Abstract
Canine histiocytic neoplasms include cutaneous histiocytoma, as well as localized and disseminated histiocytic sarcoma. These tumors have variable biologic behavior, although the malignant disorders often have a poor prognosis. Immunohistochemistry plays an essential role in differentiating histiocytic tumors from other neoplasias that may have similar histological appearances. This allows a definitive diagnosis to be established and provides a more accurate prediction of prognosis. This article reviews the biologic behavior, diagnosis, and treatment of histiocytic tumors in the dog.
Résumé
Néoplasie histiocytaire canine : exposé général. Les néoplasmes histiocytaires canins comprennent aussi bien des histiocytomes cutanés que des sarcomes histiocytaires disséminés. Ces tumeurs ont un comportement biologique variable, bien que les maladies malignes aient souvent un mauvais pronostic. L’immunohistochimie joue un rôle essentiel en différenciant les tumeurs histiocytaires des autres néoplasies qui peuvent avoir une apparence histologique similaire. Ceci permet d’établir un diagnostic définitif et permet une prédiction plus précise du pronostic. Cet article revoit le comportement biologique, le diagnostic et le traitement des tumeurs histiocytaires chez le chien.
(Traduit par Docteur André Blouin)
Introduction
Histiocytes are a subset of leukocytes that occur in tissues and serve an integral role in functioning of the immune system. These cells arise from bone marrow-derived CD34+ stem cell precursors; under the influence of various cytokines, they differentiate to form cells of the monocyte/macrophage lineage or the dendritic cell (DC) lineage (1). Once these cells have differentiated, they share many of the same surface antigens, as well as surface receptors for immunoglobulin and complement molecules (1).
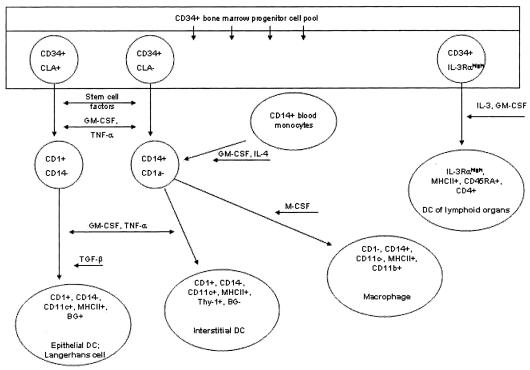
Several DC lineages have been identified in both humans and dogs, including epidermal DC/Langerhans’ cells, interstitial DCs found in many organs, and interdigitating DCs of T-cell domains in peripheral lymphoid organs (Figure 1) (2). The most well-defined DCs in the dog are found in the skin in the form of epidermal DCs (or Langerhans’ cells) and dermal DCs (part of the interstitial DC lineage) (3). Dendritic cells serve as part of the adaptive immune response by acting as potent antigen-presenting cells. After arising from the bone marrow, DCs migrate through blood to a variety of either cutaneous or mucosal epithelial sites. Once migration has occurred, the DCs reside either within epithelia or in the dermis and lamina propria. It is in these sites that antigen processing occurs. Once an antigen has been processed, DCs migrate beyond the skin to the paracortex of lymph nodes, where they present antigens to naïve T-cells in order to initiate an immune response (4).
Figure 1.
Ontogeny and phenotypic characteristics of myeloid dendritic antigen-presenting cells. DC = dendritic cells; BG = Birbeck’s granules. Used with permission, Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol 2002;39:74–83.
Cells of the monocyte/macrophage lineage are phagocytic in function. Once released from bone marrow, monocytes circulate through the blood and become fixed in tissues as mature macrophages. Mature macrophages that have arisen from histiocytes are found in various tissues throughout the body, including skin, spleen, lung (alveolar macrophages) and liver (Kupffer cells). These cells form part of the reticuloendothelial system, which also functions as part of the body’s innate immune system (5).
There are several well-documented disorders of histiocytic cell lines in both humans and dogs. These diseases range from localized, reactive, and benign processes to systemic malignancies that result in a rapid clinical progression and death. Historically, the diagnosis and treatment of these disorders in the dog has been challenging for several reasons, including a lack of histochemical markers that can reliably determine the cell of origin for each condition; the confusing terminology used to describe the different disorders; and the highly variable clinical behavior of supposedly related diseases. Although the histiocytic disorders are becoming recognized more frequently in animals, many of the steps taken in human medicine to identify related disorders and validate reliable diagnostic techniques have not yet been taken in veterinary medicine.
Current veterinary research is directed towards identifying histopathologic markers that accurately identify the cell of origin for each histiocytic disorder; immunohistochemical staining plays an increasingly important role in this respect, because it allows the pathologist to make a definitive diagnosis, thus guiding the clinician’s assessment of prognosis and recommendation of available treatments. Histiocytic tumors can be differentiated from other tumors, such as lymphoma, poorly differentiated mast cell tumors, and malignant fibrous histiocytoma (MFH). Immunohistochemical staining for these tumors involves the use of antibodies directed against the cellular antigens on the cytoplasmic surface of leukocytes known as cluster of differentiation antigens (CD antigens) (6). These antigens vary, based on the stage of a cell’s lineage-specific differentiation, and also may reflect different states of activation or inactivation in various leukocytes. Cluster of differentiation antigens are recognized by a variety of monoclonal antibodies (6). Formation of an antigen-antibody complex results in deposition of a colored precipitate on the cells expressing the antigen in question, allowing their identification on glass slides. The use of immunohistochemical staining permits specific distinction between the cells that cause canine cutaneous histiocytoma, the reactive histiocytic disorders, and the tumors of the histiocytic sarcoma complex; it also identifies cells that are not of macrophage origin, so they can be differentiated from other malignant cell types.
