Abstract
Study Objectives:
New pharmacotherapeutic treatment options are available to treat patients with 1 or more insomnia symptoms. However, these new Pharmaceuticals are subject to a variety of managed-care tools, such as prior authorizations, that may restrict access to these medications. The objective of this study was to evaluate the economic consequences to a health plan that requires prior authorization for non-benzodiazepine medications approved for the treatment of insomnia characterized by difficulties both falling and staying asleep.
Methods:
An economic model was constructed to determine the effects of a typical prior-authorization program across a hypothetical managed-care population. Model parameters were derived from national estimates and a literature review.
Results:
Economic consequences of a prior-authorization program were based on a hypothetical managed-care plan with 500,000 insured patients. An estimated acquisition cost of $300 per 100 tablets of medication requiring prior authorization, $40 to process each priorauthorization request, and prior-authorization rejection rates of 2% to 5% were considered. Using the default-model inputs of the hypothetical plan characteristics and costs, the economic model estimated a loss of $600,000 to $700,000 per year to the health plan. In a 3-way threshold sensitivity analysis when prior-authorization rejection rate was increased to 5%, the cost of each request in the prior-authorization program was decreased to $20, and the cost of a first-generation nonbenzodiazepine was decreased to a generic price (i.e. $100 per prescription), the model continued to show a net loss to managed care in each case.
Conclusions:
This model showed that requiring prior authorization for newer sleep treatments might not be a cost-saving strategy for managed-care organizations.
Citation:
Balkrishnan R; Joish VN; Bhosle MJ et al. Prior authorization of newer insomnia medications in managed care: Is it cost saving? J Clin Sleep Med 2007;3(4):393–398.
Keywords: Managed care, insomnia, sleep agents, costs, prior authorization
Prescription-drug expenditures, the fastest growing sector of healthcare spending, increased by 8.7% from 2003 to 2004, with total drug spending rising from $218.5 billion to $237.6 billion.1 These expenditure trends impart significant responsibility on managed care organizations (MCOs) to balance costs and quality of care. Various measures adopted by MCOs to contain overall prescription-drug expenditures include promoting generic drug and/or therapeutic substitutions, costs sharing, step therapy, quantity limits, and prior authorizations. Many MCOs use a tier system to encourage use of effective but less-expensive medications, such as generic equivalents, by requiring lower copayment for these drugs (Table 1). These strategies are widely used by third-party payers in the United States. In 2003, more than one half of the states in the United States used at least 4 cost-containment strategies mentioned above in the Medicaid population.2 However, benefits of such cost-cutting strategies should be investigated in order to assess potential short- and long-term unintended consequences, if any.3
Table 1.
Tier System of Therapy
| Tier | Definition |
|---|---|
| 1 | Generic drugs; a way for patients to obtain medications at the lowest copayment level |
| 2 | Preferred brand-name drugs that have no generic equivalent |
| 3 | Nonpreferred brand-name drugs; have an equally effective and less costly generic equivalent available on Tier 1 or may have 1 more preferred brand-name drug option available on Tier 2; available at a higher copayment |
| 4 | Specialty pharmaceuticals that include injectables and oral or inhalation forms of medication; includes, but is not limited to, growth hormones, low molecular weight heparins, interferons, immunologic agents, and antitumor necrosis factors |
