Abstract
Mucopolysaccharidosis type VII (Sly syndrome) is a lysosomal storage disease caused by inherited deficiency of the lysosomal enzyme β-glucuronidase. A murine model of this disorder has been well characterized and used to study a number of forms of experimental therapies, including gene therapy. We produced recombinant adenovirus that expresses human β-glucuronidase and administered this recombinant adenovirus to β-glucuronidase-deficient mice intravenously. The β-glucuronidase activities in liver and spleen were elevated to 40% and 20%, respectively, of the heterozygote enzymatic level at day 16. Expression persisted for at least 35 days. Pathological abnormalities of these tissues were also improved, and the elevated levels of urinary glycosaminoglycans were reduced in treated mice. However, the β-glucuronidase activity in kidney and brain was not significantly increased. After administration of the recombinant adenovirus directly into the lateral ventricles of mutant mice, the β-glucuronidase activity in crude brain homogenates increased to 30% of heterozygote activity. Histochemical demonstration of β-glucuronidase activity in brain revealed that the enzymatic activity was mainly in ependymal cells and choroid. However, in some regions, the adenovirus-mediated gene expression was also evident in brain parenchyma associated with vessels and in the meninges. These results suggest that adenovirus-mediated gene delivery might improve the central nervous system pathology of mucopolysaccharidosis in addition to correcting visceral pathology.
The mucopolysaccharidoses (MPSs) are a group of lysosomal storage diseases, each characterized by an inherited deficiency of one of the lysosomal acid hydrolases catalyzing degradation of glycosaminoglycans (GAG; ref. 1). The enzyme deficiency results in an accumulation of GAG in tissues. The MPSs are divided into seven distinct subgroups, each resulting from a deficiency of a different enzyme. The clinical symptoms of MPSs include coarse facies, dysostosis multiplex, joint abnormalities, hepatosplenomegaly, corneal clouding, varying degrees of central nervous system (CNS) abnormalities, and premature death. The only therapy reported to provide clinical benefit for MPS patients has been allogeneic bone marrow transplantation (2, 3). Although a number of patients have responded to bone marrow transplantation, broad application of this therapy is limited by availability of compatible donors, the high mortality and morbidity of the procedure, and the risk of graft-versus-host disease after bone marrow transplantation. Many efforts are being made to develop gene therapy as an alternative treatment for lysosomal storage disorders (4).
The deficiency of human β-glucuronidase (HBG) results in MPS type VII (MPS VII), also known as Sly syndrome, for which murine and canine models are available (5–8). Using the murine mouse model (mps/mps), enzyme replacement with infused enzyme produced improvements in visceral and CNS findings (9, 10). However, unlike Gaucher disease, the rarity of MPS VII and many similar conditions makes it unlikely that corrective enzymes can be produced at reasonable costs for treatment of humans with these disorders.
Mice with MPS VII responded well to bone marrow transplantation therapy also, though little improvement was seen in brain (11–13). In experimental gene therapy (14–20), the therapeutic gene has been transferred to hematopoietic cells (14, 18) and skin fibroblasts (16, 17, 19) by retroviral vectors ex vivo, and genetically modified cells were transplanted into mutant mice. Both approaches improved visceral symptoms but did not improve the CNS abnormalities. Gene transfer to CNS by corneal inoculation using the herpes simplex virus vector has also been reported (15). The potential of this vector is still limited by its pathogenicity to the CNS and relatively short period of expression of the transgene (21, 22). Recently, adenovirus was injected intravitreally in the MPS VII mouse and led to disappearance of storage vacuoles in the retinal pigment epithelium (20). We explored the feasibility of intravenous adenovirus-mediated gene therapy to address visceral storage in MPS VII mice by infusing an adenovirus vector that expresses HBG and measuring the level of HBG enzymatic activity in tissues, the disappearance of storage, and the excretion of urinary GAG following intravenous administration of the recombinant adenovirus. To evaluate the potential to address the CNS storage with this vector, we also determined histochemically the cells expressing HBG following intraventricular injection of virus.
MATERIALS AND METHODS
Adenovirus Preparation.
