Abstract
Background and purpose:
Controversy still exists as to whether or not inhaled β 2-adrenoceptor agonists and corticosteroids act synergistically in vivo. In this study, we have used a murine model of lung inflammation to study the synergistic effect of an inhaled β 2-adrenoceptor agonists (formoterol) and an inhaled corticosteroid (mometasone).
Experimental approach:
Actively sensitized mice were challenged with aerosolized ovalbumin, once a day, for three consecutive days. Three days after the last of the three challenges, a final allergen challenge was given. Allergen-induced increase in Penh was measured 4 h after the last challenge. A day after the last challenge, increased airway sensitivity to aerosolized methacholine was demonstrated and this was concomitant with an influx of inflammatory cells in the bronchoalveolar lavage fluids.
Key results:
Mometasone (0.1 to 3 mg kg−1) given intranasally either an hour before or after the last allergen challenge, dose-dependently inhibited all parameters. When given intranasally either one or three hours after the last allergen challenge, but not an hour before, formoterol (1.5 to 150 μg kg−1) also dose-dependently inhibited most of the parameters to different degree. A synergistic effect on the allergen-induced increase in Penh was demonstrated for mometasone and formoterol given in combination, an hour after the challenge, at the following doses: mometasone/formoterol (in μg kg−1) 1/10, 1/100, 5/10, and 5/100.
Conclusions and implications:
Our results support the hypothesis that when given as a fixed combination, inhaled corticosteroid and β 2-adrenoceptor agonist act synergistically in vivo.
Keywords: asthma, corticosteroids, β2-adrenoceptor agonists, combination
Introduction
Asthma is characterized by chronic inflammation of the airway with variable airflow limitation resulting in recurrent wheezing, chest tightness and cough. Inhaled β2-adrenoceptor agonists and inhaled corticosteroids are the most effective therapies available for asthma management. Activation of the transmembrane β2-adrenoceptor leads to an increase in intracellular cAMP, which in turn promotes relaxation of the airway smooth muscle cells. On the other hand, upon activation, the intracellular corticosteroid receptor can either downregulate pro-inflammatory gene transcription or upregulate anti-inflammatory genes. Therefore inhaled β2 agonists act as bronchodilator agents whereas inhaled corticosteroids downregulate the inflammatory reaction within the lungs of asthmatic patients.
Many clinical studies have demonstrated that the use of fixed-dose combinations provides better asthma control than increasing the dose of steroid alone. This has led to the recent introduction of fixed-dose combination inhalers containing both a β2-adrenoceptor agonist and a corticosteroid (Nelson, 2001; Kuna and Kuprys, 2002). Recent in vitro evidence suggests that in addition to their complementary beneficial effects (that is bronchial relaxation and anti-inflammatory activities), these two classes of drugs might have several positive interactions that could be of clinical relevance and lead to a synergistic effect when given as a fixed-dose combination to the patients (Nelson et al., 2003). Although a positive interaction between these two classes of drugs has been demonstrated in a model of acetaldehyde-induced airway responses in the anaesthetized guinea-pig (Rossoni et al., 2005), evidence for a true synergistic effect in conscious animals using a more relevant model of asthma has not yet been demonstrated. Hence, it is still a matter of debate as to whether the beneficial effect of a fixed-dose combination in asthma is due to an additive or a synergistic effect (Lipworth and Fardon, 2004; Metcalfe and Moodie, 2004). In this study, we have used a previously described allergen-driven model of pulmonary inflammation in mice (Cieslewicz et al., 1999; Bonneau et al., 2006) to study the potential synergistic effect of an inhaled β2-adrenoceptor agonists (formoterol) and an inhaled corticosteroid (mometasone), using the algebraic method developed by Berenbaum (1977). Our results support the hypothesis that, when given as a fixed combination, an inhaled corticosteroid and β2-adrenoceptor agonist act synergistically in vivo.
Methods
Animals
Female BALB/c mice (11 weeks old, Charles River, UK) were housed in plastic cages in an air-conditioned room at 24°C in a 12 h light–dark cycle. All animals were acclimatized in the animal unit for at least 7 days before the start of any experimental work. Food and water were available ad libitum. The studies described here were carried out in the UK and conformed to the United Kingdom Animal (scientific procedures) Act 1986. A total of 340 animals were used for the study.
