Abstract
Background and purpose:
ARL 67156, 6-N,N-Diethyl-D-β-γ-dibromomethylene adenosine triphosphate, originally named FPL 67156, is the only commercially available inhibitor of ecto-ATPases. Since the first report on this molecule, various ectonucleotidases responsible for the hydrolysis of ATP at the cell surface have been cloned and characterized. In this work, we identified the ectonucleotidases inhibited by ARL 67156.
Experimental approach:
The effect of ARL 67156 on recombinant NTPDase1, 2, 3 & 8 (mouse and human), NPP1, NPP3 and ecto-5′-nucleotidase (human) have been evaluated. The inhibition of the activity of NTPDases (using the following substrates: ATP, ADP, UTP), NPPs (pnp-TMP, Ap3A) and ecto-5′-nucleotidase (AMP) was measured by colorimetric or HPLC assays.
Key results:
ARL 67156 was a weak competitive inhibitor of human NTPDase1, NTPDase3 and NPP1 with Ki of 11±3, 18±4 and 12±3 μM, respectively. At concentrations used in the literature (50–100 μM), ARL 67156 partially but significantly inhibited the mouse and human forms of these enzymes. NTPDase2, NTPDase8, NPP3 and ecto-5′-nucleotidase activities were less affected. Importantly, ARL 67156 was not hydrolysed by either human NTPDase1, 2, 3, 8, NPP1 or NPP3.
Conclusions and implications:
In cell environments where NTPDase1, NTPDase3, NPP1 or mouse NTPDase8 are present, ARL 67156 would prolong the effect of endogenously released ATP on P2 receptors. However, it does not block any ectonucleotidases efficiently when high concentrations of substrates are present, such as in biochemical, pharmacological or P2X7 assays. In addition, ARL 67156 is not an effective inhibitor of NTPDase2, human NTPDase8, NPP3 and ecto-5′-nucleotidase.
Keywords: ecto-ATPase, NTPDase, CD39, NPP, Ecto-5′-nucleotidase, extracellular nucleotide, ARL 67156, FPL 67156
Introduction
Extracellular nucleotides such as ATP, ADP, UTP and UDP play various biological functions by activating P2X1–7, P2Y1,2,4,6,11–14 (Burnstock, 2006), cysLT1R, cysLT2R or GPR17 receptors. The latter three are G protein-coupled receptors recently reported to respond to uracil nucleotides in addition to cysteinyl leukotrienes (Ciana et al., 2006; von Kugelgen, 2006). The study of nucleotide receptors and their functions is complicated by the presence at the cell surface of many enzymes, called ectonucleotidases, that rapidly break down nucleotides into nucleosides (Zimmermann, 2000). One nucleoside in particular, adenosine, can, in turn, act as a signalling molecule via the activation of four ubiquitous receptors (A1, A2a, A2b and A3) (Jacobson and Gao, 2006). Therefore, an effect thought to be due to ATP may, in fact, involve its hydrolysis product, adenosine.
In the endeavour to develop specific tools to investigate this complex signalling system, Fisons Laboratories (now AstraZeneca, Loughborough, UK) produced a nucleotide analogue 6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate (ARL 67156). This molecule was originally named FPL 67156 or AR-c67156 (see Figure 1) and is now known as ARL 67156. It was described in 1995 by Crack et al. (1995) as a selective inhibitor of ecto-ATPase activity from blood cells. Since this original report, ARL 67156 was shown to inhibit ecto-ATPase activity in various tissues from different species: smooth muscle membranes of mouse, rat, rabbit and guinea-pig vas deferens (Khakh et al., 1995; Westfall et al., 2000b; Ghildyal and Manchanda, 2004), rat superior cervical ganglia (Connolly et al., 1998), bovine chromaffin cells (Drakulich et al., 2004) and rat parotid acinar cells (Dowd et al., 1999). However, it did not block ATPase activity from guinea-pig hearts (Erga et al., 2000) nor from rat nodose ganglia (Connolly et al., 1998). In agreement with the inhibition of ATP hydrolysis, ARL 67156 was shown to potentiate the contraction evoked by exogenous ATP in guinea-pig isolated vas deferens, in urinary bladder (Westfall et al., 1996, 1997a) and in rabbit ear artery (Crack et al., 1995) as well as the responses to endogenously released ATP induced by noradrenaline, KCl, acetylcholine or histamine (Westfall et al., 1996, 1997a). In accord with these observations, ARL 67156 failed to enhance responses to the non-hydrolysable analogue α-β-MeATP (Crack et al., 1995; Westfall et al., 1996). ARL 67156 was also reported as an effective inhibitor of UTP breakdown by superior cervical ganglion cells (Connolly and Duley, 2000) and to potentiate contractions elicited by this nucleotide in isolated tail artery of rat (McLaren et al., 1998).
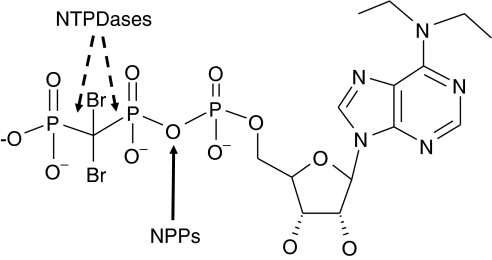
Figure 1.
Molecular structure of ARL 67156. The arrow indicates the potential cleavage site between the α- and β-phosphates of ARL 67156 by NPPs. The site of cleavage by NTPDases between the β- and γ-phosphate is blocked by −CBr2− and can therefore not be hydrolysed by these enzymes (Picher et al., 1996) as confirmed by HPLC (see Results). ARL 67156, 6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate; NPP, nucleotide pyrophosphatase/phosphodiesterase; NTPDases, nucleoside triphosphate diphosphohydrolases; HPLC, high-performance liquid chromatography.