Prior authorizations are frequently used to manage the increasing costs of pharmacy benefits. The intent of prior authorizations are to curb the inordinate and inappropriate prescribing of nonpreferred and more-expensive medications.4 The rationale of the prior-authorization method may be to target new, expensive, potentially unnecessary or dangerous medications, while encouraging the delivery of less-expensive and/or safer alternatives.4,5 Implementing a prior-authorization process has been found to be a cost-effective measure for cyclooxygenase 2 inhibitors in MCO6 and Medicaid populations.7 On the other hand, the biggest criticism to MCOs is that prior-authorization policies may deny medically necessary care, given the burden it puts on different healthcare providers (physicians, nurse practitioners, and pharmacists). For example, a study of Medicaid enrollees reported that prior-authorization and generic requirements had the highest negative effects on access to prescription drugs; prior-authorization criteria increased the likelihood of problems associated with medication access by 20%.2
MCOs are often under pressure to balance cost savings for Pharmaceuticals generated by prior-authorization programs with patient, physician, and employer-group concerns; health outcomes; legal requirements; and the administrative costs of running the program itself.8 The administrative costs of these labor-intensive prior-authorization programs are enormous, and yet 95% of the requests are approved by the health plans.8,9 The overall prior-authorization rejection rate in the Medicaid MCO has been found to be as low as 4.4%.8 According to the economic model proposed by Grant et al,10 the threshold prior-authorization denial rate (minimum rate at which the prior-authorization requests must be denied in order to have the cost-effective process) by the insurer should be higher to maintain the breakeven point. Thus, if the initial prior-authorization approval rate is high, then the anticipated costs savings may not be realized. Additional costs would also likely be incurred as a result of the enforcement of prior-authorization criteria to obtain the prescribed medication (eg, additional patients' and healthcare providers' time, greater medical care utilization due to suboptimal clinical response, increased absenteeism, and loss of productivity). Taking such issues in consideration, UnitedHealth Care, a large health plan, decided to abolish utilization management programs because they spent more than $100 million annually on reviews and approved almost 99% of prior authorizations in return with comparatively minimal total savings.9 Titlow and colleagues in their review of prior-authorization policies for Viagra®, Enbrel®, Celebrex®, and Zyban® reported that several MCOs in their study sample discontinued prior-authorization requirements because the administrative costs far exceeded any cost savings offered by these programs.11
Various parties involved in patient care may have different perspectives regarding the process. For example, physicians may view the process as time consuming and a threat to their diagnostic and treatment authority as well as unnecessary intrusion into the patient-physician relationship,12 community pharmacists may consider this as an added administrative burden, and patients may perceive prior authorization as a cost-cutting measure at the expense of the most appropriate medication.13 Sometimes the unfamiliarity of physicians with the prior-authorization process, such as completing the forms with clinical and patient data and contacting the insurers if required, may delay the entire process and may prolong patients' suffering and treatment with appropriate medications,8,9 ultimately causing dissatisfaction.
The potential advantages of prior-authorization programs in reducing medication-related costs and the long-term benefits on clinical or humanistic outcomes may not be always clear.14 Hence, it is imperative that MCOs conduct a formal cost-effective analysis to determine whether a prior authorization is meeting its intended objective of balancing costs and access prior to implementing one.
Insomnia: a Growing Problem
Insomnia may be characterized by difficulty in falling asleep (sleep onset), difficulty in staying asleep (sleep maintenance), waking too early, and/or nonrestorative sleep associated with next-day consequences.15 It is classified as being transient, short-term, or chronic, based on duration of symptoms.15–17 Chronic insomnia refers to sleep difficulties lasting at least for 3 nights per week for 1 month or more.15 Older age, female sex, and presence of comorbidities are a few of the important risk factors of insomnia.16–17 There is a high prevalence of insomnia in the United States, and it is associated with a substantial financial burden to the healthcare system.18,19