The purpose of this review of canine histiocytic neoplasia is to clarify the expected clinical presentation, biologic behavior, and available treatment options for these often confusing tumors. This review also outlines methods that can be used to differentiate histiocytic neoplasia from other malignant neoplasms. Ultimately, this will allow veterinarians to more accurately define the best treatment plan for a given disease, as well as provide the most reliable prognostic information possible to clients whose dogs are affected by these tumors.
Literature review
The literature was reviewed by using the National Center for Biotechnology Information (NCBI) PubMed database. Search terms included canine histiocytoma, histiocytic sarcoma, canine malignant histiocytosis, malignant fibrous histiocytoma, immunohistochemistry, Langerhans’ cell, and synovial cell sarcoma. All resources have been peer-reviewed, with the exception of conference proceedings and Web site information provided by Dr. Peter Moore, an internationally recognized expert on histiocytic disorders in the dog.
Canine histiocytic disorders
Histiocytic disorders affecting dogs were first described in the late 1970s. The first reports were of solitary histiocytic lesions, that came to be known as canine cutaneous histiocytomas, but these were soon followed by descriptions of other histiocytic disorders, primarily affecting Bernese mountain dogs (7,8). Since the initial studies that documented histiocytic diseases in animals, substantial research has better defined the characteristics of each of the histiocytic conditions, resulting in the identification and classification of several distinct disorders. As in humans, some of the disorders are not considered malignant, while others are highly aggressive malignancies with a high mortality rate.
Most of the histiocytic disorders are well documented in dogs. Cats, in contrast, are rarely affected by these diseases (9). In dogs, the histiocytic disorders have been classified into 3 major categories: the canine cutaneous histiocytoma; the canine reactive histiocytoses (including both cutaneous and systemic); and the histiocytic sarcoma complex (which includes both localized and disseminated histiocytic sarcoma) (10). Another tumor, which is actually a soft tissue sarcoma, is the MFH, which is composed of both histiocytic and fibroblastic cell types (11). This tumor is not histiocytic in origin, but because of its similar morphologic characteristics, it must be differentiated from histiocytic sarcoma.
Canine cutaneous histiocytoma
Canine cutaneous histiocytoma is a tumor that generally arises as a solitary lesion in young dogs (< 4 y old) (12). Brachycephalic breeds, such as boxers and bulldogs, are predisposed, although Scottish terriers, Doberman pinschers, and cocker spaniels also are reported to be overrepresented as well (12). The tumor commonly arises on the head or pinna, but may occur anywhere on the body. It has benign behavior and, generally, no treatment is necessary, although surgery may be considered in older dogs or for lesions that do not regress over long periods. Lesions usually undergo spontaneous regression within 1 to 2 mo, a process characterized by infiltration of mature lymphocytes on cytologic or histopathologic examination (13). Dogs diagnosed with solitary cutaneous histiocytomas are expected to have an excellent prognosis. Some dogs may develop multiple lesions or, in very rare cases, metastasis to a lymph node, but these are exceptional examples of aggressive behavior (12).
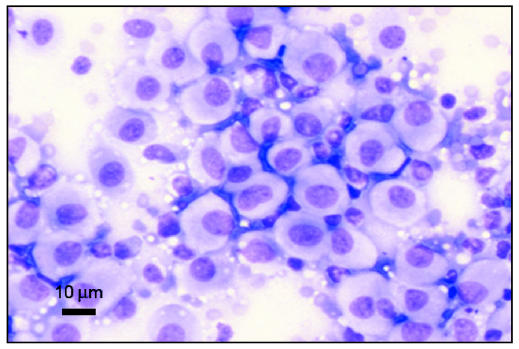
Cutaneous histiocytomas are generally diagnosed easily by cytopathologic examination (Figure 2), but histopathologic examination with immunohistochemical staining provides a definitive diagnosis. This tumor’s phenotype is identical to that of canine epidermal Langerhans’ cells, with expression of CD1a, CD1b, CD1c, major histocompatibility complex (MHC) Class II, CD11c, and E-cadherin, all of which are performed on snap-frozen sections. E-cadherin analysis can also be performed on formalin-fixed paraffin sections, which are most readily available to practitioners (3). Amongst leukocytes, E-cadherin expression is unique to Langerhans’ cells, specifically identifying canine histiocytoma as a localized epidermal Langerhans’ cell tumor (10) (see Tables 1 and 3).
Figure 2.
Photomicrograph of a cytology slide from an aspirate of a cutaneous mass on a 2-year-old dog. The mass was diagnosed as cutaneous histiocytoma. Note the pale cytoplasm and round nuclei typical of this cell type. Image courtesy of Dr. Jeff Sirninger, Louisiana State University. Bar = 10 μm.
