Abstract
The outcomes of dog’s eyes with chronic (>1 month) retinal detachment and giant retinal tears without therapy were compared with those treated with topical steroids and antiglaucoma medications, and with those that received a vitrectomy, retinal reattachment, endolaser therapy, and silicone oil tamponade. Fourteen of 16 eyes that did not receive therapy developed uveitis and secondary glaucoma, and were enucleated (4) or eviscerated (6); and 2 dogs were euthanized due to blindness and uveitis. Two eyes in 2 dogs remain without treatment, 1 and 3 years later. Fifteen of 19 eyes that received topical therapy developed nonresponsive uveitis and secondary glaucoma, and were enucleated (4) or eviscerated (9), 1 dog that was affected bilaterally was euthanized; and 3 eyes remain on topical anti-inflammatory therapy and the medication has been discontinued on 1 eye. Four of 6 eyes surgically reattached remain without clinical manifestations of uveitis and secondary glaucoma and 3 of these eyes have functional vision. Light microscopic observations completed on failed globes in the 3 groups were similar.
Résumé
Détachement chronique de la rétine et déchirures rétinienne géantes chez 34 chiens : comparaison des résultats des cas non traités, traités par médication topique ou par réattachement de la rétine après vitrectomie. Une comparaison de l’évolution du détachement chronique (>1 mois) de la rétine et des déchirures rétiniennes géantes a été réalisée entre animaux non traités et traités avec des stéroïdes topiques et une médication anti-glaucome et traités par vitrectomie, réattachement de la rétine, thérapie au laser et tamponnade à l’huile à la silicone. Quatorze des 16 yeux n’ayant pas été traités ont développé une uvéite et un glaucome secondaire : 4 ont été énucléés et 2 chiens ont été euthanasiés à cause de cécité et d’uvéite. Deux yeux chez 2 chiens étaient demeurés sans traitement 1 an et 3 ans plus tard. Quinze des 19 yeux ayant reçu une thérapie topique ont développé une uvéite qui ne répondait pas au traitement et un glaucome secondaire et ont été énucléés (4) ou éviscérés (9), 1 chien qui était atteint bilatéralement a été euthanasié et 3 yeux sont demeurés sur thérapie anti-inflammatoire topique alors que la médication a été arrêtée sur 1 œil. Quatre des 6 yeux traités par réattachement chirurgical n’ont pas manifesté d’uvéite clinique ni de glaucome secondaire et 3 de ces yeux avaient une vison fonctionnelle. Les observations en microscopie optique des globes défectueux des 3 groupes montraient des résultats similaires.
(Traduit par Docteur André Blouin)
Introduction
Retinal detachment in dogs may be partial or focal, and not perceptibly affect vision, or it may be complete and the affected eye is blind. The etiologies of retinal detachment in dogs are diverse. However, the pathogenesis always includes 1 or a combination of 3 basic mechanisms: traction detachment due to fibrous strands within the vitreous; retinal tears or holes with leakage of vitreous into the subretinal space; or exudation of fluid and inflammatory cells under the retina (1,2). After the retina detaches in dogs, the retinal pigment epithelium changes rapidly, with hypertrophy and hyperplasia, and the epithelial cells may detach from their basement membrane and become migrating cells with macrophage-like properties (3). The blood retinal barriers are easily damaged during retinal detachment, and retinal, subretinal, and vitreous hemorrhage may be present (1,2). When the detachment is chronic, hyphema and uveitis are common sequelae, due to the development of pre-iridal fibrovascular membranes (4–6). When photoreceptors are separated from the retinal pigment epithelium, the outer and inner segments of the rods and cones degenerate quite rapidly and the retinal pigment epithelium hypertrophies (7). Apoptosis is the key cellular event that occurs rapidly (within 3 d of detachment) and results in a permanent depletion of postmitotic outer nuclear cells (8). This process occurs by mitochondrial dependent pathways (8). Then, a progressive degeneration of the inner and outer segments of the photoreceptors occurs, followed by loss of the outer plexiform layer; eventually, the inner nuclear and inner plexiform layers also degenerate (7). Rapid reattachment within 24 h arrests and even reverses some of these cellular events (9). In addition, hyperoxia early in the course of retinal detachment has a predictable clinical benefit by increasing O2 diffusion to the detached retina (10–13). However, rapid reattachment (24–48 h) and the provision of hyperoxia for domestic animals with detached retinas will not likely be available in the clinical setting in the near future. Most detachments, unless bilateral, are usually subclinical and manifest with only mild pupillary dilation, until they have been present for several months when they manifest with uveitis and hyphema, when hyperoxia is of limited use.
Generally, most veterinary ophthalmic surgeons recommend surgical reattachment in the first 1–4 wk after detachment to ensure some return of vision in the affected eye (2,14–16). This time-frame is based on their experience and reports of experimental retinal detachments and reattachments that have been completed in primates (17–24), cats (25–27), and dogs (28). However, in all of these experimental studies (17–28), the retinal detachments were induced quickly with subretinal fluid injections. Generally, the more elevated the retina is off the retinal pigment epithelium combined with the presence of subretinal exudates or hemorrhage, the more rapidly the degeneration will ensue (29).
Naturally occurring disease may induce retinal degeneration that develops quite differently from experimental detachments. Recently, we reported an inherited naturally occurring, bullous retinal detaching disease in dogs in which our histologic evaluation confirmed that retinal degeneration in focal detachments extends into the photoreceptor inner segments by 4 wk, similar to experimentally induced detachments (30,31). However, in these dogs, even after 3 y, remnants of photoreceptor inner segments remain in large focal detachments (31).
