Abstract
Endocytic transport is critical for the subcellular distribution of free cholesterol and the endocytic recycling compartment (ERC) is an important organelle that stores cholesterol and regulates its trafficking. The C-terminal EHD protein, EHD1, controls receptor recycling through the ERC and affects free cholesterol distribution in the cell. We utilized embryonic fibroblasts from EHD1 knockout mice (Ehd1-/-MEF) and SiRNA in normal MEF cells to assess the role of EHD1 in intracellular transport of cholesterol. Surprisingly, Ehd1-/-MEFs displayed reduced levels of esterified and free cholesterol, which returned to normal level upon re-introduction of wild-type, but not dysfunctional EHD1. Moreover, triglyceride and cholesterol storage organelles known as ‘lipid droplets’ were smaller in size in cells lacking EHD1, indicating that less esterified cholesterol and triglycerides were being stored. Decreased cellular cholesterol and reduced lipid droplet size in Ehd1-/-MEFs correlated with ineffectual cholesterol uptake via LDL receptor, suggesting involvement of EHD1 in LDL receptor internalization.
Keywords: EHD1, cholesterol, homeostasis, recycling, lipid droplet, LDL receptor
Introduction
Since LDL receptor-mediated internalization of LDL-bound cholesterol is a major source of cellular cholesterol in non-adipose cells, the endocytic transport of this sterol plays a key role in defining its subcellular distribution. Free cholesterol can access the endocytic pathway from: (1) internalized LDL particles, (2) cholesterol internalized along with components of the plasma membrane, (3) hydrolysis of esterified LDL-derived cholesterol in either early or late endosomes [1], (4) hydrolysis of esterified cholesterol stored in lipid droplets. While LDL receptors are generally recycled back to the plasma membrane via the endocytic recycling compartment, cholesterol can either be transported to the plasma membrane via this route, or be shuttled to late endosomes/lysosomes and packaged in storage organelles called lipid droplets [2] following esterification in the ER [3,4]. Lipid droplets exist in adipose and steroidogenic cells, but are also present in most other mammalian cells [5,6]. These smaller non-adipose/non-steroidogenic lipid droplets not only contain packaged triglycerides and esterified cholesterol, but also free cholesterol on the surface layer of the droplets [7]. Esterified cholesterol in lipid droplets can also undergo hydrolysis by cholesterol esterase, rendering free cholesterol that can be effluxed and transported to various cellular membranes [8]. Recent studies have determined that much of the free cholesterol within the endocytic pathway resides in the tubulo-vesicular endocytic recycling compartment [9,10], which has also been defined as a major sterol storage organelle [11]. Moreover, overexpression of Rab11, a key GTPase involved in the regulation of recycling from the ERC, caused the accumulation of cholesterol in this compartment and inhibited cholesterol esterification [12]. Recently, a number of studies have begun to focus on the role of the C-terminal EHD proteins in endocytic transport and recycling (reviewed in [13]). In particular, the ubiquitously expressed EHD1 [14] has been found to play a critical role in the recycling of plasma membrane receptors (ie., transferrin) that are internalized either through clathrin-coated pits [15-17], or independently of clathrin (MHC-I or β1 integrins) [18,19]. C-terminal EHD proteins contain a nucleotide-binding motif, a central coiled-coil involved in oligomerization [20,21], and a conserved C-terminal EH-domain that binds to a series of proteins that contain the tripeptide asparagine-proline-phenylalanine (NPF). Recently, we have also demonstrated an interaction between EHD1 and the Rab11 effector protein, Rab11-FIP2, suggesting that EHD1 regulates the transport of Rab11-FIP2- and Rab11-containing vesicles [21]. In addition, studies in CHO cells using a ‘dominant-negative’ EHD1 mutant (G429R) have demonstrated delays in the recycling of both transferrin and dehydroergosterol, a naturally occurring sterol with a chemical structure highly similar to cholesterol [11]. Since the ERC has been described as an important station for cholesterol in the endocytic pathway [11], and since Rab11 participates in the transport of cholesterol through this organelle [12], we sought to determine the role of EHD1 on cellular cholesterol homeostasis at steady-state.
Materials and Methods
Cell culture
Mouse Embryonic Fibroblast (MEF) cells derived from EHD1 knockout mice (Ehd1-/-MEF) or their normal counterparts (Ehd1+/+MEF) have been previously described [17]. MEF and HeLa cells were maintained in DMEM (high glucose) supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, 100 U/ml penicillin and 2 mM L-glutamine at 5% CO2
Gene knockdown by RNA interference (RNAi) and transient transfection
Oligonucleotide duplexes targeting mouse-EHD1 (synthesized by Dharmacon, Lafeyette, CO), were transfected into Ehd1+/+MEF cells using Oligofectamine (Invitrogen) for 72 h essentially as described [16]. Efficacy of mouse EHD1-RNAi was confirmed by immunoblotting transfected and control cell lysates with affinity-purified rabbit polyclonal antibodies specific for EHD1. The EHD1-specific oligonucleotide sequence (AAGACATCCAGTCTCTGCCGA) (base-pairs 921-942) has no significant overlap with other mouse genes, including those that code for the other C-terminal EHD proteins.
