Abstract
Study Objective:
To examine the spectrum of sleep disorders in patients with chronic traumatic brain injury (TBI) and determine if the severity of sleep disorder is related to severity of chronic TBI.
Methods:
Patients who underwent evaluation for sleep disorder/s following a TBI were included in this retrospective analysis. Sixty adult patients with TBI (age 20–69 yr; 38 M and 22 F), who presented with sleep-related complaints 3 months to 2 years following TBI, were studied. None had sleep complaints prior to the TBI. Orophrayngeal, chin, and TMJ examinations were considered benign. The severity of injury was assessed by the Global Assessment of Functioning (GAF) scale. Polysomnograms (PSGs) were performed on 54 patients (90%), 28 of whom underwent multiple sleep latency tests (MSLTs) because they scored >11 on the Epworth Sleepiness Scale (ESS). The Beck Depression Inventory (BDI) scale was administered if there was sleep maintenance insomnia, and the Hamilton Anxiety Scale (HAS) was administered if there was sleep onset insomnia.
Results:
The TBI severity was mild in 40%, moderate in 20%, and severe in 40%. The Epworth Sleepiness Scale (ESS) score was elevated (>11) in 52%. Hypersomnia was the presenting complaint in 50%, mostly due to sleep apnea, narcolepsy, and periodic limb movement disorder (PLMD). Insomnia was the presenting complaint in 25%, half with sleep maintenance insomnia and high BDI scores, and the remainder with sleep onset insomnia and high HAS scores. Parasomnia was the presenting complaint in 25%; the most frequent parasomnia was REM behavior disorder (RBD). GAF scores were significantly correlated (p <0.05) with some of the measures of sleep disruption (stage 1, sleep efficiency, and wake during sleep), but not with others (wake before sleep, stage-shifts, PLMI, PLMA and AHI) on the PSG. Fifty-three percent (15/28) had a mean sleep onset latency <5 minutes, and 32% (9/28), also had two or more sleep onset rapid eye movement periods (SOREMPs) on the MSLT.
Conclusion:
The results of this study demonstrate that a full spectrum of common sleep disorders occurs in patients with chronic TBI. The severity of chronic TBI as measured by GAF scores is correlated with some of the measures of sleep disruption but not others, indicating a complex and multifactorial pathogenesis.
Citation:
Verma A; Anand V; Verma NP. Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med 2007;3(4):357-362.
Keywords: CHI, TBI, Daytime somnolence, EDS, OSA, insomnia, parasomnia, Epworth sleepiness scale, Hamilton anxiety scale, Beck Depression Inventory, PSG, MSLT
Traumatic brain injury occurs in 100–400 per 100,000 people per year in North America and Europe. The common causes are road traffic accidents, falls, assaults, sport injuries, and domestic accidents. Males are more often affected than women. The most common age group which suffers from traumatic brain injury is 15–35 years. It is the most frequent cause of death between ages 1–15.1 Sleep related problems secondary to chronic TBI have been described anecdotally or in case-report format since 1941. 2–10 Some commonly reported disorders include hypersomnia, narcolepsy, delayed sleep phase, insomnia, fatigue, alteration of sleepwake schedule, and movement disorders. Sleep disorders are a common finding after the acute phase of TBI. They result in daytime somnolence which in turn may lead to poor daytime performance, altered sleep-wake schedule, heightened anxiety, and poor individual sense of well being, insomnia, and depression.