Sensitization and challenge
Mice were immunized i.p. with 20 μg of ovalbumin (OA) in 0.1 ml of Alum (Serva, Heidelberg, Germany) on days 0 and 14. On days 21–23, animals were exposed, for 20 min, to an aerosol of OA (10 mg ml−1) in phosphate-buffered saline (PBS), to establish the inflammatory process within the lung, or PBS alone as a control. On day 26, a final challenge was given as an aerosol solution of OA in PBS (50 mg ml−1) or PBS alone for 20 min (Bonneau et al., 2006).
Lung function measurements
All lung function measurements were done using whole body plethysmography, using enhanced pause (Penh) as a read out, in conscious animals and expressed as area under the curve measured for 5 min (Bonneau et al., 2006).
On day 26, before the last allergen challenge, baseline Penh measurements were taken for each animal and again 4 h after the last challenge. Airway reactivity to aerosolized methacholine (0–0.6 M) was measured, in a cumulative fashion, 1 day after the last challenge.
Bronchoalveolar lavage
Two days after the last allergen challenge and following the measurement of allergen-induced increase in Penh and airway reactivity to methacholine, the animals were killed by an i.p. injection of 60 mg kg−1 pentobarbitone and bronchoalveolar lavage (BAL) was performed, by injecting four times 0.3 ml of PBS into the airway lumen, for determination of inflammatory cells numbers (Bonneau et al., 2006).
Drug treatment
Formoterol, mometasone or their combination were given, intranasally, as a solution in 50 μl of PBS containing 2% N-methyl pyronidole (Bonneau et al., 2006).
Data analysis
Results are expressed as means±s.e.mean. Statistical comparisons were performed using a Mann–Whitney test with Bonferroni correction for multiple comparisons and a P-value of less than 0.05 was considered significant (Systat V.10.2).
For comparison of the airway reactivity to aerosolized methacholine between groups, a sigmoidal curve was fitted to the dose–response data and used to calculate the concentration of methacholine that produced a 200% increase (PC200) above baseline of the Penh value (Origin V. 7.0).
The possible synergistic interaction between the two compounds was analysed by the algebraic method developed by Berenbaum (1977). On the basis of experimental data, the following coefficient was calculated: M/Me+F/Fe. M and F are the dose of mometasone and formoterol, given in combination that achieve a given quantitative effect. Me and Fe are the dose of mometasone and formoterol, given alone, that produce the same quantitative effect (equi-effective dose). A coefficient of 1 would indicate an additive effect, less than 1 a synergistic effect and greater than 1 an antagonistic effect, for the two compounds.
Drugs
Formoterol and mometasone were synthesized at the Research Department of Novartis Pharma. All other reagents were purchased from Sigma-Aldrich (Poole, UK) unless specified otherwise.
Results
Three control groups were used in the following studies: a negative control group refers to sensitized animals challenged with PBS on days 21–23 and 26 (PBS/PBS). A baseline control group refers to sensitized animals challenged with OA on days 21–23 and challenged with PBS on day 26 (PBS/OA). A positive control group refers to sensitized animals challenged with OA on days 21–23 and 26 (OA/OA).
Effect of mometasone given an hour before the last allergen challenge
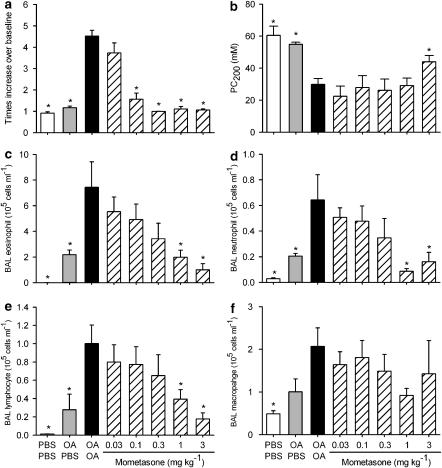
When compared with the PBS/PBS or the OA/PBS group, the OA/OA group developed an increase in Penh 4 h after the last challenge (Figure 1a). The allergen-induced increase in Penh in the OA/OA group was dose-dependently inhibited by mometasone, given an hour before the last allergen challenge, and a full inhibition was observed at a dose of 0.3 mg kg−1 (Figure 1a). When compared with the PBS/PBS and the OA/PBS groups, the OA/OA group was hypersensitive to aerosolized methacholine as demonstrated by a significantly decreased PC200 value in the latter group (Figure 1b). Mometasone inhibited the increased airway sensitivity to aerosolized methacholine at the highest dose tested, 3 mg kg−1 (Figure 1b). In the PBS/PBS group, the BAL cells were mainly macrophages with few neutrophils and lymphocytes. In the OA/PBS group, a significant increase in neutrophil, eosinophil and lymphocyte numbers was observed (Figures 1c–e). Eosinophil, neutrophil and lymphocyte numbers were further increased in the OA/OA group and this was dose-dependently inhibited by mometasone, given an hour before the last challenge (Figures 1c–e). When compared with the OA/PBS group, the macrophage numbers were not significantly different in the OA/OA group and mometasone had no effect on this cell type (Figure 1f).