Table 1.
| Marker | Cell type |
|---|---|
| CD1 (a, b, c) | Langerhans’ cells, dendritic cells |
| CD3 | T cells, used to differentiate B vs. T cell lymphoma |
| CD4 | Helper T cells, activated dendritic cells |
| CD11b | Myeloid cells (macrophages), NK1 cells |
| CD11c | Dendritic cells, NK1 cells |
| CD11d | Macrophages identified in the splenic red pulp and bone marrow |
| CD18 | Broad staining of leukocytes, prominent in histiocytic tumors |
| CD45RA | B cells and naïve T cells |
| CD79a | B cells, used to differentiate B vs. T cell lymphoma |
| MHCII | Broad expression in cells functioning in antigen presentation |
| Thy-1 (CD90) | CD34+ hematopoietic cells, specifically interstitial dendritic cells |
| E-cadherin | Specific to epidermal Langerhans cells, epithelial cells |
| Vimentin | Mesenchymal cells |
| Smooth Muscle Actin | Identifies smooth muscle cells |
NK — natural killer cells
Table 3.
Expected immunohistochemical expression patterns of neoplastic histiocytic disorders in the dog
| Markers | LCs | Interstitial DC | CCH | HS | MFH |
|---|---|---|---|---|---|
| CD1 (a,b,c) | + | + | + | + | − |
| CD11 b | − | − | − | − | +/− |
| CD11 c | + | + | + | + | +/− |
| CD11 d | − | − | − | − | − |
| CD18 | + | + | + | + | +a |
| CD4 | − | + | − | − | − |
| (if activated) | |||||
| MHC II | + | + | + | + | Unknown |
| E-cadherin | + | − | + | − | − |
| Thy-1 | − | + | − | − | − |
| Vimentin | n/a | n/a | n/a | + | + |
| SM Actin | n/a | n/a | n/a | +/− | +/−b |
LCs — Langerhans’ cells; MFH — malignant fibrous histiocytoma; CCH — canine cutaneous histiocytoma; HS–histiocytic sarcoma (localized or disseminated)
Some scattered CD18 positive cells are often seen in staining of MFH due to the infiltration of monocytes and macrophages into the tumor
In one large study, the smooth muscle actin staining was of stromal cells or vascular smooth muscle, not the actual tumor cells (26)
In macrophage disorders or malignancies, CD11b or CD11d expression is usually identified rather than CD11c
Histiocytic sarcoma complex
The histiocytic sarcoma complex of the dog, which includes both localized and disseminated histiocytic sarcoma, comprises malignant diseases characterized by infiltration of neoplastic histiocytes, most frequently observed in middle-aged Bernese mountain dogs (often with a familial association), rottweilers, flat-coated retrievers, and golden retrievers (14).
Localized histiocytic sarcoma most often occurs as a primary lesion involving the skin and subcutis of the extremities, although it may also be found in periarticular tissues surrounding large appendicular joints, spleen, lymph nodes, lung, or bone marrow (15). Affected dogs are usually presented for evaluation of a soft tissue swelling on an extremity, but the owner may have observed lameness in the affected limb, first, and discovered the mass, subsequently. This form of histiocytic sarcoma is locally invasive and local metastasis to draining lymph nodes is commonly noted (16).
Localized histiocytic sarcoma is treated either by surgical excision or by amputation of the affected limb. Curative intent radiation therapy, which may be recommended for incompletely excised or nonresectable tumors, has been reported to have some efficacy in a limited number of cases (16–18). However, the optimal prescription of radiation with respect to scheduling and total dose delivery has yet to be determined. It is also unknown if aggressive definitive courses of radiation therapy would be superior to palliative radiation protocols. Draining lymph nodes, if they are involved, may be excised at the time of surgery or treated with radiation therapy, postoperatively. Dogs with localized histiocytic sarcoma have a more favorable prognosis than dogs with disseminated disease, and euthanasia should not be automatically considered or recommended in these cases (15). However, owners should still be warned about the possibility of spread beyond the primary site (16–18). Systemic chemotherapy may be considered because of the potential for aggressive biologic behavior and metastasis (16–18). In fact, localized and disseminated histiocytic sarcoma may simply represent 2 different stages along a continuum of the same disease.
Regardless of the extent of disease, a definitive diagnosis is essential prior to the initiation of therapy, as histologically, histiocytic sarcoma can resemble other neoplasms that vary substantially with respect to recommended treatment and prognosis. For example, 35 previously diagnosed synovial cell tumors in dogs were reevaluated by histopathologic examination and immunohistochemical staining. It was determined that of the 35 original synovial cell sarcomas, 18 were definitively identified, by staining with CD18 antibody, as histiocytic sarcomas, suggesting that histiocytic sarcoma may be more prevalent in the dog than previously believed (18).