Insomnia is usually managed by behavioral and/or pharmacologic therapy.17 Among pharmacologic therapies, benzodiazepines and nonbenzodiazepines are indicated for the treatment of insomnia and have demonstrated efficacy in the management of chronic insomnia.20,21 Although benzodiazepines are effective at managing both sleep-onset and sleep-maintenance symptoms, their nonselective binding to benzodiazepine receptors and longer half-lives contribute to prolonged (next-day) sedation, adverse events, and an increased risk of tolerance and dependence.22,23 The first-generation nonbenzodiazepines, zolpidem and zaleplon, have a superior benefit-risk profile, compared with the benzodiazepines, given their selectivity for the benzodiazepine type-1 receptor and shorter half-lives, but they are not consistently effective in treating sleep-maintenance symptoms.24–26 The second-generation nonbenzodiazepines, eszopiclone and zolpidem extended-release, are indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance without specified limitations on duration of use.27,28 The second-generation nonbenzodiazepines with demonstrated efficacy in treating multiple insomnia symptoms may offer clinical benefits beyond those found with more-limited symptom coverage. In addition to providing improved clinical advantages, inclusion of the second-generation nonbenzodiazepines may also result in a significant overall cost savings to managed care.29,30
Even with the availability of approved medications for insomnia with improved efficacy and safety profiles, off-label use of other low-cost sedating agents, such as antidepressants, antipsychotics, and anticonvulsants, are still common. In fact, off-label use of the antidepressant trazodone accounts for approximately 20% of the insomnia market total prescription share,31 despite the reported risk for moderate to severe adverse events and a lack of demonstrated efficacy.22 In 2005, the National Institutes of Heath recommended that, based on limited efficacy data and potential risks, these agents cannot be recommended for use in chronic insomnia.20
Tier Systems and Prior-Authorization Requirements for Insomnia Medications
Sleep medications indicated for the treatment of insomnia, primarily the second-generation nonbenzodiazepines agents are often subject to cost-containment strategies, such as step therapy and prior authorization. Table 2 summarizes their average wholesale price, tier status, and prior-authorization requirements of the drugs approved for sleep for more than 80% of states in the US.32–35 These prior-authorization requirements, as well as tier status, vary from state to state. For example, estazolam and flurazepam are mostly under tier 1 with few exceptions, such as Virginia and Washington State, where these 2 medications are categorized as tier 2 medications. In most cases, mandatory generic substitution is used to limit more-costly branded drugs when generic equivalents are available. For example, generic triazolam is a tier 1 drug, whereas branded triazolam (Halcion®) is subjected to prior-authorization criteria or categorized as tier 2 or tier 3 drugs in most states. The second-generation nonbenzodiazepines (eg, Lunesta® [eszopiclone]), Sonata® [zaleplon], and Ambien CR® [zolpidem extended release], and melatonin agonist Rozerem® [ramelteon]) are subject to prior authorization by most MCOs in the US.33
Table 2.
Medications Available for the Treatment of Insomnia
| Generic Name | Category | Dose (mg) | AWP for 100 tablets in $USa | Tier Status × | PA required | Insurers requiring PA, % |
|---|---|---|---|---|---|---|
| Estazolam | Generic | 1 | 59.25 | Tier 1 | No | |
| (Prosom®) | Brand | 1 | 135.85 | Tier 2/Tier 3 | Yes | 15 |
| Flurazepam | Generic | 15 | 9.75 | Tier 1 | No | - |
| (Dalmane®) | 30 | 11.48 | ||||
| Brand | 30 | 187.47 | Tier 2/Tier 3 | Yes | 12- | |
| Quazepam | Brand | 7.5 | 359.94 | Tier 3 | Yes | 10- |
| (Doral®) | 15 | 393.37 | - | |||
| Temazepam | Generic | 15 | 62.13 | Tier 1 | No | - |
| (Restoril®) | 30 | 68.60 | Tier 1 | No | - | |
| Brand | 30 | 419.66 | Tier 2/Tier 3 | Yes | 7 | |
