Abstract
Background and purpose:
Platelet hyperactivity is important in the pathogenesis of cardiovascular diseases. Betel leaf (PBL) is consumed by 200-600 million betel quid chewers in the world. Hydroxychavicol (HC), a betel leaf component, was tested for its antiplatelet effect.
Experimental approach:
We tested the effect of HC on platelet aggregation, thromboxane B2 (TXB2) and reactive oxygen species (ROS) production, cyclooxygenase (COX) activity, ex vivo platelet aggregation and mouse bleeding time and platelet plug formation in vivo. The pharmacokinetics of HC in rats was also assessed.
Key results:
HC inhibited arachidonic acid (AA) and collagen-induced platelet aggregation and TXB2 production. HC inhibited the thrombin-induced TXB2 production, but not platelet aggregation. SQ29548, suppressed collagen- and thrombin-induced TXB2 production, but not thrombin-induced platelet aggregation. HC also suppressed COX-1/COX-2 enzyme activity and the AA-induced ROS production and Ca2+ mobilization. HC further inhibited the ex vivo platelet aggregation of platelet-rich plasma (>100 nmole/mouse) and prolonged platelet plug formation (>300 nmole/mouse) in mesenteric microvessels, but showed little effect on bleeding time in mouse tail. Moreover, pharmacokinetics analysis found that more than 99% of HC was metabolized within 3 min of administration in Sprague-Dawley rats in vivo.
Conclusions and implications:
HC is a potent COX-1/COX-2 inhibitor, ROS scavenger and inhibits platelet calcium signaling, TXB2 production and aggregation. HC could be a potential therapeutic agent for prevention and treatment of atherosclerosis and other cardiovascular diseases through its anti-inflammatory and antiplatelet effects, without effects on haemostatic functions.
Keywords: betel leaf, hydroxychavicol, platelet aggregation, thromboxane, cyclooxygenase, calcium
Introduction
Betel quid (BQ) chewing is popular in Taiwan, India and many other countries (Jeng et al., 2001; Warnakulasuriya et al., 2002; IARC, 2004). There are 2–2.8 million BQ chewers in Taiwan, and 200–600 million BQ chewers in the world (Jeng et al., 2001; IARC, 2004). BQ consists of areca nut (AN), the inflorescence of Piper betel (IPB) and lime, with or without betel leaf (Piper betel L., PBL). Most people chew BQ (as Lao-Hua quid) without PBL. The habit of BQ chewing is a risk factor for oral cancer, diabetes, cardiovascular disease as well as the initiator of hypertension (Mannan et al., 2000; Jeng et al., 2001; Warnakulasuriya et al., 2002; Tsai, 2003; IARC, 2004; Chiu et al., 2006). Recently, BQ chewers have tended to chew BQ with PBL instead of Lao-Hua quid, possibly because PBL contains catechin, eugenol and hydroxychavicol (4-allyl-catechol, HC), which are potentially beneficial to human health. However, whether the addition of PBL is beneficial or detrimental to human health is still controversial.
Chewing BQ containing PBL possesses less risk for oral cancer (Ko et al., 1995). PBL contains antimutagenic and chemopreventive agents (Shirname et al., 1983), and may inhibit carcinogen-induced oral tumours (Rao, 1984). Intriguingly, PBL induces vaso-relaxation (Runnie et al., 2004), inhibits low-density lipoprotein oxidation (Salleh et al., 2002), reactive oxygen species (ROS) production and platelet aggregation (Jeng et al., 2002). One of the constituents of PBL, the catechol HC, inhibits 3(H)benzopyrene–DNA interactions (Lahiri and Bhide, 1993), the nitrozation reaction (Nagabhushan et al., 1989) and the DMBA- and tobacco-specific nitrosamine-induced mutations and bone marrow micronucleated cells formation in Swiss mice (Amonkar et al., 1986, 1989; Padma et al., 1989). Recently, HC was found to be a ROS scavenger and inhibited cancer cell growth (Chang et al., 2002), indicating its possible use as a therapeutic agent.
Abnormal arachidonic acid (AA) metabolism and platelet aggregation has been linked to the pathogenesis of vascular thrombus formation and hypertension (Robbins et al., 2006). Other pro-thrombotic and hypertension factors include vessel wall damage, decreased fibrinolytic potential and activation of blood coagulation and stasis (Willoughby et al., 2002; Fogari and Zoppi, 2005). In injured vessels, platelets adhere to disrupted area and release biologically active constituents (for example, adenosine diphosphate (ADP) and thromboxane A2). Various agonists, such as thrombin, ADP and collagen, may trigger platelet aggregation (Willoughby et al., 2002; Fogari and Zoppi, 2005). Substances secreted from intracellular storage granules of activated platelets may recruit additional platelets to propagate thrombosis (Huang and Detwiler, 1986).
ROS have been shown to modulate AA- and collagen-induced platelet aggregation (Iuliano et al., 1994, 1997). Triterpenes and β-sitosterol isolated from PBL have antiplatelet actions (Saeed et al., 1993). Catechin and eugenol also inhibit cyclooxygenase (COX) activities and platelet aggregation (Dohi et al., 1991; Chen et al., 1996; Huss et al., 2002). HC, which shows structural similarity to eugenol, has antioxidative, antimutagenic, anticarcinogenic, as well as chemopreventive effects (Amonkar et al., 1986, 1989; Chang et al., 2002). Recently, we found that PBL extracts scavenged ROS and inhibited platelet aggregation and thromboxane B2 (TXB2) production (Jeng et al., 2002). We therefore investigated the antiplatelet effect of HC and its mechanisms.
Methods
All animal procedures were approved by the Ethical Committee of Animal Experiments, Chang Gung Institute of Technology.
Platelet aggregation assay
Washed rabbit platelets (3 × 108 platelets ml−1) were suspended in Tyrode's solution containing 1 mM calcium and 0.35% bovine serum albumin (Chang et al., 1998; Jeng et al., 2002). HC or dimethyl sulphoxide (DMSO), as control, was added to platelets 3 min before the addition of agonists (AA collagen or thrombin). Platelet aggregation was measured by an aggregometer (Model 600B, Payton Associates, ON, Canada) (Born and Cross, 1963) and the percent of aggregation calculated (Chang et al., 1998; Jeng et al., 2002). Similarly aspirin (100 μM) was also added 3 min before the addition of agonists for comparison.
Lactate dehydrogenase activity assay
After exposure of platelets to HC, lactate dehydrogenase (LDH) activity was measured as an index of platelet damage using LDH assay kits. LDH released was compared with the total LDH activity of platelets dissolved by 0.1% Triton X-100.
