Abstract
Objectives:
The efficacy of indiplon was evaluated by polysomnography (PSG) in an experimental model of transient insomnia consisting of the first night effect combined with a 2-hour phase advance.
Methods:
Healthy volunteers age 21–64 years (N=593; 62% female; mean (± SEM) years, 32±0.39) were randomized to double-blind treatment with a single nighttime dose of indiplon (10 mg or 20 mg) or placebo. PSG assessments included latency to persistent sleep (LPS, primary endpoint) and total sleep time (TST); self-report assessments included sleep quality (SQ); next day residual effects were evaluated by the Digit Symbol Substitution Test (DSST), Symbol Copying Test (SCT), and a Visual Analog Scale of sleepiness (VAS).
Results:
LPS mean (± SEM) values were significantly reduced on indiplon 10 mg (21.2±1.5 minutes) and indiplon 20 mg (16.8±1.1 minutes) compared to placebo (33.1±2.5minutes; p <0.0001 for both comparisons to placebo). TST mean (± SEM) values were significantly increased on indiplon 10 mg (414.5±3.9 minutes) and indiplon 20 mg (423.5±3.1 minutes) compared to placebo (402.9±3.9 minutes; p <0.005 for the 10 mg dose; p <0.0001 for the 20 mg dose). SQ was also significantly improved on both doses. There were no differences between indiplon and placebo on next day DSST, SCT, or VAS.
Conclusions:
Indiplon was effective in inducing sleep, increasing sleep duration, and improving overall sleep quality without next day residual effects in healthy volunteers in a model of transient insomnia.
Citation:
Rosenberg R; Roth T; Scharf MB et al. Efficacy and tolerability of indiplon in transient insomnia. J Clin Sleep Med 2007;3(4):374-379.
Keywords: Insomnia, indiplon, hypnotic
Transient insomnia is the most frequent form of insomnia, though precise estimates are unavailable.1,2 Transient insomnia is triggered by a wide range of situations and events, including change in sleep environment, shift work, travel across time zones, stressful life events, use of caffeine and other stimulant medications, and pain or discomfort associated with acute medical illness.3,4
Individuals who are susceptible to transient insomnia appear to exhibit a pattern of hyperarousal similar to that reported in patients diagnosed with chronic insomnia.4,7 In contrast, individuals who develop minimal-to-no insomnia when exposed to trigger situations do not exhibit hyperarousal patterns. These findings, while requiring further confirmation, raise the possibility that effective pharmacologic management of recurrent episodes of transient insomnia might prevent the hyperarousal diathesis from evolving into chronic insomnia.
The episodic course of transient insomnia makes it difficult to undertake naturalistic treatment studies. Consequently, various laboratory models have been developed that reliably induce transient insomnia, including noise, phase advancing the bedtime, and placement in an unfamiliar sleep setting such as a sleep laboratory (the “first night effect”). Characterization of the first night effect in a sleep laboratory dates back more than 40 years.8 The first night effect has been used for more than a decade to evaluate the effect of hypnotic treatment on transient/situational insomnia.9–12 Phase advancing a subject's bedtime has also been used as a laboratory model of transient insomnia in several previous clinical trials, including one study that combined both phase advance and first night effect.10,13,14
Given the high prevalence of transient insomnia and its potential to become chronic, treatment guidelines recommend considering pharmacotherapy as a treatment option.15
Indiplon is rapidly absorbed, with a Tmax of approximately 1 hour. Indiplon has linear pharmacokinetics, with dose-proportional Cmax and AUC. It is metabolized by CYP3A4 to form desmethyl indiplon and by carboxyesterase to desacetyl indiplon. There are no active metabolites. The elimination half-life is 1.5–2 hours. The PK profile of indiplon makes it a good candidate for the treatment of insomnia with a low likelihood of next day residual cognitive and psychomotor impairment.
The objective of this study was to evaluate the efficacy, tolerability, and safety of 2 doses of indiplon in healthy volunteers in a laboratory model of transient insomnia, consisting of the first night effect enhanced by a 2-hour phase advance in bedtime.