Only recently have the attempts have been made to investigate this relationship more systematically in chronic TBI11–14 The most critical study is that of Guilleminault et al who concluded that impaired daytime functioning and somnolence is present in 98% of all patients with TBI, and sleep disordered breathing is a common finding.4 Pain caused by associated cervical whiplash may be an important contributing factor. Sleep related abnormalities are more prevalent in more severely head-injured patients, such as those in coma for >24 hours, those with skull fractures, or those requiring neurosurgical intervention. Kauffman et al14 reached similar conclusions in adolescents with minor head injury and found that sleep disturbances may be long-term. This study was undertaken to extend these systematic observations in the evolving knowledge of sleep disorders in TBI survivors, as the sleep disorders make an impact on the rehabilitation of these patients15,16 and can exacerbate other symptoms such as pain, cognitive deficits, fatigue, and irritability. 16
METHODS
Study population
Patients with chronic TBI (defined as 3 months to 2 years after the injury) were evaluated by a board-certified neurologist who is also a board-certified sleep specialist. Evaluation included a detailed medical history supplemented with standard sleep-wake questionnaire, neurological examination, neck size, chin size and position, jaw alignment, and oropharyngeal examination. A flow-volume loop, considered a reflection of upper airway obstruction, was also measure
Sleepiness was measured by the Epworth Sleepiness Scale (ESS). Those with predominant sleep onset insomnia were administered the Hamilton Anxiety Scale (HAS).17 The HAS is a self-administered scale that quantifies anxiety with 14 items and a possible score of from 0–4 for each item. Those with predominant sleep maintenance insomnia were administered the Beck Depression Inventory (BDI).18 The BDI is a self-administered scale that quantifies depression with 21 items; the score varies from 0–3 for each item. 18 None of the patients had sleep related symptoms prior to the TBI. Classes of medications were anxiolytics, antidepressants, antiepileptic agents (used for their psychotropic action), stimulants, and pain medications. The medication effect and pain was not measured in this study, however. The medication dosages were not changed or discontinued prior to the testing. The severity of head injury was determined by the Global Assessment of Functioning (GAF) Scale,23 administered by a board-certified psychiatrist. A score of 100 on GAF means superior functioning whereas a score of 40 or below means some impairment in reality testing and communication or major impairment in several areas, such as work or school, family relations, judgment, thinking, or mood. 23
Polysomnography (PSG) and Multiple Sleep Latency Test (MSLT)
Single overnight PSGs were performed on 54 patients utilizing the AASM guidelines19 prior to those most recently updated in 2005. A 12-channel montage was utilized recording EEG, EOG, EKG, chin and lower extremity EMG, naso-oral airflow, thoracic and abdominal effort, and oxygen saturation by pulse oximeter. An expanded EEG montage was used when parasomnia was evaluated. Sandman Elite hardware and software were utilized. The patients were evaluated in an accredited sleep laboratory in sound attenuated rooms, monitored by an infra-red camera. The records were scored by the method of Rechtschaffen and Kales. 20 However, sleep stages 3 and 4 were scored together as delta sleep, and movement time was scored as an arousal (2–15 sec) or awakening (>15 sec). Various indices of sleep architecture analyzed were sleep efficiency, number of awakenings, number of stage shifts, percentage of sleep stages, wake before sleep (i.e. latency to 3 consecutive epochs of stage 1 or first epoch of stage 2, REM, or delta sleep), wake during sleep, latency to first REM period, and apnea-hypopnea index (AHI). Apneas and hypopneas were scored according to guidelines prevalent at that time and reproduced in Table 3. Statistical cutoffs used in Table 4 were derived from age-matched normal controls in the laboratory.
Table 3.
Scoring Criteria for Respiratory Events
| Respiratory Event | Airflow reduction | Respiratory effort | O2Desaturation | Duration |
| Obstructive apnea | >90% | Continued/Increased | - | ≥10 sec |
| Hypopnea | >50%, <90% | NA | - | ≥10 sec |
| >30%, <50% | NA | ≥3%and/or terminated by respiratory arousal | ≥10 sec | |
| Central apnea | >90% | Reduced >90% | - | ≥10 sec |
| Mixed apnea | >90% | Reduced >90% for ≥5 seconds prior to continued or increased effort | - | ≥10 sec |
Table 4.
PSG Results
| Polysomnographic data | N=54 |
|---|---|
| Impaired sleep efficiency (<85%) | 57.4% |
| Excessive awakenings (>6) | 92.5% |
| Excessive stage shifts (>60) | 83.3% |
| Excessive stage 1 (>5%) | 78.0% |
| Delayed sleep onset (>25 min) | 22.0% |
| Significant sleep maintenance insomnia (>30 min) | 75.9% |
| Reduced REM percentage (<15%) | 24.0% |
| Shortened REM latency (<67.5 min) | 13.0% |
| Increased EMG tone/frank RBD | 13.0% |
| SOREMP (<20 min) | 5.5% |
| Excessive stage 2 (>60%) | 26.0% |
| Reduced delta percentage (<15%) | 24.0% |
| Abnormal AHI (>10/hr) | 30.0% |
| Significant hypoxia (<90%) | 70.0% |
| Increased PLM index (>10/hr) | 35.0% |
| Increased PLM arousal index (>5/hr) | 9.0% |
The MSLTs were done utilizing the AASM guidelines21,22 prior to those most recently updated in 2005, in a total of 28 patients whose ESS score was >11. A fifth nap was obtained only if SOREMP occurred in 1 of the first 4 naps. The mean sleep onset latency and the presence or absence of the SOREMP/s were computed.
