Abstract
Background and purpose:
Blockade of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors is a good treatment option for a variety of central nervous system disorders. The present study evaluated the neuroprotective and anticonvulsant effects of EGIS-8332, a non-competitive AMPA receptor antagonist, as a potential drug candidate.
Experimental approach:
AMPA antagonist effects of EGIS-8332 were measured using patch-clamp techniques. Neuroprotective and anticonvulsant effects of EGIS-8332 were evaluated in various experimental models, relative to those of GYKI 53405.
Key results:
EGIS-8332 inhibited AMPA currents in rat cerebellar Purkinje cells and inhibited the AMPA- and quisqualate-induced excitotoxicity in primary cultures of telencephalon neurons (IC50=5.1-9.0 μM), in vitro. Good anticonvulsant actions were obtained in maximal electroshock-, sound- and chemically-induced seizures (range of ED50=1.4-14.0 mg kg−1 i.p.) in mice. Four days after transient global cerebral ischaemia, EGIS-8332 decreased neuronal loss in the hippocampal CA1 area in gerbils and rats. EGIS-8332 dose-dependently reduced cerebral infarct size after permanent middle cerebral artery occlusion in mice and rats (minimum effective dose=3 mg kg−1 i.p.). Side effects of EGIS-8332 emerged much above its pharmacologically active doses. A tendency for better efficacy of GYKI 53405 than that of EGIS-8332 was observed in anticonvulsant tests that reached statistical significance in few cases, while the contrary was perceived in cerebral ischaemia tests.
Conclusions and implications:
EGIS-8332 seems suitable for further development for the treatment of epilepsy, ischaemia and stroke based on its efficacy in a variety of experimental disease models, and on its low side effect potential.
Keywords: ischaemia, stroke, epilepsy, AMPA, rat, mouse, gerbil
Introduction
Glutamate is the most abundant excitatory neurotransmitter in the brain. Glutamate receptors are categorized into ionotropic and metabotropic types, based on their structure, function and pharmacology. The metabotropic glutamate receptors are coupled to G-proteins. There are three subgroups of ionotropic glutamate receptors, which are ion channels allowing cation flow into the neurons, namely N-methyl-D-aspartic acid (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainic acid receptors. Ionotropic glutamate receptors are present in neurons and astrocytes (Dingledine et al., 1999; Palmer et al., 2005).
According to the excitotoxic hypothesis, excessive stimulation of glutamate receptors leads to neuronal apoptosis and necrosis (Frandsen and Schousboe, 2003). Therefore, excitatory neurotransmitter receptors are strongly implicated in the mechanism of a number of seemingly unrelated brain disorders, such as ischaemia–hypoxia, epilepsy, traumatic brain injury, Parkinson's and motoneuron diseases, in which the activity of the glutamatergic neurotransmission is elevated. As a consequence, glutamate receptor antagonists can have therapeutic utility in the treatment of a wide range of neurological disorders (Jurányi et al., 2004; Szénási and Hársing, 2004; Weiser, 2005).
Several clinical trials aimed at decreasing the functional consequences of stroke. A number of compounds belonging to different mechanisms of action failed to achieve that goal so far, despite the same compounds producing robust neuroprotective effects in animal experiments. Early promise in this field was provided by the demonstration that NXY-059, a free-radical-trapping agent, reduced disability at 90 days but without improving other outcome measures, including neurological functioning if administered within 6 h after the onset of acute ischaemic stroke (Lees et al., 2006). Unfortunately, a later large clinical trial did not confirm the first results, so this drug finally failed in phase III. Despite the negative outcome of stroke trials, pharmacotherapy of ischaemic stroke can be effective, and thoroughly assessed neuroprotective compounds with other mechanisms of action may provide benefit for patients in carefully designed clinical trials.
NMDA receptor blockers have produced remarkable neuroprotective effects in animal models of stroke. However, these compounds failed in clinical trials mainly due to their pronounced side effects at therapeutic doses. In contrast, the side effect profile and therapeutic window of AMPA antagonists appear to be more favourable (Nielsen et al., 1999; Weiser, 2002). The first generation of selective, competitive AMPA receptor antagonists with quinoxaline-2,3-dione chemical structures, such as NBQX, PNQX and YM-90K, had poor water solubility at physiological pH and, combined with rapid kidney excretion, caused nephrotoxicity due to precipitation in the kidneys. At higher doses, respiratory depression was also observed (Turski et al., 1998; Nielsen et al., 1999; Nikam and Kornberg, 2001). As previously described, a 2,3-benzodiazepine, GYKI 52466 and its 3-N-substituted, 3,4-reduced analogue, GYKI 53405, which is one of the best-known AMPA receptor antagonists from the 2,3-benzodiazepine chemical structure, selectively antagonized AMPA receptors in a non-competitive manner (Tarnawa and Vízi, 1998). These compounds are also termed as negative allosteric modulators of AMPA receptors. Talampanel, the active enantiomer of GYKI 53405, has reached phase II clinical trial as an antiepileptic agent (Chappell et al., 2002).
We have clearly shown earlier that non-competitive AMPA antagonists prevented glutamate-induced neuronal death at any agonist concentration, but the protective effect of competitive AMPA antagonists was lost at high agonist concentration (Kovács and Szabó, 1997). Neuroprotective and antiepileptic effects of non-competitive AMPA antagonist GYKI compounds were demonstrated in several experimental models of global and focal cerebral ischaemia in rats, gerbils and mice and in different seizure tests (Chapman et al., 1991; Yamaguchi et al., 1993; Bleakman et al., 1996; Lodge et al., 1996; Gyertyán et al., 1999; Szabados et al., 2001; Kapus et al., 2004; Matucz et al., 2004; Gressens et al., 2005). Several compounds with this chemical structure reduced neuronal loss in the CA1 area of the hippocampus at 4 day after a 3 min bilateral carotid artery occlusion in gerbils, which is an acceptable model of delayed neuronal death (Kapus et al., 2004).
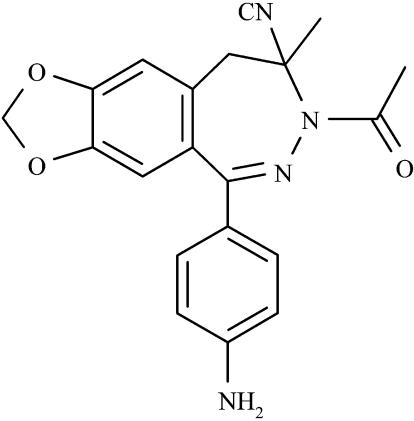
The disadvantage of 2,3-benzodiazepine AMPA antagonists is that they strongly decrease body temperature, and cause muscle relaxation at high pharmacologically active doses. We have synthesized and screened a series of new 2,3-benzodiazepines in an attempt to optimize the chemical structure. EGIS-8332 ([+/−]-7-acetyl-5-[4-aminophenyl]-7,8-dihydro-8-cyano-8-methyl-9H-1,3-dioxolo-[4,5-h]-2,3-benzodiazepine) is one of the newly developed potent non-competitive AMPA antagonists (Figure 1). The aim of the present study was to relate the efficacy of EGIS-8332 in neuroprotective and antiepileptic tests to its side effects, in order to establish its suitability for further development as a drug candidate. GYKI 53405 was also evaluated for comparison in most tests.