METHODS
Study Design
This was a double-blind, placebo-controlled, parallel-group, single dose trial designed to assess the efficacy, tolerability, and safety of 10 mg and 20 mg doses of indiplon when administered to normal healthy adult volunteers with transient insomnia induced in a sleep laboratory. The study was conducted at 18 sites in the United States. The protocol was approved by Institutional Review Board (Ethics Committee) at each site, and study conduct was consistent with the Declaration of Helsinki. The study was explained to prospective participants, who were recruited principally by advertisements in local media, and written informed consent was obtained prior to study entry or any procedures being conducted. Subjects were reimbursed for their participation.
Healthy adult subjects were screened, and then were sent home to complete a daily sleep diary for ∼7 days prior to the experimental night in the sleep laboratory (Night 1) to confirm their normal sleep patterns and establish their habitual bedtime routines. Eligible subjects were admitted to the sleep laboratory approximately 4 hours prior to their usual bedtime on the study night (Night 1). The procedure employed to experimentally induce insomnia consisted of the first night effect coupled with a 2-hour phase advance in bedtime. Eligible subjects were randomized, in a computer-generated 1:1:1 ratio, to indiplon 10 mg or 20 mg, or placebo. After 8 hours of polysomnography (PSG) recording, and after completion of next day assessments, subjects were discharged home.
Subjects
Men and women ages 21 to 64 years, inclusive, were enrolled if they met the following criteria: 1) a self-reported 3-month history of a normal nightly sleep pattern characterized by a usual lights-out time between 21:00 and 24:00, a usual latency to sleep onset of >5 and <30 minutes, a usual sleep duration between 6.5 and 9 hours/night, and no habitual napping (fewer than 2 naps per week), as well as no difficulties in daytime functioning due to sleep problems; 2) an Epworth Sleepiness Scale score <12; 3) a lights-out time between 21:00 and 24:00, inclusive, that did not deviate by more than 1.5 hours (based on sleep diary responses on at least 5 of the 7 consecutive nights before randomization); 4) good general health as determined by medical history and physical examination; and 5) willingness and ability to comply with study procedures. Female subjects had to have a negative serum pregnancy test and be using medically acceptable contraception (unless surgically sterilized or ≥2 years postmenopausal).
Key exclusion criteria included the following: 1) presence of symptoms consistent with a diagnosis of any sleep disorder, including primary insomnia, sleep apnea, narcolepsy, periodic leg movements, or restless legs syndrome; 2) current employment requiring night or shift work; 3) travel across more than 4 time zones in the 14 days prior to screening; 4) presence of clinically significant or unstable medical, neurological, or psychiatric illness within 30 days of screening; 5) any clinically significant finding on physical examination, clinical laboratory test, or electrocardiogram (ECG); 6) having a known exaggerated pharmacological sensitivity or hypersensitivity to any benzodiazepine or other drugs acting at the GABAergic receptor; 7) self-report of >5 alcoholic beverages on a single day or >14 alcoholic beverages weekly, or history of substance abuse or dependence in last year; 8) positive breathalyzer test for alcohol at screen or Night 1, or a positive drug test at screen; and 9) current use of any medications with properties affecting the central nervous system or that could affect study outcome, including anxiolytics, antidepressants, histamine-1 receptor antagonists, respiratory stimulants, decongestants, narcotic analgesics, and cytochrome P450 3A4 inhibitors and inducers.