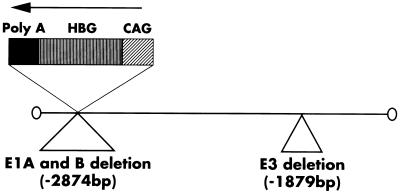
The structure of the replication-defective recombinant adenovirus carrying HBG cDNA (AxCAHBG) is shown in Fig. 1. The viruses were constructed essentially according to Saito et al. (23, 24). Briefly, to generate AxCAHBG, we first cloned HBG cDNA into a cassette cosmid pAxCAwt carrying an adenovirus type-5 genome lacking the E3, E1A, and E1B region to prevent virus replication (25). In this construct, the HBG cDNA is located downstream of the CAG (cytomegalovirus-enhancer-chicken β-actin hybrid) promoter (26). A rabbit β-globin poly(A) sequence was located downstream from the HBG cDNA. The resulting cosmid was cotransfected to 293 cells with the appropriately cleaved adenovirus genome lacking the E3 region. Recombinant virus was propagated and isolated from the 293 host cells (27) and purified by two rounds of CsCl centrifugation (28).
Figure 1.
Recombinant adenovirus AxCAHBG. The HBG cDNA was cloned downstream of the CAG (cytomegalovirus enhancer-chicken β-actin hybrid) promoter in pAxCAwt. A rabbit β-globin poly(A) sequence was located downstream of the HBG cDNA.
Mice.
Breeding pairs of (+/mps) were purchased from The Jackson Laboratory and bred. Mutants were identified by genetic analysis of DNA from tail using mismatched PCR and restriction fragment length polymorphism analysis (29, 30). Enzymatic activity of tail was measured as described below as a confirmation of the DNA diagnosis.
Infections.
Five- to 6-week-old (mps/mps) mice were used for the study (Table 1). Two different amounts of recombinant adenovirus (4.48 × 108 or 1.79 × 109 pfu) were injected through tail veins, two mice receiving each dose. One of the injected mice receiving each dose was killed at day 16 or day 35 for analysis. Urine specimens were collected at day 0 or day 35. Recombinant adenovirus (4.48 × 108 pfu) was injected into the lateral ventricle of another mouse using a 30-gauge needle. This mouse was killed at day 15 for analysis.
Table 1.
Description of each treated mps/mps mouse
| Age at injection, days | Sacrifice day after treatment | Amount of injected virus, pfu | Route of injection |
|---|---|---|---|
| 46 | 16 | 1.79 × 109 | Intravenous |
| 46 | 16 | 4.48 × 108 | Intravenous |
| 44 | 35 | 1.79 × 109 | Intravenous |
| 39 | 35 | 4.48 × 108 | Intravenous |
| 38 | 15 | 4.48 × 108 | Intraventricular |
pfu, Plaque-forming units.
Biochemical Analysis of HBG Activity.
Tail or tissues were isolated and homogenized in water using a glass homogenizer. The homogenates were spun at 14,000 × g for 10 min at 4°C in a microcentrifuge. The clear supernatant was assayed fluorometrically for HBG activity with the artificial substrate 4-methylumbelliferyl β-d-glucuronide (Sigma; ref. 31). Protein concentration was determined by the bicinchorinic acid (BCA) kit (Pierce).
GAG Determinations.
The amount of urinary GAG was determined using 1,9-dimethylmethylene blue chloride (Polysciences; ref. 32). Urinary creatinine was measured by mixing 10 μl of a 10-fold diluted urine sample with 50 μl of saturated picric acid and 50 μl of 0.2 M NaOH. Absorbance at 490 nm was read after 20 min and compared with the standard. The concentrations of GAG in liver and kidney were also determined by a published method (33) with minor modification (11).
Histological Analysis.
The activity of HBG in liver was also assayed histochemically. The tissues were isolated and submersed in OCT embedding compound (Miles) and the samples were frozen in a liquid nitrogen bath. Ten-micrometer sections were cut by cryostat. The sections were fixed in 70% acetone/30% chloral hydrate-formalin fixative (1% chloral hydrate in 20% vol/vol neutral buffered formalin). HBG histochemical staining was performed using naphthol AS-BI β-d-glucuronide and pararosaniline (29, 30). After HBG staining, the sections were counterstained by methylene green. Thin sections (0.5 μm) were obtained from 3% glutaraldehyde-fixed tissue and stained with toluidine blue.
RESULTS
HBG Enzymatic Activity in Various Tissues from Mice.