Antibodies and reagents
Polyclonal rabbit anti-EHD1, EHD2 and EHD4 peptide antibodies were raised against a sequence of amino acids found exclusively in the corresponding EHD protein. Rabbit anti-EHD1 was described previously [21]. Rabbit anti-p44-MAPK/Erk1 and p42-MAPK/Erk2 were from Cell Signaling Technology, Inc. (Danvers, MA), guinea pig anti-ADRP from Fitzgerald (Concord, MA) and anti-Myc (9E10) from Covance (Berkeley, CA). Anti-Actin was from Novus Biologicals (Littleton, CO) and human LDL, Nile Red, BODIPY-(C12)-Fatty acid, oleic acid bound to BSA, and DAPI were from Molecular Probes (Invitrogen, Eugene, OR). Secondary antibodies from Molecular Probes - Invitrogen (Eugene, OR) used in this work were: goat anti-mouse-488, goat anti-Guinea pig-568, goat anti-rabbit-488, goat anti-mouse-568 and goat anti-rabbit-568. Goat anti-mouse-HRP, donkey anti-rabbit-HRP and donkey anti-Guinea-pig-Cy2 were from Jackson ImmunoResearch (West grove, PA). Fatty-acid-free bovine serum albumin was obtained from Sigma (St. Louis, MO).
Measurement of free cholesterol by flow cytometry
Adherent MEF cells were trypsinized and transferred into ice-cold DMEM containing 5% BSA. After centrifugation, cell pellets were resuspended in 4% paraformaldehyde/PBS (v/v) for 20 min. on ice and washed with PBS. For Filipin staining, fixed cells were incubated with PBS containing 0.2% saponin and 2 μg/ml Filipin for 40 min. at room temperature. Unbound Filipin was removed by extensive washes with PBS. Cholesterol levels of at least 10,000 cells per sample were measured quantitatively utilizing a FACSVantage DIVA flow cytometry analyzer (Becton-Dickinson, San Jose, CA), by excitation at 355 nm and emission collection at 410-490 nm with a band pass filter.
Quantitative measurement of total cellular cholesterol
A fluorometric assay to measure both cholesterol and cholesteryl ester was performed using the Amplex Red Cholesterol kit from Molecular Probes (Invitrogen, Eugene, OR), according to the manufacturer’s protocol.
Immunofluorescence
MEF and HeLa cells were plated on glass cover-slips and fixed with 4% (vol/vol) paraformaldehyde/PBS at room temperature for 10 min. and immunostained as previosuly described [21]. Filipin staining was done essentially as described [12], with minor modifications. Autofluorescence of Filipin was detected by excitation with a 405 nm laser, and collecting emission with a 420-480 nm band pass filter [22,23]. Lipid storage was induced by incubating semi-confluent cells for 18 h with either 4 μg/ml Bodipy-(C12)-Fatty acid or with 100 μM Oleic acid complexed to acid-free bovine serum albumin at a molar ratio of 6:1 with fixation as described above [24]. All confocal images were acquired with a Zeiss LSM 5 Pascal microscope (Carl Zeiss, Thornwood, NY), using a 63X 1.4 numerical aperture objective.