Study Treatment and Procedures
Screening procedures, which occurred within 14 days of experimental night in the sleep laboratory (Night 1), included a complete physical and neurological examination, medical and treatment history, Epworth Sleepiness Scale, electrocardiogram (ECG), laboratory tests including breathalyzer, and a detailed sleep history. Subjects entered the sleep laboratory 4 hours before their median habitual bedtime for dosing, having finished their most recent meal at least 1.5 hours before admission. Subjects were instructed to abstain from alcohol for 24 hours and caffeine for 6 hours prior to entry to the sleep lab. The DSST and SCT were administered at the screen visit (practice test which was not scored); at Night 1 (immediately pre-dose); and the next morning, approximately 8.5 hours post-dose. After PSG electrodes were applied, a single dose of study drug was administered orally 2.5 hours before the median habitual bedtime established during the previous week. Lights out occurred 30 minutes later (2 hours before the subject's habitual bedtime). PSG recording was performed for 8 hours starting at lights out. The PSG recording was scored in accordance with standardized procedures.16 In the morning, subjective efficacy, safety, and cognitive/psychomotor assessments were performed within 30 minutes after the PSG recording concluded and the subject got out of bed. Study participation was completed at this point.
Efficacy Evaluations: PSG Parameters
The primary PSG endpoint was latency to persistent sleep (LPS), defined as the time from the beginning of the recording to the start of the first 10 minutes of persistent sleep. Secondary PSG endpoints included the following: 1) total sleep time (TST); 2) wake time after sleep onset (WASO); 3) sleep efficiency (SE); 4) number of awakenings after sleep onset (NAASO); 5) percentages of Stage 1, 2, 3/4 (NREM), and REM sleep; and 6) latency to REM sleep. The PSG recordings were conducted in accordance with the Rechtschaffen and Kales technique,16 and were scored at a central lab using standard criteria by sleep technologists blinded to study treatment.
Efficacy: Subjective Parameters
Subject-rated sleep variables consisted of: 1) latency to sleep onset (LSO); 2) subjective TST (sTST); 3) subjective number of awakenings after sleep onset (sNAASO); and 4) sleep quality, rated in the morning on a 7-point scale ranging from 1=extremely good to 7=extremely poor.
Safety
During the screening period, safety evaluations to assess patient eligibility included medical history, physical and neurological examination, vital signs, routine laboratory tests (hematology, urinalysis, and serum chemistry), 12-lead ECG assessed by the investigator but also read by a central ECG laboratory, serum pregnancy test for all women, breathalyzer and urine drug screen, Hepatitis B surface antigen and Hepatitis C antibody. Vital signs (blood pressure, heart rate, respiratory rate, and oral temperature) were repeated at each visit; weight was measured at screen and end of study. The physical and neurological examination, routine labs, and ECG were repeated at the end of the study. All observed or reported adverse events, irrespective of suspected causality by study drug, were recorded and rated as to severity.
Next day residual effects were assessed on the morning after PSG recording with the following instruments: 1) DSST, a subscale of the Wechsler Adult Intelligence Scale (WAIS) has been shown to be sensitive to cognitive and psychomotor effects of sedating medications; 2) SCT, which measures psychomotor speed and attention; 3) a patient-rated Visual Analog Scale for Sleepiness (VAS), which consists of a 100 mm horizontal line anchored with the descriptors “very alert” to “very sleepy”.
Statistical Methods
The sample size of 600 (200 per treatment arm) was designed to provide 85% power to detect an 8 minute mean difference between active drug and placebo, assuming an SD = 24, and assuming a Type I error = 0.025 (because 2 pairwise comparisons were planned). The expected mean/SD values were based on 2 previous studies using a laboratory model of transient insomnia.
Assessment of baseline comparability was conducted via analysis of variance (ANOVA), with terms for treatment and pooled site, for continuous variables. Categorical variables were analyzed via and Cochran-Mantel-Haenszel with pooled-site as a stratification variable.
Both objective assessments (LPS [the primary efficacy variable], TST, NAASO, WASO, sleep stage) and subjective assessments (LSO, sTST, sNAASO, sleep quality) were analyzed via an ANOVA with treatment and pooled-site in the model. LPS data were log-transformed prior to analysis and all subjective measures were rank transformed prior to analysis. Adjustments for multiple tests were applied to the analysis of LPS via Dunnett's test. No other adjustments for multiplicity were performed. In addition, categorical analyses of sleep quality were performed via the Wilcoxon Rank Sum test.