Statistical techniques included the calculations of Pearson's product moment correlation coefficients, 95% confidence intervals, and p values (2-tailed t tests) of the GAF scores with individual measures of sleep disruption i.e. stage 1, stage shifts, sleep efficiency, total number of awakenings, wake before and during sleep, AHI, ESS, PLMI, and PLMA.
RESULTS
During a 2-year period, 60 patients with sleep related complaints following TBI were studied. All had TBI at least 3 months but no more than 2 years prior to evaluation. Most cases were motor vehicle accident related; a few were work-related. Thirty-eight male and 22 female patients were evaluated. Twenty-seven patients had BMI >30 kg/m2. Eight patients had neck circumferences >18 inches. The oropharyngeal, chin, and TMJ examination was deemed benign in all patients. Flow-volume loop, a measure of upper airway obstruction, showed significant plateauing in only 7 patients. The GAF scores were mild (score>60) in 24, moderate (51–60) in 11, and severe (<50) in 25 patients (Table 1).
Table 1.
Patient Characteristics
| Total patients | 60 |
| Demographic Characteristics | |
| Age (years) | |
| Range | 20–69 |
| Mean | 41.03 |
| Sex | |
| Male | 38 |
| Female | 22 |
| Physiological Characteristics | |
| BMI (Kg/m2) | |
| Range | 17.6–41.26 |
| Mean | 29.95 |
| Neck size (inches) | |
| Range | 13.5–21.5 |
| Mean | 16.5 |
| TBI severity (GAF Score) | |
| Mild (>60) | 24 |
| Moderate (51–60) | 11 |
| Severe (<50) | 25 |
Excessive daytime somnolence was the presenting symptom in 50%. The ESS was scored as mild (1–11), moderate (12–15), severe (16–24), or unreliable (patient or caregiver unable to quantify) in 33%, 17%, 35%, and 15%, respectively. Thus 52% of all patients were moderately to severely sleepy during the day time.
Insomnia was present in 25%. Half of these had sleep onset insomnia and HAS scores ranging from 10 to 56 (mean 22.75). The remainder had sleep maintenance insomnia and BDI scores ranging 14 to 43 (mean 24.5).
Overnight PSG was performed in 54 patients. The sleep efficiency ranged from 31% to 99% (mean 73.2%). The number of awakenings ranged from 3–81 (mean 24). The number of times a patient shifts between various stages of sleep is also a measure of sleep disruption. It varied from 11–244 (mean 91.2). The percentage of stage 1 sleep varied from 1%–55% (mean 14.7%). The wake period before sleep onset was 0–86.4 minutes (mean 18.3 min), and wake period during sleep was 2.5–293 min (mean 79.5 min). Table 4 lists the percentage of patients with abnormal values for each of these measures of sleep disruption.
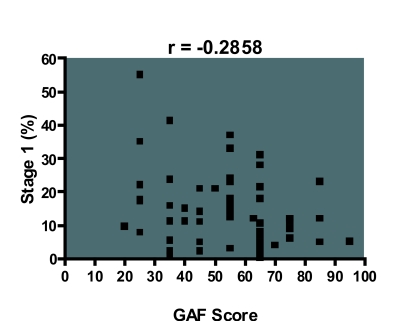
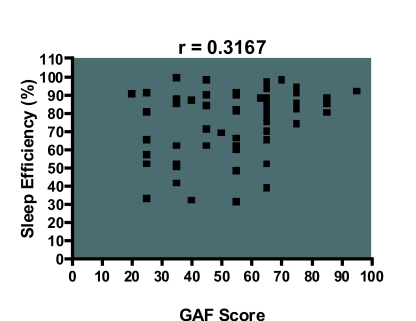
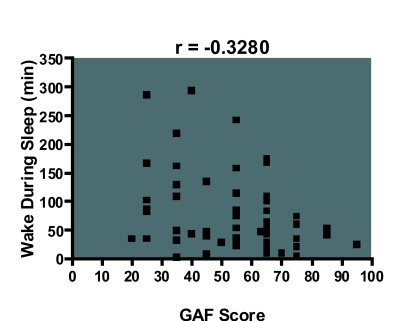
The GAF score correlated with stage 1 percentage (r = −0.285, df 50, p <0.05), sleep efficiency (r = 0.316, df 50, p<0.05) and wake during sleep (r = −0.328, df 50, p <0.05), i.e. a lower GAF score was more likely to be associated with increased stage 1 percentage, impaired sleep efficiency, and wake during sleep (Figure 1–3). However, it did not correlate with other measures of sleep disruption such as stage shifts and wake before sleep (Table 5).