It is logical to assume that naturally occurring retinal detachments with other etiologies will also vary significantly from those of an experimental model and inherited detachments. We are interested in the viability of the photoreceptors after detachment and the return of functional vision after surgical reattachment of chronic rhegmatogenous retinal detachments (>1 mo) in dogs. Most of these rhegmatogenous detachments in dogs have a giant tear, that is, by definition, one that extends over 1/4 of the retinal circumference. Many tears extend across the periphery, with the retina being draped over the optic disc. With these detachments, the separation from the retinal pigment epithelium is greatest over the tapetal region and often minimal over the nontapetal area, in which case, vitrectomy and surgical reattachment is the only treatment. This is in contrast to serous detachments that develop secondary to uveitis and are usually effectively treated by systemic immunosuppression (32,33) and to focal detachments associated with small retinal tears, or coloboma, which are often successfully treated with barrier laser or cryo-retinopexy (34,35).
It is very difficult to accurately determine the onset time of naturally occurring retinal detachments in dogs, especially when the contralateral eye is sighted. Often the veterinary ophthalmologist estimates the time, based on the history of anisocoria or blindness provided by the owner or the referring veterinarian. It is possible that previous reports (14–17) may have underestimated the time of detachment, so we have started to question the axiom that, in dogs, successful surgical retinal reattachment must be completed within 4 wk of detachment to provide any potential return of vision.
Many ocular complications develop secondary to chronic retinal detachment in dogs. Preiridal fibrovascular membranes are induced by the release of varied growth factors, including vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGFβ), from the degenerating retina (36). Hyphema and uveitis are common complications, as these preiridal vascular membranes are fragile (4–6,36). Cataracts are also common sequelae in dogs with chronic complete retinal detachment and they also commonly induce lens induced uveitis (1). The uveitis and preiridal fibrovascular membranes may lead to peripheral anterior and posterior synechiae, secondary glaucoma, and buphthalmos secondary to closed angles and pupillary block. The percentage of dogs with chronic complete retinal detachment that develop nonresponsive uveitis and secondary glaucoma has not been reported. It is our clinical impression that the majority of chronic retinal detachments and giant retinal tears in dogs eventually lead to globe-threatening complications and result in enucleation, or evisceration and intrascleral prosthesis placement. In contrast, our short-term follow-up on reattachment of chronically detached retinas has been positive, and we have noted some photopic vision, indicating a return of some cone function (37). Cone function return after detachment has been investigated recently in experimental models (38) and may be applicable to our studies.
We hypothesized that chronic (>1 mo) retinal detachment with giant tears in dogs with long-term (>1 y) follow-up with no therapy, topical medical therapy, or surgical reattachment would be poor and similar, due to preiridal fibrovascular membranes, uveitis, and secondary glaucoma. The objectives of the study included 1) documentation of the percentage of eyes that developed globe threatening complications (sustained uveitis, glaucoma) after complete retinal detachment without therapy, with topical medical management, and with surgery (vitrectomy, perfluorocarbon gas retinal reattachment, endolaser, and silicone oil replacement); and 2) evaluation of light microscopical findings of globes that developed nonresponsive uveitis and glaucoma within each category.
Materials and methods
Since this was a retrospective comparison of dogs with retinal detachment and giant retinal tears of 1 or both eyes that developed secondary to vitreous degeneration, vitreous traction bands, and retinal tears, all medically treatable exudative and serous retinal detachments, detachments that developed after intraocular surgery, and focal retinal detachments and tears that were treated with a transcorneal laser retinal pexy were excluded. Case records from the Western College of Veterinary Medicine from 2002–2005 were reviewed and 34 canine retinal detachments with giant retinal tears were documented that met the following inclusion criteria: 1) all dogs had been examined by a veterinary ophthalmologist with biomicroscopy (Osram 64222; Carl Zeiss Canada, Don Mills, Ontario), tonometry (Tonopen XL; Biorad Ophthalmic Division, Santa Clara, California, USA), indirect ophthalmoscopy (Heine Omega 200; Heine Instruments Canada, Kitchener, Ontario), and ultrasonography (GE, Logiq 3; GE Medical Systems, GE PDI, Tempe, Arizona, USA), when hyphema precluded a thorough posterior segment examination; and 2) all dogs that were treated surgically had received a routine complete blood (cell) count, serum biochemical profile, urinalysis, and systolic and diastolic blood pressure assessments prior to anesthesia induction.
Establishing the precise time that each retina had detached was difficult. To ensure that the time was estimated conservatively, the minimum time of detachment was based on the owner’s history of a dilated pupil and the referring veterinarian’s examinations identifying the detachment, or more commonly the ophthalmologist’s examinations, as many were not diagnosed by the veterinarian. When the contralateral eye was also blind or not present, the minimum time of detachment was based on the date that the owner noted blindness.
Follow-up examinations and communication with the owners had been completed by the ophthalmologist for a minimum of 12 mo (maximum 3 y), or until progressive uveitis and hyphema had necessitated enucleation or evisceration and intrascleral prosthesis placement.
The no therapy group included all dogs where topical treatments had not been utilized due to the inability to document inflammation or secondary glaucoma at the time of diagnosis or dogs whose owners had refused suggested therapy.