Next day residual effects were assessed by DSST, SCT, and VAS. Analysis of these endpoints was based on an analysis of covariance (ANCOVA) with treatment, pooled-site, and baseline scores included in the model. Finally AE rates were assessed with Chi-square tests, and SAE rates were assessed with Fisher's exact test.
RESULTS
Baseline Characteristics
The intent to treat sample of 593 subjects were randomized to one of the 3 treatment groups, all of whom received study drug or placebo and completed the study. The study sample was predominantly female in the placebo group (N = 127; 63%), and in the indiplon 10 mg (N = 113; 57%) and 20 mg (N = 125; 64%) treatment groups. The mean (± SD) age was similar in the placebo group (32.1+9.8 years), and in the indiplon 10 mg (32.2+9.8) and 20 mg (31.9±8.8) treatment groups. For all 3 treatment groups, the median bedtime was 23:00 (11:00 pm), with self-reported mean time to fall asleep of 13.9 minutes in the placebo group, 14.4 minutes in the indiplon 10 mg group, and 16.5 minutes in the indiplon 20 mg group. There were no significant between-group differences in any baseline demographic or clinical parameter (see Table 1).
Table 1.
Baseline Demographic and Sleep Characteristics of Subjects
| Placebo N=201 |
Indiplon 10 mg N=198 |
Indiplon 20 mg N=194 |
|
|---|---|---|---|
| Female, % | 63% | 57% | 64% |
| Age, years, mean ± SD | 32.1±9.8 yrs | 32.2±9.8 yrs | 31.9±8.8 yrs |
| Race, % | |||
| White | 68% | 71% | 65% |
| Black | 15% | 15% | 16% |
| Other | 17% | 14% | 19% |
| Usual bedtime (lights out), median (minimum, maximum) | 23:00 (21:00–24:00) |
23:00 (21:00–24:00) |
23:00 (21:00–24:00) |
| Usual time to fall asleep, mins, mean ± SEM | 13.9±0.4 | 14.4±0.4 | 16.5±2.1 |
| Usual duration of sleep, mins, mean ± SEM | 457±2.6 | 453±2.4 | 456±2.6 |
F-test (for age and usual sleep duration) and CMH test (for sex, race, usual time to fall asleep) found no between-group differences
Primary Endpoint: Latency to Persistent Sleep
On the experimental night in the sleep laboratory, the combined first night effect plus 2-hour phase advance of bedtime resulted in a notable increase in sleep onset latency, as indicated by an increase in LSO in the placebo group from 13.9 minutes at study entry (the usual time to fall asleep reported at screening; Table 1) to 40.4 minutes on the experimental night in the sleep lab. This difference in self-reported sleep onset time in the placebo group suggests that the experimental model was effective in inducing a clinically relevant degree of insomnia.
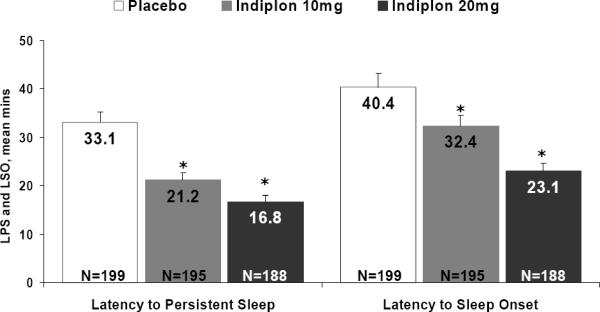
On the primary PSG outcome measure, LPS, mean (± SEM) values were significantly lower on indiplon 10 mg (21.2±1.5 minutes) and indiplon 20 mg (16.8±1.1 minutes) compared to placebo (33.1+2.5 minutes; Figure 1). Both doses of indiplon were also associated with significantly lower self-reported LSO than placebo (Figure 1). Significantly more subjects had an LPS <20 minutes on indiplon 10 mg (64%) and 20 mg (72%) compared with placebo (47%; p <0.05 for both comparisons).