Figure 1.
The chemical structure of EGIS-8332 ([+/−]-7-acetyl-5-[4-aminophenyl]-7,8-dihydro-8-cyano-8-methyl-9H-1,3-dioxolo-[4,5-h]-2,3-benzodiazepine).
Methods
Animals
Male NMRI mice were supplied by Charles River Hungary Ltd, and male DBA/2j mice were obtained from National Institute of Oncology (Budapest, Hungary). Male Sprague–Dawley rats were derived from our own colony, while male Wistar rats were supplied by Charles River Hungary Ltd. Male Mongolian gerbils were obtained from Tomker Kft. (Budapest, Hungary). The animals were kept in polycarbonate cages in thermostatically controlled rooms at 23±2°C and at relative humidity of 60±10%. The rooms were artificially illuminated with lights on 0600–1800 hours. The animals were given commercial pellet rat–mouse feed and sterile filtered tap water, ad libitum. The care and use of the experimental animals were in accordance with the 86/609/EEC directive.
In vitro experiments
Patch-clamp recordings
Cerebellar Purkinje neurons were prepared from 8-day-old rats according to Cebers et al. (1997). Cell suspensions were plated onto poly-L-lysine-precoated Corning plastic dishes of 6 cm diameter at a density of 5 × 105 cells per dish, and used on the day of preparation. Whole-cell patch-clamp studies were performed in voltage clamp mode at −70 mV at room temperature. Solutions contained (in mM): 140 KCl, 4 NaCl, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 5 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′,-tetraacetic acid (EGTA), 0.5 CaCl2, pH 7.2 in the micropipette and 140 NaCl, 5 KCl, 10 HEPES, 2 MgCl2, 2 CaCl2, 10 glucose, pH 7.3 in the reaction medium. Osmolarity of the solutions was 294±2 mosM. Test solutions containing 0.25 mM kainate, EGIS-8332 or GYKI 53405, or in combination were perfused via a polyimide tube (ID=0.4 mm) at a rate of about 1 ml min−1 to 0.5 mm from the cell studied. The flow rate was set by gravity. In the test solutions, the concentration of dimethyl sulphoxide (DMSO) did not exceed 0.08% (v/v). Borosilicate patch electrodes with tip resistance between 4.5 and 6 MΩ were attached to a CV-4 head stage and connected to an AXOPATCH-1D amplifier. Voltage control and data acquisition were achieved by Digidata 1200(2) board and Clampex version 7.0. Currents were recorded and analysed using pClamp 7.02 software package (all from Axon Instruments, Foster City, CA, USA). The data were fitted to a sigmoid dose–response curve, and IC50 values were calculated (SigmaPlot 7.0, SPSS Inc.).
Inhibition of AMPA- and quisqualate-induced cell death, in vitro
Telencephalon cells from 16-day-old embryos of Sprague–Dawley rats were prepared and grown according to Kovács and Szabó (1997). Cells were plated onto 13-mm glass plates precoated with poly-L-lysine and cultured under humidified carbogen (5% CO2/95% O2) atmosphere in Eagle's essential medium containing 4 mM L-glutamine, 12 mM D-glucose, 25 mM KCl, 10% heat-inactivated serum (from foetal calf, on the first 7 days and horse, thereafter) and antibiotics (gentamycin sulphate, 50 mg ml−1 and amphotericin B, 2.5 mg ml−1 on the first 7 days, and penicillin, 100 000 U l−1, thereafter). KCl was omitted from the culture medium from day 7. Medium was changed on the second and seventh day. Overgrowth of glial cells was diminished by the application of 10 M cytosine arabinoside for 24 h on day 6.
The experiments were performed in 17- to 18-day-old cultures. The cultures were preincubated with the test compounds for 30 min at 37°C before addition of AMPA (20 μM) or quisqualate (15 μM) (Tocris Neuramin) for 22–24 h. Final concentration of DMSO was 0.1% in the culture medium. Neuronal cell death was estimated visually by phase-contrast microscopy and quantified by measuring lactate dehydrogenase (LDH) activity in the culture medium. After 22–24 h exposure to AMPA/quisqualate and one of the antagonists, the culture medium was removed for measuring LDH activity (LDH-A), and replaced by a medium supplemented with 500 μM glutamate without horse serum to destroy all surviving cells. After 22–24 h additional incubation, the culture medium was removed for measuring LDH activity (LDH-B). Neurotoxicity was expressed as follows: [LDH-A/(LDH-A+LDH-B)] × 100. The antagonists alone had no effect on the viability of cultured telencephalic neurons. LDH activity was measured in duplicates from 60 μl aliquot of culture medium in 96-well microtiter plates at 37°C as a decrease of NADH absorbance at 340 nm using a kinetic enzyme-linked immunosorbent assay reader.
Seizure models
Maximal electroshock in mice
The experiments were performed in separate groups (n=10) of mice weighing 20–25 g according to Swinyard et al. (1952). The animals were treated with the test compounds intraperitoneally (i.p.) or per os (p.o.) at various doses or with the vehicle (control group). Maximal electroshock (MES) was administered via corneal electrodes (0.4 s, 40 mA 50 Hz AC) 30 min later after i.p. treatment and 60 min after p.o. treatment. The incidence of tonic convulsions (positive reaction) was recorded.
Audiogenic seizure in mice
The experiments were performed according to the method of Szabados et al. (2001). The test compounds or vehicle (control group) were given i.p. to groups of 9-16 mice per dose. The mice were challenged with a 14 kHz sinusoidal tone at 120 dB in a covered glass cylinder (30 cm in diameter) 15 min later. Seizure response was assessed using the following scale: (0) normal behaviour, (1) wild running, (2) clonus, (3) tonic flexor seizure, (4) tonic extensor seizure. The maximum response during the next 60 s was recorded for each animal. Lethality was also noted.
Chemoconvulsant (nicotine, bicuculline, pentylenetetrazole, picrotoxine) seizures in mice
Separate groups of mice (n=10) were injected i.p. or subcutaneous (s.c.) with test compounds 30 min before the administration of a chemoconvulsant (1.4 mg kg−1 nicotine intravenous (i.v.); 3 mg kg−1 bicuculline s.c.; 125 mg kg−1 pentylenetetrazole (PTZ) i.p.; 10 mg kg−1 picrotoxin i.p.). The animals were observed for 30 min, and the incidence of tonic convulsions (positive reaction) was recorded.
Global cerebral ischaemia models
Bilateral carotid occlusion in gerbils
Transient forebrain ischaemia was produced in ether-anaesthetized Mongolian gerbils weighing between 70 and 90 g according to the method of Gyertyán et al. (1999). The body temperature was controlled at 37±1°C with a heating pad and a heating lamp. Both common carotid arteries were carefully exposed and clamped for 3 min using aneurysm clips. Sham-operated gerbils underwent the same procedure without occluding the carotid arteries.