The medical therapy group included all dogs that had received topical prednisolone acetate 1% ophthalmic suspension (Falcon Pharmaceuticals, Alcon Laboratories, Fort Worth, Texas, USA), q6h, to control inflammation; and for the initial 1–2 wk of therapy, a cycloplegic, atropine sulphate 1% ophthalmic solution (Sandoz Canada, Boucherville, Quebec), or a mydriatic, tropicamide 1% ophthalmic solution (Sandoz Canada), as often as required, to maintain pupillary dilatation and reduce the formation of posterior synechiae by pupillary synechiae (atropine, tropicamide) and to attempt to diminish ciliary spasms (atropine). Within 1–4 wk, the steroid administration was tapered to q12h as the uveitis was controlled. Dorzolamide hydrochloride ophthalmic solution (Merck, Whitehouse Station, New Jersey, USA), q8h, was utilized to reduce the intraocular pressure if secondary glaucoma (intraocular pressure > 30 mmHg) was present or developed.
The dogs within the surgical group had had general anesthesia induced by thiopental (Abbot Laboratories, Saint-Laurent, Quebec), 10 mg/kg bodyweight (BW), IV, or propofol (Novapharm, Toronto, Ontario), 4 mg/kg BW, IV, and maintained with isoflurane (Abbot Laboratories) or sevoflurane (Abbot Laboratories). Each dog had been placed in dorsal recumbency under an operating microscope (Zeiss OPMI; Carl Zeiss Canada, Don Mills, Ontario), with an attached posterior segment viewing system (Biom; Carl Zeiss Canada). A lateral canthotomy, proptosis, and peritomy had allowed scleral exposure to the pars plana, and 3, 20-g sclerotomies had been completed for placement of a light pipe, fluid cannula, and the vitrectomy probe. The vitrectomies had been followed by manipulation of the retina with perfluorocarbon liquid (DKline; Bausch & Lomb, Waterford, Ireland) to appose them to the retinal pigment epithelium. Endolaser photocoagulations had been performed with a diode laser (Diovet Diode Laser; Iris Medical Instruments, Mountain View, California, USA) to induce 3 contiguous rows of burns around the periphery of each tear and the perimeter of the reattached retina, similar to previous reports (2,14,16). The perfluorocarbon liquids had been removed and replaced with silicone oil (Oxane 5700; Bausch & Lomb) through the working and infusion ports, respectively. The sclerotomy incisions had been closed with 6-0 polydioxanone (PDS) (Ethicon; Johnson & Johnson Medical Products, Toronto, Ontario). The conjunctival incisions had been sutured with 9-0 vicryl (Ethicon) and the canthotomy incisions with 5-0 nylon (Ethicon). Postoperatively, each dog had received prednisolone acetate 1% ophthalmic solution (Sandoz Prednisolone 1%, Sandoz Canada), nonsteroidal anti-inflammatory diclofenac 0.1% solution (Voltaren Ophtha; Novartis Ophthalmics, Mississauga, Ontario), and ciprofloxacillin 0.3% solution (Apociproflox; Apotex, Toronto, Ontario), q6h on the affected eye until the 1 mo reexamination, and cephalexin (Nu-Cephalexin; NuPharm, Richmond Hill, Ontario), 10 mg/kg BW, PO, q12h for 1 wk. If secondary glaucoma developed in these globes, dorzolamide (Trusopt 2%; Merck Frost, Kirkland, Quebec) had been given topically, q8h, until a stable intraocular pressure had been achieved. The postoperative topical antibiotic and nonsteroidal medications had been discontinued at 4 wk and the topical steroid reduced gradually over a 6- to 12-month period.
Persistent uveitis and secondary glaucoma (intraocular pressure > 30 mmHg) despite topical anti-inflammatory and anti-glaucoma therapy were the criteria for recommending evisceration and intrascleral prosthesis implantation or enucleation in each group. Buphthalmos and phthisis bulbi had been confirmed by caliper corneal measurement or ultrasonographically, and the measurements had been compared with the normal contralateral eye or an age-matched similar-sized dog (data not shown). All enucleated or eviscerated specimens had been immersion-fixed in 10% buffered formaldehyde, wax embedded, sectioned at 6 μm, and stained routinely (hematoxylin and eosin, periodic acid Schiff). The sections of each globe and eviscerated sample had been reexamined (BG). The retinas had been photographed to provide assessment of as much of the retina as possible to determine potential viability of the photoreceptors in each group at the time of enucleation or evisceration.