Figure 1.
Effect of Indiplon on Latency to Persistent Sleep and Latency to Sleep Onset
*p <0.0001; p-values based on ANOVA model using log-transformed data; adjusted for multiplicity using Dunnett's test
Secondary Efficacy Endpoints
Treatment with the 10 mg and 20 mg doses of indiplon resulted in significantly higher PSG measures of TST compared with placebo (Table 2). PSG measures of NAASO and WASO were only significant versus placebo on the 20 mg dose of indiplon (Table 2).
Table 2.
Efficacy of Indiplon on Secondary Sleep Parameters
| Placebo N=199 |
Indiplon 10 mg N=195 |
Indiplon 20 mg N=198 |
|
|---|---|---|---|
| PSG endpoints | |||
| Total sleep time (TST), mins, mean ± SEM | 402.9±3.9 | 414.5±3.9 | 423.5±3.1 |
| p-value | 0.0044 | <0.0001 | |
| Number of awakenings after sleep onset (NAASO), median | 8.0 | 7.0 | 6.0 |
| p-value | 0.0583 | 0.0084 | |
| Wake after sleep onset (WASO), mins, mean ± SEM | 49.9±2.9 | 48.7±3.5 | 42.5±2.8 |
| p-value | 0.2540 | 0.0091 | |
| Self-reported sleep endpoints | |||
| Subjective latency to sleep onset (LSO), mins, mean ± SEM | 40.4±2.8 | 32.4±4.4 | 23.1±2.4 |
| p-value | <0.0001 | <0.0001 | |
| Subjective total sleep time (sTST), mins, mean ± SEM | 401.4±5.4 | 415.7±4.6 | 429.1±4.1 |
| p-value | 0.0422 | <0.0001 | |
| Subjective number of awakenings after sleep onset (sNAASO), median | 3.0 | 2.0 | 2.0 |
| p-value | 0.0010 | <0.0001 | |
| Sleep quality, mean ± SEM | 3.4±0.08 | 3.1±0.09 | 2.7±0.08 |
| p-value | 0.0182 | <0.0001 |
p-values for self-reported assessments were based on an ANOVA model using rank-transformed data
Both doses of indiplon were associated with significantly superior outcomes compared with placebo on all self-reported sleep measures, sTST, sNAASO, and sleep quality (Table 2).
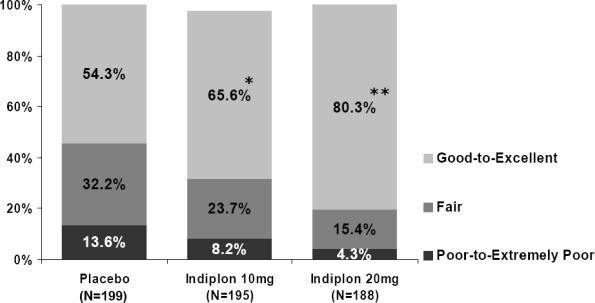
As expected in this experimental model of insomnia, a large proportion of the benefit was contributed by the effect of active treatment in reducing time to sleep onset. Improvement in PSG and self-reported sleep parameters resulted in a significantly superior subject-rated sleep quality on both doses of indiplon versus placebo (Figure 2).
Figure 2.
Subjective Ratings of Sleep Quality on Indiplon
*p <0.02; **p <0.0001; p-values from stratified Wilcoxon rank sum test (Cochran-Mantel-Haenszel test with site as a stratum).