The rectal temperature of gerbils was recorded by an electric thermometer at several time points before, during and after surgery for up to 24 h after drug injection. The area under the curve (AUC) was calculated as the area enclosed by the temperature curves of the vehicle-treated and drug-treated bilateral carotid occlusion (BCO) groups from the end of surgery to the last reading of temperature.
Behavioural evaluation: Four days after surgery, spontaneous alternation response and locomotor activity of gerbils were measured in a symmetrical Y-maze (40 cm long and 10 cm wide arms with 21.5 cm high walls). The animals were placed in the middle of the maze, and then the sequence of entries into the three arms was recorded for 5 min. By definition, the gerbil performed an arm entry when it fully entered the arm. The animal was considered to exit when it fully left the arm. An arm entry was accepted as spontaneous alternation when the animal entered the least recently visited arm. The total number of arm entries was counted and spontaneous alternation as the percentage of total arm entries was calculated for each animal.
Histological evaluation: After the behavioural test, gerbils were anaesthetized with pentobarbital (60 mg kg−1 i.p.) and perfused through the heart, first with saline for 5 min, and then with a fixative solution containing 0.1% glutaraldehyde, 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.4) for 20 min. The brain was removed from the skull and post-fixed for 1 week at 4°C in the same fixative. Six 60 μm coronal sections were cut from different levels of the dorsal hippocampus (from 0.6 to 1.2 mm caudal to bregma) by a microtome. The sections were repeatedly washed in 0.1 M phosphate buffer. The sections were stained by silver impregnation according to Gallyas et al. (1980), and then they were mounted on gelatin-coated slides and dried. Necrotic neurons were shrunken and strongly argrylophilic, somata of intact neurons appeared dark yellow. Irreversible neuronal damage in the hippocampal CA1 subfield in both hippocampi was scored on a 7 point scale: (0) undamaged, (1) <10%, (2) 10–30%, (3) 30–50%, (4) 50–70%, (5) 70–90% and (6) 90–100% cell loss. In case of asymmetrical damage, the higher score of the two sides was used in the statistical evaluation. The sections were scored without knowledge of the treatments.
Four-vessel occlusion in rats
Both vertebral arteries were occluded by electrocoagulation in male SPRD rats (180–270 g) under pentobarbital anaesthesia (60 mg kg−1 i.p.). The rats were anaesthetized with ether 24 h later, and both carotid arteries were occluded for 10 min. During surgery, the body temperature of the animals was kept at the individual preoperative levels using a heating pad and a heating lamp. Four days after surgery, the animals were deeply anaesthetized with pentobarbital, and perfused through the heart by a fixative solution. Histological evaluation was performed as described above.
Focal cerebral ischaemia models
Permanent middle cerebral artery occlusion in mice
Focal cortical ischaemia was produced according to Welsh et al. (1987). Male mice (30–35 g) were anaesthetized with 2,2,2-tribromoethanol (500 mg kg−1 i.p., 10 ml kg−1, Fluka, Buchs, Switzerland). An incision was made in the left temporo-parietal region of the head between the orbit and the ear. The temporal muscle was incised and a small burr hole was drilled into the lateral outer surface of the skull just over the middle cerebral artery (MCA). The stem of the MCA was occluded by electrocoagulation. All compounds were administered i.p. 30 min after MCA occlusion (MCAO). Two days later, the animals were deeply anaesthetized with pentobarbital (100 mg kg−1 i.p., 10 ml kg−1), perfused through the heart with a 4% solution of 2,3,5-triphenyltetrazolium chloride (Fluka) in saline. The animals were decapitated and the brains were removed and placed in saline containing 8% formalin for at least 24 h. The necrotic (non-stained) area on the brain surface was determined by means of an image analysing system (DigiCell for Windows 4.0).
Permanent middle cerebral artery occlusion in rats
Permanent focal ischaemia was produced according to Brint et al. (1988). Male Sprague–Dawley rats (180–220 g) were anaesthetized with pentobarbital (60 mg kg−1 i.p.). The temporal muscle was excised and a 2 mm burr hole was drilled 2–3 mm rostral to the fusion of the zygomatic arch with the squamosal bone. The left MCA was occluded by electrocoagulation. The left common carotid artery was isolated and simultaneously occluded by electrocoagulation. The animals were deeply anaesthetized with pentobarbital (100 mg kg−1 i.p.) 48 h later, and they were perfused through the heart with a 4% solution 2,3,5-triphenyltetrazolium chloride. The animals were decapitated and the brains were removed and placed in saline containing 8% formalin for at least 24 h. Each brain was sliced into 1 mm sections, which included the necrotic tissue. The area of necrosis was measured in each slice using a morphometric software (DigiCell for Windows 4.0). The volume of ischaemic damage (mm3) was calculated as the sum of infarct areas (mm2). The compounds were administered 30 min after MCAO.
Inclined screen (muscle relaxant effect)
The experiments were performed in separate groups of 10 mice weighing 20–25 g according to the method of Hoppe (1950). The animals were treated with the test compounds at 30 mg kg−1 i.p. The mice were placed on an inclined screen (at an angle of 60°) at different times after treatment with EGIS-8332 or GYKI 53405. Mice that failed to hang on the screen for 30 s were considered positive (myasthenic).
Behavioural effects in mice
Observational studies were carried out in mice according to the method of Irwin (1968). The compounds were administered at 30, 100 and 300 mg kg−1 i.p., and at 100, 500 and 1000 mg kg−1 p.o., and the symptoms were observed 30 min after i.p. treatment and 60 min after oral administration. Minimum effective dose (MED) was determined as the lowest dose, which produced behavioural symptoms.
Pharmacokinetics
Wistar rats, NMRI mice and gerbils were treated with EGIS-8332 at 20 mg kg−1 i.p. Plasma was obtained from mouse and gerbils (0.25 ml) and rats (0.5 ml) into heparinized vials at different time points from 15 min to 4 h after EGIS-8332 administration. [+/−]-7-propionyl-5-[4-aminophenyl]-7,8-dihydro-8-cyano-8-methyl-9H-1,3-dioxolo-[4,5-h]-2,3-benzodiazepine was used as internal standard. Plasma proteins were precipitated with perchloric acid and the compounds were extracted with chloroform after alkalization. After evaporation to dryness, the residue was dissolved in an eluent of the following composition: 50% 2 mM heptafluorobutyric acid, 35% methanol and 15% acetonitrile. The assay utilized solid-phase extraction on a C18 cartridge followed by reversed-phase Beckman System Gold HPLC with ultraviolet detection at 240 nm. Peak area (PA) of EGIS-8332 was corrected by the PA of the internal standard. Standard curves of EGIS-8332 were constructed from 0.125 to 8 μg. The assay was validated in respect to selectivity, linearity, intra-day precision and intra-day accuracy.