TXB2 assay
Platelets were incubated with HC or aspirin for 3 min and then exposed to thrombin, collagen or AA, as in the aggregation assay. Then ethylenediaminetetraacetic acid (2 mM) and indomethacin (50 μM) were added to platelet suspension. TXB2 level was measured with enzyme-linked immunosorbent assay (ELISA) kits (Jeng et al., 2002).
Effects of HC on COX enzyme activity
HC, eugenol, celecoxib (DMSO as control) and aspirin (water as control) were mixed with COX-1 or -2 enzyme for 10 min as provided by COX inhibitor screening assay kits. Then AA was added and incubated for 2 min followed by addition of 0.1 N HCl and saturated stannous fluoride solution. PGE2 production was measured by ELISA kits.
Fluorometric assay of platelet ROS production
Washed platelets were pretreated with 10 μM 2′,7′-dichlorfluorescein-diacetate (DCFH-DA) in 37°C for 30 min (Leoncini et al., 1997). After washing, 200 μl of platelets (5 × 108 ml−1) were added to 96-well plates containing 5 μl of double distiled water, DMSO diluents (control), HC (1–100 μM) and AA (100 μM). The emitted 2′,7′-dichlorfluorescein DCF fluorescence was measured with a Microplate Spectrofluorometer (Molecular Devices Corporation, Sunny Vale, CA, USA) with excitation and emission wavelength of 485 and 535 nm, respectively, for 15 min. The emitted fluorescence of resting DCFH-DA-loaded platelets was used as the basal ROS production. In some studies, higher numbers of DCFH-DA-loaded platelets (2 × 109 ml−1) were exposed to normal saline (NS) and three agonists to elucidate the extent of ROS production.
Intracellular Ca2+ mobilization
Calcium mobilization in platelets was investigated as described by Iuliano et al. (1994). Fluorescence was measured by exposure of Fura-2 loaded platelets to HC with/without agonists using a F4500 Fluorometer (Hitachi, Japan). The ratio measurement method at two excitation wavelengths, 340 and 380 nm, was used with an emission wavelength at 510 nm, and the data were automatically calculated by computer software using lysed platelets (0.2% Triton X-100) with/without 10 mM EGTA (ethylene glycol bis(β-aminoethylether)-N,N,N',N',-tetraacetic acid) to obtain maximum and minimum fluorescence.
Ex vivo platelet aggregation of platelet-rich plasma
After intravenous injection of HC (100–150 nmol mouse−1) to mice (25–27 g), blood was drawn by cardiac puncture. Whole blood containing anticoagulant (3.8% sodium citrate in 9:1 (v/v)) in an Eppendorf tube was centrifuged at 60 g for 4 min to prepare platelet-rich plasma (PRP). Ex vivo platelet aggregability of PRP by AA was measured as described.
In vivo platelet plug formation induced in mesenteric microvessels of mice
Male ICR mice (15–18 g) were anaesthetized with sodium pentobarbital (50 mg kg−1) and then given sodium fluorescein (1 mg mouse−1) and placebo (DMSO diluent control) or HC (150, 300 and 500 nmol mouse−1) through jugular vein (Chang and Huang, 1994; Chang et al., 1998). A mesenteric membrane with microvascular bed in small intestine was observed under transillumination from a halogen lamp. Five minutes after administration of sodium fluorescein, filtered light irradiation was started. The time taken to induce thrombus formation and to stop blood flow by platelet plugs in mesenteric microvessels was measured.
Measurement of mouse tail bleeding time
Male ICR mice (25–27 g) were anaesthetized and 10 min after intravenous injection of HC (100–250 nmol mouse−1), the tail of mice were transected at 2 mm from the tip and approximately 1.5 cm of the distal portion is immersed into 10 ml NS at 37°C water bath. The period from tail transection to cessation of bleeding was recorded (Chang and Huang, 1994; Chang et al., 1998).
Pharmacokinetics of HC in Sprague–Dawley rats
Three 9-week-old male Sprague–Dawley rats (275–278 g, BioLASCO Taiwan Co., Ltd.) were used for determination of HC plasma concentration. Rats were anaesthetized and HC (10 mg kg−1 body weight) was administered through jugular vein. Blood samples (0.5 ml) were collected from the carotid artery to obtain plasma at 3, 5, 10 and 20 min after HC administration.
HPLC assay
Aliquots of plasma (200 μl) were spiked with 20 μl of an internal standard (20 μg ml−1 of methyl paraben). The mixture was extracted with 600 μl of ethyl acetate and centrifuged. The supernatant was evaporated to dryness under nitrogen stream (40°C). The residue was dissolved in 100 μl of chromatographic elute and 50 μl of this solution was injected for high-performance liquid chromatography (HPLC) analysis. Separation was achieved with a Symmetry Shield ODS column (4.6 × 150 mm, 5 μ, Waters Corporation, Milford, MA, USA) with an ultraviolet detector. The mobile phase comprises 3% acetic acid/methanol (v/v, 66/34) and a flow rate of 1.4 ml min−1. The linear range of this assay was 0.05–4.00 μg ml−1 (correlation coefficients >0.999), and the recovery was >96%.
Statistical analysis
Three or more separate experiments were done. The data were analyzed and expressed as mean±s.e.m. Some inhibitory results were expressed as percent of inhibition of control. The IC50 values were calculated by regression analysis. One-way analysis of variance with post hoc Bonferroni test was used for statistical analysis. P<0.05 was taken as showing significant difference between groups.
Materials
Eugenol, aspirin, celecoxib, type I collagen, α-thrombin, AA, Fura-2 AM, LDH assay kits, DCFH-DA were from Sigma Chemical Company (St Louis, MO, USA). TXB2 ELISA kits and COX inhibitor screening assay kits were from Cayman Chemical Company (Ann Arbor, MI, USA). HC (purity of 94%) was synthesized as described (Chang et al., 2002; Jeng et al., 2004).