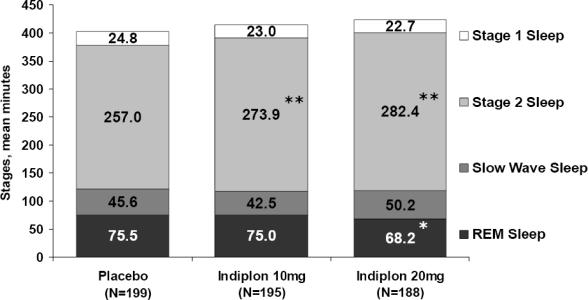
Effect of Indiplon on Sleep Architecture
Compared to placebo, treatment with indiplon had no effect on stage 3–4 sleep (Figure 3), and resulted in a modest but significant increase in stage 2 sleep (+16.9–25.4 minutes), and a small decrease in REM sleep (−7.3 minutes on the 20 mg dose). REM latency was delayed on indiplon 20 mg compared to placebo (112.0±3.6 minutes vs. 90.2±3.2 minutes; p <0.0001), while the delay on indiplon 10 mg (96.4±3.5 minutes) was not significant.
Figure 3.
Effect of Indiplon Treatment on Sleep Architecture: Mean Number of Minutes In Each Sleep Stage
*p <0.01; **p ≤0.0001; p-values based on ANOVA model using rank-transformed data
Indiplon Next day Effects
To evaluate whether indiplon was associated with next day residual effects on cognitive and psychomotor function, the DSST and SCT were administered in the evening before study treatment, and again the next morning. There were no differences between next day DSST and SCT scores for either dose of indiplon compared to placebo (Table 3). Similarly, there was no difference between indiplon and placebo in the next day VAS sleepiness score (Table 3).
Table 3.
Measures of Next Day Residual Effects
| Placebo | Indiplon 10 mg |
Indiplon 20 mg |
|
|---|---|---|---|
| N=201 | N=198 | N=194 | |
| DSST, mean ± SEM | 66.4±0.8 | 65.3±0.9 | 66.0±0.9 |
| p-value | 0.58 | 0.78 | |
| SCT, mean ± SEM | 140.6±1.7 | 139.6±1.9 | 143.5±1.9 |
| p-value | 0.58 | 0.99 | |
| VAS-sleepiness, mean ± SEM | 35.6±1.4 | 36.3±1.6 | 34.6±1.5 |
| p-value | 0.70 | 0.89 |
DSST=Digit Symbol Substitution; SCT=Symbol Copying; VAS=Visual Analog Scale (100 mm)
LS-mean data is shown for day 2 scores based on an ANCOVA adjusted for baseline covariate;
sample size may vary by N=1 due to missing data
Indiplon Safety and Tolerability
The percent of patients reporting any adverse event was comparable on indiplon 10 mg (10.1%) and placebo (10.0%), and slightly elevated on indiplon 20 mg (15.5%; Fishers χ2, 3.71; df, 2; p = 0.16). No severe adverse events were reported on either dose of indiplon. Only two treatment emergent adverse events occurred with an incidence of at least 2%: headache, 2.0% on indiplon 10 mg, 3.1% on indiplon 20 mg, and 1.0% on placebo; and nausea, 0.5% on indiplon 10 mg, 2.1% on indiplon 20 mg, and 0.5% on placebo. None of the between-group comparisons in the incidence were significant on a Fisher's exact test. No clinically significant laboratory or ECG changes were reported during the study.
DISCUSSION
The current one-night laboratory study used a combined 2-hour phase advance and first-night effect as a model of transient insomnia. Treatment with indiplon resulted in shorter PSG and self-reported time to sleep onset on both the 10 mg and 20 mg doses compared to placebo.
The sleep promoting benefit of indiplon in this one-night, transient insomnia model was also significant across the majority of secondary PSG measures, and across all self-reported sleep onset and sleep maintenance measures. In parallel, sleep quality was significantly superior to placebo on both the 10 mg and 20 mg doses of indiplon.
The effect of indiplon in the current one-night laboratory study was comparable to what has been reported in the only other double-blind, placebo-controlled trial we are aware of that employed the same combined phase-advance/first-night methodology and measured outcome using PSG.14 In that study, zolpidem improved sleep maintenance outcomes, but was not significantly different from placebo on the primary PSG outcome measure, latency to persistent sleep. This may have been attributable to either a lack of robustness of the experimental model or to a high placebo response in the study sample. As is well known, it is difficult to make definitive comparisons across studies.