Figure 1.
Effect of mometasone, given an hour before the last allergen challenge, on ovalbumin (OA)-induced, increase in Penh (a), increased airway sensitivity to aerosolized methacholine (b) and influx of bronchoalveolar lavage (BAL) eosinophils (c), neutrophils (d), lymphocytes (e) and macrophages (f). Actively sensitized animals were challenged with aerosolized OA or its vehicle PBS on days 21–23 after the first sensitization. On day 26, mice were intranasally treated with mometasone and 1 h later either challenged with an aerosolized solution of OA (OA/OA) or phosphate-buffered saline (PBS; OA/PBS). As a control, a group of sensitized animals was challenged with PBS on days 21–23 and 26 (PBS/PBS) and treated with vehicle. Results are expressed as means±s.e.mean from one experiment with 7–8 animals per group. Statistical comparisons were performed using a Mann–Whitney test with Bonferroni correction for multiple comparisons, *P<0.05 when compared with the OA/OA group.
Effect of formoterol
In a first experiment, formoterol (1.5–150 μg kg−1) was given, intranasally, an hour before the last OA challenge. Under these conditions, the drug had no or only a minimal effect on the allergen-induced increase in Penh and BAL inflammatory cell influx. In contrast, a significant decrease in the PC200 values was observed at a dose of 150 μg kg−1 (Table 1).
Table 1.
Effect of formoterol, given intranasally an hour before the allergen challenge, on allergen-induced increase in Penh, airway space cellular infiltration and increased airway sensitivity to aerosolized methacholine
| Allergen-induced increase in Penh (times increase) | BAL eosinophils (105 cells ml−1) | BAL neutrophils (105 cells ml−1) | BAL macrophages (105 cells ml−1) | BAL lymphocytes (105 cells ml−1) | PC200 (mM) | |
|---|---|---|---|---|---|---|
| PBS/PBS | 0.94±0.02* | 0.01±0.01* | 0.01±0.01* | 0.58±0.09* | 0.01±0.01* | 89.2±6.4* |
| OA/PBS | 0.98±0.03* | 3.09±0.47* | 0.08±0.04* | 2.28±0.71* | 0.86±0.09* | 63.3±9.4* |
| OA/OA | 2.70±0.21 | 9.57±0.58 | 0.60±0.16 | 3.17±0.42 | 2.01±0.22 | 42.5±10.1 |
| Formoterol | ||||||
| 1.5 μg kg−1 | 2.50±0.25 | 6.90±1.07 | 1.55±0.14* | 2.52±0.25 | 1.42±0.18 | 50.1±14.3 |
| 5 μg kg−1 | 3.32±0.56 | 7.66±1.15 | 1.62±0.45* | 2.21±0.25 | 1.29±0.18* | 44.3±12.9 |
| 15 μg kg−1 | 3.11±0.50 | 7.32±1.67 | 0.78±0.15 | 3.27±0.63 | 1.24±0.5* | 48.4±16.2 |
| 50 μg kg−1 | 2.38±0.41 | 6.43±0.54* | 0.53±0.10 | 1.54±0.20* | 1.11±0.24* | 24.5±4.2 |
| 150 μg kg−1 | 3.67±0.30 | 6.31±0.56* | 0.39±0.07 | 1.83±0.17* | 1.34±0.14* | 14.2±3.1* |
Abbreviations: BAL, bronchoalveolar lavage; OA, ovalbumin; PBS, phosphate-buffered saline; PC200, concentration of methacholine that produced a 200% increase above baseline of the Penh value.