The most common clinical signs in dogs suffering from disseminated histiocytic sarcoma are anorexia, lethargy, and weight loss (15). Lesions have been identified in every organ, although primary sites usually include the spleen, lungs, and bone marrow (16). Dogs may exhibit dyspnea due to pulmonary involvement or lameness secondary to large extremity masses. Intervertebral lesions have also resulted in ataxia and paraparesis (16). Necropsy of affected animals often reveals extensive disease involving most organs (12). Disseminated histiocytic sarcoma is difficult to differentiate from malignant histiocytosis, a multisystem disorder primarily affecting Bernese mountain dogs (15). The latter disease is characterized by disseminated lesions that simultaneously arise in multiple organs, such as skin, spleen, liver, lymph nodes, and bone marrow. Since it is virtually impossible to determine if these lesions metastasized from a primary site or all arose simultaneously, the currently accepted terminology refers to both conditions as disseminated histiocytic sarcoma (15).
Both localized and disseminated histiocytic sarcoma have the potential for highly aggressive behavior, so complete staging to determine the extent of disease is recommended prior to therapy. The minimum database for staging should include a complete blood (cell) count, a biochemical panel, and urinalysis; chest and abdominal radiographs or ultrasonographs, or both; and a bone marrow biopsy. Aspirates of regional lymph nodes should also be obtained for evaluation of metastatic disease. As already discussed, local treatments, including surgery and radiation therapy, may be appropriate for localized histiocytic sarcoma. However, disseminated histiocytic sarcoma is a rapidly progressive disease that carries a poor prognosis, even with aggressive therapy, so the current treatment recommendation is systemic chemotherapy. Responses have been reported in dogs treated with lomustine; even with an initial response to chemotherapy, median survival times remain in the 3- to 6-mo range (17). In a recent multi-institutional study, lomustine was administered at a median dose of 70.8 mg/m2 (range, 60 to 90 mg/m2) every 3–4 weeks for a median of 4 doses. The median survival time for the 59 dogs in this study was 106 d, although 3 dogs with minimal residual disease at the time of initiation of chemotherapy lived 433 d or more, after the start of the lomustine treatment (19). There are a few reports of responses to chemotherapy with doxorubicin, liposomal doxorubicin, or paclitaxel (20,21). A dog with disseminated cutaneous histiocytic sarcoma that temporarily responded to multiple protocols that included cyclophosphamide, vincristine, prednisone, mitoxantrone, dacarbazine, and etoposide has been reported (22). Many dogs are euthanized at the time of diagnosis because of poor clinical condition and poor prognosis.
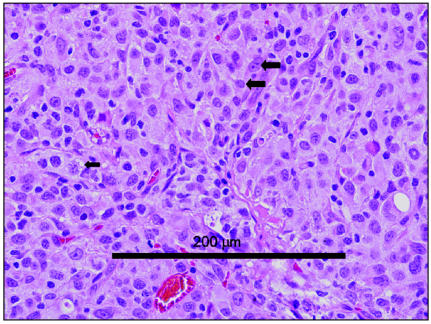
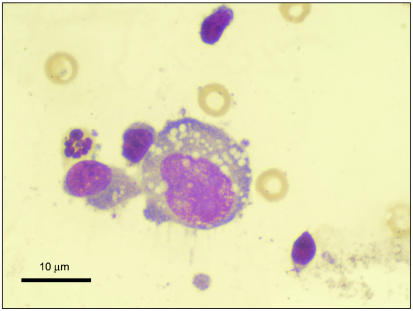
Histopathologically, tumors of the histiocytic sarcoma complex are poorly demarcated, and cell proliferation commonly results in effacement of normal tissue architecture. A population of large, vacuolated round cells is typically present, in combination with plump mesenchymal cells. Bizarre multinucleated giant cells are also a characteristic finding (16,23). Generally, a marked anisokaryosis and anisocytosis with a high mitotic rate is present (Figure 3), and it is not uncommon to identify phagocytosis of neutrophils and red blood cells or cell fragments by the tumor cells. The appearance is similar cytologically to large round cells intermixed with spindle-shaped cells (Figure 4), and multinucleated giant cells with atypical morphology are often observed. An inflammatory infiltrate may also be seen (23).
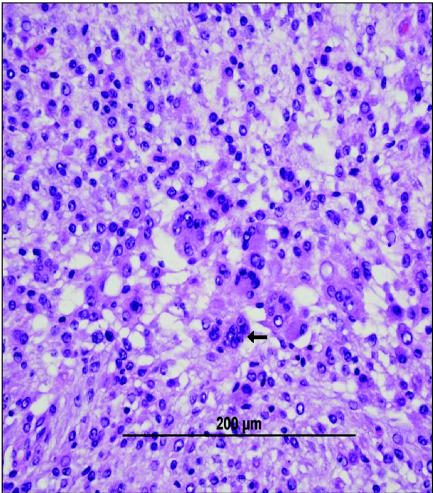
Figure 3.
Photomicrograph of a biopsy from a mass surrounding the right elbow of a 9-year-old rottweiler. Note the presence of vacuolated round cells (small arrow) as well as mesenchymal cells, along with a high degree of anisokaryosis (two large arrows). The tumor was diagnosed as a histiocytic sarcoma. Image courtesy of Dr. Scott Reed, Louisiana State University. Bar = 200 μm.
Figure 4.
Photomicrograph of the aspirate from the rottweiler in Figure 2. The photo shows a large round histiocytic cell with the typical vacuolated appearance. Image courtesy of Dr. Jeff Sirninger, Louisiana State University. Bar = 10 μm.