Results
Effect of HC on platelet aggregation
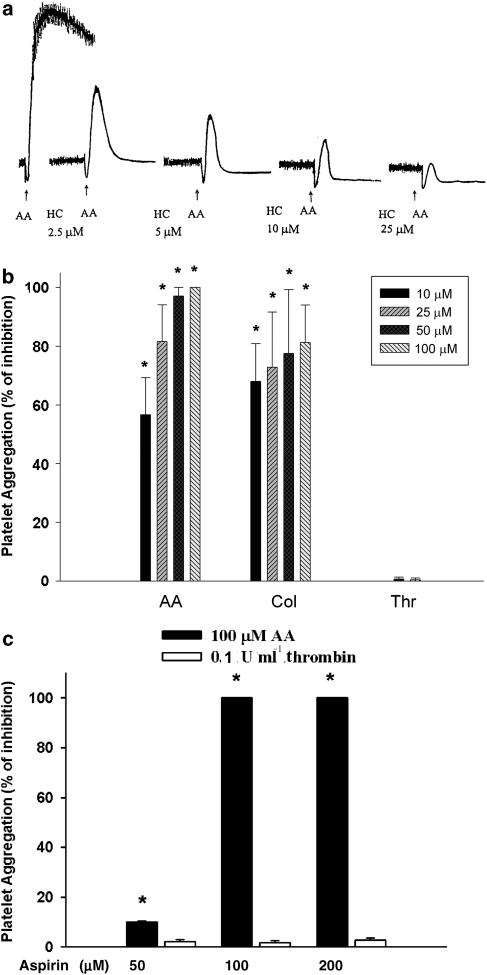
HC (>2.5 μM) inhibited the AA-induced platelet aggregation in a concentration-dependent manner (Figure 1a). Quantitatively, AA-induced platelet aggregation was completely blocked by 50–100 μM HC, whereas collagen-induced platelet aggregation was reduced by 10–100 μM of HC with 68–81% of inhibition. On the contrary, HC (<100 μM) showed little effect on platelet aggregation induced by 0.1 U ml−1 thrombin (Figure 1b), However, HC showed partial suppression of platelet aggregation induced by 0.05 U ml−1 thrombin (data not shown). Antiplatelet effects of HC were not due to cytotoxicity because no evident LDH release was noted (data not shown). As reported earlier (Carter and Heptinstall, 1985; Kariyazono et al., 2004), aspirin (100 and 200 μM) completely inhibited AA-induced platelet aggregation (100% inhibition), whereas aspirin showed little inhibitory effect (only 2–3%) on thrombin-induced platelet aggregation (Figure 1c). Moreover, SQ29548 (5–20 μM) inhibited collagen-induced platelet aggregation in a concentration-dependent manner, whereas it showed little effect on thrombin-induced platelet aggregation (data not shown).
Figure 1.
Effect of HC and aspirin (100 μM) on platelet aggregation. (a) One representative AA-induced platelet aggregation histogram and its inhibition by HC is shown. (b) Quantitative inhibitory effect of HC on platelet aggregation induced by AA (100 μM), collagen (10 μg ml−1) and thrombin (0.1 U ml−1) (% of inhibition, mean±s.e.m., n=5). Effect of (c) aspirin (100 μM) on platelet aggregation induced by AA (n=3), or thrombin (n=4). *Denotes significant difference (P<0.05) when compared with agonists-only group as analyzed by one-way ANOVA and post hoc Bonferroni test. AA, arachidonic acid; ANOVA, analysis of variance; HC, hydroxychavicol.
Effect of HC on agonists-induced TXB2 production
AA-induced platelet TXB2 production (275.7±33.8 ng ml−1) was inhibited by HC with an IC50 of 0.91±0.32 μM (Figure 2a). At concentrations of 0.5 and 1 μM, HC also inhibited collagen-induced platelet TXB2 production (164.1±34.8 ng ml−1) by 42 and 49%, respectively (IC50=1.2±0.4 μM) (Figure 2b). Similarly, HC (>0.5 μM) attenuated thrombin-induced TXB2 production (217.2±82.3 ng ml−1) (Figure 2c).
Figure 2.
Effect of HC and aspirin (100 μM) on platelet TXB2 production. Platelets were pretreated with HC and then exposed to (a) AA (n=5), (b) collagen (n=6) or (c) thrombin (n=5). (d) Platelets were pretreated with aspirin and then exposed to AA (n=3) or thrombin (n=4). Platelet TXB2 production was measured by ELISA. Results were expressed as TXB2 level (% of control, mean±s.e.m.). *Significant difference (P<0.05) when compared with agonists-only group, as analyzed by one-way ANOVA and post hoc Bonferroni test. AA, arachidonic acid; ANOVA, analysis of variance; ELISA, enzyme-linked immunosorbent assay; HC, hydroxychavicol; TXB2, thromboxane B2.
Aspirin (100 and 200 μM) almost completely inhibited the AA-induced platelet TXB2 production with 94 and 98% of inhibition, respectively. Thrombin-induced platelet TXB2 production was also inhibited by 100 and 200 μM of aspirin with 78 and 96% of inhibition, respectively (Figure 2d). Interestingly, collagen- and thrombin-induced TXB2 production was inhibited by SQ29548 at concentrations ranging from 2 to 20 μM (data not shown).
Direct COX enzyme inhibition by HC and eugenol
Eugenol suppressed COX-1 enzyme activity (IC50=59.3±10.6 μM; Table 1), but showed only slight inhibition of COX-2 activity by 19% at 500 μM. HC inhibited COX-1 enzyme activity by 33–58% at concentrations of 50–100 μM (IC50=79.8±2.8 μM). Intriguingly, HC (20–50 μM) also inhibited COX-2 enzyme activity in a concentration-dependent manner (IC50=64.8±10.5 μM) (Table 1). Under similar experimental condition, aspirin inhibited COX-1 activity with an IC50 concentration of 52.0±3.2 μM, whereas celecoxib inhibited COX-2 activity with an IC50 of 21.5±5.3 μM (Table 1).
Table 1.
Inhibition of COX-1 and COX-2 enzyme activities by HC, eugenol, aspirin and celecoxib
| Chemical/concentrations | COX-1 activity | COX-2 activity |
|---|---|---|
| (% of control) | (% of control) | |
| Eugenol (μM) | ||
| 20 | 107.8±4.2 | 109.3±14.9 |
| 50 | 79.1±9.2 | 102.7±12.7 |
| 100 | 20.2±6.1a | 90.8±14.0 |
| 200 | 2.8±0.6a | 95.9±16.5 |
| 500 | 1.3±0.2a | 80.8±19.4 |
| HC (μM) | ||
| 20 | 90.3±0.8 | 77.8±6.5a |
| 50 | 67.3±5.2a | 56.8±3.2a |
| 100 | 41.8±2.3a | 44.0±3.2a |
| 200 | 6.8±0.5a | 8.8±0.8a |
| 500 | 1.0±0.1a | 2.3±0.3a |
| Aspirin (μM) | ||
| 10 | 80.8±2.5a | ND |
| 50 | 48.5±2.9a | ND |
| 100 | 15.9±1.9a | ND |
| 200 | 10.4±1.2a | ND |
| Celecoxib (μM) | ||
| 2.5 | ND | 66.7±6.6 |
| 5 | ND | 69.1±7.3 |
| 10 | ND | 58.3±7.4 |
| 25 | ND | 38.2±10.5a |
| 50 | ND | 26.1±11.1a |
Abbreviations: ANOVA, analysis of variance; COX-1, cyclooxygenase-1; HC, hydroxychavicol; ND, not determined.