Results
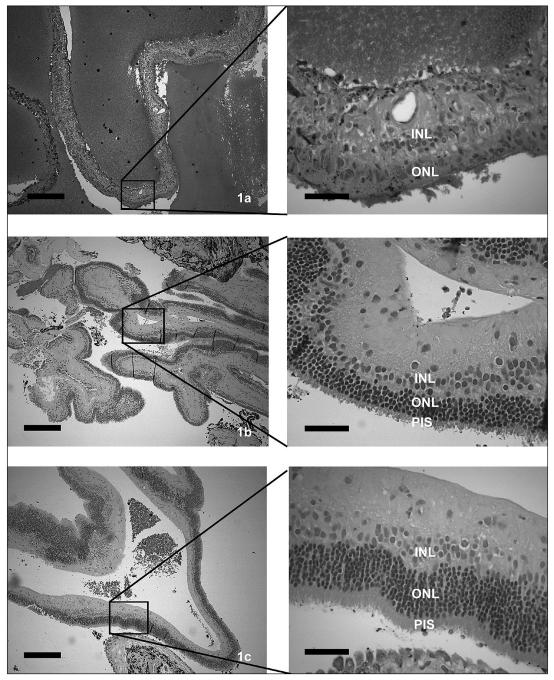
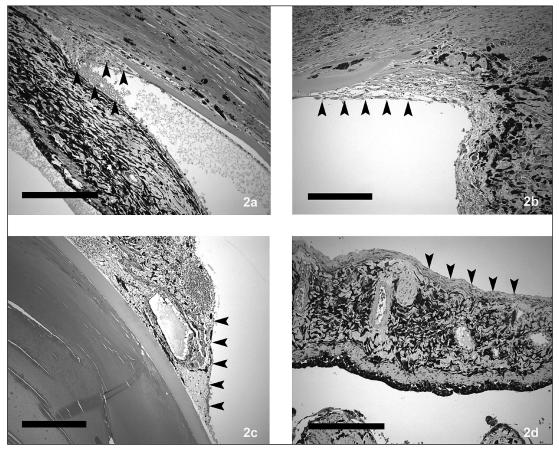
Sixteen eyes in 11 dogs with retinal detachments and giant retinal tears were present in the no treatment group (Table 1). These included an English springer spaniel, a toy poodle, a miniature poodle, a bichon frisé, a papillon, a bearded collie, a basset hound, a Boston terrier, an American cocker spaniel, and 2 shih tzus. The average age at diagnosis was 7.7 y; 7 were males and 4 were females. The estimated range of time of detachment prior to inclusion was 4 wk to 4 y (mean 25 wk). Ten of the 16 eyes developed uveitis and secondary glaucoma or had these clinical signs worsen within 4 wk to 5 mo (mean 4.2 wk) of diagnosis, resulting in enucleation (n = 4) or evisceration (n = 6) of the affected globes. Of the remaining dogs without therapy, 2 that had bilateral detachments were euthanized at the owners’ request at 1 mo and at 1 y because of blindness. Only 2 eyes in 2 dogs remain 1 y and 3 y postconfirmation of diagnosis, and both manifest with mild conjunctival hyperemia. Light microscopy was completed on 7 of the 10 enucleated or eviscerated eyes and each revealed preiridal fibrovascular membranes, retinal pigment epithelial hypertrophy, and retinal degeneration, consistent with chronic retinal detachment (retinal pigment epithelial hypertrophy and loss of photoreceptors, outer nuclear layers, outer plexiform layers, and inner plexiform); uveitis; cortical cataracts; vitreous degeneration; and secondary glaucoma. The retinal degeneration within this group varied within each eye and within the group from extensive where the retina was reduced to a glial scar, to mild where moderate amounts of inner segments remained in large areas of the sections examined (Figures 1a–c). Preiridal fibrovascular membranes were common (Figures 2a–d), often spanned the filtration angle, and may have induced secondary glaucoma, which was common, based on a lack of ganglion cells and nerve fiber layers.
Table 1.
Signalment, estimated time of detachment and outcome of no treatment, topical therapy, and surgical attachment of chromic (>1 mo) retinal detachment in dogs
| Group Number of eyes (dogs) | No treatment 16 (11) | Topical therapy 19 (17) | Surgical attachment 6 (6) |
|---|---|---|---|
| Age | 7.7 y | 8.2 y | 5.7 y |
| Sex | 7 males, 4 females | 10 males, 7 females | 3 males, 3 females |
| (Estimated range of time of detachment) and (mean) at inclusion into treatment group | (4 wk–4 y), (25 wk) | (4 wk–1 y), (8 wk) | (4 wk–4 y), (16 wk) |
| Outcome: uncontrolled uveitis or glaucoma | 14 | 15 | 2 |
| Number of eyes treated with an evisceration and intrascleral prosthesis | 6 | 9 | 1 |
| Number of eyes enucleated | 4 | 4 | 1 |
| The mean time until enucleation or evisceration of globes with for non-responsive uveitis or glaucoma | 4 wk | 15 wk | 4 wk |
| Number of dogs euthanized | 2 (bilaterally affected) | 1 (bilaterally affected) | 0 |
| Eyes remaining with maximum follow-up of 3 y | 2 | 3 | 4 |
Figures 1a–c.
The retinal degeneration related to retinal detachment and secondary glaucoma varied significantly within dogs that received no treatment from a glial scar (1a), to relatively mild outer retinal degeneration where photoreceptor inner segments remain (1b,c). Inner nuclear layer = INL, outer nuclear layer = ONL, photoreceptor inner segments = PIS. (Low power figures bar = 250 μm, high power figures bar = 50 μm).
Figures 2a–d.
Preiridal fibrovascular membranes (outlined by arrowheads) were common in dogs with retinal detachment that was not treated or treated, and varied from barely detectable to extensive. (Figures 2a and 2d bar = 250 μm, Figures 2b and 2c bar = 100 μm).