Actively sensitized animals were challenged with aerosolized OA or its vehicle PBS on days 21–23 after the first sensitization. On day 26, mice were either challenged with an aerosolized solution of OA (OA/OA) or PBS (OA/PBS). As a control, a group of sensitized animals was challenged with PBS on days 21–23 and 26 (PBS/PBS). Results are expressed as means±s.e.mean from one experiment with 7–8 animals per group. Statistical comparisons were performed using a Mann–Whitney test with Bonferroni correction for multiple comparisons.
P<0.05 when compared with the OA/OA group.
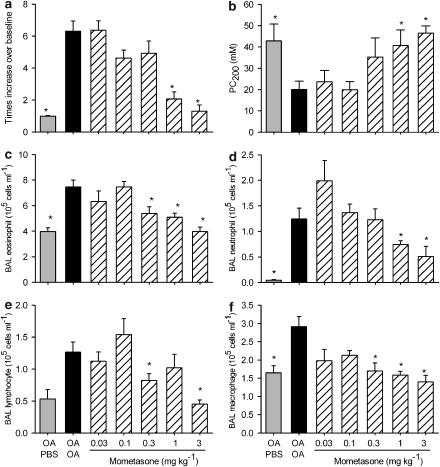
In contrast to the pre-allergen challenge treatment regime, when given either 1 or 3 h after the last allergen challenge, formoterol dose-dependently inhibited the allergen-induced increase in Penh measured 4 h after the challenge (Figure 2a). The efficacy of the drug was similar whether it was given 1 or 3 h after the challenge, with a maximal inhibition of 55% (Figure 2a). One day after the last OA challenge, the animals from the OA/OA group were hypersensitive to aerosolized methacholine when compared with the OA/PBS group, and a significant inhibition was only evident for the highest dose of formoterol (150 μg kg−1) in the animals treated 3 h after the challenge (Figure 2b).
Figure 2.
Effect of formoterol, given 1 or 3 h after the last allergen challenge, on ovalbumin (OA)-induced, increase in Penh (a), increased airway sensitivity to aerosolized methacholine (b) and influx of bronchoalveolar lavage (BAL) eosinophils (c), neutrophils (d), lymphocytes (e) and macrophages (f). Actively sensitized animals were challenged with aerosolized OA or its vehicle phosphate-buffered saline (PBS) on days 21–23 after the first sensitization. On day 26, mice were either challenged with an aerosolized solution of OA (OA/OA) or PBS (OA/PBS) and were intranasally treated with formoterol 1 h (horizontally hatched columns) or 3 h (diagonally hatched columns) later. Results are expressed as means±s.e.mean from one experiment with 7–8 animals per group. Statistical comparisons were performed using a Mann–Whitney test with Bonferroni correction for multiple comparisons, *P<0.05 when compared with the OA/OA group.
When compared with the OA/PBS group, the animals in the OA/OA group had a significant increase in the number of eosinophils, neutrophils and lymphocytes and a significant inhibition was observed only when formoterol was given 3 h after the challenge (Figures 2c–e). Although macrophage numbers were not significantly increased by the OA challenge, formoterol, when given 3 h after the challenge but not if given 1 h after, did significantly inhibit the number of this cell type (Figure 2f).
Effect of mometasone given an hour after the allergen challenge
As formoterol was shown to be inactive when given before the last OA challenge, and since both compounds had to be given at the same time in the combination experiment, it was necessary to establish the dose–response data for mometasone when given after the allergen challenge. Mometasone, given an hour after the last allergen challenge, dose-dependently inhibited the allergen-induced increase in Penh with about a 10-fold loss of potency when compared with the pre-challenge treatment schedule (Figure 3a). The increased airway sensitivity to aerosolized methacholine (Figure 3b) and the inflammatory cell influx (Figures 3c–f) was dose-dependently inhibited with a similar potency, when compared with the pre-challenge treatment schedule.
Figure 3.
Effect of mometasone, given 1 h after the last allergen challenge, on ovalbumin (OA)-induced, increase in Penh (a), increased airway sensitivity to aerosolized methacholine (b) and influx of bronchoalveolar lavage (BAL) eosinophils (c), neutrophils (d), lymphocytes (e) and macrophages (f). Actively sensitized animals were challenged with aerosolized OA or its vehicle phosphate-buffered saline (PBS) on days 21–23 after the first sensitization. On day 26, mice were either challenged with an aerosolized solution of OA (OA/OA) or PBS (OA/PBS) and were intranasally treated with mometasone or its vehicle 1 h later. Results are expressed as means±s.e.mean from one experiment with 7–8 animals per group. Statistical comparisons were performed using a Mann–Whitney test with Bonferroni correction for multiple comparisons, *P<0.05 when compared with the OA/OA group.