Results were expressed as percentage of COX-1 and -2 enzyme activity relative to control (mean±s.e.m., respective control as 100%, n=3–5).
The IC50 values for HC and COX-1 was 79.8±2.8 μM; for HC and COX-2 was 64.8±10.5 μM; for eugenol and COX-1 was 59.3±10.6 μM; for aspirin and COX-1 was 52.0±3.2 μM; for celecoxib and COX-2 was 21.3±5.3 μM in our experimental conditions.
Denotes significant difference when compared with control by one-way ANOVA and post hoc Bonferroni test.
Inhibition of platelet ROS production by HC
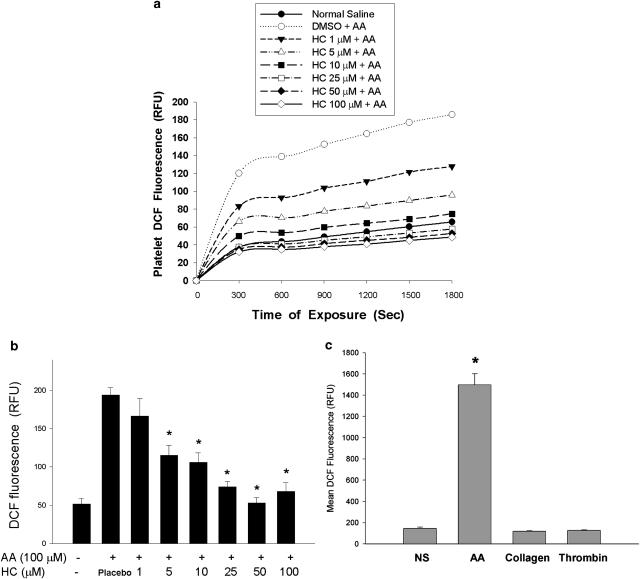
Platelet DCF fluorescence increased following addition of AA. Resting platelets also generated a low amount of ROS with a gradual increase in DCF fluorescence (Figure 3a). HC suppressed AA-induced ROS production by decrease in DCF fluorescence (in relative fluorescent unit (RFU)) from 194 to 74–115 by 5–25 μM HC (IC50=11.1±2.6 μM) (Figure 3b). With increased numbers of DCFH-DA-loaded platelets (2 × 109 platelets ml−1), an elevated DCF fluorescence in platelets was noted after exposure to AA (an average RFU of 1498), whereas exposure of platelets to collagen and thrombin showed undetectable changes in DCF fluorescence (Figure 3c). No direct effect of HC on DCF emitted fluorescence was detected (data not shown).
Figure 3.
Effect of HC on AA-induced ROS production. DCFH-DA-labeled platelets were pretreated with DMSO diluent (placebo) or HC followed by addition of AA (100 μM). The DCF fluorescence in platelets was measured. (a) One representative platelet DCF fluorescence following exposure to saline (NS) only, AA with/without HC (1–100 μM) for 30 min. (b) Quantitative DCF fluorescence of platelets after exposure to NS, DMSO diluent, AA with/without HC (as relative fluorescent unit, RFU) (mean±s.e.m.) (n=5), *denotes significant difference (P<0.05) relative to AA-induced group (placebo). (c) DCF fluorescence of platelets (2 × 109 platelets ml−1) after exposure to normal saline (NS) or agonists (mean±s.e.m.) (n=6). *Significant difference (P<0.05) when compared with NS (control) group. AA, arachidonic acid; DCFH-DA, 2′,7′-dichlorfluorescein-diacetate; DMSO, dimethyl sulphoxide; HC, hydroxychavicol.
Inhibition of Ca2+ mobilization in platelets by HC
Ca2+ is crucial for platelet aggregation (Holmsen, 1994; Rosado and Sage, 2002), and we found that Ca2+ mobilization induced by collagen in platelets was inhibited by HC (>2.5 μM) with a decrease in Fura-2 fluorescence (Figure 4a). Quantitatively, HC (>2.5 μM) inhibited the AA- (IC50=3.9±0.8 μM) and collagen-induced Ca2+ mobilization (Figure 5b, panels 1 and 2), whereas HC showed little effect on thrombin-induced Ca2+ mobilization (Figure 4b, panel 3).
Figure 4.
Inhibition of Ca2+ mobilization by HC. (a) One representative record of fluorescence changes measured in Fura-2 AM-loaded platelets (2 × 108 ml−1) after stimulation by collagen (Col) and inhibition by HC. (b) Quantitative inhibition of agonists-induced Ca2+ mobilization in platelets by HC toward AA (panel 1), collagen (panel 2) and thrombin (panel 3) (% of inhibition, mean±s.e.m.) (n=5) *Significant difference (P<0.05) when compared with respective agonists group. AA, arachidonic acid; HC, hydroxychavicol.
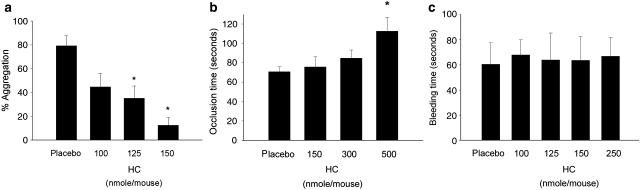
Figure 5.
(a) Inhibition of ex vivo AA-induced aggregation of PRP by HC (% of inhibition, n=9). (b) The time taken (s) for mesenteric microvessel platelet plug formation and its inhibition by HC in mice (mean±s.e.m., n=10). *Significant difference vs placebo group (P<0.05), (c) effect of HC on tail bleeding time (s) was recorded (n=7). *Significant difference (P<0.05) when compared with placebo group. AA, arachidonic acid; HC, hydroxychavicol; PRP, platelet-rich plasma.
Inhibition of ex vivo platelet aggregability of PRP by HC
HC (100–150 nmol mouse−1) suppressed ex vivo platelet activation by AA (Figure 5a). As the circulating blood volume of mice is estimated to be approximately 78–80 ml kg−1 (BVA/Frame/RSPCA/UFAW Joint Working Group of Refinements, 1993), the initial HC concentration in blood of mice (25–27 g) would be approximately 50–75 μM.