Results and Discussion
Knock-down of EHD1 reduces cellular levels of total cholesterol
To examine the impact of EHD1 on steady-state levels of free, non-esterified cholesterol, we utilized both primary and SV40-transformed mouse embryonic fibroblast (MEF) cells, derived from mice with a targeted knock-out for Ehd1 (Ehd1-/-MEF) [17]. Loss of EHD1 did not affect the levels of the other EHD paralogs (Fig. 1G) as seen by immunoblotting with antibodies specific for EHD1, EHD2 and EHD4 (EHD3 is poorly expressed in MEF cells). Actin was used as a loading control. Assessment of the cellular free cholesterol was performed with Filipin, an intrinsically fluorescent antifungal, sterol-binding polyene macrolide, (Fig. 1A-C, transformed MEFs; Fig. 1D-E, primary MEFs). In Ehd1+/+MEF cells (Fig. 1A and D), free cholesterol was seen throughout the cell, with concentrations evident particularly at the plasma membrane (arrows) and perinuclear region. In contrast, Ehd1-/-MEFs (Fig. 1B and E ) showed markedly reduced levels of cellular cholesterol. To further validate these findings, we used SiRNA knockdown reduce expression of EHD1 in Ehd1+/+MEFs. The efficacy of this treatment was confirmed by immunoblot analysis (Fig.1F), showing >90% knockdown of endogenous EHD1 as compared to the p44/Erk1 and p42/Erk2 controls. SiRNA knock-down cells showed significantly decreased the levels of cellular free cholesterol (Fig. 1C). To measure the differences in the free cholesterol levels observed microscopically, we used flow cytometry to detect levels of Filipin in permeabilized cells. As shown (Fig. 1H), the level of Filipin staining in Ehd1-/-MEFs exhibited a mean count of 74.8, comprising only 37% of the mean count for Ehd1+/+MEF cells (202 counts). Total cholesterol levels (esterified and free), extracted from an equivalent number of Ehd1+/+ and Ehd1-/-MEFs were also examined (Fig. 1I), demonstrating a similar ˜two-fold reduction.
Fig. 1. Decreased free cellular cholesterol in MEF cells lacking EHD1.
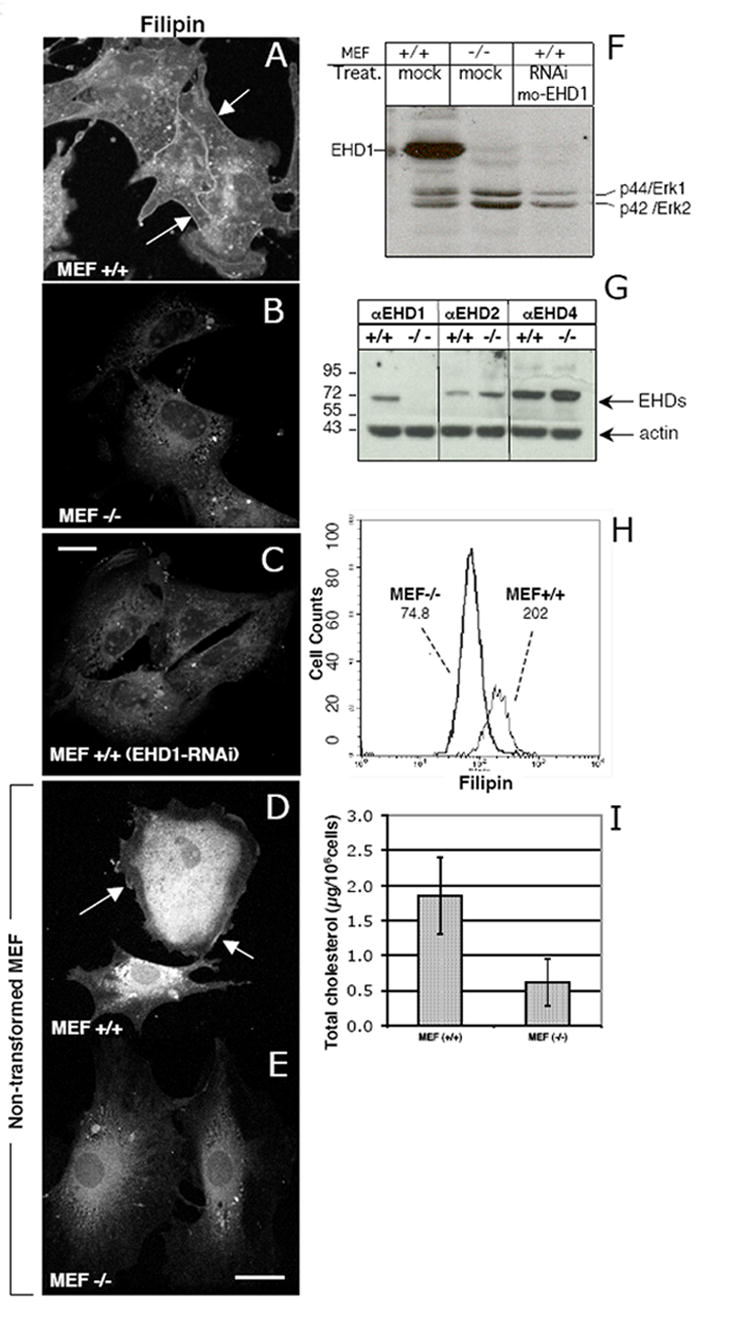
(A-C) SV40-transformed MEF cells, and (D and E) primary, non-transformed MEF cells were plated on glass cover–slips for 24 h. Ehd1+/+ MEF cells (C) were treated with EHD1-RNAi for 48 h, whereas Ehd1+/+ and Ehd1-/- MEF cells were mock-treated (A-B, respectively). Cells were fixed and free cholesterol was visualized by Filipin staining (A-E). Arrows depict plasma membrane cholesterol. (F) Lysates from mock-treated Ehd1+/+ and Ehd1-/-MEF cells, and Ehd1+/+ MEF cells treated with EHD1-RNAi (as in C) were lysed and separated by SDS-PAGE and immunoblotted with rabbit anti-EHD1 and with anti-Erk 1/2 to show levels of protein loading. (G) The protein levels of other C-terminal EHD paralogs were analyzed in Ehd1+/+ and Ehd1-/-MEF cells. Lysates with equivalent protein concentrations were separated by SDS PAGE and immunoblotted with antibodies for EHD1, EHD2, EHD4, and with anti-actin as a control. (H) Flow cytometry analysis of free cholesterol was performed on at least 10,000 Ehd1+/+ and Ehd1-/- MEF cells stained with Filipin in the presence of saponin to permeabilize the cells. Mean fluorescence of each cell population is depicted in the graph. (I) Total cellular cholesterol levels (cholesterol and cholesteryl-ester) of Ehd1+/+ and Ehd1-/- MEF cells were measured by fluorometric assay. The mean and standard deviation are from 3 independent experiments, each utilizing 1 × 106 cells. Bar, 10 μm.