Effect of the combination of mometasone and formoterol given an hour after the last allergen challenge
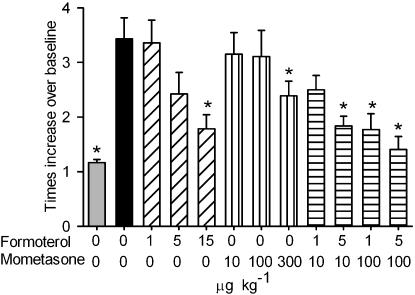
To study a possible synergistic effect of mometasone and formoterol in this model, animals were either treated with three doses of each of the drugs alone (formoterol: 1, 5 and 15 μg kg−1; mometasone: 10, 100 and 300 μg kg−1) or with four different combinations (formoterol/mometasone in μg kg−1: 1/10, 5/10, 1/100 and 5/100). As formoterol was shown to be inactive when given before the last OA challenge, and since both compounds had to be given at the same time, all animals were treated an hour after the last allergen challenge.
Both formoterol and mometasone dose-dependently inhibited the allergen-induced increase in Penh. A significant, but incomplete inhibition was observed at doses of 15 and 300 μg kg−1 for formoterol and mometasone, respectively. All combinations, apart from 1 μg kg−1 formoterol together with 10 μg kg−1 mometasone, significantly inhibited the allergen-induced increase in Penh (Figure 4). Berenbaum's analysis demonstrated a synergistic effect for all four combinations tested when compared with the single entities with a coefficient of 0.23, 0.45, 0.24 and 0.32 for the formoterol/mometasone combinations of 1/10, 5/10, 1/100 and 5/100 μg kg−1, respectively. Formoterol, up to the highest dose tested, 15 μg kg−1, had no effect on the OA-induced increased airway sensitivity to aerosolized methacholine. In contrast, mometasone did fully inhibit this response at a dose of 300 μg kg−1. None of the combinations tested had a significant effect on the increased airway sensitivity to aerosolized methacholine (Table 2). All the inflammatory cell types were increased after the OA challenge, but only eosinophils were inhibited by the highest dose of mometasone (300 μg kg−1) and neutrophils by the combination of formoterol 5 μg kg−1 together with mometasone 100 μg kg−1 (Table 2). Since neither formoterol nor mometasone, at the doses used, had a significant effect on the increased airway sensitivity to aerosolized methacholine or the inflammatory cell influx, Berenbaum's analysis could not be applied.
Figure 4.
Effect of formoterol, mometasone or their combination, given 1 h after the last allergen challenge, on ovalbumin (OA)-induced, increase in Penh. Actively sensitized animals were challenged with aerosolized OA or its vehicle phosphate-buffered saline (PBS) on days 21–23 after the first sensitization. On day 26, mice were either challenged with an aerosolized solution of OA (OA/OA) or PBS (OA/PBS), and were intranasally treated with formoterol, mometasone or their combinations mometasone or its vehicle 1 h later. Results are expressed as means±s.e.mean from one experiment with 7–8 animals per group. Statistical comparisons were performed using a Mann–Whitney test with Bonferroni correction for multiple comparisons, *P<0.05 when compared with the OA/OA group.
Table 2.