In the past decade following the original publication on ARL 67156, several enzymes responsible for the hydrolysis of nucleotides have been identified, cloned and characterized (Robson et al., 2006). Among these nucleotidases, four members of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family, namely NTPDase1, NTPDase2, NTPDase3 and NTPDase8, and two members of the ecto-nucleotide pyrophosphatases/phosphodiesterases (E-NPP) family, NPP1 and NPP3, are located at the cell surface and hydrolyse extracellular nucleotides and derivatives (Zimmermann, 2000; Kukulski et al., 2005; Stefan et al., 2005; Robson et al., 2006). NTPDases dephosphorylate a variety of nucleoside triphosphates (for example, ATP and UTP) and diphosphates (for example, ADP and UDP) in the presence of divalent cations (Ca2+ or Mg2+), with different specificity and ability. NTPDase1 (also called CD39 or vascular ATPDase) dephosphorylates ATP to AMP, removing one phosphate at a time with a modest appearance of ADP. In contrast, NTPDase2 (CD39L1, ecto-ATPase) hydrolyses ATP to ADP, with minimal AMP accumulation. NTPDase3 (CD39L3, HB6) and NTPDase8 (hepatic ATPDase) are functional intermediates as they convert ATP to AMP with a transient accumulation of ADP (Kukulski et al., 2005). As for NPP1 (PC-1), NPP2 (PD-1α, autotaxin) and NPP3 (CD203c, PD-1β, B10, gp130RB13−6), they release nucleoside 5′-monophosphate from a variety of nucleotides and nucleotide derivatives (Cimpean et al., 2004; Stefan et al., 2006). For example, these enzymes catalyse the hydrolysis of ATP to AMP and pyrophosphate (PPi), diadenosine 5′,5′″-P1,P3-triphosphate (Ap3A) to AMP and ADP, etc. Compared to NPP1 and NPP3, NPP2 is a rather poor phosphodiesterase (Gijsbers et al., 2003) that bears an intrinsic lysophospholipase-D activity (Jansen et al., 2005). In addition, NPP2 was recently shown to be a zymogen (pre-pro-enzyme) proteolytically cleaved and secreted rather than a membrane protein (Jansen et al., 2005). Finally, monophosphonucleosides (for example, AMP) are hydrolysed to nucleosides (for example, adenosine) and Pi by ecto-5′-nucleotidase (CD73), a glycosyl phosphatidylinositol-anchored enzyme located at the cell surface (Sträter, 2006).
In the recent literature, there is a confusion regarding the specificity of ARL 67156, as it was described as a ‘non specific inhibitor of ectonucleotidases' (Farahbakhsh, 2003; He et al., 2005), a ‘non specific ecto-triphosphate nucleotidase [sic] inhibitor' (Farahbakhsh, 2003), an ‘inhibitor of the ecto-NTPDases' (Westfall et al., 2000a), a ‘specific inhibitor of NTPDase1' (Machida et al., 2005), an ‘inhibitor of NTPDase1 and NTPDase2 activities in several tissues' (Farahbakhsh, 2003), or even a ‘selective ecto-5′-nucleotidase inhibitor' (Silva et al., 2006). In fact, the only clues regarding the identity of the enzyme(s) influenced by ARL 67156, so far, are its capacity to block ATP, ADP and UTP hydrolysis (Crack et al., 1995; Connolly and Duley, 2000; Laitinen et al., 2001), three excellent substrates of NTPDases (Kukulski et al., 2005), and a report in 2002 by Sesti et al. (2002) showing that ARL 67156 decreased ATPase and ADPase activity (between 25–37%) of an artificial recombinant soluble form of human NTPDase1 (solCD39) when used at twice the concentration of substrate. Nevertheless, it remains unclear whether ARL 67156 can also affect the native membrane-bound NTPDase1. Indeed, the membrane environment of NTPDases is important for their activity and substrate specificity as they are altered in the presence of detergent or when the transmembrane domains are removed (Wang et al., 1998; Mukasa et al., 2005). Hitherto, the identity of the ectonucleotidases influenced by ARL 67156 remains uncharacterized (Westfall et al., 1997b; Zimmermann, 2000).
In this work, we have investigated the specificity of ARL 67156 on the ectonucleotidases present at the cell surface, namely NTPDase1, 2, 3 and 8, NPP1 and NPP3, and ecto-5′-nucleotidase. We also looked at the capacity of human NTPDases and NPPs to enzymatically cleave the P–C–P bond or the diphosphoester bond between α- and β-phosphates of ARL 67156, respectively (Figure 1).
Methods
Plasmids
The plasmids used in this study have all been described in published reports: human NTPDase1 (GenBank accession no. U87967) (Kaczmarek et al., 1996), human NTPDase2 (NM_203468) (Knowles and Chiang, 2003), human NTPDase3 (AF034840) (Smith and Kirley, 1998), human NTPDase8 (AY430414) (Fausther et al., 2007), mouse NTPDase1 (NM_009848) (Enjyoji et al., 1999), mouse NTPDase2 (AY376711) (Kukulski et al., 2005), mouse NTPDase3 (AY376710) (Lavoie et al., 2004), mouse NTPDase8 (AY364442) (Bigonnesse et al., 2004), human ecto-5′-nucleotidase (DQ186653) (Lecka et al., manuscript in preparation), human NPP1 (NM_006208) (Buckley et al., 1990) and human NPP3 (NM_005021) (Jin-Hua et al., 1997).
Cell transfection and preparation of membrane fraction
COS-7 and HEK 293T cells were transfected in 10 cm plates using Lipofectamine (Invitrogen, Burlington, ON, Canada), as described previously (Kukulski et al., 2005). Briefly, 80–90% confluent cells were incubated for 5 h at 37°C in Dulbecco's modified Eagle's medium, nutriment mix F-12 (DMEM/F-12) in the absence of fetal bovine serum (FBS) with 6 μg of plasmid DNA and 24 μl of Lipofectamine reagent. The reaction was stopped by the addition of an equal volume of DMEM/F-12 containing 20% FBS and the cells were harvested 44–72 h later.
For the preparation of protein extracts, transfected cells were washed three times with Tris-saline buffer at 4°C, collected by scraping in the harvesting buffer (in mM, 95 NaCl, 0.1 phenylmethylsulphonyl fluoride (PMSF) and 45 Tris at pH 7.5), and washed twice by 300 g centrifugation for 10 min at 4°C. Cells were resuspended in the harvesting buffer containing 10 μg ml−1 aprotinin and sonicated. Nucleus and cellular debris were discarded by centrifugation at 300 g for 10 min at 4°C and the supernatant (crude protein extract) was aliquoted and stored at –80°C until used for activity assays. Protein concentration was estimated by the Bradford microplate assay using bovine serum albumin (BSA) as a standard (Bradford, 1976).
Enzymatic assays
NTPDases (EC 3.6.1.5)
Activity was measured as described previously (Kukulski et al., 2005) in 0.2 ml of incubation medium (5 mM CaCl2 and 80 mM Tris, pH 7.4) or Tris-Ringer buffer (in mM, 120 NaCl, 5 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 5 glucose, 80 Tris, pH 7.4) at 37°C with or without ARL 67156 (Sigma-Aldrich, Oakville, ON, Canada (lots #084K4610 and #098H4727) or Tocris, Ellisville, MO, USA (lot #3 and #4)). NTPDase protein extracts were added to the incubation mixture and pre-incubated at 37°C for 3 min. The reaction was initiated by the addition of 10–500 μM ATP, ADP or UTP and stopped after 15 min with 50 μl of malachite green reagent. The released inorganic phosphate (Pi) was measured at 630 nm according to Baykov et al. (1988). The type of inhibition was determined by Dixon and Cornish-Bowden plots of four independent experiments and Ki using nonlinear regression.