Effects of HC on platelet plug formation in mesenteric microvessels and mice tail bleeding time
HC prolonged platelet plug formation induced by irradiation of mesenteric microvessels containing sodium fluorescein. At doses of 150 and 300 nmol mouse−1, HC elevated the occlusion time from 71 s (control) to 76 and 85 s, respectively (Figure 5b). An elevation of microvessel occlusion time to 113 s was noted when 500 nmol mouse−1 of HC was given. Moreover, HC (100–250 nmol mouse−1) showed little effect on mice tail bleeding time (Figure 5c).
Pharmacokinetics of HC in Sprague–Dawley rats
Our HPLC system for measuring HC gave a linear response over the concentration range of 0.05–4.00 μg ml−1 HC, as shown in Figure 6. Rat plasma samples prepared 3 min after giving HC were analyzed by HPLC. The observed retention time was 15.5 and 12.1 min for HC and internal standard (IS), respectively. A decrease in HC elution peak in plasma taken 10 min after HC administration was noted.
Figure 6.
HPLC analysis of plasma HC concentrations. (a) Calibration curve of HC in plasma indicated a linear response over the concentration range of 0.05–4.00 μg ml−1. (b) One representative HPLC record showing the concentration of HC in one sample of rat plasma prepared 3 min after HC administration, (c) HPLC record showing plasma concentration of HC in one rat, 10 min after HC administration. Peak of HC was identified after 15.5 min of elution. HC, hydroxychavicol; HPLC, high-performance liquid chromatography.
The mean plasma concentrations (n=3) for each time point after a single intravenous injection of HC ranged from 1.394 μg ml−1 (0.43% of its original level) at 3 min to 0.049 μg ml−1 at 20 min (Table 2). HC concentration in plasma was below the limit of quantitation by 20 min after injection.
Table 2.
Plasma HC concentration and percentage of HC after a single intravenous injection of 10 mg kg−1 in rats
| Rat code | Body weight (g) |
Plasma HC concentration (μg ml−1) |
Percentage of dose (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 min | 5 min | 10 min | 20 min | 40 min | 3 min | 5 min | 10 min | 20 min | 40 min | ||
| 1040601001 | 276 | 1.329 | 0.965 | 0.307 | 0.041 | <LOQ | 0.41 | 0.30 | 0.10 | 0.01 | <LOQ |
| 1040601003 | 278 | 1.638 | 1.193 | 0.493 | 0.065 | <LOQ | 0.51 | 0.37 | 0.15 | 0.02 | <LOQ |
| 1040601006 | 275 | 1.216 | 0.840 | 0.319 | 0.040 | <LOQ | 0.38 | 0.26 | 0.10 | 0.01 | <LOQ |
| Mean (n=3) | 276 | 1.394 | 0.999 | 0.373 | 0.049 | — | 0.43 | 0.31 | 0.12 | 0.02 | — |
| s.d. (n=3) | 2 | 0.218 | 0.179 | 0.104 | 0.014 | — | 0.068 | 0.055 | 0.032 | 0.004 | — |
Abbreviation: LOQ, limit of quantitation.
Discussion and conclusions
In this study, we found that HC was a antiplatelet agent through its inhibition of COX-1, platelet TXB2 production and calcium mobilization in vitro and its inhibition of platelet plug formation in vivo. All these features may potentially prevent thrombosis and the progression of hypertension and stroke (Willoughby et al., 2002; Fogari and Zoppi, 2005; Robbins et al., 2006). Platelets may adhere to disrupted blood vessels and release some active constituents (Huang and Detwiler, 1986; Willoughby et al., 2002; Fogari and Zoppi, 2005), which induce thrombotic plug formation (Marcus and Safier, 1993). In this study, inhibition of platelet aggregation and TXB2 production by HC can partly explain the antiplatelet effect of PBL (Jeng et al., 2002). HC showed inhibition of thrombin-induced TXB2 production but had little effect on platelet aggregation. Aspirin also inhibited the thrombin-induced platelet TXB2 production but not aggregation in this study. Moreover, recent reports also suggest that aspirin is not able to inhibit thrombin-induced platelet aggregation (Li et al., 2003; Rotondo et al., 2004). Possibly thrombin-induced platelet aggregation is not solely mediated via thromboxane production and signaling via TXA2-positive feedback, but by multiple signaling pathways through different thrombin receptors at different dosages (Yamada et al., 1983; Covic et al., 2000; Willoughby et al., 2002; Rotondo et al., 2004). Triterpenes and β-sitosterol in PBL also have antiplatelet and anti-inflammatory effects (Saeed et al., 1993), suggesting the potential medicinal use of PBL.
Recently we found that SQ29548, a TXA2 receptor antagonist, suppressed AA-induced platelet aggregation, whereas it showed little effect on AA-induced TXB2 production (Jeng et al., 2007). In this study, we further found that SQ29548 inhibited collagen-induced platelet aggregation and TXB2 production, indicating that collagen-induced platelet aggregation was mainly derived from positive feedback activation of TXA2 receptors. TXA2 receptor activation was necessary for both AA- and collagen-induced platelet aggregation. Similarly, thrombin-induced TXB2 production was mediated by initial TXA2 production and then feedback TXA2 receptor activation. However, thrombin-induced TXA2 production and TXA2 receptor activation are not essential for platelet aggregation. Our results also suggest that AA- and collagen-induced platelet aggregation are differentially regulated by TXA2 production and TXA2 receptor activation. Effect of collagen on platelet activation can be mediated by surface glycoprotein Ia/IIa and VI receptor, whereas platelet activation by thrombin is mediated by protease-activated receptors (PAR-1, PAR-3 or PAR-4) and glycoprotein Ib (Colman, 1990; Krotz et al., 2002; Maurice et al., 2004). Interestingly, HC also inhibited the AA-, collagen- and thrombin-induced platelet TXB2 production.
Prior reports have found that eugenol as a COX-1 inhibitor has antiplatelet effects and inhibits COX-2 activity with an IC50 of 64–129 μM by different reports (Dohi et al., 1991; Chen et al., 1996; Huss et al., 2002). In this study, eugenol slightly inhibited COX-2 activity at a concentration of 500 μM. Interestingly, HC inhibited both COX-1 (IC50=79.8 μM) and COX-2 (IC50=64.8 μM) activities, suggesting the functional –OH group in HC is important. HC is more effective in killing KB cells than eugenol, whereas the suppressive effect of HC towards COX-1 and COX-2 enzyme activity is slightly less than aspirin and celecoxib. In our study, the IC50 concentration for celecoxib on COX-2 activity is higher than values reported in the literature (Gierse et al., 1999; Kato et al., 2001). The IC50 concentration for HC on COX enzyme activity is also higher than the value on platelet aggregation and thromboxane production. This could be due to differences in assay conditions such as the amount and origin of COX as well as the incubation periods used in different reports. As tissue inflammation is critical in the atherosclerotic process (Ross, 1999; Blake and Ridker, 2001), HC could be potentially used in prevention or treatment of cardiovascular diseases through its anti-inflammatory effect.