Figure 1.
Scatter diagram showing that a lower GAF score is associated with higher stage 1 percentage. These results were significant at the alpha level of 0.05.
Figure 2.
Scatter diagram showing that a lower GAF score is associated with lower sleep efficiency. These results were significant at the alpha level of 0.05.
Figure 3.
Scatter diagram showing that a lower GAF score is associated with increased wake period during sleep. These results were significant at the alpha level of 0.05.
Table 5.
GAF Scores Versus Measures of Sleep Disruption.
| Response variable | Pearson's Product Moment Correlation Coefficient (r) | P value (two- tailed t test) | 95% Confidence Interval |
|---|---|---|---|
| Wake before sleep (min) | –0.0378 | 0.7817 | –0.2979 to 0.2274 |
| Wake during sleep (min) | –0.328 | 0.0136* | –0.5440 to –0.0711 |
| Stage 1 (%) | –0.285 | 0.0327* | –0.5105 to –0.0247 |
| Sleep efficiency (%) | 0.316 | 0.0174* | 0.0585 to 0.5351 |
| Number of stage changes | –0.0763 | 0.5758 | –0.3327 to 0.1904 |
| Number of awakenings | –0.093 | 0.4943 | –0.3477 to 0.1740 |
| ESS | 0.0621 | 0.6619 | –0.2145 to 0.3295 |
| PLM index | –0.0889 | 0.5146 | –0.3438 to 0.1782 |
| PLMA Index | –0.0126 | 0.9265 | –0.2747 to 0.2512 |
| AHI | 0.0526 | 0.7000 | –0.2133 to 0.3113 |
p <0.05.
df:50,
Stage 2 sleep ranged from 20%–85% (mean 52.7%). Delta sleep stage ranged from 0%–50% (mean 20.6%). Latency to first REM period ranged from 0–352.5 min (mean 129.6 min). Sleep onset REM (<20 min) occurred in 3/54 patients. REM sleep varied from 0%–34.4% (mean 13.6%). Parasomnias were diagnosed using the ICSD-R criteria (2001)24 and were present in 25% (15/60) of all patients (Table 2), and 13% (7/54) had an increase in muscle tone in chin EMG and/or frank REM behavior disorder on PSG. Table 4 lists the percentage of patients with abnormal stage distribution, SOREMPS, or abnormal REM sleep.
Table 2.
Presenting Symptoms
| Symptoms | N=60 |
|---|---|
| Hypersomnia | 30 |
| Insomnia | 15 |
| Sleep onset insomnia | 7 |
| Sleep maintenance insomnia | 8 |
| Parasomnia | 15 |
| Acting out dreams | 5 |
| Nightmares | 4 |
| Sleep paralysis | 3 |
| Sleep walking | 5 |
| Nocturnal enuresis | 3 |
| Cataplexy | 2 |
| Nocturnal eating | 2 |
Twenty percent had snoring and/or pauses in breathing by history, and 30% had an AHI >10/hr. The apnea-hypopnea index (AHI) varied from 0–75.7/hr (mean 9.26/hr). Apneas and hypopneas were predominantly obstructive in 74%. Thirty-eight patients (70%) were found to have lowest oxygen saturation of below 90%. The periodic limb movement index varied from 0–169/hr (mean 10/hr); the PLM arousal index varied from 0–32/hr (Table 4). GAF scores did not correlate with these indices (Table 5).
In the MSLT, the sleep onset latency ranged from 0.6–20 min (mean 5.7 min). Fifty-three percent (15/28) had a mean sleep onset latency <5 min, and 32% (9/28) had this finding and ≥2 sleep onset REM (<15 min). On subsequent HLA testing, 2 of these 9 patients with both shortened sleep latency and ≥2 SOREMPs had positive HLA-DRB1-15 and DQB1*0602 antigens.