For intact cells, activity at the cell surface of transiently transfected cells were carried out in 0.25 ml of incubation medium containing 145 mM NaCl in 24-well plates. The reaction was stopped by sampling an aliquot of 0.2 ml promptly mixed with 50 μl of malachite reagent and Pi determined as indicated above. The activity obtained from cells transfected with a control plasmid was subtracted from the one obtained with NTPDase-transfected cells.
NPPs (EC 3.1.4.1; EC 3.6.1.9)
Evaluation of the effect of ARL on human NPP1 and NPP3 activity was carried out with para-nitrophenyl thymidine 5′-monophosphate (pnp-TMP) and Ap3A as substrates (Belli and Goding, 1994; Vollmayer et al., 2003). The reactions were carried out at 37°C in 0.2 ml of the following incubation mixture, in mM, 1 CaCl2, 140 NaCl, 5 KCl and 50 Tris, pH 8.5, with or without ARL 67156 (100 μM) or ATP (100 μM). Human NPP1 or NPP3 extract was added to the incubation mixture and pre-incubated at 37°C for 3 min. Reaction was initiated by the addition of 100 μM pnp-TMP or Ap3A. For pnp-TMP, the production of para-nitrophenol was measured at 410 nm, 15 min after the initiation of the reaction. For Ap3A, the reaction was stopped after 30 min by transferring an aliquot of 0.1 ml from the reaction mixture to 0.125 ml ice-cold 1 M perchloric acid. The samples were centrifuged for 5 min at 13 000 g. Supernatants were neutralized with 1 M KOH (4°C) and centrifuged for 5 min at 13 000 g. An aliquot of 20 μl was separated by reverse-phase high-performance liquid chromatography (HPLC) to evaluate the nucleotide content of each reaction sample (see below). The type of inhibition and Ki was calculated by plotting the data of three independent experiments using pnp-TMP as substrate according to Dixon and Cornish-Bowden methods.
Ecto-5′-nucleotidase (EC 3.1.3.5)
Activity was measured at 37°C for 15 min in 0.2 ml of incubation solution (in mM, 1 CaCl2, 1 MgCl2, 200 NaCl, 10 KCl and 100 Tris, pH 7.5), with or without ARL 67156. Reaction was started by the addition of 100 μM AMP and stopped with 50 μl malachite green reagent after 15 min. Released inorganic phosphate was measured as described above for NTPDases.
Evaluation of ARL 67156 as a potential substrate for human NTPDases and NPPs
The potential hydrolysis of ARL 67156 and its parent compound β,γ-MeATP by human NTPDase1, 2, 3 and 8, and human NPP1 and NPP3 was evaluated. In this experiment, ATP, ARL 67156 or β,γ-MeATP was incubated with the indicated NTPDase or NPP, in the appropriate reaction medium, as described above under the respective enzyme assays. Reactions were stopped after 60 min by the addition of an equivalent volume of ice-cold CHCl3 and vigorous mixing. The samples were immediately centrifuged for 5 min at 13 000 g, the aqueous phases were collected and aliquots of 20 μl separated by reverse-phase HPLC to evaluate the disappearance of ARL 67156 and appearance of new peaks expected to correspond to hydrolysis products (6-N,N-diethyl-ADP and 6-N,N-diethyl-AMP). HPLC conditions are described below.
Separation and quantification of nucleotides and dinucleotides by HPLC
An aliquot of 20 μl of the reaction products (described above) was used for nucleotide analysis by HPLC using a 15 cm × 4.6 mm, 3 μm SUPELCOSIL LC-18-T column (Supelco, Bellefonte, PA, USA). β,γ-MeATP and its hydrolysis product AMP were separated with a mobile phase composed of 25 mM tetrabutyl ammonium (TBA), 5 mM ethylenediaminetetraacetic acid, 100 mM KH2PO4/K2HPO4, pH 7.0 and 2% methanol (v/v), at a flow rate of 0.75 ml min−1 for the first 11 min and 1.4 ml min−1 for the following 14 min. Separated nucleotides were detected by ultraviolet absorption at 260 nm, identified and quantified by the comparison of the retention time with the appropriate standards. Ap3A was eluted using a step gradient of MeOH in the same mobile phase at a flow rate of 1 ml min−1. The step gradient was carried out for 25 min with 2% MeOH, then 10% MeOH for 15 min, and finally the column was re-equilibrated with 2% MeOH for 15 min before the next run. We measured the potential hydrolysis of ARL 67156 using a mobile phase composed of 8 mM TBA, 100 nM KH2PO4, 25% AcN, pH 6.0 at a flow rate of 1.4 ml min−1, by measuring ARL 67156 decrease and by verifying the appearance of a new peak corresponding to the expected degradation product 6-N,N-diethylAMP, detected by UV absorption at 277 nm. Since this molecule is not available commercially, we evaluated the retention time of 6-N,N-dimethylAMP, kindly provided by Dr Bilha Fischer (Bar-Ilan University, Ramat Gan, Israel), assuming that it would have an elution time close to 6-N,N-diethylAMP. The retention time of 6-N,N-dimethylAMP and ARL 67156 was 6 and 19 min, respectively.
Reagents
ADP, ATP, BSA, KCl, malachite green, para-nitrophenol, pnp-TMP, PMSF, TBA and UTP were purchased from Sigma-Aldrich. Ammonium molybdate, NaCl, Tris and Tween 20 were provided by EMD Chemicals (Gibbstown, NJ, USA). Acetonitrile (AcN), CaCl2 and perchloric acid (HClO4) were acquired from Fischer Scientific (Ottawa, ON, Canada). ARL 67156 was obtained from both Tocris and Sigma-Aldrich (for further details see Enzymatic assays and Results). Chloroform (CHCl3) was from Laboratoire MAT (Québec, QC, Canada).
Results
The ecto-ATPases inhibited by ARL 67156 are expected to be NTPDases and/or NPPs (Westfall et al., 1997b; Vollmayer et al., 2003). NTPDase1, 2, 3 and 8 that are bound to the plasma membrane have been tested while NTPDase4–7, that are mainly associated with intracellular organelles (Kukulski et al., 2005; Robson et al., 2006), have been excluded from this study. In addition, another family of ectonucleotidases, the E-NPPs, was tested. Protein extracts from COS-7 or HEK 293T cells transiently transfected with an expression vector encoding mouse or human recombinant NTPDase1, 2, 3 or 8 or human NPP1 or 3 were used as a source of enzymes.