HC, as an antioxidant in PBL and IPB, suppressed the ROS-induced chemiluminescence and DNA breaks (Chang et al., 2002). Various agonists may stimulate platelet ROS production and aggregation, via regulating AA metabolism or via COX inhibition (Iuliano et al., 1992, 1994, 1997). In the presence of haemoglobin, ROS-induced platelet aggregation is enhanced (Iuliano et al., 1992). We found that resting platelets also generated a low amount of ROS. Intriguingly, AA stimulates platelet ROS production, which is inhibited by HC, supporting HC as a ROS scavenger (Chang et al., 2002). Collagen, but not thrombin, has been shown to induce platelet superoxide production, which mediates ADP production but not initial platelet aggregation (Krotz et al., 2002). However, little platelet ROS production by collagen and thrombin was detected. Moreover, the IC50 value (11.1±2.6 μM) for HC on AA-induced ROS formation in platelets showed slight differences to AA-induced platelet thromboxane production in this study. One explanation is that AA-induced thromboxane production is not solely mediated by ROS production. The other possible reason is that platelet ROS production can be mediated by COX as well as other enzymes such as platelet isoforms of NADPH oxidase, xanthine oxidase, mitochondrial respiration, and so on (Krotz et al., 2004), whereas HC inhibits not all of the enzymes responsible for platelet ROS formation. SOD enhanced platelet aggregation and TXB2 production in AA- and collagen-primed platelets via dismutation of superoxide radicals to oxygen and H2O2 (Finazzi-Agro et al., 1982). However, higher ROS levels suppress thromboxane synthesis (Hornberger and Patscheke, 1990), suggesting a need for more studies on basal level of redox status and the inducible ROS on platelet functions. As ROS production by leucocytes may affect platelet functions, which link to angina pectoris and myocardial infarction (Miller et al., 1994), HC could be used for prevention and treatment of these diseases.
Various agonists can trigger platelet aggregation, by activation of receptors followed by phospholipase C activation and production of inositol-1,4,5-trisphosphate/diacylglycerol leading to an increase in cytosolic Ca2+ (Holmsen, 1994; Rosado and Sage, 2002). Interestingly, HC inhibited AA- and collagen-induced, but not thrombin-induced, platelet Ca2+ mobilization, comparable to their inhibition of platelet aggregation, suggesting that the antiplatelet effect of HC were associated with inhibition of calcium signaling.
An occlusive platelet thrombus may form in sites with chronic inflammation, leading to blood flow stasis (Willoughby et al., 2002). Antithrombotic effects by therapeutic agents can be mediated by their antiplatelet or anticoagulation effects. Administration of HC to mice in vivo inhibited platelet aggregation, measured ex vivo. This confirms that HC would exhibit an antiplatelet effect in vivo. HC further prolonged the occlusion time for irradiation-induced platelet plug formation in mice, suggesting that HC prevented thrombus formation in vivo. Since mice blood was approximately 78–80 ml kg−1 body weight (BVA/Frame/RSPCA/UFAW Joint Working Group of Refinements, 1993), the effective HC concentration in vivo was approximately 50–100 μM. Aspirin has been used to reduce stroke and myocardial infarction via its antiplatelet effect. However, aspirin increases the risk of gastroduodenal ulcer and bleeding. Interestingly, HC administration showed no marked delay in bleeding time, implying that HC was an antiplatelet agent without increasing the risk of any bleeding tendency.
IPB contains 9.74 mg g−1 weight of HC and salivary concentration of HC in chewers was 4.6 mM (Huang et al., 1993). From our result, more than 99% of HC was metabolized by platelets and other vascular cells within 3 min in rats. As the antiplatelet effect of HC lasted for more than 5 min after administration, this effect of HC could be due to an irreversible event or due to its metabolites. In conclusion, PBL is known to exert antioxidative, anticarcinogenic and antiplatelet effects (Shirname et al., 1983; Padma et al., 1989; Jeng et al., 2002). One of its constituents, HC, could be a preventive or therapeutic agent for hypertension or stroke via its anti-inflammatory, antiplatelet and antithrombotic properties, without impairing haemostatic functions.
Acknowledgments
We thank Professor Tur-Fu Huang for critical comments on this paper and Miss Yi-Hsuen Chang for experimental assistance. This study is supported by National Science Council (NSC) and Chang Gung Memorial Hospital, Taiwan.
Abbreviations
- AA
arachidonic acid
- AN
areca nut
- BQ
betel quid
- COX
cyclooxygenase
- DCFH-DA
2′,7′-dichlorfluorescein-diacetate
- HC
hydroxychavicol
- IPB
Piper betel inflorescence
- LDH
lactate dehydrogenase
- PBL
betel leaf
- PRP
platelet-rich plasma
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TXB2
thromboxane B2
Conflict of interest
The authors state no conflict of interest.