DISCUSSION
Our study population closely mirrors the age and sex distribution of TBI in general adult population. The age range was 20–69 years with a mean of 41 years, and sex distribution was roughly 2:1, similar to general adult population with TBI. That sleep disorder was related to TBI is suggested by a lack of history of sleep complaints prior to head trauma, benign oropharyngeal, chin, and TMJ examination, and normal flow volume loop. The results of our study suggest that TBI may be associated with virtually all common sleep related symptoms and disorders. The GAF scores correlated with some measures of sleep disruption such as stage 1 percentage, wake during sleep, and number of awakenings but not with other measures, such as stage shifts, wake before sleep, AHI, PLMI, and PLMA, indicating a complex and multifactorial pathogenesis.
The possible pathogenetic mechanisms of TBI causing sleep disorders include: direct brain injury, indirect brain injury, collateral damage to neck and back and resulting pain interfering with sleep,13 weight gain (secondary to head trauma or medications used to treat head trauma or its sequelae such as posttraumatic mood, anxiety or stress disorder), pre-existing genetic propensity for narcolepsy, which may be clinically aggravated or precipitated by head-trauma10, a pre-existing anatomical abnormality of sleep-related brain mechanisms, oropharyngeal abnormality aggravated by head trauma or resulting weight gain, anatomical abnormalities caused by head trauma such as jaw dislocation, TMJ problems, and brainstem and forebrain lesions induced by TBI.
Direct brain injury was first described by Strich in 196125 as diffuse degeneration of white matter subsequently termed the diffuse axonal injury (DAI). This was later determined in animal experiments to be the consequence of inertial loading of the head by prolonged coronal angular acceleration26 with brunt of abnormality in septum pellucidum, corpus callosum, deep gray matter and dorso-lateral pons and midbrain, areas closely associated with sleep-wake mechanisms. The biochemical basis of this in-jury is excitotoxicity, 27 inflammation,28 free radicals/eicasanoids,1 hyperglycolysis,29 hyperglycemia,29 and apolipoprotein E e4 synthesis.30 These mechanisms most likely operate in sleep disorders associated with mild head injury.
Secondary brain injury (by an extradural, subdural or intraparenchymal hematoma or parenchymal contusion of cerebral hemispheres), or the involvement of brainstem (with contusion, distortion, compression, or hemorrhage) can potentially compromise sleep-wake centers or mechanisms. The raised intracranial pressure during the acute and sub-acute stages of TBI either secondary to cerebral edema or cerebral hyperemia may contribute indirectly. Systemic hypotension, pyrexia, and hypoxia are other potential variables that may be indirectly responsible. Secondary brain injury is likely to be a major factor in patients with moderate to severe head injury with sleep disorders. Discrete sleep syndromes such as posttraumatic narcolepsy, posttraumatic pathologic hypersomnolence, or posttraumatic delayed sleep phase syndrome also appear to be the result of discrete, localized damage to brain in a strategic location.
Excessive daytime somnolence was the most common presenting symptom in our study. Masel et al, in a study to evaluate the prevalence, demographics, and causes of excessive daytime sleepiness in TBI survivors after acute phases of their injury, 31 found that hypersomnia was a common finding, with a relatively high prevalence of sleep apnea-hypopnea syndrome, periodic limb movement disorder, and posttraumatic hypersomnia. In another study, Kaufman et al, in a retrospective analysis to evaluate long-term sleep disturbances in 19 adolescents after minor head injury, reported that minor head injury was associated with lower sleep efficiency, more wake time, and with more awakenings lasting more than 3 minutes.14 Daytime somnolence was reported by 50% of all patients (subjectively confirmed by Epworth sleepiness scale score >11 in 52% of all patients). Daytime somnolence was severe (mean sleep onset latency <5 min on MSLT) in 53% (15/28) of all patients who underwent MSLT. The etiology of this daytime somnolence was multifactorial. An increased PLM arousal index was responsible in a minority of patients. Medication used to treat TBI or its consequences may be directly or indirectly responsible for EDS, but was not evaluated in this study because of its retrospective nature. The most common reason for daytime somnolence appeared to be nocturnal sleep disruption (excessive stage 1, excessive awakenings, excessive stage shifts, reduced delta and REM sleep, and decreased sleep efficiency in various combinations in 24%–92.5% of all patients). Thirty percent of patients had clinically significant sleep related breathing disorders, likely contributing to daytime sleepiness. Our results are consistent with the results obtained by Webster et al, in a study to investigate the nature and occurrence of sleep related breathing disorders in adults with TBI. The authors found that the rate of sleep apnea in their study population was significantly higher than would be predicted based on population norms.32 Clinically definite narcolepsy likely secondary to TBI contributed to daytime somnolence in 32% patients who had MSLT. Hormonal and traumatic factors have long been considered as responsible for development of narcoleptic symptoms at or around puberty in patients who are genetically predisposed to have narcolepsy. Baumann et al, in a prospective study described low levels of CSF hypocretin-1 in acute traumatic brain injury patients. The authors also suggested that the deficiency may be responsible for increased frequency of sleep disturbances in traumatic brain injury survivors as well.33 Lankford et al10 theorized that even mild to moderate head trauma may unmask a genetic propensity to develop narcolepsy, similar to at least 2 patients in our study.