Re-introduction of wild-type but not mutant EHD1 rescues free cholesterol levels in Ehd1-/-MEF cells
To further examine the role of EHD1 in cellular cholesterol homeostasis, we undertook ‘rescue’ experiments by re-introducing wild-type and mutant EHD1 into Ehd1-/-MEF cells (Fig. 2). While untransfected Ehd1-/-MEFs (Fig. 2B; upper cells) showed decreased levels of Filipin staining compared to Ehd1+/+MEFs (Fig. 2A), Ehd1-/-MEFs that were transfected with wild-type Myc-EHD1 (Fig. 2B (star), C and inset) displayed levels of Filipin staining approximating those of the control Ehd1+/+MEF cells (Fig. 2A). Moreover, Ehd1-/-MEF cells transfected with two previously described dysfunctional EHD1 mutants, Myc-EHD1 ΔEH and Myc-EHD1 G65R (Fig. 2D-G; stars)[15,18], showed no significant difference in Filipin staining when compared with untransfected Ehd1-/-MEF counterparts. The cellular distribution of EHD1 mutants is depicted in Fig 2E and G as well as in the corresponding insets (Fig. 2 inset for G and E). These results support a role for EHD1 in cellular cholesterol homeostasis.
Fig. 2. Introduction of wt EHD1, but not mutant EHD1, into MEF -/- cells rescues cellular free cholesterol levels.
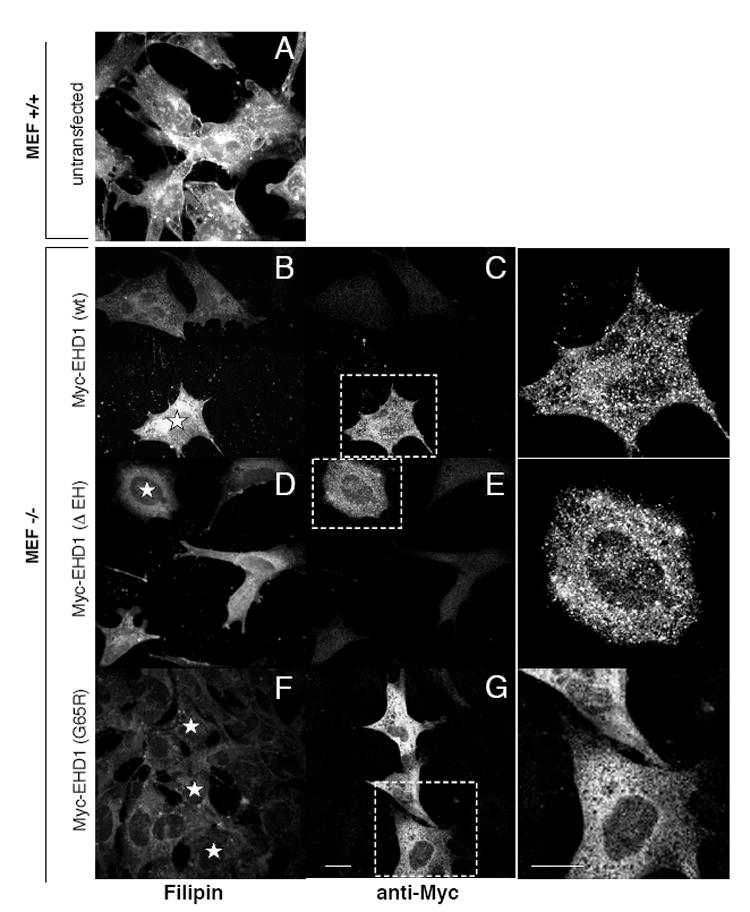
Ehd1+/+ MEF cells (A) and Ehd1-/- MEF cells (B-G) were plated on glass cover-slips for 18 h. Ehd1-/- MEF cells were then transfected with wild-type Myc-EHD1 (B, C), Myc-EHD1 ΔEH (D, E), and myc-EHD1 G65R (F-G) for 48 h in the presence of 5 mM butyric acid for the last 18 h of transfection to enhance expression of the transfected proteins. After fixation and permeabilization, cells were stained with Filipin. Myc-EHD1 transfected proteins were identified with anti-Myc antibodies, followed by 568-Alexa goat anti-mouse IgG. Transfected cells stained with anti-Myc are enlarged to illustrate a more detailed staining pattern (outlined by ‘dashed rectangles’; insets for C, E and G). Stars depict transfected cells. Bar 10 μm.