Statistics
Results are presented as means±s.e.m. In seizure tests percentage inhibition, ED50 values and 95% confidence limits were obtained by log-probit analysis and the effects of the two compounds were compared as described by Litchfield and Wilcoxon (1953). Statistical significance was assessed using two-way analysis of variance (ANOVA) followed by Duncan's test in MCAO tests. Body temperature of gerbils was compared using two-way ANOVA for repeated measures followed by Duncan's test. Differences between histological results in drug-treated and vehicle-treated groups of gerbils were analysed by Mann–Whitney U-test. Differences in hypermotility and spontaneous alternation among the groups were evaluated by Kruskal–Wallis ANOVA. In case of a significant group effect (P<0.05), Mann–Whitney U-test was used for paired comparisons.
Chemicals
EGIS-8332 and GYKI 53405 were synthesized at the Division of Chemical Research of EGIS Pharmaceuticals PLC. EGIS-8332 and GYKI 53405 were dissolved using DMSO, 40% hydroxypropyl-β-cyclodextrin (HPCD, Sigma-Aldrich, Budapest, Hungary) solution in saline, and 5 M HCl solution (Merck, Darmstadt, Germany) for experiments in vivo. The volume of DMSO or HPCD solution was not more than 10% of the total volume, and pH was adjusted to 5.0 with 1 M NaOH. For experiments in vitro, the test compounds were dissolved in DMSO to produce a concentration of 5–100 mM, and further diluted with the medium to the final concentrations. Sodium pentobarbital was obtained from CEVA-Phylaxia (Budapest, Hungary). All other chemicals and cell culture reagents were purchased from Sigma Hungary Ltd, except for kainate (Tocris, Bristol, UK).
Results
In vitro experiments
Both EGIS-8332 and GYKI 53405 inhibited whole cell AMPA currents in patch-clamp studies. EGIS-8332 was more active at AMPA receptors than GYKI 53405 in cerebellar Purkinje cells, as IC50 of EGIS-8332 was lower than that of GYKI 53405 (9.0±1.4 vs 16.3±5.0 μM, P<0.05). EGIS-8332 inhibited the quisqualate-induced (IC50: 7.2±0.9 μM) and the AMPA-induced (IC50: 5.1±0.4 μM) excitotoxicity in primary telencephalon cell culture. GYKI 53405 also protected against quisqualate-induced (IC50: 7.2±1.2 μM) and AMPA-induced (IC50: 9.3±1.1 μM) excitotoxicity. GYKI 53405 and EGIS-8332 had similar efficacy in both tests.
Seizure models
MES in mice
Both EGIS-8332 and GYKI 53405 protected mice against generalized extensor seizures caused by MES in a dose-dependent manner (Table 1). EGIS-8332 and GYKI 53405 blocked convulsions at similar doses both after i.p. and p.o. administrations.
Table 1.
Anticonvulsant effects of EGIS-8332 and GYKI 53405 in MES and AS tests in mice
| Compound |
MES |
AS tonus | AS clonus | |
|---|---|---|---|---|
| ED50 | ED50 | ED50 | ED50 | |
| mg kg−1 i.p. | mg kg−1 p.o. | mg kg−1 i.p. | mg kg−1 i.p. | |
| EGIS-8332 | 4.8 (3.69–6.30) | 7.2 (3.65–14.32) | 1.4 (0.98–1.96) | 2.4 (1.39–4.02) |
| GYKI 53405 | 4.9 (3.21–7.46) | 3.2 (2.07–5.09) | 1.4 (1.02–1.91) | 2.4 (1.73–3.26) |
Abbreviations: AS, audiogenic; ED50, half-maximal effective dose; i.p., intraperitoneal; MES, maximal electroshock, p.o., per os.
The ED50 values for the inhibition of tonic hindlimb extensor seizure in MES test and for clonic or tonic convulsions in AS test were calculated according to Litchfield and Wilcoxon (1953). 95% confidence intervals are shown in brackets.
Audiogenic seizure in mice
EGIS-8332 and GYKI 53405 prevented sound-induced clonic and tonic convulsions in a dose-dependent manner. The anticonvulsant activity of EGIS-8332 was similar to that of GYKI 53405 in the audiogenic seizure (AS) test (Table 1).
Seizures induced by nicotine, bicuculline, PTZ and picrotoxine in mice
EGIS-8332 and GYKI 53405 displayed a dose-related anticonvulsant activity against several chemically induced seizures. EGIS-8332 produced the most effective anticonvulsant action in the PTZ model, and it was the least effective against the nicotine-induced seizures. EGIS-8332 blocked PTZ-induced convulsions at similar doses to that of GYKI 53405 (Table 2). The potency of GYKI 53405 was greater than that of EGIS-8332 in nicotine-, bicuculline- and picrotoxine-induced seizure tests (P<0.05).
Table 2.
Anticonvulsant effects of EGIS-8332 and GYKI 53405 in nicotine, bicuculline, PTZ and picrotoxin seizure models in mice
| Compound | Nicotine | Bicuculline | PTZ | Picrotoxin |
|---|---|---|---|---|
| ED50 | ED50 | ED50 | ED50 | |
| mg kg−1 i.p. | mg kg−1 i.p. | mg kg−1 s.c. | mg kg−1 s.c. | |
| EGIS-8332 | 14.0 (11.23–17.39) | 6.9 (5.58–8.60) | 3.1 (2.10–4.71) | 7.2 (5.27–9.95) |
| GYKI 53405 | 6.0* (4.61–7.83) | 2.8* (2.07–3.76) | 2.6 (1.68–3.88) | 0.7* (0.45–1.11) |
Abbreviations: ED50, half-maximal effective dose; i.p., intraperitoneal; PTZ, pentylenetetrazole; s.c., subcutaneous.
The ED50 for the inhibition of tonic hindlimb extensor seizure values were calculated according to Litchfield and Wilcoxon (1953). 95% confidence intervals are shown in brackets.
P<0.05 vs the group treated with EGIS-8332.
Global cerebral ischaemia models
Bilateral carotid occlusion in gerbils
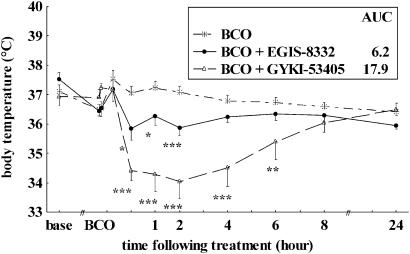
Occlusion of both carotid arteries for 3 min killed the majority of pyramidal cells in the CA1 area of the hippocampus, and increased the number of arm entries in a Y maze (hypermotility) at 4 days after ischaemia in all experiments (Table 3). BCO also impaired spontaneous alternation response, a measure of spatial memory, in two out of four experiments. Treatment of gerbils with EGIS-8332 at 45 min after ischaemia decreased hippocampal CA1 neuronal cell death by 37 and 67% at 10 or 20 mg kg−1 i.p., respectively. GYKI 53405 reduced pyramidal cell death by 35 and 53% at 10 or 20 mg kg−1 i.p., respectively, that is, the neuroprotective effects of the two compounds were similar. The higher dose of EGIS-8332 and both doses of GYKI 53405 inhibited hypermotility but neither compound reversed the ischaemia-induced impairment in spontaneous alternation response (Table 3). EGIS-8332 induced a smaller and shorter lasting decrease in body temperature than GYKI 53405 (P<0.001), reflected also by a lower AUC for EGIS-8332 than for GYKI 53405 (Figure 2).