Insomnia was the second most common presenting symptom in our study. It was present in 20 patients (33%) and was the presenting complaint in 15 patients (25%). Parcell et al, in a retrospective study to explore the sleep complaints in patients in the chronic stages of TBI, found a significantly higher frequency of reported sleep changes after TBI.34 The authors also reported more nighttime awakenings and longer sleep onset latency in TBI survivors. In another study, using questionnaires in patients with prior TBI, Ouellet et al found that half of the patients had insomnia symptoms and 29.4% of the participants satisfied the criteria for a diagnosis of insomnia syndrome.35 Half of all our TBI patients with insomnia had sleep onset insomnia; heightened anxiety scores on Hamilton anxiety scale (range 14–43, mean Hamilton anxiety scale score 24.5), appeared to a factor in its causation, secondary to either post-traumatic stress disorder or to posttraumatic anxiety disorder. It was verified on overnight polysomnography in 22% percent as delayed sleep onset. Sleep maintenance insomnia was the complaint in the other half of patients presenting with insomnia. Objectively, it was prevalent in far higher number, in 76% of all cases, although the first-night effect was not excluded. Posttraumatic mood disorder, as suggested by high Beck Depression Inventory scores (score range 10–57, mean BDI score 22.75), was obviously a factor, but it was also influenced by factors such as sleep disordered breathing, periodic limb movements, narcolepsy and other parasomnias such as RBD. Collateral damage elsewhere as a contributing factor, such as neck and back pain, has been emphasized by Guilleminault et al 13 but was not measured in our study.
Parasomnias were a presenting complaint in about 25% (15/60) of our patients. An increase in EMG tone and/or frank REM behavior disorder was diagnosed in 13% (7/54) of those who had polysomnography. REM behavior disorder, characterized by acting out of dreams and with potential to injure self or others in the process, is also most likely due to the damage to brainstem mechanisms (peri-locus coeruleus area sending excitatory impulses to nucleus reticularis magnocellularis in the medulla via the tegmentoreticular tract, and the latter hyperpolarizing the spinal motoneurones via the ventrolateral reticulospinal tract during normal REM sleep). This disorder was first described in experimental animals36 and was predicted to occur in human beings and since then has been described in TBI, in addition to Parkinsonism, alcoholism, and in response to medications.37 All of these may have indirectly contributed to RBD in our patients. These factors were not quantified however. Regardless, none had symptoms of it prior to head trauma and thus, head trauma contributed to the development of RBD directly or indirectly.
Weight gain is another variable that can contribute to or cause sleep disorder by restricting the chest expansion in sleep, compromising normal or previously abnormal upper airways, or causing general lethargy. It is far more common to gain weight than to lose weight in chronic TBI. In our patient population, 45% of the patients exceeded a BMI of 30kg/m2 and most of them indicated that they gained significant weight in the time period following TBI. Possible mechanisms for weight gain after traumatic brain injury include direct injury to hypothalamic feeding and satiety centers; inactivity secondary to pain or neurological handicap; as a feature of posttraumatic mood disorder; or due to antiepileptic drugs, antidepressants, and psychotropic drugs used to treat post-traumatic complications.
Limitations of this study include its retrospective nature, and lack of exclusion of the first-night effect and the possible effects of medication and pain. Also, HAS and BDI were not obtained in all patients regardless of whether they had sleep onset or sleep maintenance insomnia. In addition, the sample size may have been inadequate, masking the relationship between severity of TBI and some of the measures of sleep disruption. Finally, GAF scores may not be the best measure of severity of chronic TBI; alternative scales such as Rancho Los Amigos Scale38 may be better indicators and were not used in this study.