In the current study, a modest efficacy advantage was observed in favor of the 20 mg dose of indiplon compared to the 10 mg dose across the majority of sleep onset, sleep duration, and sleep maintenance measures. In this relatively young healthy volunteer study sample, the incidence of adverse events was low on both doses of indiplon, with only headache and nausea occurring in the 2%–3% range.
Consistent with its short elimination half-life of 1.5–2 hours, indiplon was not associated with any increase, compared with placebo, in next morning residual effects as measured by the VAS sleepiness scale. Furthermore, there was no next day residual impairment in cognitive or psychomotor function on indiplon as measured by the DSST and the SCT. Results from additional studies of indiplon, in the dosage range of 5–20 mg, confirm its lack of a next day residual effect, even when testing was performed within 4–6 hours of dosing.17–19
The current study provided the opportunity to evaluate the effect of indiplon on sleep architecture. Sleep stages were essentially preserved on both doses of indiplon, with a modest (17–25 minute versus placebo) increase in the duration of stage 2 sleep and a similarly modest (−7.3 minutes on the 20 mg dose) decrease in the duration of REM being the only significant effects. Notable was the absence of any clinically significant effect on slow wave sleep. These findings are consistent with similar PSG data reported for zolpidem and other second-generation hypnotics, as distinguished from nonselective benzodiazepine hypnotics, which have been shown to significantly reduce slow wave sleep.20,21
In summary, subjects treated with indiplon demonstrated significantly shorter sleep latencies as well as better sleep maintenance and sleep quality when treated with indiplon in a one-night, laboratory model utilizing both phase-advance and first-night effects to generate transient insomnia. Both doses were similarly well tolerated, with very low incidence of adverse events and no next day residual cognitive or psychomotor impairment. Future research is needed to evaluate the role of indiplon in the treatment of naturally occurring episodes of transient insomnia.
ACKNOWLEDGMENTS
The authors wish to acknowledge the individual study investigators for their participation in this trial:
Jed Black, M.D., Michael Bonnet, M.D., Martin Cohn, M.D., Bruce Corser M.D., Cynthia Dorsey, Ph.D., Milton Erman, M.D., June Fry, M.D., Max Hirshkowitz, Ph.D., Steven Hull, M.D., Andrew Jamieson, M.D., Alan Lankford, Ph.D., Angela Randazzo, Ph.D., Russell Rosenberg, Ph.D., Martin Scharf, Ph.D., David Seiden, M.D., Edward Stepanski, Ph.D., Stephen Thein, Ph.D., Gary Zammit, Ph.D.
The authors wish to acknowledge Phil Jochelson, M.D. for contributions to the original study design; Rick Landin, Ph.D. and Richard Zhang, Ph.D. for assistance with the statistical analysis; and Edward Schweizer, M.D. for assistance in the preparation of the manuscript.
Footnotes
Disclosure Statement
This study was a multi-site investigation supported by Neurocrine Biosciences, Inc. Dr. Roth is a consultant for Abbott, Accadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, BMS, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotech, Forest, GlaxoSmithKline, Hypnion, Johnson & Johnson, King, Ludbeck, McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Orginer, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Somaxon, Syrex, Takeda, Transoral, Vanda, Vivometric, Wyeth, Yamanuchi, and Xenoport; has received research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, ScheringPlough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport; and has participated in speaking engagements supported by Sanofi and Takeda. Dr. Scharf is a member of Neurocrine Biosciences Insomnia Advisory Board. Dr. Lankford has served on the advisory board the speaker's bureau for Neurocrine Biosciences, Inc. Dr. Farber is employed by Neurocrine Biosciences. Inc. Dr. Rosenberg has indicated no other financial conflicts of interest.