Table 3.
Effects of EGIS-8332 and GYKI 53405 on pyramidal cell death in the CA1 area of the hippocampus, spontaneous alternation response and locomotor activity measured in a Y-maze following a 3-min BCO in Mongolian gerbils
| Groups | Ischaemic damage (score) | Spontaneous alternation (% of all entries) | Locomotor activity (number of arm entries) |
|---|---|---|---|
| Sham operation | 72.3±2.7 | 35.1±1.8 | |
| BCO | 4.87±0.14 | 51.1±2.5+++ | 49.0±3.2+ |
| BCO+EGIS-8332, 10 mg kg−1 i.p. | 3.00±0.59** | 54.9±3.5++ | 39.4±3.7 |
| Sham operation | 58.5±2.0 | 42.6±3.0 | |
| BCO | 4.71±0.18 | 51.4±5.9 | 60.1±5.4+ |
| BCO+EGIS-8332, 20 mg kg−1 i.p. | 1.57±0.65** | 54.9±3.6 | 41.6±6.4* |
| Sham operation | 57.6±3.3 | 38.5±3.0 | |
| BCO | 4.90±0.10 | 54.6±2.9 | 60.7±2.8++ |
| BCO+GYKI 53405, 10 mg kg−1 i.p. | 3.20±0.59** | 56.5±4.2 | 41.7±4.4** |
| Sham operation | 62.3±3.5 | 36.5±3.2 | |
| BCO | 4.70±0.21 | 46.8±3.0+ | 57.7±4.3++ |
| BCO+GYKI 53405, 20 mg kg−1 i.p. | 2.22±0.76** | 49.2±2.8+ | 43.8±4.1* |
Abbreviations: ANOVA, analysis of variance; BCO: bilateral carotid artery-occluded, vehicle-treated animals; i.p., intraperitoneal; Sham: sham-operated, vehicle-treated animals.
The compounds or vehicle were administered 45 min after BCO (n=8–15/group). Behavioural variables were measured on day 4 after surgery.
Data shown in the table are means±s.e.m.; EGIS-8332 and GYKI 53405 were administered after BCO at doses indicated.
P<0.05,
P<0.01,
P<0.001 compared with the sham-operated group.
P<0.05,
P<0.01 compared with the BCO group (Kruskal–Wallis ANOVA followed by Mann–Whitney U-test).
Figure 2.
Effects of EGIS-8332 and GYKI 53405 on body temperature of Mongolian gerbils subjected to 3 min of BCO. The compounds were administered at 20 mg kg−1 i.p. 45 min after BCO. *P<0.05, **P<0.01, ***P<0.001 vs BCO; AUC (n=9–14). AUC, area under the curve; BCO, bilateral carotid occlusion.
Four-vessel occlusion in rats
Both EGIS-8332 and GYKI 53405 decreased hippocampal CA1 pyramidal cell death (Control=3.69±0.52 (n=13) vs EGIS-8332=2.18±0.60 (n=11), 41%, P<0.05; Control=4.70±0.60 (n=10) vs GYKI 53405=2.40±0.75 (n=10), 49%, P< 0.05) at the dose of 30 mg kg−1 i.p. given 30 min after reperfusion in rats. The neuroprotective effects of EGIS-8332 and GYKI 53405 were similar in the 4VO test.
Focal cerebral ischaemia models
Permanent MCAO in mice
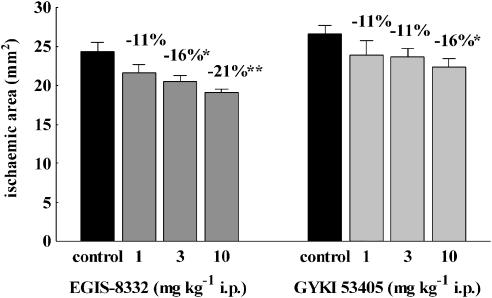
Both compounds decreased the area of cerebral infarction in the focal cerebral ischaemia test in mice (Figure 3). The MED of EGIS-8332 was lower (3 mg kg−1 i.p.) than that of GYKI 53405 (10 mg kg−1 i.p.), and EGIS-8332 was more effective than GYKI 53405 (P<0.05).
Figure 3.
Effects of various doses of EGIS-8332 and GYKI 53405 on the area of brain infarction after permanent MCAO in mice. The compounds were administered 30 min after the start of ischaemia. *P<0.05, **P<0.01 vs control (n=5–11). MCAO, middle cerebral artery occlusion.
Permanent MCAO in rats
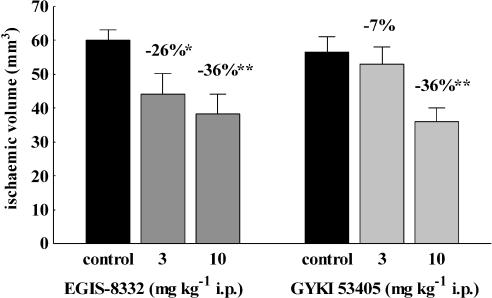
Both compounds decreased the volume of brain infarctions in the MCAO test in rats (Figure 4). The MED of EGIS-8332 was lower (3 mg kg−1 i.p.) than that of GYKI 53405 (10 mg kg−1 i.p.), but the effects of the two compounds were not statistically different at any dose administered.
Figure 4.
Effects of various doses of EGIS-8332 and GYKI 53405 on the volume of brain infarction after permanent MCAO in rats. The compounds were administered 30 min after the start of ischaemia. *P<0.05, **P<0.01 vs control (n=7–12). MCAO, middle cerebral artery occlusion.
Inclined screen (muscle relaxant effect)
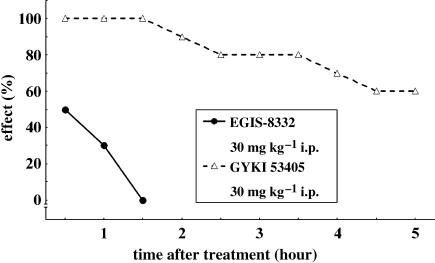
EGIS-8332 produced a weak muscle relaxant effect in mice while GYKI 53405 provoked strong ataxia for a long period of time (Figure 5).
Figure 5.
Time course of the muscle relaxant effects of EGIS-8332 and GYKI 53405 measured in the inclined screen test in mice. The results are expressed as the percentage of mice that failed to hang on the screen for 30 s.
Simplified Irwin test in mice
Both compounds caused ataxia and ptosis, and impaired reflex responses in mice. The lowest dose of EGIS-8332 that caused neurobehavioural changes was 100 mg kg−1 both after p.o. and i.p. administration. Treatment with GYKI 53405 caused side effects at 30 and 100 mg kg−1 after i.p. and p.o. administration, respectively. The effects of the two compounds were not different in the Irwin test.