Effects of formoterol, mometasone and their combinations, given an hour after the allergen challenge, on increased airway sensitivity to aerosolized methacholine and airway space cellular infiltration
| PC200 (mM) | Eosinophils (105 cells ml−1) | Neutrophils (103 cells ml−1) | Lymphocytes (105 cells ml−1) | Macrophages (105 cells ml−1) | |
|---|---|---|---|---|---|
| OA/PBS | 39.4±7.4* | 4.7±0.7* | 5.5±2.3* | 0.7±0.2* | 2.0±0.3* |
| OA/OA | 22.1±4.6 | 10.0±0.8 | 29.7±7.5 | 1.6±0.2 | 4.9±0.5 |
| Formoterol (μg kg−1) | |||||
| 1 | 23.6±4.9 | 11.2±1.4 | 18.1±4.4 | 1.2±0.2 | 3.5±0.4 |
| 5 | 23.8±2.2 | 10.3±1.9 | 13.7±3.6 | 1.9±0.3 | 3.0±0.6 |
| 15 | 27.3±3.1 | 12.3±1.5 | 46.4±14.9 | 2.1±0.4 | 4.4±0.9 |
| Mometasone (μg kg−1) | |||||
| 10 | 30.1±7.1 | 9.3±0.8 | 46.1±7.2 | 1.7±0.2 | 5.1±0.5 |
| 100 | 33.7±4.1 | 9.9±1.8 | 42.2±13.1 | 1.6±0.5 | 4.1±1.0 |
| 300 | 41.2±6.7* | 5.7±0.8* | 35.5±6.3 | 1.2±0.2 | 3.6±0.8 |
| Formoterol/mometasone (μg kg−1) | |||||
| 1/10 | 33.1±3.3 | 7.2±1.2 | 19.9±4.3 | 1.2±0.3 | 3.2±0.6 |
| 5/10 | 29.6±3.2 | 8.8±1.0 | 16.6±5.0 | 1.2±0.3 | 3.6±0.5 |
| 1/100 | 33.1±4.0 | 7.9±1.8 | 15.0±5.0 | 1.8±0.6 | 3.4±0.6 |
| 5/100 | 33.0±7.4 | 9.7±1.5 | 6.5±3.1* | 1.5±0.4 | 3.1±0.8 |
Abbreviations: OA, ovalbumin; PBS, phosphate-buffered saline; PC200, concentration of methacholine that produced a 200% increase above baseline of the Penh value.
Actively sensitized animals were challenged with aerosolized ovalbumin or its vehicle PBS on days 21–23 after the first sensitization. On day 26, mice were either challenged with an aerosolized solution of ovalbumin (OA/OA) or PBS (OA/PBS). Animals were intranasally treated with formoterol, mometasone or their combination. Results are expressed as means±s.e.mean from one experiment with 7–8 animals per group. Statistical comparisons were performed using a Mann–Whitney test with Bonferroni correction for multiple comparisons.
P<0.05 when compared with the OA/OA group.
Discussion
In this study, we have shown that formoterol and mometasone, two clinically used drugs, act synergistically to inhibit the allergen-induced increase in Penh observed in allergen-sensitized and challenged mice.
Because we wanted to measure all parameters (lung function and airway inflammation) within the same animal, the lung function measurements were done using unrestrained barometric whole body plethysmography and expressed as Penh value. The use of Penh to assess lung function is controversial (Adler et al., 2004; Schwarze et al., 2005) and we recognized that Penh is not a substitute for established parameters of lung mechanics. However, a good correlation between airway resistance and Penh has been demonstrated in BALB/c mice, the strain used in our study (Adler et al., 2004). Moreover, it was previously demonstrated that the airway response to allergen challenge in the present model was equivalent whether it was measured using Penh in conscious mice or airway resistance in anaesthetized animals (Cieslewicz et al., 1999).
There is growing evidence in vitro that β2-adrenoceptor agonists and corticosteroids have complementary and synergistic effects. In both primary lung fibroblasts (Eickelberg et al., 1999) and primary bronchial smooth muscle cells (Roth et al., 2002) from humans, β2-adrenoceptor agonists have been shown to induce ligand-independent activation of the corticosteroid receptor. Importantly, the combination of low doses of β2-adrenoceptor agonists and corticosteroids resulted in a synchronized activation of the corticosteroid receptor suggesting a synergistic effect of the two drugs in inhibiting cellular proliferation (Roth et al., 2002). This synchronized activation of the corticosteroid receptor by β2-adrenoceptor agonists has recently been confirmed in healthy volunteers (controls) and patients with mild asthma, where combination therapy with low dose of inhaled corticosteroid and inhaled β2-adrenoceptor agonist augmented the activation of the corticosteroid receptor when compared with the single entities (Usmani et al., 2005). On the other hand, corticosteroids also have positive effects on the β2-adrenoceptor. As such, β2-adrenoceptor transcription is increased by corticosteroid treatment in human lung tissue in vitro (Mak et al., 1995) or in human nasal mucosa in vivo (Baraniuk et al., 1997). A positive interaction between corticosteroids and β2-adrenoceptor agonists has also been demonstrated on the inhibition of the release of cytokines from human airway smooth muscle (Pang and Knox, 2000) and airway epithelial cells (Korn et al., 2001). All these studies showed positive interactions between steroids and the β2-adrenoceptor, but none of them clearly demonstrated a synergistic effect between these two classes of compound.