| Triazolam | Generic | 0.125 | 40.41 | Tier 1 | No | |
| (Halcion®) | Brand | 0.125 | 141.17 | Tier 2/Tier 3 | Yes | 10- |
| Zolpidem | Brand | 5 | 262.86 | Tier 2/Tier 3 | Yes | 11 |
| (Ambien®) | 10 | 323.32 | ||||
| Zolpidem extended release | Brand | 6.25 | 353.63 | Tier 2/Tier 3 | Yes | 16 |
| (Ambien CR®) | ||||||
| Zaleplon | Brand | 5 | 247.99 | Tier 2/Tier 3 | Yes | 11 |
| (Sonata®) | 10 | 305.02 | ||||
| Ramelteon | Brand | 8 | 281.30 | Tier 2/Tier 3 | Yes | 16 |
| (Rozerem®) | ||||||
| Eszopiclone | Brand | 1 | 370.47 | Tier 2/Tier 3 | Yes | 17 |
| (Lunesta®) | 2 | 370.47 |
AWP refers to average wholesale price; PA, prior authorization; 1. Source: Martin et al (2004); Lippmann et al (2001); Vermeeren (2004); IMS Market scan report, (2006); Red Book (2005); 2. Brand names indicated in parenthesis;
3. Costs found in 2005 Red book; 4. × Tier status indicated for majority (more than 80%) of the insurers
Even though most insurers have prior-authorization criteria on medications indicated to treat insomnia,8 once a request is made by an appropriate agent (eg, physician), it is approved in more than 95% of the cases.8, 9 However, prior authorizations can also be rejected. Both medication- and patient-related factors influence prior-authorization rejections. Factors such as therapeutic category, formulary status of the medication, and patients' age have high impacts on prior-authorization rejection rate.8,34
METHODS
In order to understand whether or not requiring prior authorization for newer insomnia medications is beneficial to a MCO, a hypothetical model was developed using national estimates and market-scan reports.33,35–37 The model is illustrated in the following sections. Estimates from a recently conducted set of studies by Balkrishnan and Rasu35–37 were used to obtain percentages of insured patients receiving some type of pharmacologic treatment for insomnia. Based on the previous literature,8 a baseline prior-authorization rejection rate of 2% was assumed for insomnia medications.
Cost Impact Model Assumptions
The model assumed a hypothetical managed care plan (eg, MCO) with an enrollment of 500,000 members. Using national epidemiologic rates, 25,000 patients (500,000 × 5%) were estimated to be prescribed at least 1 medication for their sleep problems (Table 3). Total market share for second-generation nonbenzodiazepines was assumed to be approximately 20%.33 If a patient was denied a second-generation nonbenzodiazepine under prior authorization, any 1 of the following cases could occur: patient is prescribed a first-generation nonbenzodiazepine for insomnia (zaleplon and zolpidem) not under prior authorization and covered by plan; or patient is prescribed a generic benzodiazepine (eg, temazepam) not under prior authorization and covered by plan; or patient is prescribed low-dose sedating antidepressant (eg, trazodone) that is not indicated for insomnia and seldom under prior-authorization requirement.
Table 3.
Estimates Used in the Model Building
| (I) Insomnia-related outpatient visits in National Medical Survey data (1996–2001) | |
| Patients with insomnia | 94.6 million/5 years |
| Patients with insomnia/year | 19 million/year |
| Insured population (enrolled in MCO) with insomnia | 26% or 4.9 million |
| (II) Estimates of patients receiving some insomnia medication in US MCO settings29 | |
| Prescribed medication therapy only | 48.4% |
| Prescribed both behavioral and medication therapy | 14.3% |
| MCO enrollees with some insomnia medication prescribed | 62.7% |
| (III) From I and II | |
| Total insured population enrolled in MCO with some medication prescribed for insomnia | 3,000,000 (4,900,000 × 62.7%) or 5% of the entire HMO population30 |
| (IV) Case of a hypothetical MCO | |
| Total umber of enrollees in an MCO | 500,000 |
| Number MCO enrollees with some insomnia medication | 25,000 (500,000 × 5%) |
| Market share of second-generation nonbenzodiazepines | 20% |
| Number of prescriptions for medication × (including refills)/year | 4 |
| Number of prescriptions for medication ×/year | 20,000 (25000 × 20% × 4) |
Where, medication X = second-generation nonbenzodiazepine, a new insomnia medication under prior-authorization requirement. MCO refers to managed care organization.