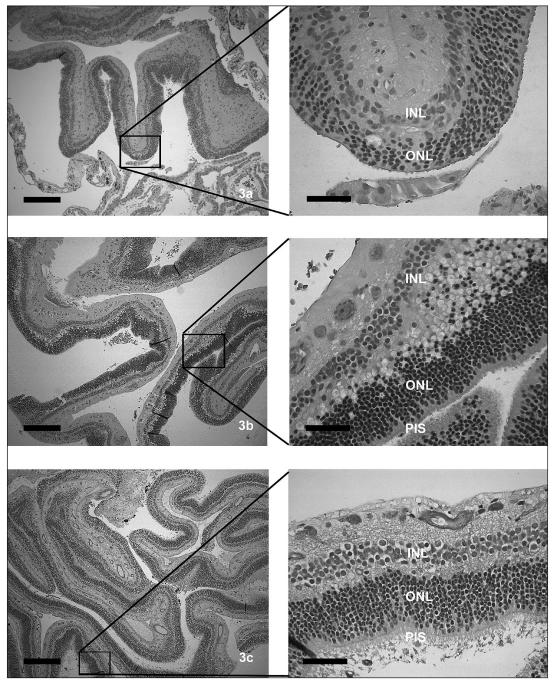
Nineteen eyes in 17 dogs were present in the topical therapy group, including 3 bichon frisés, 3 Jack Russell terriers, 2 Shetland sheepdogs, 2 Boston terriers, 1 Belgian shepherd, 1 toy poodle, 1 miniature schnauzer, 1 American cocker spaniel, 1 miniature poodle, 1 shih tzu, and 1 Yorkshire terrier. The average age at diagnosis was 8.2 y and there were 10 males and 7 females. The estimated range of time of detachment was 4 wk to 4 y (mean 8 wk). Thirteen eyes in 12 dogs developed non-responsive uveitis and secondary glaucoma and were enucleated (n = 4) or eviscerated (n = 9) within 1 to 20 mo (mean 15 wk). One dog (bilaterally affected) was euthanized for an unrelated neurologic disorder and blindness, 3 continue to receive topical medication at 1.5, 3, and 4 y, and the last dog in this group was taken off medication at 1 y postdiagnosis and there is no evidence of uveitis or secondary glaucoma. Light microscopic findings were available for 9 eyes and revealed retinal degeneration that varied within each globe and within the group from extreme, as a glial scar, to mild, where photoreceptor inner segments remained throughout retinal sections examined (Figures 3a–c). Most of the retinas examined also had marked inner retinal degeneration with loss of the nerve fiber layer, ganglion cells, inner plexiform layers, preiridal fibrovascular membranes, all consistent with glaucoma. These light microscopic findings were remarkably similar to those of the no-treatment group.
Figures 3a–c.
Retinal degeneration secondary to chronic retinal detachment and giant retinal tears in dogs that received topical therapy varied from extensive (3a) to relatively mild (3c). Inner nuclear layer = INL, outer nuclear layer = ONL, photoreceptor inner segments = PIS. (Low power figures bar = 250 μm, high power figures bar = 50 μm).
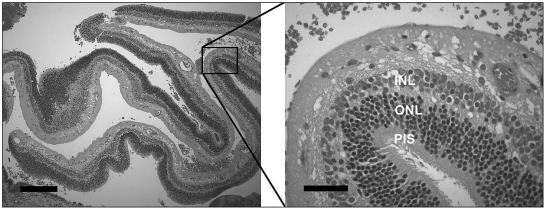
Retinal reattachment surgery was completed on 6 dogs, including 1 bichon frisé, 1 papillion, 1 basset hound, 1 Boston terrier, and 2 shih tzus (Table 1). The average age at diagnosis was 5.7 y and the minimum time of retinal detachment with giant retinal tears prior to reattachment surgery ranged from 1 to 6 mo (mean 16 wk). All of these dogs also had vitreous degeneration and mild uveitis prior to surgery. Each retina was successfully reattached; however, in 2 dogs, fibrous epiretinal membranes were carefully stripped from the detached and coiled-up retinas. In addition, mild to moderate anterior segment hyphema developed during each of these posterior segment surgeries. Surprisingly 4 dogs, including 1 of the shih tzus, the Boston terrier, the bichon frisé, and the papillon regained some functional vision (able to traverse a photopic maze, find toys, and negotiate stairs) within 3.3 mo postreattachment surgery. However, the shih tzu developed significant silicone oil leakage into the anterior chamber and, at approximately 4 mo post-reattachment, the silicone oil was removed. The silicone leakage into the anterior chamber continued, the retina detached again, and the dog became blind again, so the eye was eviscerated. The other shih tzu developed posterior synechiae, pupillary occlusion, iris bombe, and secondary glaucoma, which was undiagnosed for 2 wk. Although the retina was attached, no functional vision could be detected and the elevated intraocular pressure was resistant to medical management. The affected eye was eviscerated and an intrascleral prosthesis was implanted. Light microscopic examination of that eye revealed retinal degeneration consistent with chronic retinal detachment; however, large portions of the retina had photoreceptor inner segments present. In addition, there was mild lymphocytic plasmacytic uveitis, mild preiridal fibrovascular membranes, and degeneration of the nerve fiber, ganglion cell, inner plexiform, and inner nuclear layers, consistent with secondary glaucoma (Figure 4).
Figure 4.
The retinal degeneration present in the 1 eye examined histologically after surgical reattachment exhibited variable retinal degeneration. Photoreceptor inner segments are present in this eye, which was eviscerated due to iris bombe and secondary glaucoma. Note the loss of ganglion cells, which developed secondary to glaucoma in this dog. This retina was attached at the time of evisceration. Inner nuclear layer = INL, outer nuclear layer = ONL, photoreceptor inner segments = PIS. (Low power figure bar = 250 μm, high power figure bar = 50 μm).
Complications associated with surgical retinal reattachment in the remaining 4 dogs included focal corneal ulceration (n = 2 eyes), migration of silicone oil (n = 2 eyes), medically controlled glaucoma (n = 1 eye), and mild cataract formation (n = 2 eyes). In the affected eye of 3 dogs, all retinas remain attached and functional vision is present; uveitis and glaucoma were controlled or not present at follow-up examinations by the veterinary ophthalmologist for 1–3 y. The eyes with reattached retinas remain on topical steroids (n = 3 eyes), q24h, and dorzolamide, if required (n = 1 eye), to maintain normal intraocular pressures.