References
- Amonkar AJ, Nagabhushan M, D'Souza AV, Bhide SV. Hydroxychavicol: a new phenolic antimutagen from betel leaf. Food Chem Toxicol. 1986;24:1321–1324. doi: 10.1016/0278-6915(86)90065-7. [DOI] [PubMed] [Google Scholar]
- Amonkar AJ, Padma PR, Bhide SV. Protective effect of hydroxychavicol, a phenolic component of betel leaf, against the tobacco-specific carcinogens. Mutat Res. 1989;210:249–253. doi: 10.1016/0027-5107(89)90085-7. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- Born GVR, Cross MJ. The aggregation of blood platelets. J Physiol. 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BVA/Frame/RSPCA/UFAW Joint Working Group of Refinements Removal of blood from laboratory mammals and birds. Lab Anim. 1993;27:1–22. doi: 10.1258/002367793781082412. [DOI] [PubMed] [Google Scholar]
- Carter AJ, Heptinstall S. Platelet aggregation in whole blood: the role of thromboxane A2 and adenosine diphosphate. Thromb Haemost. 1985;54:612–616. [PubMed] [Google Scholar]
- Chang MC, Huang TF. In vivo effect of a thrombin-like enzyme on platelet plug formation induced in mesenteric microvessels of mice. Thromb Res. 1994;73:31–38. doi: 10.1016/0049-3848(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Chang MC, Lin HK, Peng HC, Huang TF. Antithrombotic effect of crotalin, a platelet membrane glycoprotein Ib antagonist from venom of Crotalus atrox. Blood. 1998;91:1582–1589. [PubMed] [Google Scholar]
- Chang MC, Uang BJ, Wu HL, Lee JJ, Hahn LJ, Jeng JH. Inducing the cell cycle arrest and apoptosis of oral KB carcinoma cells by hydroxychavicol: roles of glutathione and reactive oxygen species. Br J Pharmacol. 2002;135:619–630. doi: 10.1038/sj.bjp.0704492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Wang MH, Chen IJ. Antiplatelet and calcium inhibitory properties of eugenol and sodium eugenol acetate. Gen Pharmacol. 1996;27:629–633. doi: 10.1016/0306-3623(95)02089-6. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Wu SC, Tseng CD, Yen MF, Chen THH. Progression of prehypertension, stage 1 and 2 hypertension (JNC 7): a population-based study in Keelung, Taiwan (Keelung Community based integrated screening No. 9) J Hypertension. 2006;24:821–828. doi: 10.1097/01.hjh.0000222750.82820.19. [DOI] [PubMed] [Google Scholar]
- Colman RW. Aggregin: a platelet ADP receptor that mediates activation. FASEB J. 1990;4:1425–1435. doi: 10.1096/fasebj.4.5.2407587. [DOI] [PubMed] [Google Scholar]
- Covic L, Gresser AL, Kuliopulos A. Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry. 2000;39:5458–5467. doi: 10.1021/bi9927078. [DOI] [PubMed] [Google Scholar]
- Dohi T, Anamura S, Shirakawa M, Okamoto H, Tsujimoto A. Inhibition of lipoxygenase by phenolic compounds. Jpn J Pharmacol. 1991;55:547–550. doi: 10.1254/jjp.55.547. [DOI] [PubMed] [Google Scholar]
- Finazzi-Agro A, Menichelli A, Persiani M, Biancini G, Del Principe D. Hydrogen peroxide release from human blood platelets. Biochim Biophys Acta. 1982;718:21–25. doi: 10.1016/0304-4165(82)90004-6. [DOI] [PubMed] [Google Scholar]
- Fogari R, Zoppi A. Is the effect of antihypertensive drugs on platelet aggregatility and fibrinolysis clinically relevant. Am J Cardiovasc Drugs. 2005;5:211–223. doi: 10.2165/00129784-200505040-00001. [DOI] [PubMed] [Google Scholar]
- Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Kinetic basis for selective inhibition of cyclooxygenases. Biochem J. 1999;339:607–614. [PMC free article] [PubMed] [Google Scholar]
- Holmsen H. Significance of testing platelet functions in vitro. Eur J Clin Invest. 1994;24 Suppl 1:3–8. doi: 10.1111/j.1365-2362.1994.tb02418.x. [DOI] [PubMed] [Google Scholar]
- Hornberger W, Patscheke H. Primary stimuli of ecosanoid release inhibit arachidonyl-CoA synthetase and lysophospholipid acyl transferase. Eur J Biochem. 1990;187:175–181. doi: 10.1111/j.1432-1033.1990.tb15292.x. [DOI] [PubMed] [Google Scholar]
- Huang EM, Detwiler TC.Stimulus–response coupling mechanisms Biochemistry of Platelets 1986Academic Press: New York; 1–50.In: Phillips DR, Shuman MA (eds). [Google Scholar]
- Huang LS, Wang CK, Sheu MJ, Kao LS.Phenolic compounds of piper betle flower as flavoring and neuronal activity modulating agents Phenolic Compounds in Food and their Effects on Health I: Analysis, Occurrence and Chemistry 1993. In Ho CT, Lee CY, Huang MT (eds).Chapter 16, ACS Symposium Series No. 506. American Chemical Society, Washington, DC
- Huss U, Ringbom T, Perera P, Bohlin L, Vasange M. Screening of ubiquitous plant constituents for COX-2 inhibition with a scintillation proximity based assay. J Nat Prod. 2002;65:1517–1521. doi: 10.1021/np020023m. [DOI] [PubMed] [Google Scholar]
- IARC Betel-quid and areca nut chewing and some areca-nut-derived nitrosamines IARC Monographs 2004International Agency for Research on Cancer, WHO, Lyon, France; vol. 85 [PMC free article] [PubMed] [Google Scholar]
- Iuliano L, Colavita AR, Leo R, Pratico D, Violi F. Oxygen free radicals and platelet activation. Free Radical Biol Med. 1997;22:999–1006. doi: 10.1016/s0891-5849(96)00488-1. [DOI] [PubMed] [Google Scholar]
- Iuliano L, Pedersen JZ, Pratico D, Rotilio G, Violi F. Role of hydroxyl radicals in the activation of human platelets. Eur J Biochem. 1994;221:695–704. doi: 10.1111/j.1432-1033.1994.tb18782.x. [DOI] [PubMed] [Google Scholar]
- Iuliano L, Violi F, Pedersen JZ, Pratico D, Rotilio G, Balsano F. Free radical-mediated platelet activation by hemoglobin released from red blood cells. Arch Biochem Biophys. 1992;299:220–224. doi: 10.1016/0003-9861(92)90267-z. [DOI] [PubMed] [Google Scholar]
- Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001;37:477–492. doi: 10.1016/s1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- Jeng JH, Chen SY, Liao CH, Tung YY, Lin BR, Hahn LJ, et al. Modulation of platelet aggregation by areca nut and betel leaf ingredients: roles of reactive oxygen species and cyclooxygenase. Free Radical Biol Med. 2002;32:860–871. doi: 10.1016/s0891-5849(02)00749-9. [DOI] [PubMed] [Google Scholar]