Conclusion
Sleep disorders are a common finding after the acute phase of TBI. They result in daytime somnolence which in turn may lead to poor daytime performance, altered sleep-wake schedule, heightened anxiety, and poor individual sense of well being, insomnia and depression. The results of this study confirm that sleep changes and deranged sleep architecture are common in chronic TBI patients. The sleep disorders seen in this population are similar to those seen in the general population. GAF scores correlated with some, but not all measures of sleep disruption, indicating a complex and multifactorial pathogenesis.
Regardless of the limitations noted previously, this study is an important contribution. It identifies and describes the spectrum of sleep disorders in chronic TBI. Sleep disturbances can compromise the rehabilitation process and the ability to return to work. 16 A high index of suspicion may lead to a diagnosis and subsequent treatment of these disorders and contribute to physical and cognitive rehabilitation of these patients.
ACKNOWLEDGEMENTS
We thank Dr. Maryanne Guidice, Dr. Thomas Park, and Dr. Anthony Petrilli for referring their Chronic TBI patients to our clinic for evaluation. We thank Dr. Park for providing the GAF scores and Trevor Szymanski for the statistical analysis.
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Verma, Anand, and Verman have indicated no financial conflicts of interest.
REFERENCES
- 1.Parker F, Andrews PJD, Azouvi P, Aghakhani N, Perrouin-Verbe B. Acute Traumatic brain injury. Continuum. 2001;7:7–31. [Google Scholar]
- 2.Gill AW. Idiopathic and traumatic narcolepsy. Lancet. 1941;1:474–6. [Google Scholar]
- 3.Amico G, Pasquali F, Pittaluga E. Pickwickian-narcoleptic disorders after brain concussion. Riv Sper Freniatr Med Leg Alien Ment. 1972;29:74–85. [PubMed] [Google Scholar]
- 4.Guilleminault C, Faull K, Miles L, Van den Hoed J. Post-traumatic excessive daytime sleepiness: a review of 20 patients. Neurology. 1982;33:1584–9. doi: 10.1212/wnl.33.12.1584. [DOI] [PubMed] [Google Scholar]
- 5.Prigatano GP, Stahl ML, Orr WC, Zeiner HK. Sleep and dreaming disturbances in closed head injury patients. J Neurol Neurosurg Psychiatry. 1982;45:78–80. doi: 10.1136/jnnp.45.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons LC, Ver Beek D. Sleep-awake patterns following cerebral concussion. Nurs Res. 1982;31:260–4. [PubMed] [Google Scholar]
- 7.Drake ME. Jactitio nocturna after head injury. Neurology. 1986;36:867–8. doi: 10.1212/wnl.36.6.867. [DOI] [PubMed] [Google Scholar]
- 8.Patten SB, Lauderdale WM. Delayed sleep disorder after traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 1992;31:100–2. doi: 10.1097/00004583-199201000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Biary N, Singh B, Bahou Y, al Deeb SM, Sharif H. Post-traumatic paroxysmal nocturnal hemidystonia. Mov Disord. 1994;9:98–9. doi: 10.1002/mds.870090116. [DOI] [PubMed] [Google Scholar]
- 10.Lankford DA, Wellman JJ, O'Hara C. Post-traumatic narcolepsy in mild to moderate closed head injury. Sleep. 1994;17(suppl 8):S25–S28. doi: 10.1093/sleep/17.suppl_8.s25. [DOI] [PubMed] [Google Scholar]
- 11.Perlis ML, Artiola L, Giles DE. Sleep complaints in post concussion syndrome. Percept Mot Skills. 1997;84:595–9. doi: 10.2466/pms.1997.84.2.595. [DOI] [PubMed] [Google Scholar]
- 12.Clinchot DM, Bogner J, Mysiw WJ, Fugate L, Corrigan J. Defining sleep disturbance after brain injury. Am J Phys Med Rehabil. 1998;77:291–5. doi: 10.1097/00002060-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Yuen KM, Gulevich BA, Karadeniz D, Leger D, Philip P. Hypersomnia after head-neck trauma-a medicolegal dilemma. Neurology. 1999;54:653–9. doi: 10.1212/wnl.54.3.653. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman Y, Tzischinsky O, Epstein R, Etzioni A, Lavie P, Piller G. Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2001;24:129–34. doi: 10.1016/s0887-8994(00)00254-x. [DOI] [PubMed] [Google Scholar]
- 15.Worthington AD, Melia Y. Rehabilitation is compromised by arousal and sleep disorders: results of a survey of rehabilitation centres. Brain Inj. 2006;20:327–32. doi: 10.1080/02699050500488249. [DOI] [PubMed] [Google Scholar]
- 16.Ouellet MC, Savard J, Morin CM. Insomnia following traumatic brain injury: a review. Neurorehabil Neural Repair. 2004;18:187–98. doi: 10.1177/1545968304271405. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton MC. Hamilton anxiety scale [HAMA] 1959.