HBG activity levels in liver, spleen, kidney, and brain are shown in Table 2. Mouse β-glucuronidase enzymatic activities in tissues from control heterozygote (mps/+) mice are also shown. Enzymatic activities in all tissues from homozygote (mps/mps) mice were nearly null. At 16 days after intravenous virus injection (1.79 × 109 pfu), the enzymatic level in liver was increased to 40% of the heterozygote level. The HBG activity in spleen was increased to 20% and 10% of heterozygote activity at day 16 when 1.79 × 109 pfu virus and 4.48 × 108 pfu virus were injected, respectively. The HBG enzymatic activity in liver and spleen subsequently fell but was detectable up to 35 days after injection. In contrast to liver and spleen, the increase in enzymatic activity in kidney was minimal.
Table 2.
β-Glucuronidase activity in organs after intravenous injections of virus
| Genotype | Amount of virus, pfu | Days after injection | Activity in organs, units/mg
|
|||
|---|---|---|---|---|---|---|
| Liver | Spleen | Kidney | Brain | |||
| +/− | 0 | — | 56.9 | 100.3 | 9.93 | 7.0 |
| −/− | 0 | — | 0.10 | 0.10 | 0.05 | 0.13 |
| −/− | 4 × 108 | 16 | 8.4 | 8.9 | 0.17 | 0.11 |
| −/− | 1.7 × 109 | 16 | 21.2 | 16.8 | 0.22 | 0.12 |
| −/− | 4 × 108 | 35 | 2.0 | 1.9 | 0.06 | 0.06 |
| −/− | 1.7 × 109 | 35 | 3.0 | 2.0 | 0.08 | 0.11 |
Histochemical Demonstration of HBG Activity in Liver.
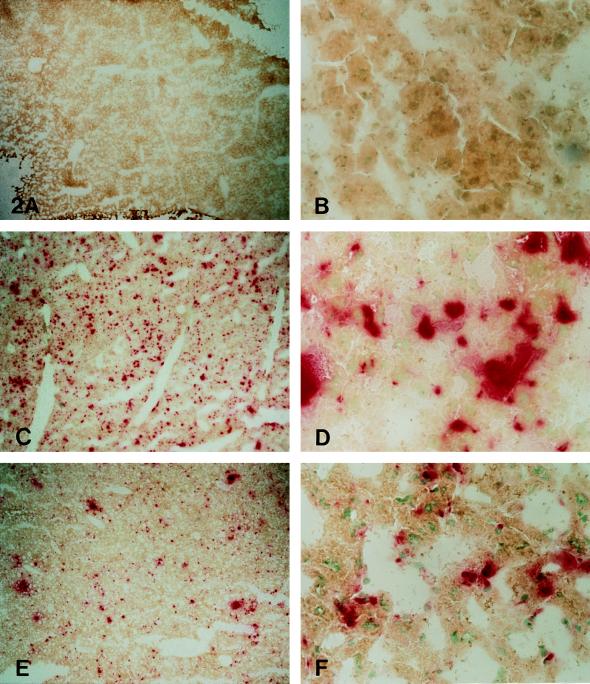
HBG activity is shown histochemically in Fig. 2. Liver from untreated mice revealed no positive stain (Fig. 2 A and B). At 16 days after injection of 1.79 × 109 pfu (Fig. 2 C and D), many cells were positive for HBG activity. The positive cells associated with sinusoids may be sinus lining cells. Occasional large polygonal cells morphologically consistent with hepatocytes also showed HBG activity. The number of HBG-positive cells was decreased at 35 days after injection, but enzyme activity was still present (Fig. 2 E and F).
Figure 2.
Histochemical staining for β-glucuronidase activity in the liver of an untreated MPS VII mouse (A and B) shows no enzyme activity. Sixteen days after receiving 1.79 × 109 pfu of recombinant adenovirus, the liver from an MPS VII mouse (C and D) shows numerous β-glucuronidase-containing cells. Their distribution and location suggest that many are sinus lining cells. Scattered large polygonal cells morphologically consistent with hepatocytes also contained β-glucuronidase. Thirty-five days after receiving 1.79 × 109 pfu of recombinant adenovirus (E and F), β-glucuronidase-containing cells are still seen, although they are less numerous than at 16 days after injection. (A, C, and E, ×20; B, D, and F, ×210.)
HBG Activity in Brain.