Discussion
The results of this study add some significant findings to the existing literature. The unexpected return of some vision in 4 eyes, after chronically detached retinas had been reattached, without clinical manifestations of uveitis (hyphema, aqueous flare) and secondary glaucoma and with minimal topical medications at 1–3 y were not expected or previously published. Many veterinary ophthalmologists assume that most globes with chronic retinal detachments eventually fail due to chronic uveitis and secondary glaucoma and that there will be no return of vision if they are surgically reattached. However, the percentage of globes that fail due to uveitis and secondary glaucoma, with or without therapy, has not been reported. Similarly, surgical reattachment of chronically detached retinas in dogs has not been reported, although there are several publications describing the uveitis and secondary glaucoma associated with retinal detachments (1,2,4–6). The development of sustained uveitis and glaucoma and similarities in outcomes of eyes with detached retinas without therapy and those with topical therapy also has not been reported. It is interesting that the retinal degeneration was similar in each group and yet varied significantly within each eye. Some eyes within each of the treatment groups had significant sections of retina with inner segments of photoreceptors that might be capable of regrowing the outer segments, which potentially could restore some vision, despite having been detached for many months, perhaps as a result of the close proximity of portions of the nontapetal retina to the retinal pigment epithelium ventral to the optic disc.
The impact of this study is limited by the small number (n = 6) of chronically detached retinas that were surgically managed, but it is sufficient to encourage retinal reattachment wherever possible, as the outcome of no therapy and topical therapy are uniformly poor, and to encourage further studies on reattachment of chronic retinal detachments. A little vision for a dog significantly affects its quality of life and is superior to darkness and chronic uveitis and secondary glaucoma. The successful surgical management of most retinal detachments, contrasted with the failures in no treatment and topical therapy groups, warrants referral of retinal detachment whenever possible to a veterinary retinal surgeon. Only 2/16 eyes with no therapy and 1/19 eyes with topical treatment had minimal uveitis and remained normotensive within 3 y of diagnosis.
No breed or sex predilection could be identified in any category, based on the small number of dogs in each group, although the bichon frisé, shih tzu, and Boston terriers may be over represented, as in previous reports (14,16).
Enucleation or evisceration and intrascleral prosthesis were completed in a majority of the globes in the no therapy group. The complications in the dogs that received no therapy were expected; we had anticipated the development of preiridal fibrovascular membranes, uveitis, cataract, and secondary glaucoma. We initially assumed that the retinal detachments in this group were more acute than those in the topical therapy group, given the limited uveitis and lack of secondary glaucoma present at the time of diagnosis. However, this assumption was incorrect given the estimated times of detachment that we assessed retrospectively (Table 1); with more time, uveitis and secondary glaucoma developed, and the light microscopic findings in 7 of the globes that failed without therapy were very similar to those in globes that failed with topical medical therapy, since they included uveitis, preiridal fibrovascular membranes, varying retinal degeneration, and secondary glaucoma.
The topical therapy group was predetermined by the presence of inflammation, including hyphema and often secondary glaucoma, which usually had lead to the initial referral. We had expected that these would worsen quickly and become nonresponsive to medical management. It is also not surprising that the topical medical management did not deter the development of anterior segment neovascular membranes, secondary to retinal degeneration. Corticosteroids are not known to inhibit neovascularization or the angiogenic cytokines that induce the anterior segment membranes. This topical therapy was used only to palliate the uveitis. Results in a 4th treatment group with long-term antiangiotic medications, coupled with systemic anti-inflammatory drugs, would have been interesting but currently are not available. As expected, nonresponsive uveitis worsened and secondary glaucoma often developed, resulting in evisceration or enucleation of most of the affected eyes. Nine eviscerated and enucleated globes from this group were examined histologically, whereupon preiridal fibrovascular membranes, uveitis, and secondary glaucoma were confirmed. In addition, 2 dogs in this group were euthanized due to blindness and unrelated disorders. Only 4 globes within the topical medical management group remain and they continue to exhibit conjunctival and episcleral hyperemia, suggesting ongoing uveitis despite topical anti-inflammatory therapy. Enucleation or evisceration and intrascleral prostheses for these 4 had been recommended, but the owners declined surgical therapy. Only 1 dog in this group has been taken off topical therapy and the uveitis remains in remission.
Results of surgical reattachment of chronic (>1 mo) retinal detachments and pre- and postsurgical therapy were similar to those in previous reports of surgical retinal reattachment in dogs (14,16). The vitrectomies were completed with relative ease, given the vitreous degeneration present in each dog. Fibrous epiretinal membranes were encountered in 2 dogs, the basset hound and the Boston terrier. These were difficult to remove completely and precluded complete reattachment of the peripheral retinal edges in these 2 dogs. Despite this, complications have not been noted in these dogs at the 1 and 2-year follow-up examinations (no retinal slippage, further detachment, or uveitis and glaucoma). Hyphema was noted in 6/6 dogs during the vitrectomy and especially during silicone oil transfer, which is unusual. We usually do not experience hyphema during surgical reattachment of acutely detached retinas. The etiologies of the hyphema during surgery were not confirmed; however, preiridal fibrovascular membranes may have contributed. The hyphema was distressing at the time; however, it cleared postoperatively, relatively quickly, often within a week. One dog developed pupillary blockade and secondary glaucoma that were complications of the hyphema and uveitis that contributed to posterior synechiae, iris bombe, and anterior synechiae. Unfortunately, this complication was not confirmed by an ophthalmologist for 2 wk. Prompt reexamination, injection of anterior chamber tissue plasminogen activator, and medical or surgical management may have resolved this condition and precluded the development of iris bombe and secondary glaucoma.