Distribution and size of lipid droplets are altered in MEF cells lacking EHD1
Given that loss of EHD1 alters cellular cholesterol levels, we next examined whether loss of EHD1 impacts the formation of lipid droplets. Under standard culture conditions, non-adipocyte cells generally contain very small lipid droplets, but the addition of fatty acids is known to induce formation of large, detectable lipid droplets [25,26]. Lipid droplet biogenesis was induced in Ehd1+/+ and Ehd1-/-MEF cells by the addition of oleic acid for 18 h. After fixation, stored lipids were detected by Nile Red, while lipid droplet membranes were stained with Adipophilin Related Protein (ADRP; green) (Fig. 3A-F). Comparison of the lipid droplet distribution and size in Ehd1+/+ (Fig. 3A-C) vs. Ehd1-/-MEF cells (Fig. 3D-F) revealed dramatic differences, with droplets being greatly reduced in size in the Ehd1-/-MEF cells. However, levels of ADRP remained intact in Ehd1-/-MEF cells, as measured by immunoblot analysis (Fig. 3K). We hypothesize that the reduced levels of cholesterol in Ehd1-/-MEF cells diminished the need for storage of esterified cholesterol, resulting in decreased lipid droplet size.
Fig. 3. Lack of EHD1 alters size and distribution of lipid droplets in Ehd1-/- MEF cells, and EHD1 and lipids droplets partially co-localize in Ehd1+/+MEF cells.
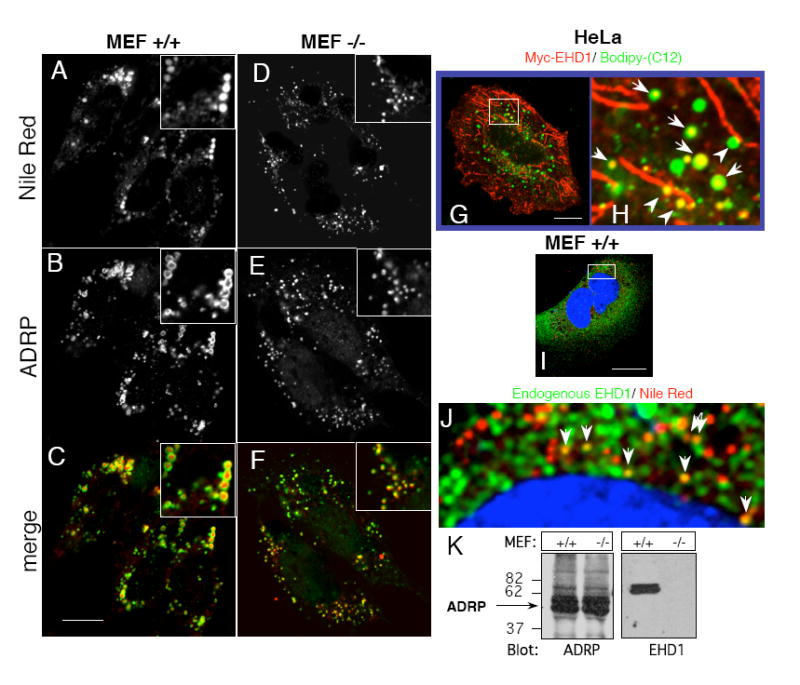
(A-C) Ehd1+/+ MEFs and (D-F) Ehd1-/- MEFs were plated on glass cover-slips for 24 h in fatty-acid free medium containing 100 μM oleic acid. After fixation and permeabilization, lipid droplets were co-stained with Nile Red and anti-ADRP, followed by Cy2-Donkey anti-Guinea pig IgG. Insets depict the size of a cluster of lipid droplets. (G and inset; H) Myc-EHD1-transfected HeLa cells were grown on glass cover-slips in fatty-acid free medium containing 4 μg/ml Bodipy-(C12)-Fatty acid (visualized in green) for 18 h at 37°C. After fixation, the cells were incubated with anti-Myc followed by 568-goat anti-mouse IgG to detect Myc-EHD1 (red). (I and inset; J) Untransfected Ehd1+/+ MEF cells were incubated with fatty-acid free medium supplemented with 100 μM oleic acid for 18 h at 37°C, and fixed. The cells were co-stained (in the presence of saponin) with Nile Red to visualize lipid droplets, DAPI to mark the nuclei (blue), and with rabbit anti-EHD1 followed by 488-Alexa anti-rabbit IgG to detect endogenous EHD1 (green). Arrows mark EHD1 localized to Nile Red labeled lipid droplets, and arrowheads depict alignment of lipid droplets with EHD1-containing tubular membranes. (K) Equal numbers of Ehd1+/+ and Ehd1-/-MEFs were lysed, separated on SDS-PAGE and immunoblotted with either anti-ADRP (left panel), or anti-EHD1 (right panel). Bars, 10 μm.