ARL 67156 is a non-hydrolysable ATP analogue
We first tested whether ARL 67156 could be hydrolysed by ecto-nucleotidases. Theoretically, this molecule is not expected to be hydrolysed by NTPDases, as the phosphodiester bond (P–O–P) present in ATP is substituted by a phosphomethyl bond (P–C–P). However, this resistance to hydrolysis was only verified for bovine NTPDase1 with the parent compound β,γ-MeATP (Picher et al., 1996). To confirm the absence of ARL 67156 hydrolysis by NTPDases, we incubated 500 μM of ARL 67156 in parallel with the parent compound β,γ-MeATP, and with ATP, a substrate of NTPDase1, 2, 3 and 8, with large amount of transfected HEK 293T cell lysate (12 μg) for 60 min. The resulting samples were separated and analysed using reverse-phase HPLC. In these conditions, ATP was hydrolysed by over 85% by human NTPDase1, 2, 3 and 8 (100, 92, 97, 85%, respectively), but no significant decrease in ARL 67156 nor in β,γ-MeATP concentration could be detected. In addition, no new peaks corresponding to potential hydrolysis product could be observed (data not shown).
In contrast, as NPP1 and NPP3 generate nucleoside 5′-monophosphate from a variety of dinucleotides, nucleotides and nucleotide derivatives (Goding, 2000; Stefan et al., 2006), it could therefore be expected that these enzymes would hydrolyse ARL 67156 to 6-N,N-diethyl-adenosine 5′-monophosphate and (PO3)CBr2(PO3), as depicted in Figure 1. Consequently, we evaluated by HPLC the hydrolysis of ARL 67156 in parallel with the parent compound β,γ-MeATP as a control. In these conditions, no conversion of ARL 67156 could be detected by either recombinant human NPP1 or NPP3, even after incubating 100 μM of ARL 67156 in the presence of large amount of transfected HEK 293T cell lysate (25 μg) for 2 h, while 16–23% of the parent compound β,γ-MeATP was hydrolysed to AMP by both NPPs.
It is noteworthy to mention that in our preliminary experiments, when we used the usual HClO4 precipitation followed by KOH neutralization, the peak corresponding to ARL 67156 on HPLC profiles was completely replaced by two unknown peaks with lower retention times, suggesting a degradation of ARL 67156 under acidic condition.
Effect of ARL 67156 on ecto-NTPDases
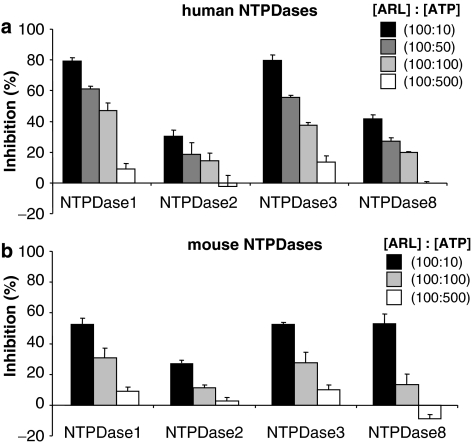
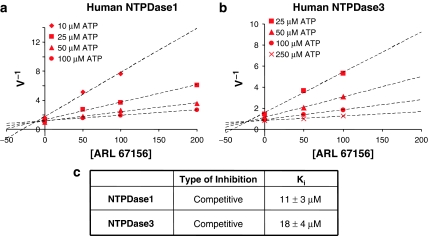
The inhibition of ATP hydrolysis by NTPDases was evaluated in two different buffers. In Tris-Ca2+ buffer, at the concentration generally used in the literature (100 μM), ARL 67156 was not able to block the hydrolysis of 500 μM ATP by any of the human recombinant NTPDases (Figure 2a). However, at a higher ARL 67156/ATP ratio (100 μM/10 μM), an efficient inhibition of human NTPDase1 and 3 was observed, while NTPDase2 and 8 were less affected (Figure 2a). Similar results were obtained in Ringer buffer for human NTPDases (data not shown). Figure 3 shows that both human NTPDase1 and 3 were inhibited in a competitive manner with Ki of 11±3 and 18±4 μM, respectively, as determined by Dixon and Cornish-Bowden representations (data not shown for the latter) and nonlinear regression. Note that no differences were seen with different batches of ARL 67156 purchased from Sigma or Tocris (see Methods for details) when using human NTPDase1 protein extracts (data not shown).
Figure 2.
Effect of ARL 67156 on human and mouse NTPDases. ATPase activity of protein extracts from HEK 293T or COS-7 cells transfected with human or mouse NTPDase1, 2, 3 or 8 was tested with or without ARL 67156. The concentration of ARL 67156 was set at 100 μM and the concentration of ATP ranged from 10 to 500 μM, as indicated. Reaction was carried out for 15 min in the presence of 5 mM CaCl2 and 80 mM Tris, pH 7.4. In each of these assays, less than 10% of the substrate was hydrolysed. The modest increases in activity of human NTPDase2 and mouse NTPDase8 at 500 μM ATP are not statistically different from the controls. (a) Human NTPDases. (b) Mouse NTPDases. The means±s.e.m. of 3–10 experiments, each performed in triplicate, are shown. ARL 67156, 6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate; NTPDases, nucleoside triphosphate diphosphohydrolases.
Figure 3.
Determination of the kinetic parameters for the inhibition of human NTPDase1 and NTPDase3 by ARL 67156. (a and b) Dixon plot of a representative experiment (of four) is shown with ATP concentration ranging from 10 to 100 μM for human NTPDase1 (a) or 25–250 μM for human NTPDase3 (b). In both panels, ARL 67156 concentration was 0, 50, 100 or 200 μM, as indicated. (c) Inhibition type and the Ki (mean±s.e.m.) obtained from four independent experiments, each performed in triplicate, are indicated. NTPDases, nucleoside triphosphate diphosphohydrolases; ARL 67156, 6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate.
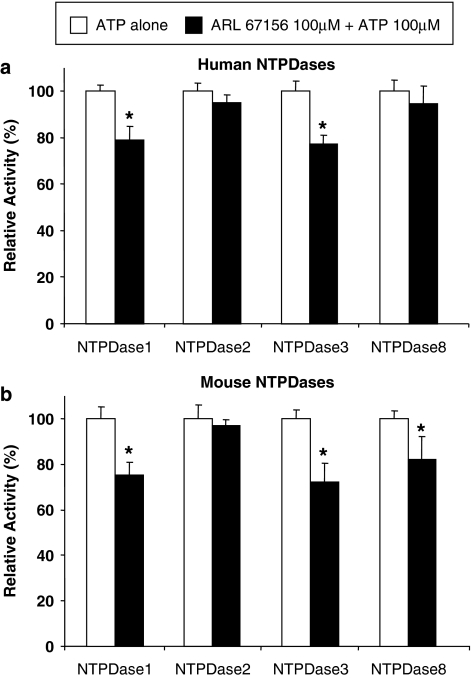
Importantly, when used at the same concentration, ARL 67156 blocked the hydrolysis of UTP by both human NTPDase1 and 3 more efficiently than the hydrolysis of ATP, while the inhibition of ADP hydrolysis was in between (Table 1). Mouse NTPDases were similarly inhibited, although to a lesser extent compared to their human counterpart. At 100 μM ARL and 10 μM ATP, a maximal inhibition of approximately 50% for NTPDase1 and 3 was observed (Figure 2b) compared to ∼80% for their human orthologs (Figure 2a). We also confirmed that ARL 67156 (100 μM) decreased ATP hydrolysis (100 μM) similarly on intact COS-7 cells transfected with plasmid encoding these enzymes. Figure 4 shows that NTPDase1 and 3 were both significantly inhibited by ARL 67156 for both species, although slightly less than with protein extracts (Figure 2).