Although our data clearly show a synergistic effect of formoterol and mometasone in vivo, the precise cell type and/or inflammatory events affected by the two drugs is not clear. Both corticosteroids (Nocker et al., 1999) and β2-adrenoceptor agonists (Greiff et al., 1998; Proud et al., 1998) are known to inhibit plasma leakage, and, therefore, one can hypothesize that in our model, the increase in Penh is driven by an increased vascular leakage and that the synergistic effect seen with formoterol and mometasone is linked to their anti-plasma leakage properties. We did not assess plasma leakage in the present study, but a previous study has shown that following allergen challenge, mice only develop an early plasma exudation (within 15 min post challenge) and that no late exudation phase can be observed, even following repeated allergen challenges (Erjefalt et al., 1998). Since we measured the increase in Penh at 4 h after the allergen challenge, it is unlikely to be linked to plasma exudation.
In our model and in line with its clinical profile, formoterol, given after the allergen challenge, has only a marginal inhibitory effect on the eosinophil influx and inhibits the allergen-induced airway increased airway sensitivity to aerosolized methacholine. This is the reason why the synergistic effect of the mometasone/formoterol combination could not be assessed on these two parameters. Indeed, to study synergy using the method described by Berenbaum (1977), both components should be able to inhibit the parameter studied when given on their own. Nevertheless, our data suggest a positive interaction between the two drugs on the allergen-induced airway neutrophilia, since both drugs were inactive when given on their own and a significant inhibition was observed with a combination of 5 μg kg−1 formoterol and 100 μg kg−1 mometasone. Knowing that steroids are not very effective in promoting the resolution of neutrophilic inflammation, this observation could have significant clinical implications for patients with severe asthma where neutrophil is thought to be the dominant inflammatory cell (Kamath et al., 2005).
It is noteworthy that when given as a single entity an hour before the allergen challenge, formoterol had a deleterious effect on the airway reactivity to aerosolized methacholine. A possible explanation for this phenomenon could be related to the bronchodilating property of this class of compound that would facilitate the penetration of the antigen within the small airways, thereby, enhancing the airway reactivity to aerosolized methacholine. In support of this interpretation is the fact that when given either 1 or 3 h after the allergen challenge such a deleterious effect is not seen with formoterol.
In summary, our results demonstrate that coadministration of formoterol and mometasone, in a murine model of allergen-induced lung inflammation, acts synergistically when compared to the single administration of each drug. This observation supports the concept that when given as a fixed-dose combination to asthmatic patients, these drugs act synergistically.
Abbreviations
- BAL
bronchoalveolar lavage
- OA
ovalbumin
- PBS
phosphate-buffered saline
- PC200
concentration of methacholine that produced a 200% increase above baseline of the Penh value
Conflict of interest
All authors are permanent employees of Novartis Pharma. Novartis Pharma, together with Schering-Plough, is developing a fixed-dose combination inhaler containing formoterol and mometasone.
References
- Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol. 2004;97:286–292. doi: 10.1152/japplphysiol.00821.2003. [DOI] [PubMed] [Google Scholar]
- Baraniuk JN, Ali M, Brody D, Maniscalco J, Gaumond E, Fitzgerald T, et al. Glucocorticoids induce beta2-adrenergic receptor function in human nasal mucosa. Am J Respir Crit Care Med. 1997;155:704–710. doi: 10.1164/ajrccm.155.2.9032216. [DOI] [PubMed] [Google Scholar]
- Berenbaum MC. Synergy, additivism and antagonism in immunosuppression. A critical review. Clin Exp Immunol. 1977;28:1–18. [PMC free article] [PubMed] [Google Scholar]
- Bonneau O, Wyss D, Ferretti S, Blaydon C, Stevenson CS, Trifilieff A. Effect of adenosine A2A receptor activation in murine models of respiratory disorders. Am J Physiol. 2006;290:L1036–L1043. doi: 10.1152/ajplung.00422.2005. [DOI] [PubMed] [Google Scholar]