Pharmacokinetics
Pharmacokinetic analysis of EGIS-8332 was performed after i.p. administration in order to get some preliminary data on the plasma levels attained under conditions in which the majority of tests were conducted in this study. Initial plasma concentration (C0) of EGIS-8332 was similar in the three species, amounting to 19 μg ml−1 in the mouse and rat and to 24 μg ml−1 in the gerbil. The rate of decline in plasma concentration of EGIS-8332 during the elimination phase (T1/2) was the slowest in the mouse (77 min) followed by the rat (55 min) and the gerbil (37 min), that is, metabolic stability of the compound was acceptable in all three species.
Discussion
EGIS-8332, a non-competitive AMPA receptor antagonist with a 2,3-benzodiazepine chemical structure, inhibited whole cell AMPA currents in the micromolar concentration range in cerebellar Purkinje cells. Similar results were obtained in cultured telencephalon neurons of the rat (Vegh et al., 2007). The antagonist effect of EGIS-8332 on AMPA receptors was stronger than that of GYKI 53405 in the present study. We have shown previously that EGIS-8332 blocked AMPA receptors with similar efficacy in patch clamp, spreading depression and population spike tests, whereas AMPA antagonist activity of GYKI 53405 varied much more in these experiments (Kapus et al., 2004).
Excessive stimulation of glutamate receptors causes excitotoxicity, which is one of the main mechanisms of ischaemia-induced brain necrosis (Frandsen and Schousboe, 2003; Szénási and Hársing, 2004). EGIS-8332 and GYKI 53405 inhibited both the quisqualate- and AMPA-induced excitotoxicity with similar efficacy in a primary culture of rat telencephalone neurons. Similarly results were reported for GYKI 53655 (Kovács and Szabó, 1997).
The 2,3-benzodiazepine AMPA antagonists, including GYKI 53405, have anticonvulsant activity in a variety of seizure models (Chapman et al., 1991; Yamaguchi et al., 1993; Szabados et al., 2001; Gressens et al., 2005). AMPA antagonists protected against ASs in mice, maximal electroconvulsive shock in mice and rats, and several chemoconvulsants in rats and mice (Grasso et al., 1999; Ábraham et al., 2000). Talampanel (GYKI 53773, LY-300164), the active enantiomer of GYKI 53405, produced good anticonvulsant effects in amygdala-kindled rats (Borowicz et al., 1999). Talampanel caused a 21% reduction in seizure frequency in patients with refractory partial seizures in phase II clinical trials (Chappell et al., 2002). In the current study, treatment with EGIS-8332 and GYKI 53405 resulted in strong anticonvulsant effects in six different seizure models in mice. Efficacy of the two compounds varied from test to test. However, it was evident that the anticonvulsant effect of GYKI 53405 was superior to that of EGIS-8332. Therefore, EGIS-8332 is potentially useful for the treatment of epilepsy but epilepsy may not be a preferred therapeutic indication of EGIS-8332.
AMPA receptor antagonists are excellent anti-ischaemic agents. Global cerebral ischaemia causes delayed neuronal loss leading to impaired learning and memory. Both the quinoxaline and 2,3-benzodiazepine type AMPA antagonists rescued a large fraction of pyramidal cells in the CA1 area of the hippocampus after BCO in the gerbil and after four-vessel occlusion (4VO) in the rat (Kawasaki-Yatsugi et al., 1997; Gyertyán et al., 1999; Nielsen et al., 1999; Kapus et al., 2004). Non-competitive AMPA antagonists decreased hypermotility and increased spontaneous alternation response, a measure of spatial memory, after global cerebral ischaemia in gerbils (Gyertyán et al., 1999; Kapus et al., 2004). Our current results confirm previous findings as both EGIS-8332 and GYKI 53405 produced dose-dependent neuroprotective effects in the gerbil BCO model, as revealed by the histological evaluation of the CA1 area of the hippocampus. Both compounds attenuated or even fully prevented the ischaemia-induced hypermotility at 4 days after BCO. The two compounds also produced good neuroprotective effects after a 10-min 4VO in rats. The efficacy of EGIS-8332 and GYKI 53405 was comparable in the two global ischaemia tests. However, GYKI 53405 decreased the body temperature of gerbils to a much greater extent than did EGIS-8332. A strong effect on body temperature complicates interpretation of the neuroprotective effect of compounds as hypothermia has been repeatedly shown to attenuate ischaemic injury (Colbourne et al., 2003; Harukuni and Bhardwaj, 2006), although our previous results did not support this possibility (Gyertyán et al., 1999). Furthermore, the duration of ischaemia was relatively short in both global ischaemia models in our study in comparison to that applied in other studies. However, other laboratories use halothane or isoflurane for anaesthesia, and volatile anaesthetics cause preconditioning and neuroprotection (Li et al., 2002; Zheng and Zuo, 2005; Statler et al., 2006). To our best knowledge, ether does not have the same effect. Therefore, gas narcosis can attenuate the ischaemia-induced neuronal necrosis, that is, a shorter ischaemia in ether anaesthesia can bring about similar injury to that caused by a longer ischaemia in gas narcosis.
Focal cerebral ischaemia simulates human ischaemic stroke. Studies from various laboratories have shown the efficacy of AMPA antagonists, including 2,3-benzodiazepine type AMPA antagonists in rodent stroke models (Ábraham et al., 2000; Matucz et al., 2004; Elger et al., 2005; Erdo et al., 2005, 2006). We have shown that both EGIS-8332 and GYKI 53405 exerted significant, dose-dependent neuroprotective effects after permanent MCAO in rats and mice. EGIS-8332 was a better anti-ischaemia agent than GYKI 53405 in permanent MCAO tests, because its MED was lower than that of GYKI 53405 in both species. However, EGIS-8332 and GYKI 53405 similarly decreased the volume of cerebral infarction after transient MCAO in rats (Matucz et al., 2004). Altogether, EGIS-8332 appeared to be a better anti-stroke agent than GYKI 53405, but the difference in efficacy between the two compounds was small. The therapeutic time window of neuroprotection by EGIS-8332 and GYKI 53405 was 2 h in permanent and 3 h in transient focal cerebral ischaemia in rats (Matucz et al., 2006).
The side effect profile of 2,3-benzodiazepine type AMPA antagonists is well known. Several attempts have been made to synthesize 2,3-benzodiazepines with altered chemical structures, intended to decrease or avoid muscle relaxation. In this respect, EGIS-8332 was a good compound because it induced a small and short-lasting effect in the inclined screen test in mice, while GYKI 53405 caused a strong and long-lasting muscle relaxation. Since EGIS-8332 was a better neuroprotective agent than GYKI 53405 in the same species, the contradictory results cannot be explained by pharmacokinetic differences between the two compounds. The results of the behavioural test further supported the favourable side effect profile of EGIS-8332 in mice. Although few doses were employed in this preliminary testing, the results suggested a tendency for a higher MED for EGIS-8332 than for GYKI 53405 after i.p. administration.