REFERENCES
- 1.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon M. Epidemiological study of insomnia in the general population. Sleep. 1996;19(3) Suppl:S7–15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Caulet M, Guilleminault C. Complaints about nocturnal sleep: how a general population perceives its sleep, and how this relates to the complaint of insomnia. Sleep. 1997;20:715–23. doi: 10.1093/sleep/20.9.715. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 5.Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5:5–15. doi: 10.1016/s1098-3597(03)90031-7. [DOI] [PubMed] [Google Scholar]
- 6.Roth T, Hajak G, Ustun TB. Consensus for the pharmacological management of insomnia in the new millennium. Int J Clin Pract. 2001;55:42–52. [PubMed] [Google Scholar]
- 7.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 8.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 9.Roehrs T, Vogel G, Sterling W, Roth T. Dose effects of temazepam in transient insomnia. Arzneimittelforschung. 1990;40:859–62. [PubMed] [Google Scholar]
- 10.Vogel G. Clinical uses and advantages of low doses of benzodiazepine hypnotics. J Clin Psychiatry. 1992;53:19–22. [PubMed] [Google Scholar]
- 11.Roth T, Roehrs T, Vogel G. Zolpidem in the treatment of transient insomnia: a double-blind, randomized comparison with placebo. Sleep. 1995;18:246–51. doi: 10.1093/sleep/18.4.246. [DOI] [PubMed] [Google Scholar]
- 12.Erman MK, Loewy D, Scharf MB. Comparison of temazepam 7.5 mg with temazepam 15 mg for the treatment of transient insomnia. Curr Med Res Opin. 2004;20:441–9. doi: 10.1185/030079904125003151. [DOI] [PubMed] [Google Scholar]
- 13.Walsh JK, Schweitzer PK, Sugerman JL, Muehlbach MJ. Transient insomnia associated with a 3-hour phase advance of sleep time and treatment with zolpidem. J Clin Psychopharmacol. 1990;10:184–9. [PubMed] [Google Scholar]
- 14.Erman MK, Erwin CW, Gengo FM, et al. Comparative efficacy of zolpidem and temazepam in transient insomnia. Hum Psychopharmacol. 2001;16:169–76. doi: 10.1002/hup.238. [DOI] [PubMed] [Google Scholar]
- 15.Walsh JK, Benca RM, Bonnet M. National Heart, Lung, and Blood Institute Working Group on Insomnia. Insomnia: assessment and management in primary care. Am Fam Physician. 1999;59:3029–39. [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A. A manual of standardized terminology techniques and scoring system for sleep stages of human subjects. Washington DC: US Government Printing Office, National Institute of Health Publication No 204; 1968. [Google Scholar]
- 17.Scharf M, Rosenberg R, Cohn M, Zammit GK, Jochelson P. Poster presentation at annual American Psychiatric Association meeting. New York, NY: 2004. May, Efficacy and safety of indiplon in elderly patients with chronic insomnia. [Google Scholar]
- 18.Walsh JK, Roth T, Lankford DA, Rosenberg R, Jochelson P. Poster presentation at annual American Psychiatric Association meeting. New York, NY: 2004. May, Efficacy and safety of 35 days of treatment with indiplon in adults with chronic insomnia. [Google Scholar]
- 19.Garber M, Burke J, Farber R, Jochelson P. Poster presentation at annual American Psychiatric Association meeting. New York, NY: 2004. May, Residual effects of middle of the night dosing: a placebo-controlled crossover study of indiplon, zolpidem, and zopiclone in healthy volunteers. [Google Scholar]
- 20.Feinberg I, Fein G, Walker JM, Price LJ, Floyd TC, March JD. Flurazepam effects on slow-wave sleep: stage 4 suppressed but number of delta waves constant. Science. 1977;198:847–8. doi: 10.1126/science.21453. [DOI] [PubMed] [Google Scholar]
- 21.Bixler EO, Kales A, Soldatos CR, Scharf MB, Kales JD. Effectiveness of temazepam with short-intermediate-, and long-term use: sleep laboratory evaluation. J Clin Pharmacol. 1978;18:110–18. doi: 10.1002/j.1552-4604.1978.tb02430.x. [DOI] [PubMed] [Google Scholar]