Table 1.
Comparative effect of ARL 67156 on the biochemical activity of human NTPDases
| Enzyme | % of inhibition (100 μM substrate and 100 μM ARL 67156) |
||
|---|---|---|---|
| ATP (n=4–9) | ADP (n=3) | UTP (n=3) | |
| NTPDase1 | 48±5 | 70±4 | 89±2 |
| NTPDase2 | 15±5 | 1±6 | 25±6 |
| NTPDase3 | 42±5 | 69±3 | 73±3 |
| NTPDase8 | 20.1±0.4 | 21±4 | 38±1 |
Abbreviations: ARL 67156, 6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate; NTPDase, nucleoside triphosphate diphosphohydrolase.
Figure 4.
Effect of ARL 67156 on ATP hydrolysis by NTPDases expressing cells. Activity of intact COS-7 cells transfected with NTPDase1, 2, 3 or 8 was measured in 24-well plates. Cells were preincubated 3 min with or without 100 μM ARL 67156, then the reaction was started with 100 μM ATP. (a) Human NTPDases. (b) Mouse NTPDases. The means ±s.e.m. of four independent experiments, each performed in triplicate, are shown; a star (*) indicates significant differences as evaluated by Student's t-test analysis (P-values <0.05). ARL 67156, 6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate; NTPDases, nucleoside triphosphate diphosphohydrolases.
Note that only minor inhibition for either human or mouse NTPDase2 was observed even in the presence of ten-fold excess of ARL 67156 compared to ATP (100 μM vs 10 μM; Figure 2). Human NTPDase8 was hardly affected (Figures 2a and 4a). A modest but significant inhibition of mouse NTPDase8 could be seen with concentrations equal to that of ATP, with both protein extract and intact cells (Figures 2b and 4b). The inhibition of mouse NTPDase8 was more obvious at a ten-fold excess of ARL 67156 with the protein extract (Figure 4b). As the inhibition of mouse and human NTPDase8 was slightly different, we also tested the hydrolysis of ADP in the presence of ARL 67156 for these two enzymes. Interestingly, 100 μM ARL 67156 blocked hydrolysis of ADP (100 μM) by mouse NTPDase8 by 51±1%, while ATP hydrolysis was inhibited by only 14±7% (data not shown). No significant differences between inhibition of ATP and ADP hydrolysis were observed with the human ortholog (Table 1).
Effect of ARL 67156 on NPP1 and NPP3 activity
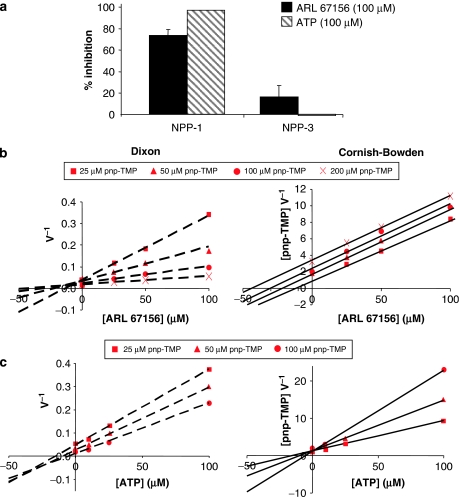
Indirect evidence suggested that NPPs could also be affected by ARL 67156: (1) ARL 67156 potentiated the contraction evoked by Ap4A in guinea-pig vas deferens (Westfall et al., 1997b) and NPPs hydrolyse ApnA (Luthje and Ogilvie, 1985; Vollmayer et al., 2003), and (2) Farahbakhsh showed that pre-treatment with pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonate (PPADS) and ARL 67156 had similar effect on nonpigmented epithelium Ca2+ mobilization in response to UTP and proposed that NPP1 was the enzyme affected (Farahbakhsh, 2003) since PPADS was shown to block NPP1 (Grobben et al., 2000). Considering that both NPP1 and NPP3 could hydrolyse ATP to AMP+PPi, we investigated whether ARL 67156 could inhibit these enzymes. However, the ATPase activity measured in transfected HEK 293T or COS-7 cell extracts was only 25% higher than in controls (transfected with inactive NPP3 plasmid or untransfected cell extracts). Therefore, to determine whether ARL 67156 inhibits ecto-NPP activity, and since ATP was poorly hydrolysed, we compared the capacity of ARL 67156 and ATP, both at 100 μM, to block the hydrolysis of the nucleotide analogue pnp-TMP (100 μM) by human NPP1 and NPP3. Human NPP1 activity was decreased by 73±5% in the presence of ARL 67156, and by 94±4% with ATP, while human NPP3 remained unaffected (Figure 5a). Further kinetic analysis of human NPP1 using Dixon and Cornish-Bowden representations showed a competitive inhibition for ARL 67156 with a Ki of 12±3 μM, and a mixed type of inhibition for ATP (Figures 5b and c). ARL 67156 could also inhibit NPP1 hydrolysis of the more physiological substrate Ap3A, showing about 40% inhibition in the presence of ARL 67156. Again, NPP3 activity was unaffected by ARL 67156 (data not shown).
Figure 5.
Effect of ARL 67156 and ATP on pnp-TMP hydrolysis by human NPP1 and NPP3. (a) Cell lysates from HEK 293T cells transfected with human NPP1 or NPP3 were preincubated with or without 100 μM ATP or 100 μM ARL 67156 and the reaction started by the addition of 100 μM pnp-TMP. The activity was evaluated by measuring the absorbance of pnp produced at 410 nm. The average ±s.e.m. of three independent experiments, each performed in triplicate, is shown. (b and c) Dixon and Cornish-Bowden plots of a representative experiment out of three is presented for both ARL 67156 (b) and ATP (c) inhibition of pnp-TMP hydrolysis by human NPP1. Concentrations used for pnp-TMP ranged from 25 to 200 μM and ARL 67156 or ATP concentrations from 0 to 100 μM for panels b and c, respectively. ARL 67156, 6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate; pnp-TMP, para-nitrophenyl thymidine 5′-monophosphate.
Effect of ARL 67156 on ecto-5′-nucleotidase
Finally, we addressed the possibility that ARL 67156, being an analogue of ATP, could influence the activity of ecto-5′-nucleotidase. Indeed, nucleotides like ADP and ATP in micromolar concentration as well as α,β-MeADP in nanomolar concentration are highly effective competitive inhibitors of this enzyme (Sträter, 2006). At the same concentration as the substrate, 100 μM, ARL 67156 inhibited human ecto-5′-nucleotidase by 28±3%, while ATP and ADP were more effective inhibitors of AMP hydrolysis giving an inhibition of 40 and 70%, respectively (data not shown).