Immunohistochemical staining has been used to help in identifying the cell of origin of histiocytic sarcoma, and in determining if there is a difference in cell phenotype between the localized and disseminated forms of the disease. Unfortunately, there are no known immunohistochemical or histopathological means of differentiating between the localized and disseminated forms of histiocytic sarcoma (16). Recently, various tissues from 39 dogs diagnosed with either localized or disseminated histiocytic sarcoma were stained immunohistochemically by using a panel of leukocyte-specific antibodies; the tumor cell phenotype was determined to be consistent with a myeloid dendritic antigen- presenting cell origin (16). This indicates that histiocytic sarcomas arise from the dendritic lineage of histiocytes (either interstitial or interdigitating dendritic cells), not the macrophage lineage (16). All of the tumors in this study, whether localized or disseminated, consistently expressed CD18 (a leukocyte marker; Figure 5), CD1abc, CD11c, and MHC II, which is consistent with a dendritic cell origin. Because they lack expression of E-cadherin, the staining pattern of these cells is inconsistent with a Langerhans’ cell origin. Lack of Thy-1 and CD4 expression also means their phenotype is inconsistent with the cells of reactive histiocytosis. Lack of expression of CD79a or CD3 rules out lymphoid origin, while lack of expression of CD11b rules out macrophage origin (see Tables 1, 2, and 3) (16).
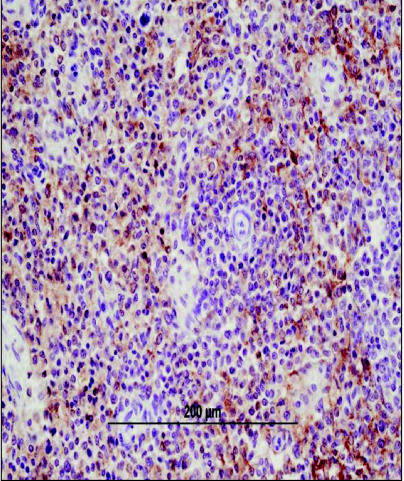
Figure 5.
Photomicrograph of splenic tissue exhibiting diffuse CD18 staining by immunohistochemistry. The CD18 positive cells are identified by their golden-brown staining pattern. CD18 staining is performed on formalin-fixed tissue and although it is a broad leukocyte marker, staining is prominent in histiocytic tumors. Image courtesy of Dr. Tim Morgan, Louisiana State University. Bar = 200 μm.
Table 2.
Expected immunohistochemical expression patterns for histiocytic sarcoma
| Tissue type | Expression pattern |
|---|---|
| Snap-frozen tissue | CD1+ CD4 − CD11c+ CD11d − MCH II+ Thy−1 +/− |
| Formalin-fixed tissue | CD3 − CD11d − CD18 + CD45 RA − CD79a − E-cadherin − |
It is not yet possible to determine the exact sublineages of dendritic cells from which histiocytic sarcomas arise. However, use of immunophenotyping in conjunction with histopathologic examination is very helpful in differentiating these tumors from lymphomas, poorly differentiated mast cell tumors, and malignant fibrous histiocytomas (10).
Malignant fibrous histiocytoma
Malignant fibrous histiocytoma is a soft tissue sarcoma that is often confused with histiocytic sarcoma. This tumor was first diagnosed in humans in the 1960s, and later recognized as the most common soft tissue sarcoma to occur in adult life (24). They were initially reported to have a high rate of local recurrence after surgical excision and a metastatic rate as high as 42% (25). However, more recent work has revealed that many MFHs after being reviewed histopathologically and immunohistochemically need to be reclassified. Many tumors are reclassified as leiomyosarcomas, myxofibrosarcomas, or undifferentiated sarcomas of unknown cell origin (26). In fact, the most current immunohistochemical and molecular studies performed on so-called MFHs in humans suggest that MFH is not a true clinical entity at all, but rather a group of undifferentiated pleomorphic sarcomas. Accordingly, MFH has been removed from the latest World Health Organization classification of tumors (27).
Since the first documented reports of MFH in 2 dogs in 1979, the diagnosis has become more and more common in veterinary medicine (28), most often in dogs on the limb or trunk, but also in the spleen and other organs (28,29). Affected animals are usually middle-aged to older, and rottweilers and golden retrievers are reported to be predisposed (11). Tumor behavior is believed by many authors to be similar to other soft tissue sarcomas; that is, locally invasive with a low to moderate rate of metastasis (~15%) (30). Gleiser et al (28) described MFH in 2 dogs and 3 cats, where all the tumors involved the skin or subcutis and no distant metastases were identified in any of the animals (28). Hendrick (29) reported 6 dogs with MFH of the spleen, only 1 of which had evidence of hepatic metastasis at the time of death; 3 of the dogs were still alive at the time of the writing (29). Based on these reports, optimal therapy for MFH would appear to include complete surgical resection, followed by definitive radiation therapy, as needed, for residual disease (30). Unless histopathologic study reveals evidence of a high grade tumor, the indication for chemotherapy is questionable and a favorable prognosis is expected (75% 5-year survival rate) (30).