Recent reports suggest that the EHD1 paralog, EHD2, can associate with lipid droplets [27,28]. To determine whether EHD1 localizes to lipid droplets, HeLa cells were transfected with Myc-EHD1 and incubated overnight with labeled fatty acid (Bodipy-C12). As depicted in Fig. 3G, a portion of Myc-EHD1 (red) localized to (green) fatty acid-labeled lipid droplets (see inset Fig. 3H; arrows) and in some cases aligned with tubular Myc-EHD1-containing membranes (inset Fig. 3H; arrowheads). In MEF cells, where the distribution of endogenous EHD1 is mostly punctate, partial co-localization of endogenous EHD1 (green) was also observed with Nile Red labeled lipid droplets loaded with oleic acid (Fig. 3I and inset; J). The nucleus was stained with DAPI (blue). The partial localization of EHD1 to lipid droplets suggests its potential involvement in cholesterol storage in these organelles.
Delivery of LDL-derived cholesterol to lipid droplets is reduced in EHD1 knockout cells
One of the primary modes by which cholesterol levels are regulated in non-hepatic cells is by the uptake of cholesterol-laden low density lipoprotein (LDL) particles via the LDL receptor [1]. Since EHD1 has been implicated in: (I) recycling of receptors to the plasma membrane [ 15,16,18], and (II) in the internalization of certain receptors [29], we hypothesized that EHD1 might affect cellular cholesterol homeostasis by regulating LDL transport via its receptor. To examine the potential link between EHD1 and LDL-derived cholesterol delivery to lipid droplets, we supplemented Ehd1+/+ and Ehd1-/-MEFs with LDL in media containing de-lipidated serum (Fig.4), and then compared free cholesterol levels and lipid droplet size by co-staining the cells with Filipin and antibodies directed against ADRP, respectively. As a comparative control, another set of cells was grown in complete media without the addition of LDL or any exogenous lipids. As depicted at steady-state (Fig4 A-D), Filipin levels were detectably stronger in Ehd1+/+MEFs (Fig. 4A) compared to Ehd1-/-MEFs (Fig. 4C), consistent with our data from Figures 1 and 2. In addition, both Ehd1+/+ and Ehd1-/-MEFs at steady state displayed very small lipid droplets (Fig. 4B and D ), most likely since neither LDL loaded with exogenous lipids nor fatty acids had been added to the media. However, upon LDL uptake, Ehd1+/+MEF cells showed markedly enhanced Filipin staining (Fig. 4E), indicating that free cholesterol had been internalized along with LDL. Accordingly, ADRP-coated lipid droplets were also enlarged (Fig. 4F; G is a higher magnification of a single cell), suggesting that excess esterified cholesterol was being stored in lipid droplets. On the other hand, Ehd1-/-MEF cells showed little increase in Filipin staining even after 18 h LDL uptake (Fig. 4H), and little or no change in lipid droplet size was seen with ADRP staining (Fig. 4I; J is a higher magnification of a single cell). These results are consistent with a role for EHD1 in the regulation of cellular cholesterol homeostasis and lipid droplet biogenesis.
Fig. 4. Reduced delivery of LDL-derived cholesterol to lipid droplets in cells lacking EHD1.