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Chesson AL, Jr, Ferber RA, Fry JM, et al. Practice parameters for the indications for polysomnography and related procedures. Sleep. 1997;20:406–22. doi: 10.1093/sleep/20.6.423. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. Manual of standardized terminology: Technique and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Rearch Institute; 1968. [Google Scholar]
- 21.Carskadon MA, Dement WC, Mitler MM, et al. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 22.Thorpy MJ. The clinical use of the multiple sleep latency test. The Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1992;15:268–276. doi: 10.1093/sleep/15.3.268. [DOI] [PubMed] [Google Scholar]
- 23.Frances A, Pincus HA, First MB. Diagnostic and statistical manual of mental disorders - IV. Washington DC: American Psychiatric Association; 1994. The Global Assessment of Functioning Scale (GAF) [Google Scholar]
- 24.American Academy of Sleep Medicine. International classification of sleep disorders, revised: diagnostic and coding manual. Rochester, MN: American Academy of Sleep Medicine; 2001. [Google Scholar]
- 25.Strich SJ. Shearing of nerve fibres as a cause for brain damage due to head injury. Lancet. 1961;2:443–8. [Google Scholar]
- 26.Copes WS, Champion HR, Sacco WJ. Progress in characterizing anatomic injury. J Trauma. 1990;30:1200–7. doi: 10.1097/00005373-199010000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Mody I, MacDonald JF. NMDA receptor-dependent excitotoxicity: the role of intracellular calcium release. Trends Pharmacol Sci. 1995;16:356–9. doi: 10.1016/s0165-6147(00)89070-7. [DOI] [PubMed] [Google Scholar]
- 28.McKeating EG, Andrews PJ. Cytokines and adhesion molecules in acute brain injury. Br J Anaeth. 1998;80:77–84. doi: 10.1093/bja/80.1.77. [DOI] [PubMed] [Google Scholar]
- 29.Hovda DA. Effect of hyperglycemia after cortical injury. Crit Care Med. 1997;25:1267–8. doi: 10.1097/00003246-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Teasdale GM, Nicoll JR, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome of head injury. Lancet. 1997;350:1069–71. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- 31.Masel BE, Scheibel RS, Kimbark T, Kuna ST. Excessive daytime sleepiness in adults with brain injuries. Arch Phys Med Rehabil. 2001;82:1526–32. doi: 10.1053/apmr.2001.26093. [DOI] [PubMed] [Google Scholar]
- 32.Webster JB, Bell KR, Hussey JD, Natale TK, Lakshminarayan S. Sleep apnea in adults with traumatic brain injury: a preliminary investigation. Arch Phys Med Rehabil. 2001;82:316–21. doi: 10.1053/apmr.2001.20840. [DOI] [PubMed] [Google Scholar]
- 33.Baumann CR, Stocker R, Imhof HG, et al. Hypocretin-1(orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–9. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- 34.Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after traumatic brain injury. Arch Phys Med Rehabil. 2006;87:278–85. doi: 10.1016/j.apmr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Ouellet MC, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J Head Trauma Rehabil. 2006;21:199–212. doi: 10.1097/00001199-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hendricks JC, Morrison AR, Mann GL. Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res. 1982;239:81–105. doi: 10.1016/0006-8993(82)90835-6. [DOI] [PubMed] [Google Scholar]
- 37.Mahowald MW, Schenk CH. REM sleep behavior disorder. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: WB Saunders; 1989. pp. 389–401. [Google Scholar]
- 38.Hagen C, Malkmus D, Durham P. Rehabilitation of the head injured adult: comprehensive physical management. Dowey, CA: Professional Staff Association of the Rancho Los Amigos Hospital, Inc; 1979. Levels of cognitive functioning. [Google Scholar]