In contrast, however, some reports have suggested that canine MFH is more aggressive than the typical soft tissue sarcoma: the clinical course may be similar to that described in humans in whom there is a high rate of local recurrence and metastasis, in which case, affected dogs would be expected to have a poor prognosis (31–33). Unfortunately, immunohistochemical staining to determine the cell of origin was not performed in most of these early studies, and histopathologic similarities between this tumor and histiocytic sarcoma or leiomyosarcoma make it possible that some of these more aggressive tumors were, in fact, of a different cell origin (33). Ten cases of MFH were identified retrospectively, and the affected dogs were reported to have a median survival time of only 61 d (31). However, no immunohistochemical staining was performed on any of these specimens (31). A recent report describing MFH in 14 flat-coated retrievers, known to be predisposed to histiocytic sarcoma, suggests that MFH has a more aggressive course in these dogs compared with that in other breeds (33).
Morphologically, MFHs are composed of a population of mesenchymal cells that are often fibroblastic in appearance and may be arranged in a storiform pattern (11). Many MFHs also have scattered multinucleated giant cells resembling the giant cells present in histiocytic sarcomas, but it is unclear if these cells are part of the malignant population (Figure 6). Large populations of reactive monocytes and macrophages have been observed infiltrating the site of tumor implantation in severe combined immunodeficiency (SCID) mice bearing human MFH (34), leading the authors of this report to conclude that these cells were not actually part of the malignant population, a feeling shared by many pathologists in the human field with respect to naturally occurring tumors in humans (35).
Figure 6.
Histopathology of a malignant fibrous histiocytoma removed from the left lateral thorax of a 6-year-old rottweiler. A large population of mesenchymal cells was identified. Occasional multinucleate giant cells were noted (arrow). Image courtesy of Dr. Tim Morgan, Louisiana State University. Bar = 200 μm.
Definitive immunohistochemical staining patterns have not been clearly identified for MFH in humans. The pleomorphic sarcomas that were once considered MFH have been identified as staining positive for vimentin and potentially smooth muscle actin, but CD18 staining has not been done consistently (36). The difficulty in establishing a definitive staining pattern has carried over into veterinary medicine, and no standardized antibody panel has been recommended for analysis of these tumors. In dogs, histiocytic sarcomas are known to be uniformly CD18 positive and usually vimentin positive, whereas MFH are reported to exhibit a vimentin positive, CD18 negative phenotype (16,18,33). Although positive staining for smooth muscle actin was used to identify 2 canine MFH in 1 study (18), in the largest study utilizing immunohistochemical staining to differentiate between tumors of different cell types, it was discovered that the smooth muscle actin was staining vascular smooth muscle or stromal cells, not neoplastic cells (33). In addition, scattered CD18 positive staining should be expected in MFH because of tumor infiltration by leukocytes of the monocyte/macrophage lineage (Figure 7). Interestingly, the immunohistochemical phenotype that is reported for MFH in both humans and dogs is almost identical to that of leiomyosarcoma, and many of the tumors once thought to represent MFH in humans have been reclassified as leiomyosarcomas (26,36). Some veterinary pathologists also suggest that MFH may not be a distinct entity in animals but, in fact, may be a group of sarcomas with differing cell origins but similar histopathological features (37,38). Certainly, the degree of variation in immunohistochemical staining patterns reported for MFH makes it likely that this tumor is not a distinct entity in the dog. Once the appropriate staining patterns have been firmly established, immunohistochemical analysis will provide an even more valuable tool for differentiating so-called MFH, histiocytic sarcoma, leiomyosarcoma, and other tumors with similar morphologic characteristics. This, in turn, will allow veterinarians to determine the best therapeutic options and provide the most precise prognostic information to the client.
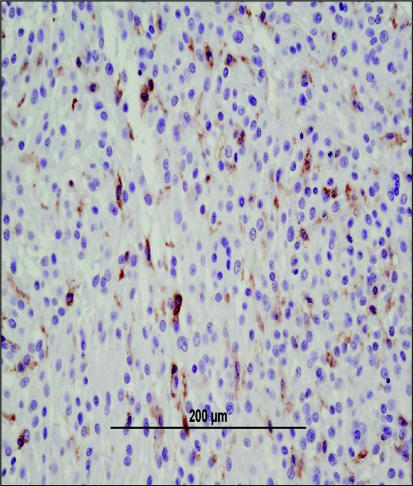
Figure 7.
Immunohistochemical staining of tissue from the same dog identified in Figure 5 with an MFH. Occasional CD18 positive cells can be identified within the tissue; in this tumor the CD18-positive cells are consistent with non-malignant macrophages infiltrating tumor tissue. Image courtesy of Dr. Tim Morgan, Louisiana State University. Bar = 200 μm.