- Jeng JH, Wang YJ, Chang WH, Wu HL, Li CH, Uang BJ, et al. Reactive oxygen species are crucial for hydroxychavicol toxicity toward KB epithelial cells. Cell Mol Life Sci. 2004;61:83–96. doi: 10.1007/s00018-003-3272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng JH, Wu HL, Lin BR, Lan WH, Chang HH, Ho YS, et al. Antiplatelet effect of sanguinarine is correlated to calcium mobilization, thromboxane and cAMP production. Atherosclerosis. 2007;191:250–258. doi: 10.1016/j.atherosclerosis.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Kariyazono H, Nakamura K, Arima J, Ayukawa O, Onimaru S, Masuda H, et al. Evaluation of antiplatelet aggregatory effects of aspirin, cilostazol and ramatroban on platelet-rich plasma and whole blood. Blood Coagul Fibrinolysis. 2004;15:157–167. doi: 10.1097/00001721-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Kato M, Nishida S, Kitasato H, Sakata N, Kawai S. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: investigation using human peripheral monocytes. J Pharm Pharmacol. 2001;53:1679–1685. doi: 10.1211/0022357011778070. [DOI] [PubMed] [Google Scholar]
- Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. 1995;24:450–453. doi: 10.1111/j.1600-0714.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- Krotz F, Sohn HY, Gloe T, Zahler S, Rieninger T, Schiele TM, et al. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100:917–924. doi: 10.1182/blood.v100.3.917. [DOI] [PubMed] [Google Scholar]
- Krotz F, Sohn HY, Pohl U. Reactive oxygen species. Players in the platelet game. Arterioscler, Thromb Vasc Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- Lahiri M, Bhide SV. Effect of four plant phenols, β-carotene and α-tocopherol on 3(H)benzopyrene–DNA interaction in vitro in the presence of rat and mouse liver postmitochondrial fraction. Cancer Lett. 1993;73:35–39. doi: 10.1016/0304-3835(93)90185-c. [DOI] [PubMed] [Google Scholar]
- Leoncini G, Signorello MG, Piana A, Carrubba M, Armani U. Hyperactivity and increased hydrogen peroxide formation in platelets of NIDDM patients. Thromb Res. 1997;86:153–160. doi: 10.1016/s0049-3848(97)00058-3. [DOI] [PubMed] [Google Scholar]
- Li N, Hu H, Hjemdahl P. Aspirin treatment does not attenuate platelet or leukocyte activation as monitored by shole blood flow cytometry. Thromb Res. 2003;111:165–170. doi: 10.1016/j.thromres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Mannan N, Boucher BJ, Evans SJ. Increased waist size and weight in relation to consumption of Areca catechu (betel-nut): a risk factor for increased glycemia in Asians in east London. Br J Nutr. 2000;83:267–275. doi: 10.1017/s0007114500000349. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Safier LB. Thromboregulation: multicellular modulation of platelet reactivity in haemostasis and thrombosis. FASEB J. 1993;7:516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- Maurice P, Legrand C, Fauvel-Lafeve F. Platelet adhesion and signaling induced by the octapeptide primary binding sequence (KOGEOGPK) from type III collagen. FASEB J. 2004;18:1339–1347. doi: 10.1096/fj.03-1151com. [DOI] [PubMed] [Google Scholar]
- Miller CC, Hale P, Pentland AP. Ultraviolet B injury increase prostaglandin synthesis through atyrosine kinase dependent pathway. J Biol Chem. 1994;269:3529–3533. [PubMed] [Google Scholar]
- Nagabhushan M, Amonkar AJ, D'Souza AV, Bhide SV. Hydroxycahvicol: a new anti-nitrosating phenolic compound from betel leaf. Mutagenesis. 1989;4:200–204. doi: 10.1093/mutage/4.3.200. [DOI] [PubMed] [Google Scholar]
- Padma PR, Amonkar AJ, Bhide SV. Antimutagenic effects of betel leaf extract against the mutagenicity of two tobacco-specific N-nitrosamines. Mutagenesis. 1989;4:154–156. doi: 10.1093/mutage/4.2.154. [DOI] [PubMed] [Google Scholar]
- Rao AR. Modifying influences of betel quid ingredients on B(a)P-induced carcinogenesis in the buccal pouch of hamster. Int J Cancer. 1984;33:581–586. doi: 10.1002/ijc.2910330506. [DOI] [PubMed] [Google Scholar]
- Robbins IM, Kawut SM, Yung D, Reilly MP, Lloyd W, Cunningham G, et al. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;27:578–584. doi: 10.1183/09031936.06.00095705. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. The ERK cascade, a new pathway involved in the activation of store-mediated calcium entry in human platelets. Trends Cardiovasc Med. 2002;12:229–234. doi: 10.1016/s1050-1738(02)00161-5. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: an inflammatory disease. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rotondo S, Krauze-Brzosko K, Manarini S, Evangelista V, Cerletti C. Licofelone, an inhibitor of cyclooxygenase and 5-lipooxygenase, specifically inhibits cyclooxygenase-1-dependent platelet activation. Eur J Pharmacol. 2004;488:79–83. doi: 10.1016/j.ejphar.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Runnie I, Salleh MN, Mohamed S, Head RJ, Abeywardena MY. Vasorelaxation induced by commom edible tropical plant extracts in isolated rat aorta and mesenteric vascular bed. J Ethnopharmacol. 2004;92:311–316. doi: 10.1016/j.jep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Saeed SA, Farnaz S, Simjee RU, Malik A. Triterpenes and B-sitosterol from Piper betle: isolation, antiplatelet and anti-inflammatory effects. Biochem Soc Transact. 1993;21:462S. doi: 10.1042/bst021462s. [DOI] [PubMed] [Google Scholar]
- Salleh MN, Runnie I, Roach PD, Mohamed S, Abeywardena MY. Inhibition of low-density lipoprotein oxidation and up-regulation of low-density lipoprotein receptor in HepG2 cells by tropical plant extracts. J Agric Food Chem. 2002;50:3693–3697. doi: 10.1021/jf011593f. [DOI] [PubMed] [Google Scholar]
- Shirname LP, Menon MM, Nair J, Bhide SV. Correlation of mutagenicity and tumorigenicity of betel quid and its ingredients. Nutr Cancer. 1983;5:87–91. doi: 10.1080/01635588309513783. [DOI] [PubMed] [Google Scholar]
- Tsai CC.The association between areca/betel quid chewing, cigarette smoking, alcohol drinking and asthma, heart disease and peptic ulcer in Taiwan 2003Kaohsiung Medical University; Master thesis [Google Scholar]
- Warnakulasuriya S, Trivedy C, Peters TJ. Areca nut use: an independent risk factor for oral cancer: the health problem is under recognized. BMJ. 2002;324:799–800. doi: 10.1136/bmj.324.7341.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby S, Holmes A, Losalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nursing. 2002;1:273–288. doi: 10.1016/s1474-5151(02)00038-5. [DOI] [PubMed] [Google Scholar]
- Yamada K, Shuto K, Nakamizo N. Inhibition of platelet aggregation by a new agent, 2,2′-dithiobis-(N-2-hydroxypropyl benzamide) (KF4939) Thromb Res. 1983;29:197–206. doi: 10.1016/0049-3848(83)90141-x. [DOI] [PubMed] [Google Scholar]