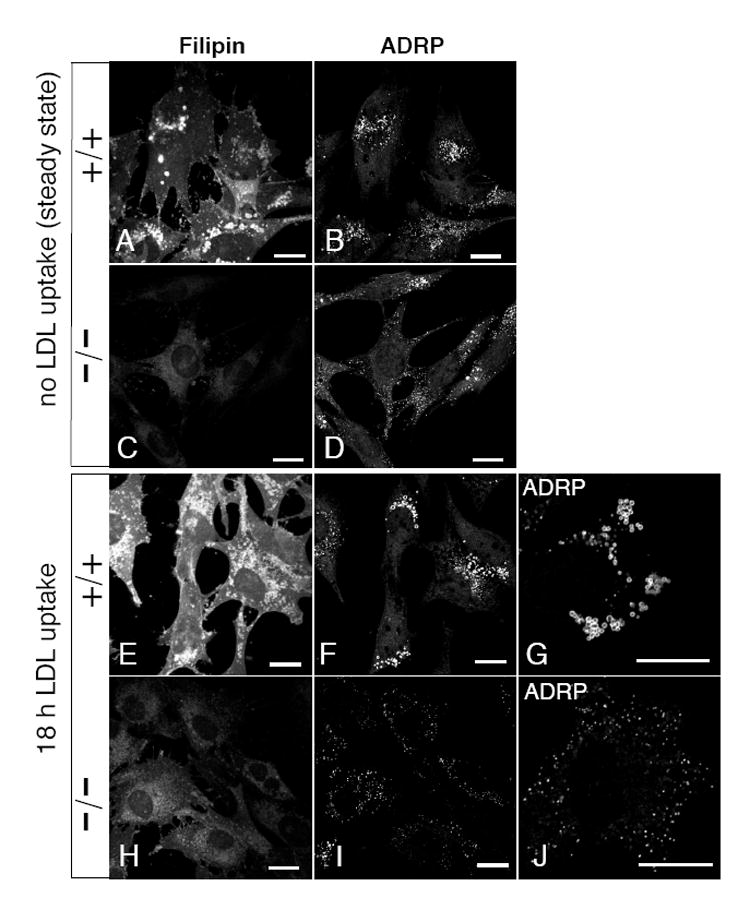
Ehd1+/+ MEF cells (A, B, E-G) and Ehd1-/-MEF cells (C, D, H-J) were plated on glass cover-slips for 18 h in complete media. For LDL uptake (E-J) the complete media was replaced by media containing fatty-acid free serum, and supplemented with 30 μg/ml serum-derived LDL for 18 h at 37°C prior to fixation. As a control (A-D) MEF cells growing in complete medium (without LDL) were fixed. All cover slips were permeabilized and stained with Filipin and anti-ADRP, followed by 568-conjugated goat-anti-Guinea pig IgG. Insets (G) and (J) depict ADRP stain of lipid droplets in a single-cell shown at higher magnification. Bars, 10 μm.
A number of possible explanations could account for the dramatically reduced size of the lipid droplets. One possibility is that loss of EHD1 leads to impaired activity of the ACAT enzyme required for esterification of cholesterol [2]. Alternatively, Ehd1-/-MEFs might have enhanced efflux of cholesterol from lipid droplets due to activity of the enzyme nCEH, which is expressed in a range of cell types and completes the cholesterol-ester cycle by hydrolyzing cholesteryl-esters back to cholesterol and fatty acids [30]. Our present work show that the size of lipid droplets (which tends to reflect the overall levels of intracellular cholesterol) was largely dependent upon LDL uptake. Since little or no increase in lipid droplet size was observed upon incubation of Ehd1-/-MEF cells with LDL, we hypothesize that impaired internalization of LDL-derived cholesterol is a possible cause for the decreased levels in esterified and free cholesterol in cells lacking EHD1 and for their reduced size of lipid droplets.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM074876-01) and American Heart Association (04600001Z) to S.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Chang TY, Chang CC, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 3.Maxfield FR, Wustner D. Intracellular cholesterol transport. J Clin Invest. 2002;110:891–898. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soccio RE, Breslow JL. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24:1150–1160. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- 5.Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol. 1999;10:51–58. doi: 10.1006/scdb.1998.0275. [DOI] [PubMed] [Google Scholar]
- 6.Brown DA. Lipid droplets: proteins floating on a pool of fat. Curr Biol. 2001;11:R446–449. doi: 10.1016/s0960-9822(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 7.Atshaves BP, Storey SM, McIntosh AL, Petrescu AD, Lyuksyutova OI, Greenberg AS, Schroeder F. Sterol carrier protein-2 expression modulates protein and lipid composition of lipid droplets. J Biol Chem. 2001;276:25324–25335. doi: 10.1074/jbc.M100560200. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, St Clair RW, Rudel LL. Mobilization of cytoplasmic CE droplets by overexpression of human macrophage cholesteryl ester hydrolase. J Lipid Res. 2003;44:1833–1840. doi: 10.1194/jlr.M300162-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee S, Zha X, Tabas I, Maxfield FR. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 11.Hao M, Lin SX, Karylowski OJ, Wustner D, McGraw TE, Maxfield FR. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J Biol Chem. 2002;277:609–617. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- 12.Holtta-Vuori M, Tanhuanpaa K, Mobius W, Somerharju P, Ikonen E. Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol Biol Cell. 2002;13:3107–3122. doi: 10.1091/mbc.E02-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naslavsky N, Caplan S. C-terminal EH-domain-containing proteins: consensus for a role in endocytic trafficking, EH? J Cell Sci. 2005;118:4093–4101. doi: 10.1242/jcs.02595. [DOI] [PubMed] [Google Scholar]