The results of the pharmacokinetic analysis revealed that EGIS-8332 exhibited good metabolic stability in all three species studied. Based on the initial plasma concentrations, it can be concluded that the effective concentration of EGIS-8332 is in the micromolar range after a dose of 10–20 mg kg−1 i.p., since EGIS-8332 produced good neuroprotective effects at the same doses in both global and focal ischaemia tests.
GYKI 53405 was a better antiepileptic agent than EGIS-8332 in mice. On the contrary, EGIS-8332 was a better anti-ischaemic agent than GYKI 53405 in the MCAO test in mice, and EGIS-8332 caused much weaker muscle relaxations than GYKI 53405 in the same species. EGIS-8332 induced less hypothermia than GYKI 53405 in gerbils, although the two compounds accomplished almost equipotent neuroprotective actions in the BCO test. EGIS-8332 reduced the volume of cerebral infarction at lower doses than did GYKI 53405 after permanent MCAO in rats, but the efficacy of the two compounds was similar in other ischaemia tests in rats. Such divergent results are surprising, taking into consideration that two AMPA antagonists with very similar chemical structures produced all of the above effects.
There is no clear explanation for this discrepancy but one possible hypothesis can be put forward. The AMPA receptor is a heterotetrameric complex assembled usually from two out of four different subunits (GluR1 to GluR4), which exist in flip and flop splice variants (Palmer et al., 2005). The GluR2 subunit prevents Ca2+ permeability of the AMPA receptor, allowing only Na+ influx from the extracellular space. Owing to the prominent role of intracellular Ca2+ in the regulation of cellular functions, Ca2+ permeability of the AMPA receptors is of great importance, especially in pathophysiological states (Dingledine et al., 1999; Colbourne et al., 2003; Frandsen and Schousboe, 2003). Therefore, the significance of the profile of a compound at AMPA receptors seems obvious. However, there is very limited information on the subtype selectivity of different AMPA receptor antagonists. Further, the subtypes of AMPA receptors involved in specific pharmacological responses are also poorly defined. Our observations suggest that subtype selectivity of EGIS-8332 and GYKI 53405 may be different and that small differences in subtype selectivity may alter their activity in various tests.
In conclusion, EGIS-8332 is a non-competitive AMPA receptor antagonist with a 2,3-benzodiazepine chemical structure. EGIS-8332 demonstrated good neuroprotective effects in several animal models of global and focal brain ischaemia, in vivo. The favourable neuroprotective effect of EGIS-8332 was accompanied with a weak side effect liability, indicating good clinical possibilities in the treatment of a wide range of human central nervous system disorders. Based on the current results, EGIS-8332 may be especially suitable for the treatment of stroke.
Acknowledgments
We thank József Baranyai and Gábor Tolmár for expert technical assistance.
Abbreviations
- 4VO
four-vessel occlusion
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AS
audiogenic seizure
- AUC
area under the curve
- BCO
bilateral carotid occlusion
- DMSO
dimethyl sulphoxide
- HPCD
hydroxypropyl-β-cyclodextrin
- LDH
lactate dehydrogenase
- MCA
middle cerebral artery
- MCAO
middle cerebral artery occlusion
- MED
minimum effective dose
- MES
maximal electroshock
- NMDA
N-methyl-D-aspartic acid
- PTZ
pentylenetetrazole
Conflict of interest
The authors state no conflict of interest.
References
- Ábraham G, Sólyom S, Csuzdi E, Berzsenyi P, Ling I, Tarnawa I, et al. New non competitive AMPA antagonists. Bioorg Med Chem. 2000;8:2127–2143. doi: 10.1016/s0968-0896(00)00133-4. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Ballyk BA, Schoepp DD, Palmer AJ, Bath CP, Sharpe EF, et al. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- Borowicz KK, Luszczki J, Szadkowski M, Kleinrok Z, Czuczwar SJ. Influence of LY 300164, an antagonist of AMPA/kainate receptors, on the anticonvulsant activity of clonazepam. Eur J Pharmacol. 1999;380:67–72. doi: 10.1016/s0014-2999(99)00541-5. [DOI] [PubMed] [Google Scholar]
- Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8:474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- Cebers G, Zhivotovsky B, Ankarcrona M, Liljequist S. AMPA neurotoxicity in cultured cerebellar granule neurons: mode of cell death. Brain Res Bull. 1997;43:393–403. doi: 10.1016/s0361-9230(97)00025-7. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Smith SE, Meldrum BS. The anticonvulsant effect of the non-NMDA antagonists, NBQX and GYKI 52466, in mice. Epilepsy Res. 1991;9:92–96. doi: 10.1016/0920-1211(91)90018-b. [DOI] [PubMed] [Google Scholar]
- Chappell AS, Sander JW, Brodie MJ, Chadwick D, Lledo A, Zhang D, et al. A crossover, add-on trial of talampanel in patients with refractory partial seizures. Neurology. 2002;58:1680–1682. doi: 10.1212/wnl.58.11.1680. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci USA. 2003;100:2906–2910. doi: 10.1073/pnas.2628027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Elger B, Huth A, Neuhaus R, Ottow E, Schneider H, Seilheimer B, et al. Novel alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor antagonists of 2,3-benzodiazepine type: chemical synthesis, in vitro characterization, and in vivo prevention of acute neurodegeneration. J Med Chem. 2005;48:4618–4627. doi: 10.1021/jm0580003. [DOI] [PubMed] [Google Scholar]
- Erdõ F, Berzsenyi P, Andrási F. The AMPA-antagonist talampanel is neuroprotective in rodent models of focal cerebral ischemia. Brain Res Bull. 2005;66:43–49. doi: 10.1016/j.brainresbull.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Erdõ F, Berzsenyi P, Német L, Andrási F. Talampanel improves the functional deficit after transient focal cerebral ischemia in rats. A 30-day follow up study. Brain Res Bull. 2006;68:269–276. doi: 10.1016/j.brainresbull.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Frandsen A, Schousboe A. AMPA receptor-mediated neurotoxicity: role of Ca2+ and desensitization. Neurochem Res. 2003;28:1495–1499. doi: 10.1023/a:1025666207754. [DOI] [PubMed] [Google Scholar]
- Gallyas F, Wolff JR, Böttcher H, Záborszki L. A reliable and sensitive method to localize terminal degeneration and lysosomes in the central nervous system. Stain Technol. 1980;55:299–306. doi: 10.3109/10520298009067258. [DOI] [PubMed] [Google Scholar]