Much to our surprise some functional vision returned in 4/6 dogs that were operated on, and 3 remain visual after 3 y. These dogs were able to navigate a photopic maze with the normal eye patched or when the other eye was blind. They were always noticeably more tentative in scotopic conditions and they failed maze examinations in dim light. This may support a lack of functional return of rod function or, simply, very little functioning retina. The timing of the return of vision in these dogs was varied; in most dogs, it was slow and confirmed only several months postsurgery by the ophthalmologist. We stress that functional vision in these dogs (traversing a maze in phototopic conditions) is not to be interpreted as normal vision. The menace response and counting fingers in humans are often the last detectable signs to be abolished prior to blindness and the ability to traverse a maze slowly with 1 eye is not normal vision. However, this amount of vision should not to be ignored in dogs. The owners noted an improvement in their dogs’ quality of life (fewer errors, bumping into things) and in their ability to find and play with toys, get into favorite chairs, and traverse stairs. Although the number is small, the majority (4/6) of the surgically treated eyes are complication free 3 y after surgical therapy of chronic retinal detachments and giant retinal tears.
In conclusion, most dogs’ eyes with retinal detachment and giant retinal tears develop nonresponsive uveitis and secondary glaucoma, whether they receive topical therapy or not. Light microscopy confirmed uveitis and secondary glaucoma. Despite the chronicity of these detachments, several of these globes in each group had large areas of the retina that contained potentially viable photoreceptor inner segments at the time of enucleation or evisceration. Successful surgical reattachment of chronically detached retinas is possible in dogs and some vision was restored in 3/6 dogs within 3 y postsurgery. CVJ
References
- 1.Martin CL. Ophthalmic Disease in Veterinary Medicine. London: Manson Publ; 2005. [Google Scholar]
- 2.Smith PJ. Surgery of the posterior segment. In: Gelatt KN, editor. Veterinary Ophthalmology. 3. Philadelphia: Lippincott, Williams and Wilkins; 1999. pp. 935–980. [Google Scholar]
- 3.Fisher SK, Anderson DH. Cellular responses of the retinal pigment epithelium to retinal detachment and reattachment. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. 2. New York: Oxford Univ Pr; 1998. pp. 406–419. [Google Scholar]
- 4.Hendrix DV, Nasisse MP, Cowen P, Davidson MG. Clinical signs, concurrent diseases, and risk factors associated with retinal detachments in dogs. Prog Vet Comp Ophthalmol. 1993;3:87–91. [Google Scholar]
- 5.Nelms SR, Nasisse MP, Davidson MG, Kirschner SE. Hyphema associated with retinal disease in dogs: 17 cases (1986–1991) J Am Vet Med Assoc. 1993;202:289–1292. [PubMed] [Google Scholar]
- 6.Peiffer RL, Wilcock BP, Yin H. The pathogenesis and significance of pre-iridal fibrovascular membrane in domestic animals. Vet Pathol. 1990;27:41–45. doi: 10.1177/030098589002700106. [DOI] [PubMed] [Google Scholar]
- 7.Wilcock BP. The eye and ear. In: Jubb KVF, Kennedy PG, Palmer N, editors. Pathology of Domestic Animals. 4. Toronto: Academic Pr; 1993. pp. 441–529. [Google Scholar]
- 8.Hisatomi T, Sakamoto T, Goto Y, et al. Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr Eye Res. 2002;24:161–172. doi: 10.1076/ceyr.24.3.161.8305. [DOI] [PubMed] [Google Scholar]
- 9.Lewis GP, Charteris DG, Sethi CS, et al. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412–2420. [PubMed] [Google Scholar]
- 10.Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photo-receptors on the choroid in the normal and detached retina. Invest Ophthamol Vis Sci. 2000;41:3117–3123. [PubMed] [Google Scholar]
- 11.Sakai T, Lewis GP, Linberg KA, Fisher SK. The ability of hyperoxia to limit the effects of experimental detachment in cone dominated retina. Invest Ophthalmol Vis Sci. 2001;42:3264–3273. [PubMed] [Google Scholar]
- 12.Lewis GP, Talaga KC, Linberg KA, Avery RL, Fisher SK. The efficacy of delayed oxygen therapy in the treatment of experimental retinal detachment. Am J Ophthalmol. 2004;137:1085–1095. doi: 10.1016/j.ajo.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Linsenmeier RA. Hyperoxia improves oxygen consumption in the detached feline retina. Invest Ophthalmol Vis Sci. 2007;48:1335–1341. doi: 10.1167/iovs.06-0842. [DOI] [PubMed] [Google Scholar]
- 14.Vainisi SJ, Wolfer JC. Canine retinal surgery. Vet Ophthalmol. 2004;7:291–306. doi: 10.1111/j.1463-5224.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 15.Dziezyc J, Wolf ED, Barrie KP. Surgical repair of rhegmatogenous retinal detachments in dogs. J Am Vet Med Assoc. 1986;188:902–904. [PubMed] [Google Scholar]