Discussion
In the present work, we showed that among the ectonucleotidases that break down extracellular nucleotides involved in P2 receptor signalling, ARL 67156 was a weak competitive inhibitor of NTPDase1 (CD39), NTPDase3 and NPP1, with Ki of 11, 18 and 12 μM, respectively (Figures 3 and 5). ARL 67156 was not an effective inhibitor of NTPDase2, NPP3 and ecto-5′-nucleotidase (CD73), although it could also reduce the activity of these enzymes. ARL 67156 affected human and mouse NTPDase8 differently. While it inhibited ADP hydrolysis by mouse NTPDase8 more effectively than ATP hydrolysis (data not shown), it was a poor inhibitor of human NTPDase8. Previous reports showed a pIC50 of 4.62 (∼24 μM) for the ecto-ATPase activity of human blood cells (Crack et al., 1995) and 5.1 (∼8 μM) for rat vas deferens (Khakh et al., 1995). In a recent report using a capillary electrophoresis method, Iqbal et al. (2005) showed the effect of ARL 67156 on three rat NTPDases. NTPDase1 and 3 were inhibited with a Ki of 27 and 112 μM, while NTPDase2 was inefficiently blocked by ARL 67156 (Ki>1 mM). Although the Ki values measured in the latter study are slightly higher than the one evaluated here for the human orthologs, the inhibition of rat NTPDase1, 2 and 3 by ARL 67156 is in agreement with the data presented here on the human and mouse orthologs. These minor differences may be due to the species, the assay conditions and/or the method used for Ki determination. In this paper, Iqbal et al. focus their study on the description of a new technique for the rapid versatile and automated screening of ectonucleotidase inhibitors.
These data suggest that ARL 67156 would delay nucleotide hydrolysis in tissues where NTPDase1, NTPDase3 or NPP1 are present but would exert a minor effect in tissues expressing NTPDase2, NPP3 or ecto-5′-nucleotidase. In the case of NTPDase8, ARL 67156 would be expected to slow down nucleotide hydrolysis in mouse, but not in human, tissues. Therefore, in tissues where ARL 67156 was described to block ATP hydrolysis such as vas deferens, superior cervical ganglia, chromaffin cell, urinary bladder, ear and tail arteries, and parotid acinar cells (Crack et al., 1995; Khakh et al., 1995; Westfall et al., 1996, 1997a, 2000b; Connolly et al., 1998; McLaren et al., 1998; Dowd et al., 1999; Drakulich et al., 2004; Ghildyal and Manchanda, 2004), NTPDase1, NTPDase3 and/or NPP1 would be expected to play a pivotal role in extracellular nucleotide hydrolysis. Indeed the inhibition of ATP hydrolysis in ear and tail arteries by ARL 67156 could be explained by NTPDase1 as it is the major ectonucleotidase expressed by the vascular endothelium (Kaczmarek et al., 1996; Marcus et al., 1997; Enjyoji et al., 1999). In other tissues with high ectonucleotidase activities and where ARL 67156 had no effect, NTPDase2, NPP3, or potentially NTPDase8 depending of the species, would be expected to be more important as for example in heart and nodose ganglia (Connolly et al., 1998; Erga et al., 2000). Hence, the lack of inhibition by ARL 67156 of the activity from guinea-pig hearts (Erga et al., 2000) could be explained by NTPDase2 that is highly expressed in this tissue (Kegel et al., 1997; Sévigny et al., 2002). The complete correlation between ARL 67156 inhibition and the identity of the ectonucleotidases involved in a given tissue will be possible when the complete localization of these enzymes has been accomplished; this is already thoroughly investigated with specific antibodies in our laboratory and others (for more information see the recent review of Robson et al. (2006)). Finally, ARL 67156 had a modest effect on ecto-5′-nucleotidase and would thereby not be expected to interfere with AMP hydrolysis directly. However, ARL 67156 could delay the formation of adenosine indirectly by decreasing both the formation of ecto-5′-nucleotidase substrate (AMP) and the breakdown of its potent inhibitors (ATP and ADP) as a consequence of NTPDase1 and NTPDase3 inhibition.
The data presented here also suggest that ARL 67156 would not be efficient in blocking any ectonucleotidase in experiments where high concentrations of ATP are involved, as in the activation of P2X7 receptors. This would happen even in the presence of NTPDase1, NTPDase3 and/or NPP1, as millimolar concentration of ARL 67156 would still be expected to be insufficient to inhibit these enzymes. Furthermore, high concentrations of ARL 67156 were reported to affect P2U (P2Y2 or P2Y4), P2T (mainly P2Y12) and P2X (most probably P2X1 (Benham and Tsien, 1987) but possibly other P2X) receptors from rabbit ear artery with a pA2 of 3.3 or less (⩾500 μM) (Crack et al., 1995). A more recent study showed that at a concentration 300-fold higher than the one needed to block ecto-ATPase activity, ARL 67156 affected P2Y receptors on bovine chromaffin cells (Drakulich et al., 2004). An effect on other P2 receptors among the 15 P2 receptors (P2Y1,2,4,6,11–14 and P2X1–7) (Burnstock, 2006) and the three cysteinyl leukotrienes/uracil nucleotides G protein-coupled receptors (Ciana et al., 2006; von Kugelgen, 2006) can also not be excluded as there are no studies reporting the effect of ARL 67156 on all known nucleotide receptors.
An important characteristic of ARL 67156, though, is its high stability towards the action of several ectonucleotidases compared to ATP. ARL 67156 differs from ATP by two modifications, (1) the phosphodiester bond (P–O–P) between the β- and γ-phosphates is substituted by a phosphodibromomethyl bond (P–CBr2–P) and (2) two ethyl groups are added to the primary amine in position 6 of the adenine ring (Figure 1). The phosphate chain modification theoretically confers resistance to hydrolysis by NTPDases. In agreement with these assumptions, in conditions where over 85% of ATP was hydrolysed, we did not observe any hydrolysis of ARL 67156 or of the parent compound β,γ-MeATP by human NTPDase1, 2, 3 and 8. Alternatively, NPP1 and NPP3 could theoretically hydrolyse this compound since they cleave their substrates after the α-phosphate (that is, ATP → AMP+PPi), which remains unaltered in ARL 67156. In our hands, we could not see any hydrolysis of ARL 67156 even when it was incubated with large amount of human NPP1 or NPP3. Since in these conditions, the parent compound β,γ-MeATP was efficiently hydrolysed to AMP, this suggest that either the modification on the adenine ring or the two bromides on the carbon between the terminal phosphates adjacent to the cleavage site would be responsible for this resistance to NPP hydrolysis. Nevertheless, the higher chemical stability compared to ATP and the inability of both NTPDases and NPPs to hydrolyse ARL 67156 suggests that it can remain available for a long period of time, although it was unstable in highly acidic conditions, as suggested by the disappearance of ARL 67156 peak in HPLC after 0.5 N HClO4 treatment of the samples.
