van Patterson L, Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Herve P, Eddahibi S, Adnot S. 5-Hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y, Nakanishi Y, Walsh EJ, Wilson DP, Welsh DG, Cole WC. Heteromultimeric TRPC6–TRPC7 channels contribute to arginine vasopressin-induced cation current of A7r5 vascular smooth muscle cells. Circ Res. 2006;98:1520–1527. doi: 10.1161/01.RES.0000226495.34949.28. [DOI] [PubMed] [Google Scholar]
- McGoon MD, Vlietstra RE. Vasodilator therapy for primary pulmonary hypertension. Mayo Clin Proc. 1984;59:672–677. doi: 10.1016/s0025-6196(12)62055-2. [DOI] [PubMed] [Google Scholar]
- McKay RR, Szymeczek-Seay CL, Lievremont JP, Bird GS, Zitt C, Jungling E, et al. Cloning and expression of the human transient receptor potential 4 (TRP4) gene: localization and functional expression of human TRP4 and TRP3. Biochem J. 2000;351:735–746. [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, et al. Large store-operated calcium-selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L, Callewaert G, De Smedt H, Parys JB. 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium. 2001;29:111–116. doi: 10.1054/ceca.2000.0163. [DOI] [PubMed] [Google Scholar]
- Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563:409–419. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenylborate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol AV, Eisner DA, Wray S. The role of the sarcoplasmic reticulum as a Ca2+ sink in rat uterine smooth muscle cells. J Physiol. 1999;520:153–163. doi: 10.1111/j.1469-7793.1999.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Leno E, Csutora P, Trepakova E, Bolotina VM. Ca2+-independent phospholipase A2 is a novel determinant of store-operated Ca2+ entry. J Biol Chem. 2003;278:11909–11915. doi: 10.1074/jbc.M210878200. [DOI] [PubMed] [Google Scholar]
- Smith GD, Lee RJ, Oliver JM, Keizer J. Effect of Ca2+ influx on intracellular free Ca2+ responses in antigen-stimulated RBL-2H3 cells. Am J Physiol (Cell Physiol) 1996;270:C939–C952. doi: 10.1152/ajpcell.1996.270.3.C939. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta TA, Feng W, Molinski TF, Pessah IN. Hydroxylated xestospongins block IP3-induced Ca2+ release and sensitize Ca2+-induced Ca2+ release mediated by ryanodine receptors. Mol Pharmacol. 2005;69:532–538. doi: 10.1124/mol.105.019125. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Csutora P, Hunton DL, Marchase RB, Cohen RA, Bolotina VM. Calcium influx factor (CIF) directly activates store-operated cation channels in vascular smooth muscle cells. J Biol Chem. 2000;275:26158–26163. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Bird GS, Mori Y, Putney JW., Jr Native TRPC7 channel activation by an inositol trisphosphate receptor-dependent mechanism. J Biol Chem. 2006;281:25250–25258. doi: 10.1074/jbc.M604994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol (Cell Physiol) 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol (Lung Cell Mol Physiol) 2005;288:L1059–L1069. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- Waurick R, Knapp J, Van Aken H, Boknik P, Neumann J, Schmitz W. Effect of 2,3-butanedione monoxime on force of contraction and protein phosphorylation in bovine smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:484–492. doi: 10.1007/pl00005380. [DOI] [PubMed] [Google Scholar]
- Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol (Lung Cell Mol Physiol) 2005;289:L5–L13. doi: 10.1152/ajplung.00044.2005. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Mason HS, Ng LC, Montague S, Johnston L, Nicholson N, et al. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol. 2005;144:252–264. doi: 10.1038/sj.bjp.0706077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Mason HS, Smith GD, Nicholson N, Johnston L, Janiak R, et al. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol. 2002;543:917–931. doi: 10.1113/jphysiol.2002.021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kamimura N, Takeo T, Suga S, Wakui M, Maruyama T, et al. 2-Aminoethoxydiphenylborate modulates kinetics of intracellular Ca2+ signals mediated by inositol 1,4,5-trisphosphate-sensitive Ca2+ stores in single pancreatic acinar cells of mouse. Mol Pharmacol. 2000;58:1368–1374. doi: 10.1124/mol.58.6.1368. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, et al. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]