- 16.Vainisi SJ, Packo KH. Management of giant retinal tears in dogs. J Am Vet Med Assoc. 1995;206:491–495. [PubMed] [Google Scholar]
- 17.Machemer R, Laqua H. Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation) Am J Ophthalmol. 1975;80:1–23. doi: 10.1016/0002-9394(75)90862-4. [DOI] [PubMed] [Google Scholar]
- 18.Machemer R. Experimental retinal detachment in the owl monkey II. Histology of the retina and pigment epithelium. Am J Ophthalmol. 1968;66:396–409. doi: 10.1016/0002-9394(68)91523-7. [DOI] [PubMed] [Google Scholar]
- 19.Kroll AJ, Machemer R. Experimental retinal detachment in the owl monkey III. Electron microscopy of retina and pigment epithelium. Am J Ophthalmol. 1968;66:410–427. doi: 10.1016/0002-9394(68)91524-9. [DOI] [PubMed] [Google Scholar]
- 20.Machemer R. Experimental retinal detachment in the owl monkey IV. The reattached retina. Am J Ophthalmol. 1968;66:1075–1101. doi: 10.1016/0002-9394(68)90816-7. [DOI] [PubMed] [Google Scholar]
- 21.Kroll AJ, Machemer R. Experimental retinal detachment in the rhesus monkey. Electron microscopy of rods and cones. Am J Ophthalmol. 1969;69:58–77. doi: 10.1016/0002-9394(69)94935-6. [DOI] [PubMed] [Google Scholar]
- 22.Kroll AJ, Machemer R. Experimental retinal detachment in the owl monkey V. Electron microscopy of the reattached retina. Am J Ophthalmol. 1969;69:117–130. doi: 10.1016/0002-9394(69)90016-6. [DOI] [PubMed] [Google Scholar]
- 23.Machemer R, Kroll AJ. Experimental retinal detachment in the owl monkey VII. Photoreceptor protein renewal in normal and detached retina. Am J Ophthalmol. 1971;71:690–695. doi: 10.1016/0002-9394(71)90432-6. [DOI] [PubMed] [Google Scholar]
- 24.Kroll AJ, Machemer R. Experimental retinal detachment in the owl monkey VIII. Photoreceptor protein renewal in early retinal reattachment. Am J Ophthalmol. 1971;72:356–366. doi: 10.1016/0002-9394(71)91306-7. [DOI] [PubMed] [Google Scholar]
- 25.Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. Retinal detachment in the cat: The pigment epithelial-photoreceptor interface. Invest Ophthalmol Vis Sci. 1983;24:906–926. [PubMed] [Google Scholar]
- 26.Anderson DH, Guerin CJ, Erickson PA, Stern WH, Fisher SK. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183. [PubMed] [Google Scholar]
- 27.Lewis GP, Erickson PA, Guerin CJ, Anderson DH, Fisher SK. Changes in the expression of specific Mueller cell proteins during long-term retinal detachment. Exp Eye Res. 1989;49:93–111. doi: 10.1016/0014-4835(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 28.Fraunfelder FT, Potts AM. An experimental study of retinal detachments. Am J Ophthalmol. 1966;62:561–568. doi: 10.1016/0002-9394(66)91343-2. [DOI] [PubMed] [Google Scholar]
- 29.Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94:762–773. doi: 10.1016/0002-9394(82)90301-4. [DOI] [PubMed] [Google Scholar]
- 30.Grahn BH, Philibert H, Cullen CL, Houston DM, Semple HA, Schmutz SM. Mulifocal retinopathy of great Pyrenees dogs. Vet Ophthalmol. 1998;1:211–221. doi: 10.1046/j.1463-5224.1998.00041.x. [DOI] [PubMed] [Google Scholar]
- 31.Grahn BH, Cullen CL. Retinopathy of Great Pyrenees dogs: Fluorescein angiography, light microscopy and transmitting and scanning electron microscopy. Vet Ophthalmol. 2001;4:191–199. doi: 10.1046/j.1463-5216.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 32.Andrew WE, Abrams KL, Brooks DE, Kublis PS. Clinical features of steroid responsive retinal detachments in twenty-two dogs. Vet Comp Ophthalmol. 1997;7:82–87. [Google Scholar]
- 33.Gwin RM, Wyman M, Ketring K, Winston S. Idiopathic uveitis and exudative retinal detachment in the dog. J Am Anim Hosp Assoc. 1980;16:163–170. [Google Scholar]
- 34.Vainisi SJ, Peyman GA, Wolf DE, West SC. Treatment of serous retinal detachments associated with optic disc pits in dogs. J Am Vet Med Assoc. 1989;195:1233–1236. [PubMed] [Google Scholar]
- 35.Grahn BH, Szentimrey D, Pharr JW, Farrow CS, Fowler JD. Ocular and orbital porcupine quills in the dog: A review and case series. Can Vet J. 1995;36:488–493. [PMC free article] [PubMed] [Google Scholar]
- 36.Wilcock BP. Eye, eyelids, conjunctiva and orbit. In: McGavin MD, Zachary JF, editors. Pathological Basis of Veterinary Disease. 4. St Louis: Mosby Elsevier; 2007. pp. 1349–1413. [Google Scholar]
- 37.Grahn BH, Barnes L, Breaux C, Sandmeyer L. Chronic retinal detachment with giant retinal tears, comparison of three treatment groups (abstract) Proc Am Coll Vet Ophthalmol. 2006:30. [Google Scholar]
- 38.Sakai T, Calderone JB, Lewis GP, Linberg K, Fisher SK, Jacobs GH. Cone photoreceptor recovery after experimental detachment and reattachment surgery: An immunocytochemical, morphological and electrophysiological study. Invest Ophthalmol Vis Sci. 2003;44:416–425. doi: 10.1167/iovs.02-0633. [DOI] [PubMed] [Google Scholar]