Interestingly, when we investigated the capacity of NPP1 and NPP3 to hydrolyse ATP, we measured only a low appearance of AMP and pyrophosphate (PPi). These observations combined with the fact that other reports on NPPs used only more sensitive techniques, that is radiolabelled ATP or the synthetic substrate pnp-TMP, suggest that the breakdown of ATP by NPP1–3 (Cimpean et al., 2004) would be less important than the one by NTPDases, at least when using the recombinant enzymes.
In conclusion, we showed that ARL 67156 is a weak competitive inhibitor of NTPDase1, NTPDase3 and NPP1, and is not an effective inhibitor of NTPDase2, NPP3 and ecto-5′-nucleotidase. In addition, ARL 67156 is also a weak inhibitor of mouse NTPDase8, especially on ADPase activity, but not of human NTPDase8. On the one hand, our results suggest that in cell environment at the concentration usually used (50–100 μM), ARL 67156 would prolong the effect of ATP on P2 receptors if NTPDase1, NTPDase3 or NPP1 are the dominant ectonucleotidases in the system investigated. On the other hand, our biochemical data suggest that in assays where large concentrations of exogenous nucleotides are used, or on cells expressing NTPDase2 or NPP3, ARL 67156 would be unable to block ATP hydrolysis. Hence, ARL 67156 can be used with some precaution. Highly effective broad range and specific inhibitors of NTPDases and NPPs still need to be developed to allow the functional study of these enzymes and of P2 receptor signalling in tissues expressing ectonucleotidases.
Acknowledgments
We thank Dr AF Knowles, Dr TL Kirley, Dr JW Goding and Dr K Sano for providing plasmids encoding human NTPDase2, NTPDase3, NPP1 and NPP3, respectively. A special thank to Dr M Singh Rana for the cloning of human ecto-5′-nucleotidase (manuscript in preparation). We also thank Dr B Fischer and I Kogan for the kind gift of the 6,N,N-dimethyl AMP molecule. This work was supported by grants from the Canadian Institutes of Health Research (CIHR), and The Arthritis Society of Canada (TAS01/0078). SAL was the recipient of a scholarship from the ‘Fonds de la Recherche en Santé du Québec' (FRSQ), EGL of both ‘Fonds de Recherche sur l'Arthrite et les Maladies Rhumatismales de l'Université Laval' (FRAMR) and FRSQ, and JS of a New Investigator award from the CIHR.
Abbreviations
- Ap3A
diadenosine 5′,5′″-P1,P3-triphosphate
- ARL 67156 (FPL 67156)
6-N,N-diethyl-D-β-γ-dibromomethylene adenosine triphosphate
- E-NPP
ecto-nucleotide pyrophosphatase/phosphodiesterase
- E-NTPDase
ecto-nucleoside triphosphate diphosphohydrolase
- pnp-TMP
para-nitrophenyl thymidine 5′-monophosphate
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonate
Conflict of interest
The authors state no conflict of interest.
References
- Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- Belli SI, Goding JW. Biochemical characterization of human PC-1, an enzyme possessing alkaline phosphodiesterase I and nucleotide pyrophosphatase activities. Eur J Biochem. 1994;226:433–443. doi: 10.1111/j.1432-1033.1994.tb20068.x. [DOI] [PubMed] [Google Scholar]
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bigonnesse F, Levesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, et al. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buckley MF, Loveland KA, McKinstry WJ, Garson OM, Goding JW. Plasma cell membrane glycoprotein PC-1. cDNA cloning of the human molecule, amino acid sequence, and chromosomal location. J Biol Chem. 1990;265:17506–17511. [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147 Suppl 1:S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimpean A, Stefan C, Gijsbers R, Stalmans W, Bollen M. Substrate-specifying determinants of the nucleotide pyrophosphatases/phosphodiesterases NPP1 and NPP2. Biochem J. 2004;381:71–77. doi: 10.1042/BJ20040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly GP, Demaine C, Duley JA. Ecto-nucleotidases in isolated intact rat vagi, nodose ganglia, and superior cervical ganglia. Adv Exp Med Biol. 1998;431:769–776. doi: 10.1007/978-1-4615-5381-6_147. [DOI] [PubMed] [Google Scholar]
- Connolly GP, Duley JA. Ecto-nucleotidase of cultured rat superior cervical ganglia: dipyridamole is a novel inhibitor. Eur J Pharmacol. 2000;397:271–277. doi: 10.1016/s0014-2999(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Crack BE, Pollard CE, Beukers MW, Roberts SM, Hunt SF, Ingall AH, et al. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br J Pharmacol. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd FJ, Li LS, Zeng W. Inhibition of rat parotid ecto-ATPase activity. Arch Oral Biol. 1999;44:1055–1062. doi: 10.1016/s0003-9969(99)00100-4. [DOI] [PubMed] [Google Scholar]
- Drakulich DA, Spellmon C, Hexum TD. Effect of the ecto-ATPase inhibitor, ARL 67156, on the bovine chromaffin cell response to ATP. Eur J Pharmacol. 2004;485:137–140. doi: 10.1016/j.ejphar.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, II, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- Erga KS, Seubert CN, Liang HX, Wu L, Shryock JC, Belardinelli L. Role of A(2A)-adenosine receptor activation for ATP-mediated coronary vasodilation in guinea-pig isolated heart. Br J Pharmacol. 2000;130:1065–1075. doi: 10.1038/sj.bjp.0703386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahbakhsh NA. Ectonucleotidases of the rabbit ciliary body nonpigmented epithelium. Invest Ophthalmol Vis Sci. 2003;44:3952–3960. doi: 10.1167/iovs.02-1213. [DOI] [PubMed] [Google Scholar]
- Fausther M, Lecka J, Kukulski F, Lévesque SA, Pelletier J, Zimmermann H, et al. Cloning, purification and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastroenterol Liver Physiol. 2007;292:G785–G795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildyal P, Manchanda R. Effects of cooling and ARL 67156 on synaptic ecto-ATPase activity in guinea pig and mouse vas deferens. Auton Neurosci. 2004;115:28–34. doi: 10.1016/j.autneu.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Gijsbers R, Aoki J, Arai H, Bollen M. The hydrolysis of lysophospholipids and nucleotides by autotaxin (NPP2) involves a single catalytic site. FEBS Lett. 2003;538:60–64. doi: 10.1016/s0014-5793(03)00133-9. [DOI] [PubMed] [Google Scholar]