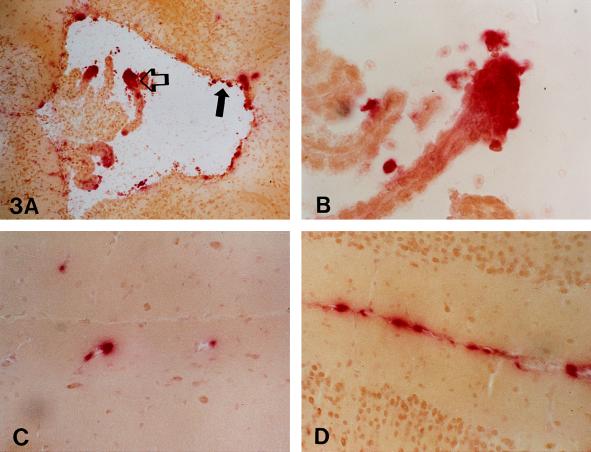
HBG activity in brain homogenate from mice that received virus intravenously did not increase (Table 2). Histochemically, virtually no positive cells were observed in brain from mice that received virus intravenously (data not shown). In brain from the mouse that received virus intraventricularly, most of the β-glucuronidase activity was demonstrable in the ependyma and choroid lining of the third ventricle (Fig. 3 A and B). Scattered HBG-positive cells were also seen associated with vessels in the parenchyma (Fig. 3C) and in the meninges (Fig. 3D).
Figure 3.
The ependyma (solid arrow) and choroid lining (open arrow) of the third ventricle (A and B) contained histochemically demonstrable β-glucuronidase activity 15 days after 4.48 × 108 pfu of recombinant adenovirus was injected into the lateral ventricle. In the same mouse, scattered β-glucuronidase-positive cells were also seen in cells associated with vessels (C) in the parenchyma. The meninges (D) contained perivascular and parenchymal cell β-glucuronidase activity. (A, ×50; B, ×210; C and D, ×130.)
Histological Evidence of Correction.
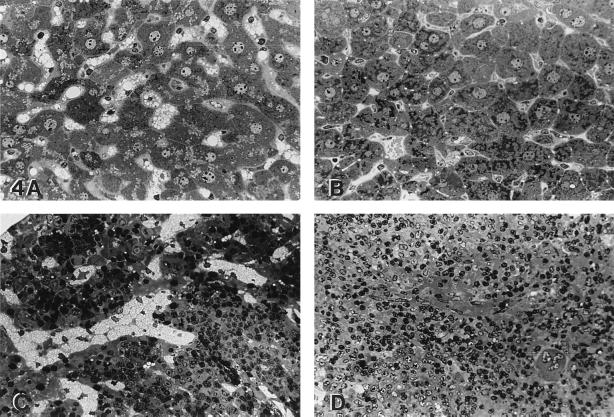
Light microscopic examination of liver and spleen is shown in Fig. 4. The extensive lysosomal storage in Kupffer cells and the small amount of storage in hepatocytes were reduced at day 35 in treated animals (Fig. 4 A and B). In spleen, the abundant lysosomal storage in red and white pulp had almost disappeared at day 35 (Fig. 4 C and D). The disappearance of storage was observed even at the lower dose of virus injected (4.48 × 108 pfu; data not shown). Electron microscopic findings confirmed the reduction or disappearance of lysosomal findings in liver and spleen (data not shown).
Figure 4.
The liver of an untreated, age-matched MPS VII mouse (A) showed lysosomal distention in Kupffer cells and a lesser amount of storage in hepatocytes. Thirty-five days after receiving recombinant adenovirus (B), both hepatocyte storage and Kupffer cell storage were markedly reduced. The untreated MPS VII mouse spleen (C) contained abundant lysosomal storage in sinus lining cells in the red pulp. Thirty-five days after recombinant adenovirus (D), there was a similar marked reduction in the amount of lysosomal storage. (A–D, toluidine blue; A–D, ×190.)
Effect of Treatment on GAG Content of Liver, Kidney, and Urine.
GAG contents were determined in liver, kidney, and urine (Table 3). The GAG content in liver was reduced in treated mice. Although treated animals showed little change in HBG activity in kidney, GAG content in kidney after treatment was decreased in three of four treated mice, though not to the level of GAG in kidney of the heterozygote. Untreated homozygote (mps/mps) mice also excreted increased amounts of GAG in their urine compared with normal and heterozygote mice. After intravenous injection of both doses of AxCAHBG virus, the level of GAG in urine collected at day 35 was reduced (Table 3).