Source: Balkrishnan et al (2005); Rasu (2005); Rasu et al (2005); IMS Market scan report (2006)
In each of the above scenarios, administrative costs of conducting the prior-authorization program would be incurred by the plan in addition to cost of the alternative medication. Assuming sleep medications are prescribed 4 times a year,36 a total of 20,000 prescriptions (25000 × 20% × 4) was assumed for the hypothetical MCO annually. The average estimated acquisition costs (average wholesale price minus 15%) to the MCO for second-generation nonbenzodiazepines (eg, zolpidem extended release, 12.5 mg), first-generation nonbenzodiazepines (eg, zaleplon, 10 mg), and generics (eg, temazepam, 30 mg) were estimated to be $300, $260, and $48 per member per prescription, respectively.22 The cost of prior authorization was assumed to be $40 per administration per request.
RESULTS
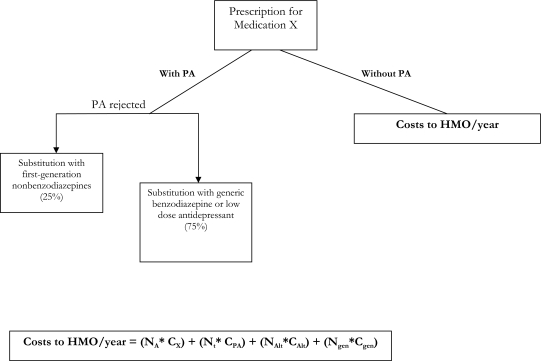
For the base case, assuming a market share of 20%33 for second-generation nonbenzodiazepines and a 2% prior-authorization rejection rate (with the assumption that 25% rejected due to availability, therefore substitution of first-generation nonbenzodiazepines, and 75% due to availability, therefore substitution of generic benzodiazepine or low-dose trazodone), the total costs per year to the hypothetical MCO were calculated using the model presented in Figure 1. Based on this model, the estimated annual costs to the MCO with and without prior authorization for newer insomnia medication was $6,720,400 ([19,600 × 300] + [20,000 × 40] + [100 × 260] + [300 × 48]), and $6,000,000 (20,000 × 300), respectively. Thus, the total annual loss to the MCO associated with prior-authorization requirement was $720,400 ($6,720,400 $6,000,000).
Figure 1.
Annual costs to a health maintenance organization (HMO) with and without prior-authorization (PA) requirements for Medication X (a new insomnia medication—eg, zolpidem extended-release, a second-generation nonbenzodiazepine).
Where,
NA= Number of prescriptions of second-generation nonbenzodiazepines for which PA was approved in the entire year (20000 x 98% PA approval rate= 19,600)
Cx= EAC of second-generation nonbenzodiazepine per member per prescription (PMPP)
Cprior-authorization= Costs of PA for each prescription ($40)
Nt = Total number of prescriptions of medication X prescribed in entire year
NAlt = Number of alternative medication prescribed for entire year (assuming 25% of medication X prescriptions for which PA was rejected and patient received first-generation nonbenzodiazepine)
CAlt = EAC of first-generation nonbenzodiazepine prescription PMPP
Ngen= Number of generic medication prescribed for entire year (assuming 75% of medication X prescriptions for which PA was rejected and patient received less-expensive generic medication)
Cgen = EAC of generic prescription PMPP
Sensitivity Analysis
A 1-way sensitivity analysis was performed to determine the robustness of our assumptions. Model inputs that were thought to be the most sensitive to model assumptions were varied 1 at a time using liberal estimates. When the prior-authorization rejection rate was increased to 5%, or the cost of the prior-authorization program per request was decreased to $20, the model continued to show a net loss in each case. (Table 4).
Table 4.
Results of 1-way Threshold Sensitivity Analysis
| Parameters | Net savings, $ |
|---|---|
| Prior-authorization rejection rate: 5% | 601,000 |
| Cost of each prior-authorization request: $20 | 320,400 |
| Cost of first-generation benzodiazepine: $100/prescription | 704,400 |
Note: The analysis was performed by varying each parameter 1 at a time in the original model.