- Cieslewicz G, Tomkinson A, Adler A, Duez C, Schwarze J, Takeda K, et al. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest. 1999;104:301–308. doi: 10.1172/JCI7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M, et al. Ligand-independent activation of the glucocorticoid receptor by beta 2-adrenergic receptor agonists in primary human LUNG Fibroblasts and Vascular Smooth Muscle Cells. J Biol Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Erjefalt JS, Andersson P, Gustafsson B, Korsgren M, Sonmark B, Persson CG. Allergen challenge-induced extravasation of plasma in mouse airways. Clin Exp Allergy. 1998;28:1013–1020. doi: 10.1046/j.1365-2222.1998.00372.x. [DOI] [PubMed] [Google Scholar]
- Greiff L, Wollmer P, Andersson M, Svensson C, Persson CG. Effects of formoterol on histamine induced plasma exudation in induced sputum from normal subjects. Thorax. 1998;53:1010–1013. doi: 10.1136/thx.53.12.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AV, Pavord ID, Ruparelia PR, Chilvers ER. Is the neutrophil the key effector cell in severe asthma. Thorax. 2005;60:529–530. doi: 10.1136/thx.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn SH, Jerre A, Brattsand R. Effects of formoterol and budesonide on GM-CSF and IL-8 secretion by triggered human bronchial epithelial cells. Eur Respir J. 2001;17:1070–1077. doi: 10.1183/09031936.01.00073301. [DOI] [PubMed] [Google Scholar]
- Kuna P, Kuprys I. Symbicort Turbuhaler: a new concept in asthma management. Int J Clin Pract. 2002;56:797–803. [PubMed] [Google Scholar]
- Lipworth BJ, Fardon TC. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. J Allergy Clin Immunol. 2004;113:178–179. doi: 10.1016/j.jaci.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Mak JC, Nishikawa M, Barnes PJ. Glucocorticosteroids increase beta 2-adrenergic receptor transcription in human lung. Am J Physiol. 1995;268:L41–L46. doi: 10.1152/ajplung.1995.268.1.L41. [DOI] [PubMed] [Google Scholar]
- Metcalfe S, Moodie P. Seretide meta-analysis missed important features and overstates any advantages over concurrent LABA/ICS devices. J Allergy Clin Immunol. 2004;113:568–569. doi: 10.1016/j.jaci.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Nelson HS. Advair: combination treatment with fluticasone propionate/salmeterol in the treatment of asthma. J Allergy Clin Immunol. 2001;107:398–416. doi: 10.1067/mai.2001.112939. [DOI] [PubMed] [Google Scholar]
- Nelson HS, Chapman KR, Pyke SD, Johnson M, Pritchard JN. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. J Allergy Clin Immunol. 2003;112:29–36. doi: 10.1067/mai.2003.1558. [DOI] [PubMed] [Google Scholar]
- Nocker RE, Weller FR, Out TA, de Riemer MJ, Jansen HM, van der Zee JS. A double-blind study on the effect of inhaled corticosteroids on plasma protein exudation in asthma. Am J Respir Crit Care Med. 1999;159:1499–1505. doi: 10.1164/ajrccm.159.5.9806116. [DOI] [PubMed] [Google Scholar]
- Pang L, Knox AJ. Synergistic inhibition by beta(2)-agonists and corticosteroids on tumor necrosis factor-alpha-induced interleukin-8 release from cultured human airway smooth-muscle cells. Am J Respir Cell Mol Biol. 2000;23:79–85. doi: 10.1165/ajrcmb.23.1.3985. [DOI] [PubMed] [Google Scholar]
- Proud D, Reynolds CJ, Lichtenstein LM, Kagey-Sobotka A, Togias A. Intranasal salmeterol inhibits allergen-induced vascular permeability but not mast cell activation or cellular infiltration. Clin Exp Allergy. 1998;28:868–875. doi: 10.1046/j.1365-2222.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, Razzetti R, Civelli M, Bongrani S, Berti F. Positive interaction of the beta2-agonist CHF 4226.01 with budesonide in the control of bronchoconstriction induced by acetaldehyde in the guinea-pigs. Br J Pharmacol. 2005;144:422–429. doi: 10.1038/sj.bjp.0706096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Johnson PRA, Rudiger JJ, King GG, Ge Q, Burgess JK, et al. Interaction between glucocorticoids and [beta]2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–1299. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- Schwarze J, Hamelmann E, Gelfand EW. Barometric whole body plethysmography in mice. J Appl Physiol. 2005;98:1955–1957. doi: 10.1152/japplphysiol.01279.2004. [DOI] [PubMed] [Google Scholar]
- Usmani OS, Ito K, Maneechotesuwan K, Ito M, Johnson M, Barnes PJ, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172:704–712. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]