- Grasso S, De Sarro G, De Sarro A, Micale N, Zappala M, Puia G, et al. Synthesis and anticonvulsant activity of novel and potent 2,3-benzodiazepine AMPA/kainate receptor antagonists. J Med Chem. 1999;42:4414–4421. doi: 10.1021/jm991086d. [DOI] [PubMed] [Google Scholar]
- Gressens P, Spedding M, Gigler G, Kertész S, Villa P, Medja F, et al. The effects of AMPA receptor antagonists in models of stroke and neurodegeneration. Eur J Pharmacol. 2005;519:58–67. doi: 10.1016/j.ejphar.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Gyertyán I, Gigler G, Simó A. The neuroprotective and hypothermic effect of GYKI-52466, a non-competitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-antagonist on histological and behavioural variables in the gerbil global ischemia model. Brain Res Bull. 1999;50:179–186. doi: 10.1016/s0361-9230(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Hoppe JO. A pharmacological investigation of 2,5-bis-(3-diethylaminopropylamino) benzoquinone-bis-benzylchloride (WIN 2747): a new curarimimetic drug. J Pharmacol Exp Ther. 1950;100:333–345. [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Jurányi Z, Sziray N, Markó B, Lévay G, Hársing LG., Jr AMPA receptor blockade potentiates the stimulatory effect of L-DOPA on dopamine release in dopamine-deficient corticostriatal slice preparation. Crit Rev Neurobiol. 2004;16:129–139. doi: 10.1615/critrevneurobiol.v16.i12.140. [DOI] [PubMed] [Google Scholar]
- Kapus G, Kertész S, Gigler G, Simó A, Vegh M, Barkóczy J, et al. Comparison of the AMPA antagonist action of new 2,3-benzodiazepines in vitro and their neuroprotective effects in vivo. Pharm Res. 2004;21:317–323. doi: 10.1023/b:pham.0000016245.74809.41. [DOI] [PubMed] [Google Scholar]
- Kawasaki-Yatsugi S, Yatsugi S, Koshiya K, Shimizu-Sasamata M. Neuroprotective effect of YM90K, an AMPA-receptor antagonist, against delayed neuronal death induced by transient global cerebral ischemia in gerbils and rats. Jpn J Pharmacol. 1997;74:253–260. doi: 10.1254/jjp.74.253. [DOI] [PubMed] [Google Scholar]
- Kovács AD, Szabó G. GYKI 53665, a 2,3-benzodiazepine, non-competitively protects cultured neurones against AMPA toxicity. Eur J Pharmacol. 1997;331:93–96. doi: 10.1016/s0014-2999(97)01046-7. [DOI] [PubMed] [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- Li J, Zheng S, Zuo Z. Isoflurane decreases AMPA-induced dark cell degeneration and edematous damage of Purkinje neurons in the rat cerebellar slices. Brain Res. 2002;958:399–404. doi: 10.1016/s0006-8993(02)03700-9. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Jr, Wilcoxon F. The reliability of graphic estimates of relative potency from dose–per cent effect curves. J Pharmacol Exp Ther. 1953;108:18–25. [PubMed] [Google Scholar]
- Lodge D, Bond A, O'Neill MJ, Hicks CA, Jones MG. Stereoselective effects of 2,3-benzodiazepines in vivo: electrophysiology and neuroprotection studies. Neuropharmacology. 1996;35:1681–1688. doi: 10.1016/s0028-3908(96)00155-4. [DOI] [PubMed] [Google Scholar]
- Matucz E, Móricz K, Gigler G, Simó A, Barkóczy J, Lévay G, et al. Reduction of cerebral infarct size by non-competitive AMPA antagonists in rats subjected to permanent and transient focal ischemia. Brain Res. 2004;1019:210–216. doi: 10.1016/j.brainres.2004.05.098. [DOI] [PubMed] [Google Scholar]
- Matucz E, Móricz K, Gigler G, Benedek A, Barkóczy J, Lévay G, et al. Therapeutic time window of neuroprotection by non-competitive AMPA antagonists in transient and permanent focal cerebral ischemia in rats. Brain Res. 2006;1123:60–67. doi: 10.1016/j.brainres.2006.09.043. [DOI] [PubMed] [Google Scholar]
- Nielsen EO, Varming T, Mathiesen C, Jensen LH, Moller A, Gouliaev AH, et al. SPD 502: a water-soluble and in vivo long-lasting AMPA antagonist with neuroprotective activity. J Pharmacol Exp Ther. 1999;289:1492–1501. [PubMed] [Google Scholar]
- Nikam SS, Kornberg BE. AMPA receptor antagonists. Curr Med Chem. 2001;8:155–170. doi: 10.2174/0929867013373877. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Holubkov R, Dixon CE, Clark RS, et al. Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res. 2006;1076:216–224. doi: 10.1016/j.brainres.2005.12.106. [DOI] [PubMed] [Google Scholar]
- Swinyard EA, Brown WC, Goodman LS. Comparative assays of antiepileptic drugs in mice and rats. J Pharmacol Exp Ther. 1952;106:319–330. [PubMed] [Google Scholar]
- Szabados T, Gigler G, Gacsályi I, Gyertyán I, Lévay G. Comparison of anticonvulsive and acute neuroprotective activity of three 2,3-benzodiazepine compounds, GYKI 52466, GYKI 53405, and GYKI 53655. Brain Res Bull. 2001;55:387–391. doi: 10.1016/s0361-9230(01)00516-0. [DOI] [PubMed] [Google Scholar]
- Szénási G, Hársing LG., Jr Pharmacology and prospective therapeutic usefulness of negative allosteric modulators of AMPA receptors. Drug Discovery Today: Therapeutic Strategies. 2004;1:69–76. [Google Scholar]
- Tarnawa I, Vízi ES. 2,3-Benzodiazepine AMPA antagonists. Restor Neurol Neurosci. 1998;13:41–57. [PubMed] [Google Scholar]
- Turski L, Huth A, Sheardown M, McDonald F, Neuhaus R, Schneider HH, et al. ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma. Proc Natl Acad Sci USA. 1998;95:10960–10965. doi: 10.1073/pnas.95.18.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegh MG, Kovacs AD, Kovacs G, Szabo G, Tihanyi K, Harsing LG, Jr, et al. The new 2,3-benzodiazepine derivative EGIS-8332 inhibits AMPA/kainate ion channels and cell death. Neurochem Int. 2007;50:555–563. doi: 10.1016/j.neuint.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Weiser T. AMPA receptor antagonists with additional mechanisms of action: new opportunities for neuroprotective drugs. Curr Pharm Des. 2002;8:941–951. doi: 10.2174/1381612024607135. [DOI] [PubMed] [Google Scholar]
- Weiser T. AMPA receptor antagonists for the treatment of stroke. Curr Drug Targets CNS Neurol Disord. 2005;4:153–159. doi: 10.2174/1568007053544129. [DOI] [PubMed] [Google Scholar]
- Welsh FA, Sakamoto T, McKee AE, Sims RE. Effect of lactacidosis on pyridine nucleotide stability during ischemia in mouse brain. J Neurochem. 1987;49:846–851. doi: 10.1111/j.1471-4159.1987.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning decreases glutamate receptor overactivation-induced Purkinje neuronal injury in rat cerebellar slices. Brain Res. 2005;1054:143–151. doi: 10.1016/j.brainres.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Donevan SD, Rogawski MA. Anticonvulsant activity of AMPA/kainate antagonists: comparison of GYKI 52466 and NBOX in maximal electroshock and chemoconvulsant seizure models. Epilepsy Res. 1993;15:179–184. doi: 10.1016/0920-1211(93)90054-b. [DOI] [PubMed] [Google Scholar]