- Goding JW. Ecto-enzymes: physiology meets pathology. J Leukoc Biol. 2000;67:285–311. doi: 10.1002/jlb.67.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobben B, Claes P, Roymans D, Esmans EL, Van Onckelen H, Slegers H. Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br J Pharmacol. 2000;130:139–145. doi: 10.1038/sj.bjp.0703289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells. Purinergic Signal. 2005;1:135–144. doi: 10.1007/s11302-005-6208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Vollmayer P, Braun N, Zimmermann H, Müller CE. A capillary electrophoresis method for the characterization of ecto-nucleoside triphosphate diphosphohydrolase (NTPDases) and the analysis of inhibition by in capillary enzymatic microreaction. Purinergic Signal. 2005;1:349–358. doi: 10.1007/s11302-005-8076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Stefan C, Creemers JW, Waelkens E, Van Eynde A, Stalmans W, et al. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J Cell Sci. 2005;118:3081–3089. doi: 10.1242/jcs.02438. [DOI] [PubMed] [Google Scholar]
- Jin-Hua P, Goding JW, Nakamura H, Sano K. Molecular cloning and chromosomal localization of PD-Ibeta (PDNP3), a new member of the human phosphodiesterase I genes. Genomics. 1997;45:412–415. doi: 10.1006/geno.1997.4949. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E, Koziak K, Sévigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Kegel B, Braun N, Heine P, Maliszewski CR, Zimmermann H. An ecto-ATPase and an ecto-ATP diphosphohydrolase are expressed in rat brain. Neuropharmacology. 1997;36:1189–1200. doi: 10.1016/s0028-3908(97)00115-9. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Michel AD, Humphrey PPA. Inhibition of ecto-ATPase and Ca-ATPase in rat vas deferens by P2 purinoceptor antagonists. Br J Pharmacol. 1995;115:2P. [Google Scholar]
- Knowles AF, Chiang WC. Enzymatic and transcriptional regulation of human ecto-ATPase/E-NTPDase 2. Arch Biochem Biophys. 2003;418:217–227. doi: 10.1016/j.abb.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Kukulski F, Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, et al. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen JT, Uri A, Raidaru G, Miettinen R. [(35)S]GTPgammaS autoradiography reveals a wide distribution of G(i/o)-linked ADP receptors in the nervous system: close similarities with the platelet P2Y(ADP) receptor. J Neurochem. 2001;77:505–518. doi: 10.1046/j.1471-4159.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- Lavoie EG, Kukulski F, Lévesque SA, Lecka J, Sévigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-3. Biochem Pharmacol. 2004;67:1917–1926. doi: 10.1016/j.bcp.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Luthje J, Ogilvie A. Catabolism of Ap3A and Ap4A in human plasma. Purification and characterization of a glycoprotein complex with 5′-nucleotide phosphodiesterase activity. Eur J Biochem. 1985;149:119–127. doi: 10.1111/j.1432-1033.1985.tb08901.x. [DOI] [PubMed] [Google Scholar]
- Machida T, Heerdt PM, Reid AC, Schafer U, Silver RB, Broekman MJ, et al. Ectonucleoside triphosphate diphosphohydrolase 1/CD39, localized in neurons of human and porcine heart, modulates ATP-induced norepinephrine exocytosis. J Pharmacol Exp Ther. 2005;313:570–577. doi: 10.1124/jpet.104.081240. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, et al. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren GJ, Burke KS, Buchanan KJ, Sneddon P, Kennedy C. Evidence that ATP acts at two sites to evoke contraction in the rat isolated tail artery. Br J Pharmacol. 1998;124:5–12. doi: 10.1038/sj.bjp.0701772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa T, Lee Y, Knowles AF. Either the carboxyl- or the amino-terminal region of the human ecto-ATPase (E-NTPDase 2) confers detergent and temperature sensitivity to the chicken ecto-ATP-diphosphohydrolase (E-NTPDase 8) Biochemistry. 2005;44:11160–11170. doi: 10.1021/bi050019k. [DOI] [PubMed] [Google Scholar]
- Picher M, Sévigny J, D'Orleans-Juste P, Beaudoin AR. Hydrolysis of P2-purinoceptor agonists by a purified ectonucleotidase from the bovine aorta, the ATP-diphosphohydrolase. Biochem Pharmacol. 1996;51:1453–1460. doi: 10.1016/0006-2952(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti C, Broekman MJ, Drosopoulos JH, Islam N, Marcus AJ, Levi R. EctoNucleotidase in cardiac sympathetic nerve endings modulates ATP-mediated feedback of norepinephrine release. J Pharmacol Exp Ther. 2002;300:605–611. doi: 10.1124/jpet.300.2.605. [DOI] [PubMed] [Google Scholar]
- Sévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, et al. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- Silva G, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension. 2006;47:563–567. doi: 10.1161/01.HYP.0000197954.93874.ef. [DOI] [PubMed] [Google Scholar]
- Smith TM, Kirley TL. Cloning, sequencing, and expression of a human brain ecto-apyrase related to both the ecto-ATPases and CD39 ecto-apyrases1. Biochim Biophys Acta. 1998;1386:65–78. doi: 10.1016/s0167-4838(98)00063-6. [DOI] [PubMed] [Google Scholar]
- Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal. 2006;2:361–370. doi: 10.1007/s11302-005-5303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sträter N. Ecto-5′-nucleotidase: structure function relationships. Purinergic Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayer P, Clair T, Goding JW, Sano K, Servos J, Zimmermann H. Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. Eur J Biochem. 2003;270:2971–2978. doi: 10.1046/j.1432-1033.2003.03674.x. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wang TF, Ou Y, Guidotti G. The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J Biol Chem. 1998;273:24814–24821. doi: 10.1074/jbc.273.38.24814. [DOI] [PubMed] [Google Scholar]
- Westfall TD, Kennedy C, Sneddon P. Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156. Br J Pharmacol. 1996;117:867–872. doi: 10.1111/j.1476-5381.1996.tb15273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TD, Kennedy C, Sneddon P. The ecto-ATPase inhibitor ARL 67156 enhances parasympathetic neurotransmission in the guinea-pig urinary bladder. Eur J Pharmacol. 1997a;329:169–173. [PubMed] [Google Scholar]
- Westfall TD, McIntyre CA, Obeid S, Bowes J, Kennedy C, Sneddon P. The interaction of diadenosine polyphosphates with P2x-receptors in the guinea-pig isolated vas deferens. Br J Pharmacol. 1997b;121:57–62. doi: 10.1038/sj.bjp.0701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TD, Menzies JR, Liberman R, Waterston S, Ramphir N, Westfall DP, et al. Release of a soluble ATPase from the rabbit isolated vas deferens during nerve stimulation. Br J Pharmacol. 2000a;131:909–914. doi: 10.1038/sj.bjp.0703662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TD, Sarkar S, Ramphir N, Westfall DP, Sneddon P, Kennedy C. Characterization of the ATPase released during sympathetic nerve stimulation of the guinea-pig isolated vas deferens. Br J Pharmacol. 2000b;129:1684–1688. doi: 10.1038/sj.bjp.0703271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]