- 14.Mintz L, Galperin E, Pasmanik-Chor M, Tulzinsky S, Bromberg Y, Kozak CA, Joyner A, Fein A, Horowitz M. EHD1--an EH-domain-containing protein with a specific expression pattern. Genomics. 1999;59:66–76. doi: 10.1006/geno.1999.5800. [DOI] [PubMed] [Google Scholar]
- 15.Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 16.Naslavsky N, Boehm M, Backlund PS, Jr, Caplan S. Rabenosyn-5 and EHD1 Interact and Sequentially Regulate Protein Recycling to the Plasma Membrane. Mol Biol Cell. 2004;15:2410–2422. doi: 10.1091/mbc.E03-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapaport D, Auerbach W, Naslavsky N, Pasmanik-Chor M, Galperin E, Fein A, Caplan S, Joyner AL, Horowitz M. Recycling to the plasma membrane is delayed in EHD1 knockout mice. Traffic. 2006;7:52–60. doi: 10.1111/j.1600-0854.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 18.Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- 20.Lee DW, Zhao X, Scarselletta S, Schweinsberg PJ, Eisenberg E, Grant BD, Greene LE. ATP Binding regulates oligomerization and endosome association of RME-1 family proteins. J Biol Chem. 2005;280:280–290. doi: 10.1074/jbc.M412751200. [DOI] [PubMed] [Google Scholar]
- 21.Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD Proteins and Rab11-FIP2: A Role for EHD3 in Early Endosomal Transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller CP, Stephany DA, Winkler DF, Hoeg JM, Demosky SJ, Jr, Wunderlich JR. Filipin as a flow microfluorometry probe for cellular cholesterol. Cytometry. 1984;5:42–54. doi: 10.1002/cyto.990050108. [DOI] [PubMed] [Google Scholar]
- 23.Hassall DG, Graham A. Changes in free cholesterol content, measured by filipin fluorescence and flow cytometry, correlate with changes in cholesterol biosynthesis in THP-1 macrophages. Cytometry. 1995;21:352–362. doi: 10.1002/cyto.990210407. [DOI] [PubMed] [Google Scholar]
- 24.Garcia A, Subramanian V, Sekowski A, Bhattacharyya S, Love MW, Brasaemle DL. The amino and carboxyl termini of perilipin a facilitate the storage of triacylglycerols. J Biol Chem. 2004;279:8409–8416. doi: 10.1074/jbc.M311198200. [DOI] [PubMed] [Google Scholar]
- 25.Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta. 2000;1483:251–262. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 26.Wolins NE, Rubin B, Brasaemle DL. TIP47 associates with lipid droplets. J Biol Chem. 2001;276:5101–5108. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- 27.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic Analysis of Proteins Associated with Lipid Droplets of Basal and Lipolytically Stimulated 3T3-L1 Adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 28.Vessal M, Mishra S, Moulik S, Murphy LJ. Prohibitin attenuates insulin-stimulated glucose and fatty acid oxidation in adipose tissue by inhibition of pyruvate carboxylase. Febs J. 2006;273:568–576. doi: 10.1111/j.1742-4658.2005.05090.x. [DOI] [PubMed] [Google Scholar]
- 29.Rotem-Yehudar R, Galperin E, Horowitz M. Association of insulin-like growth factor 1 receptor with EHD1 and SNAP29. J Biol Chem. 2001;276:33054–33060. doi: 10.1074/jbc.M009913200. [DOI] [PubMed] [Google Scholar]
- 30.Riddle MC, Fujimoto W, Ross R. Two cholesterol ester hydrolases. Distribution in rat tissues and in cultured human fibroblasts and monkey arterial smooth muscle cells. Biochim Biophys Acta. 1977;488:359–369. doi: 10.1016/0005-2760(77)90195-3. [DOI] [PubMed] [Google Scholar]