Future directions and conclusions
The ability to definitively diagnose the histiocytic diseases and differentiate reactive from malignant conditions has been greatly enhanced by the recent immunohistochemical distinctions found amongst these disorders. However, it is still unclear how to best differentiate histiocytic sarcoma from presumed MFH, both morphologically and from an immunohistochemical perspective. This distinction is significant, because the recommended treatment and survival times for these diseases may vary greatly: local therapy alone can result in long-term control in cases of so called MFH, while histiocytic sarcoma often exhibits highly malignant behavior and effective treatment protocols have yet to be identified for animals with aggressive local disease or metastasis. Based on recent work, a panel of antibodies to detect CD1, CD4, CD11c, CD11d, MCH II, and Thy-1 is suggested to diagnose histiocytic sarcomas (Table 2) (16). However, many of these antibodies can only be utilized for the staining of snap- frozen tissue samples, since they will not react with formalin-fixed samples. A different panel is recommended for formalin- fixed tissues, including antibodies to CD3, CD11d, CD18, CD45RA, CD79a, and E-cadherin (Table 2) (16). These panels are recommended for tumors of lymphoid origin, histiocytic tumors, mast cell tumors, leiomyosarcomas, and so-called MFHs (10). It is important for veterinary oncologists and pathologists to work together to establish the most appropriate antibody panels for each tumor type, based on their expected expression patterns. In addition, it is necessary to establish explicit definitions of the histopathological features of each tumor, so that the diagnosis of histiocytic sarcoma and the soft tissue sarcomas, formerly designated MFH, becomes unambiguous. Once this distinction has been made, veterinary oncologists can determine if there truly is a subset of animals with aggressive so-called MFH, or if the majority of these tumors are, in fact, histiocytic sarcomas or leiomyosarcomas that have been misdiagnosed. Consideration should be given to following the human example and discarding the diagnosis of MFH altogether, because of the confusion that surrounds this terminology. CVJ
References
- 1.Janeway CA, Travers P, Walport M. Principles of innate and adaptive immunity. In: Janeway CA, Travers P, editors. Immunobiology: The Immune System in Health and Disease. Garland Publ; New York: 1999. pp. 11–20. [Google Scholar]
- 2.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 3.Moore PF, Schrenzel MD, Affolter VK, Olivry T, Naydan D. Canine cutaneous histiocytoma is an epidermotropic Langerhans cell histiocytosis that expresses CD1 and specific beta 2-integrin molecules. Am J Pathol. 1996;148:1699–1708. [PMC free article] [PubMed] [Google Scholar]
- 4.Moore PF. Histiocytes in skin disease. In: Kwotchka K, Willemse T, von Tscharner C, editors. Advances in Veterinary Dermatology. Vol. 3. Oxford: Butterworth-Heinemann; 1998. pp. 77–94. [Google Scholar]
- 5.Janeway CA, Travers P, Walport M. Infection and innate immunity. In: Janeway CA, Travers P, editors. The Immune System in Health and Disease. New York: Garland Publ; 1999. pp. 363–374. [Google Scholar]
- 6.Kuby J. Cells and organs of the immune system. In: Goldsby KTRA, Osborne BA, editors. Immunology. New York: WH Freeman; 2000. pp. 25–31. [Google Scholar]
- 7.Moore PF. Systemic histiocytosis of Bernese mountain dogs. Vet Pathol. 1984;21:554–563. doi: 10.1177/030098588402100602. [DOI] [PubMed] [Google Scholar]
- 8.Glick AD, Holscher M, Campbell GR. Canine cutaneous histiocytoma: Ultrastructural and cytochemical observations. Vet Pathol. 1976;13:374–380. doi: 10.1177/030098587601300507. [DOI] [PubMed] [Google Scholar]
- 9.Kraje AC, Patton CS, Edwards DF. Malignant histiocytosis in 3 cats. J Vet Intern Med. 2001;15:252–256. doi: 10.1892/0891-6640(2001)015<0252:mhic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Moore PF. The histiocytic disease complex. Proc Annu Meet Coll Vet Intern Med, Minneapolis, Minnesota. 2004:437–438. [Google Scholar]
- 11.Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In: Meuten DJ, editor. Tumors in Domestic Animals. 4. Ames, Iowa: Iowa State Univ Pr; 2002. pp. 89–91. [Google Scholar]
- 12.Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In: Meuten DJ, editor. Tumors in Domestic Animals. Ames, Iowa: Iowa State Univ Pr; 2002. pp. 109–111. [Google Scholar]
- 13.Gross TL, Ihrke PJ, Walder EJ. In: Veterinary Dermatopathology: A Macroscopic Evaluation of Canine and Feline Skin Diseases. Gross TL, Ihrke PJ, Walder EJ, editors. St. Louis, Missouri; Mosby: 1992. pp. 215–221. [Google Scholar]
- 14.Pool RR, Thompson KG. Tumors of joints. In: Meuten DJ, editor. Tumors in Domestic Animals. 4. Ames, Iowa: Iowa State Pr; 2002. pp. 237–238. [Google Scholar]
- 15.Moore PF. The UC Davis Canine Histiocytosis site. [Last accessed April 23, 2005];Histiocytic sarcoma and malignant histiocytosis. Available from: http://www.histiocytosis.ucdavis.edu/