Table 3.
Effect of treatment on liver, kidney, and urinary GAG levels
| Organ | Day post injection | Amount of injected virus | GAG* |
|---|---|---|---|
| Liver | Untreated control (−/−) | — | 2595 |
| 16 | 4.48 × 108 | 1389 | |
| 16 | 1.79 × 109 | 399 | |
| 35 | 4.48 × 108 | 336 | |
| 35 | 1.79 × 109 | 319 | |
| Heterozygote (+/−) | — | 821 | |
| Kidney | Untreated control (−/−) | — | 3585 |
| 16 | 4.48 × 108 | 3601 | |
| 16 | 1.79 × 109 | 2363 | |
| 35 | 4.48 × 108 | 2517 | |
| 35 | 1.79 × 109 | 2243 | |
| Heterozygote (+/−) | — | 652 | |
| Urine* | Untreated control (−/−; n = 2) | 1674, 758 | |
| 35 | 4.48 × 108 | 488 | |
| 35 | 1.79 × 109 | 469 | |
| Untreated +/+ normal | — | 163 | |
| Untreated +/− | — | 63 |
Urinary GAG is expressed in μg/mg creatinine; GAG levels in liver and kidney are expressed in μg/mg protein.
DISCUSSION
Several efforts have been made to develop gene therapy for MPS disorders using the murine MPS VII model. Approaches have included retroviral-mediated gene transfer to hematopoietic cells (14, 18) and fibroblasts (16, 17, 19). Reimplantation of transfected cells after retroviral-mediated gene transfer to hematopoietic cells and fibroblasts did improve somatic and visceral findings. However, CNS pathology was not altered by either approach. Moreover, gene transfer to hematopoietic stem cells in human trials showed very low efficiency (1/3,000 to 1/1,000,000 cells; ref. 34). Until efficiency of gene transfer to stem cells can be improved, this approach to gene therapy for MPS disorders has limited application in humans.
Herpes simplex virus-mediated gene transfer to brain by corneal inoculation was also tried, but this approach did not change CNS pathology (15). More recently, adenovirus was injected intravitreally and subretinally and resulted in the disappearance of storage vacuoles in the retinal pigment epithelium (20).
The promising results from some of these approaches to gene transfer stimulated us to evaluate using an adenovirus vector delivered by direct injection into the tail vein or into the cerebral ventricles. Intravenous injection is a routine procedure in clinics, and intrathecal injections, corresponding to intraventricular injections, are common clinical procedures. Our results indicated that the pathologies of visceral organs, including liver and spleen, were improved by a single intravenous injection of recombinant virus. Moreover, even the smaller amount of virus (4.48 × 108 pfu) administered intravenously corrected the pathology of liver and spleen.
Nonetheless, there are limitations to this approach. Adenovirus vectors induce immune responses in the recipient, both to the virus and to virus-infected cells. This is caused by expression of small amounts of viral proteins together with therapeutic gene products, which activate the host-cytotoxic T cells, which, in turn, destroy the virus-infected cells (35–38). Possibly, this problem may be overcome by newly developed adenovirus vectors (39, 40).
Other limitations of adenovirus vectors include the difficulty in obtaining detectable gene transfer upon a second administration of virus (41), and the transient duration of expression. These disorders require life-long expression of the transgene. Immunosuppressants have been tried as means of inhibiting antibody production to get more prolonged expression (42). Recently, Yang et al., using a mouse model (43), demonstrated that interleukin 12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy. The MPS VII mice provide an attractive model to explore such methods to overcome limitations of adenovirus-mediated gene therapy.
Another important consideration in gene therapy for MPS is that CNS storage needs to be addressed in most of these disorders. Neither retrovirus-mediated gene transfer to hematopoietic cells nor transfer to fibroblasts has produced changes in CNS pathology in MPS VII mice, nor has expression after herpes simplex virus-mediated gene transfer to CNS by corneal inoculation. We attempted to evaluate access to the CNS by adenovirus-mediated gene delivery, injecting virus directly into the lateral ventricles. This resulted mainly in ependymal and meningeal cell infection. However, adenovirus was recently shown to infect parenchymal cells (mainly glial cells) when osmotic pressure was altered to open the blood brain barrier (44).