The sensitivity analysis represents the only tool available to researchers to assess uncertainty around a set of cost-effectiveness estimates. However, a 1-way sensitivity analysis may be overtly simplistic and not represent reality. The 3-way sensitivity analyses accounts for more-realistic situations in which more than factor accounts for a decision or estimate and, therefore, is likely to produce savings that are more conservative, because of the complex interactions between all 3 factors, and accounting for the more “purified” estimate of each factor, after adjusting for the effect of other factors related to uncertainty..38 A 3-way sensitivity analysis using all 3 liberal estimates (5% rejection, $100 for first-generation medication, and $20 for prior authorization) at 1 time also showed a net loss of $161,000 to the managed care organization having a PA program in place.
DISCUSSION
Although prior authorizations are considered to be an effective measure to control prescription drug costs, insurers must assess the cost benefits before mandating prior authorization for certain medications, as the program may in fact be expensive to the insurer. In our analysis of newer sleep agents, based on estimates from nationally representative data, we found that prior authorization may not be a cost-saving strategy for health maintenance organizations at the prior-authorization rejection rates of 5% or less. These results are consistent with the economic model proposed by Grant et al,10 suggesting that prior-authorization programs for newer insomnia medications are cost effective only at a very high prior-authorization rejection rates, which may not be the real-world case. Although these analyses were performed using an MCO perspective, a further consideration of patient, physician, and pharmacists' time and indirect costs (eg, worker presenteeism/absenteeism) into the model might show greater losses to the society as a whole. Beyond economic losses, prior-authorization criteria on second-generation nonbenzodiazepines may deny some patients from deriving desired therapeutic benefit due to the improved efficacy or pharmacokinetics of second-generation nonbenzodiazepines. Like all chronic conditions, both the efficacy and safety profiles of medications are important to consider in the selection of therapy. For insomnia, the nonbenzodiazepines have a superior safety profile, compared with the older benzodiazepines (eg, residual sedation, tolerability, and abuse potential).20 The second-generation nonbenzodiazepines, eszopiclone28 and zolpidem extended-release,27 offer further improvements in their clinical profile through demonstrated efficacy in both sleep-onset and -maintenance symptoms.
Some limitations to this study require mention. Lack of head-to-head comparisons of insomnia medications may limit some of the conclusions regarding clinical benefits of first- versus second-generation nonbenzodiazepines. Study assumptions are based on expert advice, healthcare systems or plans, and estimates from the literature that may not be generalizable to all MCOs. Thus, caution is warranted when applying the results of this study to another setting.
The idea of prior authorization may be to encourage the prescriber to “think twice” before prescribing an expensive medication. Thus, some money may be saved if a physician decides not to go through the hassle of prior authorization in the first place. Implementing prior authorization on expensive medications may affect physicians' decisions to prescribe that particular medication.
Chronic conditions like insomnia require effective pharmacotherapy in order to achieve successful disease management. Although, the nonbenzodiazepines may be more expensive than traditional benzodiazepines or low-dose antidepressants, there is no published literature reporting inordinate or inappropriate prescribing of this class of medications. Therefore, given the intent of prior authorization, as mentioned in the Federal guidelines,5 newer insomnia medications like the second-generation nonbenzodiazepines may not be the ideal candidates for prior-authorization requirements. Additionally, insomnia management with optimal pharmacotherapy has been shown to curb overall healthcare cost in a large health maintenance organization population.30 Such evidences should be investigated thoroughly using real-world data to support or eliminate prior-authorization programs.
Footnotes
Disclosure Statement
This study was funded by Sanofi-Aventis. Dr. Balkrishnan is a paid consultant for Sanofi-Aventis. Dr. Joish is an employee of Sanofi-Aventis. Drs. Bhosle, Rasu, and Nahata have indicated no other conflicts of interest.