- 16.Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74–83. doi: 10.1354/vp.39-1-74. [DOI] [PubMed] [Google Scholar]
- 17.Fidel J, Schiller I, Hauser B, et al. Histiocytic sarcomas in flat-coated retrievers: A summary of 37 cases (November 1998 – March 2005) Vet Comp Oncol. 2006;4:63–74. doi: 10.1111/j.1476-5810.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 18.Craig LE, Julian ME, Ferracone JE. The diagnosis and prognosis of synovial tumors in dogs: 35 cases. Vet Pathol. 2002;39:66–73. doi: 10.1354/vp.39-1-66. [DOI] [PubMed] [Google Scholar]
- 19.Skorupski KA, Clifford CC, Paoloni MC, et al. CCNU for the treatment of dogs with histiocytic sarcoma. J Vet Intern Med. 2007;21:121–126. doi: 10.1892/0891-6640(2007)21[121:cfttod]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Vail DM, Kravis LD, Cooley AJ, Chun R, MacEwen EG. Preclinical trial of doxorubicin entrapped in sterically stabilized liposomes in dogs with spontaneously arising malignant tumors. Cancer Chemother Pharmacol. 1997;39:410–416. doi: 10.1007/s002800050591. [DOI] [PubMed] [Google Scholar]
- 21.Poirier VJ, Hershey AE, Burgess KE. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222. doi: 10.1892/0891-6640(2004)18<219:eatopt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Uno Y, Momoi Y, Watari T, et al. Malignant histiocytosis with multiple skin lesions in a dog. J Vet Med Sci. 1993;55:1059–1061. doi: 10.1292/jvms.55.1059. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DW, Dungworth DL. Tumors of the respiratory tract. In: Meuten DJ, editor. Tumors in Domestic Animals. Ames, Iowa: Iowa State Univ Pr; 2002. pp. 397–399. [Google Scholar]
- 24.O’Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964;17:1445–1455. doi: 10.1002/1097-0142(196411)17:11<1445::aid-cncr2820171112>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Enzinger FM, Weiss SW. Malignant fibrohistiocytic tumors. In: Weiss SW, Enzinger FM, editors. Soft Tissue Tumors. St. Louis, Missouri: Mosby; 1988. pp. 269–300. [Google Scholar]
- 26.Daugaard S. Current soft-tissue sarcoma classifications. Eur J Cancer. 2004;40:543–548. doi: 10.1016/j.ejca.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher CDM, Mertens F. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon: IARC Pr; 2002. [Google Scholar]
- 28.Gleiser CA, Raulston GL, Jardine JH, Gray KN. Malignant fibrous histiocytoma in dogs and cats. Vet Pathol. 1979;16:199–208. doi: 10.1177/030098587901600205. [DOI] [PubMed] [Google Scholar]
- 29.Hendrick MJ, Brooks JJ, Bruce EH. Six cases of malignant fibrous histiocytoma of the canine spleen. Vet Pathol. 1992;29:351–354. doi: 10.1177/030098589202900410. [DOI] [PubMed] [Google Scholar]
- 30.McKnight JA, Mauldin GN, McEntee MC, Meleo KA, Patnaik AK. Radiation treatment for incompletely resected soft-tissue sarcomas in dogs. J Am Vet Med Assoc. 2000;217:205–210. doi: 10.2460/javma.2000.217.205. [DOI] [PubMed] [Google Scholar]
- 31.Waters CB, Morrison WB, DeNicola DB, Widmer WR, White MR. Giant cell variant of malignant fibrous histiocytoma in dogs: 10 cases (1986–1993) J Am Vet Med Assoc. 1994;205:1420–1424. [PubMed] [Google Scholar]
- 32.O’Brien RT, Hendrick MJ, Evans SM, Brooks JJ. Pathological and radiographical features of multicentric malignant fibrous histiocytoma in two dogs. J Comp Pathol. 1991;105:423–430. doi: 10.1016/s0021-9975(08)80111-9. [DOI] [PubMed] [Google Scholar]
- 33.Morris JS, McInnes EF, Bostock DE, Hoather TM, Dobson JM. Immunohistochemical and histopathologic features of 14 malignant fibrous histiocytomas from flat-coated retrievers. Vet Pathol. 2002;39:473–479. doi: 10.1354/vp.39-4-473. [DOI] [PubMed] [Google Scholar]
- 34.Hatano H, Tokunaga K, Ogose A, et al. Origin of histiocyte-like cells and multinucleated giant cells in malignant fibrous histiocytoma: neoplastic or reactive? Pathol Int. 1999;49:14–22. doi: 10.1046/j.1440-1827.1999.00819.x. [DOI] [PubMed] [Google Scholar]
- 35.Schneider P, Busch U, Meister H, Qasem Q, Wunsch PH. Malignant fibrous histiocytoma (MFH). A comparison of MFH in man and animals. A critical review. Histol Histopathol. 1999;14:845–860. doi: 10.14670/HH-14.845. [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa T, Hasegawa F, Hirose T, Sano T, Matsuno Y. Expression of smooth muscle markers in so called malignant fibrous histiocytomas. J Clin Pathol. 2003;56:666–671. doi: 10.1136/jcp.56.9.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thoolen RJ, Vos JH, van der Linde-Sipman JS, et al. Malignant fibrous histiocytomas in dogs and cats: an immunohistochemical study. Res Vet Sci. 1992;53:198–204. doi: 10.1016/0034-5288(92)90110-n. [DOI] [PubMed] [Google Scholar]
- 38.Kerlin RL, Hendrick MJ. Malignant fibrous histiocytoma and malignant histiocytosis in the dog — convergent or divergent phenotypic differentiation? Vet Pathol. 1996;33:713–716. doi: 10.1177/030098589603300614. [DOI] [PubMed] [Google Scholar]