Another approach to correcting CNS storage in the MPS VII mouse is introducing neural progenitor cells into the mutant mouse brain that migrated extensively and produced some improvement in CNS pathology (45). It will be of interest to determine whether this approach to correcting CNS storage or the adenovirus-mediated gene therapy described here can modify the CNS pathology (46) and behavioral abnormalities of the MPS VII mouse (47). Bony abnormalities form still another important component of the phenotype of the MPS VII mouse. Additional experiments are necessary to determine whether improvements in bone findings such as those reported for enzyme replacement therapy (10) are induced by intravenous administration of recombinant adenovirus.
Acknowledgments
We thank Dr. I. Saito and Dr. Y. Kanegae (Institute of Medical Science, University of Tokyo, Tokyo) for providing us the adenovirus vector, Dr. J. Miyazaki (Institute of Medical Genetics, Kumamoto University Medical School, Kumamoto, Japan) for providing CAG promoter, and Dr. J. A. Barranger (Department of Human Genetics, University of Pittsburgh, Pittsburgh) for helpful suggestions for this study. We also wish to thank Dr. S. Tanaka (Department of Molecular and Cellular Biology, The Jikei University School of Medicine) for the histological study. This work was supported by a grant from the Ministry of Human Health and Welfare (Japan) and by National Institutes of Health Grants GM34182 and DK40163.
Footnotes
Abbreviations: MPS VII, mucopolysaccharidosis type VII; GAG, glycosaminoglycan(s); CNS, central nervous system; HBG, human β-glucuronidase; pfu, plaque-forming units.
References
- 1.Neufeld E F, Muenzer J. In: The Metabolic and Molecular Bases of Inherited Disease. 7th Ed. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2465–2494. [Google Scholar]
- 2.Hobbs J R, Hugh-Jones K, Barrett A J, Byrom N, Chambers D, Henry K, James D C, Lucas C F, Rogers T R, Benson P F, Tansley L R, Patrick A D, Mossman J, Young E P. Lancet. 1981;ii:709–712. doi: 10.1016/s0140-6736(81)91046-1. [DOI] [PubMed] [Google Scholar]
- 3.Barranger J A. N Engl J Med. 1984;311:1629–1631. doi: 10.1056/NEJM198412203112509. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson S. Blood. 1991;78:2481–2492. [PubMed] [Google Scholar]
- 5.Sly W S, Quinton B A, McAlister W H, Rimoin D L. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 6.Birkenmeier E H, Davisson M T, Beamer W G, Ganschow R E, Vogler C A, Gwynn B, Lyford K A, Maltais L M, Wawrzyniak C A. J Clin Invest. 1989;83:1258–1266. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogler C, Birkenmeier E H, Sly W S, Levy B, Pegors C, Kyle J W, Beamer W G. Am J Pathol. 1989;136:207–217. [PMC free article] [PubMed] [Google Scholar]
- 8.Haskins M E, Desnick R J, DiFerrante N, Jezyk P F, Patterson D F. Pediatr Res. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Vogler C, Sands M, Higgins A, Levy B, Grubb J, Birkenmeier E H, Sly W S. Pediatr Res. 1993;34:837–840. doi: 10.1203/00006450-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Sands M S, Vogler C, Kyle J W, Grubb J H, Levy B, Galvin N, Sly W S, Birkenmeier E H. J Clin Invest. 1994;93:2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poorthuis B J H M, Romme A E, Willemsen R, Wagemaker G. Pediatr Res. 1994;36:187–193. doi: 10.1203/00006450-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Birkenmeier E H, Barker J, Vogler C A, Kyle J W, Sly W S, Gwynn B, Levy B, Pegors C. Blood. 1991;78:3081–3092. [PubMed] [Google Scholar]
- 13.Sands M S, Barker J E, Vogler C, Levy B, Gwynn B, Galvin N, Sly W S, Birkenmeier E. Lab Invest. 1993;68:676–686. [PubMed] [Google Scholar]
- 14.Wolfe J H, Sands M S, Barker J E, Gwynn B, Rowe L B, Vogler C A, Birkenmeier E H. Nature (London) 1992;36:749–753. doi: 10.1038/360749a0. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe J H, Deshmane S L, Fraser N W. Nat Genet. 1993;1:379–384. doi: 10.1038/ng0892-379. [DOI] [PubMed] [Google Scholar]
- 16.Moullier P, Marechal V, Danos O, Heard J M. Transplantation. 1993;56:427–432. doi: 10.1097/00007890-199308000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Moullier P, Bohl D, Heard J M, Danos O. Nat Genet. 1993;4:154–159. doi: 10.1038/ng0693-154. [DOI] [PubMed] [Google Scholar]
- 18.Marechal V, Naffakh N, Danos O, Heard J M. Blood. 1993;82:1358–1365. [PubMed] [Google Scholar]
- 19.Moullier P, Bohl D, Cardoso J, Heard J M, Danos O. Nat Med. 1995;1:353–357. doi: 10.1038/nm0495-353. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Davidson B L. Proc Natl Acad Sci USA. 1995;92:7700–7704. doi: 10.1073/pnas.92.17.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink D J, Sternberg L R, Weber P C, Mata M, Goins W F, Glorioso J C. Hum Gene Ther. 1992;3:11–19. doi: 10.1089/hum.1992.3.1-11. [DOI] [PubMed] [Google Scholar]
- 22.Johnson P A, Miyanohara A, Levine F, Cahill T, Friedman T. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito I, Oya Y, Yamamoto K, Yuasa T, Shimojo H. J Virol. 1985;54:711–719. doi: 10.1128/jvi.54.3.711-719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I. Nucleic Acids Res. 1995;23:3816–3821. doi: 10.1093/nar/23.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 27.Graham F L, Smiley J, Russell W C, Nairn R. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 28.Kanegae Y, Makimura M, Saito I. Jpn J Med Sci Biol. 1994;47:157–166. doi: 10.7883/yoken1952.47.157. [DOI] [PubMed] [Google Scholar]
- 29.Sands M S, Birkenmeier E H. Proc Natl Acad Sci USA. 1993;90:6567–6571. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe J H, Sands M S. In: Protocols for Gene Transfer in Neuroscience: Toward Gene Therapy of Neurologic Disorders. Lowenstein P R, Enquist L W, editors. Essex, U.K.: Wiley; 1996. pp. 263–274. [Google Scholar]
- 31.Glaser J H, Sly W S. J Lab Clin Med. 1973;82:969–977. [PubMed] [Google Scholar]
- 32.Whitley C B, Ridnour M D, Draper K A, Dutton C M, Neglia J P. Clin Chem. 1989;35:374–379. [PubMed] [Google Scholar]
- 33.Farndale R W, Sayers C A, Barrett A J. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 34.Kohn D B, Weinberg K I, Nolta J A, Heiss L N, Lenarsky C, et al. Nat Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Ertl H C J, Wilson J M. Immunity. 1994;1:433–422. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 38.Engelhardt J F, Ye X, Doranz B, Wilson J M. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 40.Engelhardt J F, Litzky L, Wilson J M. Hum Gene Ther. 1994;5:1217–1229. doi: 10.1089/hum.1994.5.10-1217. [DOI] [PubMed] [Google Scholar]
- 41.Fang B, Eisensmith R C, Li X H, Finegold M J, Shedlovsky A, Dove W, Woo S L. Gene Ther. 1994;1:247–254. [PubMed] [Google Scholar]
- 42.Fang B, Eisensmith R C, Wang H, Kay M A, Cross R E, Landen C N, Gordon G, Bellinger D A, Read M S, Hu P C, Brinkhous K M, Woo S L C. Hum Gene Ther. 1995;6:1039–1044. doi: 10.1089/hum.1995.6.8-1039. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Trinchieri G, Wilson J M. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 44.Doran S E, Ren X D, Betz A L, Pagel M A, Neuwelt E A, Roessler B J, Davidson B L. Neurosurgery. 1995;36:965–970. doi: 10.1227/00006123-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Snyder E Y, Taylor R M, Wolfe J H. Nature (London) 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 46.Levy B, Galvin N, Vogler C, Birkenmeier E H, Sly W S. Acta Neuropathol. 1996;92:562–568. doi: 10.1007/s004010050562. [DOI] [PubMed] [Google Scholar]
- 47.Chang P L, Lambert D T, Pisa M A. NeuroReport. 1993;4:504–510. doi: 10.1097/00001756-199305000-00011. [DOI] [PubMed] [Google Scholar]