REFERENCES
- 1.Smith C, Cowan C, Heffler S, Catlin A. National health spending in 2004: recent slowdown led by prescription drug spending. Health Aff. 2006;25:186–96. doi: 10.1377/hlthaff.25.1.186. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham PJ. Medicaid cost containment and access to prescription drugs. Health Aff. 2005;24:780–9. doi: 10.1377/hlthaff.24.3.780. [DOI] [PubMed] [Google Scholar]
- 3.Olson BM. Approaches to pharmacy benefit management and the impact of consumer cost sharing. Clin Ther. 2003;25:250–72. doi: 10.1016/s0149-2918(03)90035-x. [DOI] [PubMed] [Google Scholar]
- 4.Fallik B. The Academy of Managed Care Pharmacy's concepts in managed care pharmacy: prior authorization and the formulary exception process. J Manag Care Pharm. 2005;11:358–61. doi: 10.18553/jmcp.2005.11.4.358a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjan JN. Medicaid and the unconstitutional dimensions of prior authorization. Mich Law Rev. 2002;101:602–47. [PubMed] [Google Scholar]
- 6.Shaw E, Stacy J, Arledge MD, Howell-Smith D. Pharmacoeconomic modeling of prior-authorization intervention for COX-2 specific inhibitors in a 3-tier copay plan. J Manag Care Pharm. 2003;9:327–34. doi: 10.18553/jmcp.2003.9.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med. 2004;351:2187–94. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- 8.LaPensee KT. Analysis of a prescription drug prior authorization program in a Medicaid health maintenance organization. J Manag Care Pharm. 2003;9:36–44. doi: 10.18553/jmcp.2003.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Healthcare abolished prior authorization, relies on data analysis. Managed Care Outlook. 1999;12:1. [Google Scholar]
- 10.Grant WC, Yoder DM, Mullins CD. Threshold denial rates in prior authorization prescription programs. Expert Rev Pharmacoeconom Outcomes Res. 2004;4:165–9. doi: 10.1586/14737167.4.2.165. [DOI] [PubMed] [Google Scholar]
- 11.Titlow K, Randel L, Clancy CM, Emanuel EJ. Drug coverage decisions: the role of dollars and values. Health Aff. 2000;19:240–7. doi: 10.1377/hlthaff.19.2.240. [DOI] [PubMed] [Google Scholar]
- 12.Hausman K. Psychiatrist sues state Medicaid plan over prior-authorization requirement. Psychiatr News. 2004;39:2. [Google Scholar]
- 13.Reissman D. What is the real cost of prior authorization? Drug Benefit Trends. 2000;12:22–24. [Google Scholar]
- 14.MacKinnon NJ, Kumar R. Prior authorization programs: s critical review of the literature. J Managed Care Pharm. 2001;7:297–302. [Google Scholar]
- 15.Neubauer DN. Insomnia. Prim Care. 2005;32:375–88. doi: 10.1016/j.pop.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Hatoum HT, Kania CM, Kong SX, Wong JM, Mendelson WB. Prevalence of insomnia: a survey of the enrollees at five managed care organizations. Am J Manag Care. 1998;4:79–86. [PubMed] [Google Scholar]
- 17.NCSDR. Overview of the Findings of the National Commission on Sleep Disorders Research (1992) [Accessed on: March 21,2006];NCSDR. 1998 [On-line] Available at: http://www.Stanford.edu/∼dement/overview-ncsdr.html.
- 18.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 19.Hohagen F, Kappler C, Schramm E, et al. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening—temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994;17:551–4. [PubMed] [Google Scholar]
- 20.Lippmann S, Mazour I, Shahab H. Insomnia: therapeutic approach. South Med J. 2001;94:866–73. [PubMed] [Google Scholar]
- 21.Erman MK. Therapeutic options in the treatment of insomnia. J Clin Psychiatry. 2005;66(9) Suppl:18–23. [PubMed] [Google Scholar]
- 22.Rosenberg RP. Sleep maintenance insomnia: strengths and weak-nesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18:49–56. doi: 10.1080/10401230500464711. [DOI] [PubMed] [Google Scholar]
- 23.Dundar Y, Dodd S, Strobl J, Boland A, Dickson R, Walley T. Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis. Hum Psychopharmacol. 2004;19:305–22. doi: 10.1002/hup.594. [DOI] [PubMed] [Google Scholar]
- 24.Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction. 2003;98:1371–8. doi: 10.1046/j.1360-0443.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 25.Prescribing information for zolpidem tartrate extended-release tablets. [Accessed on: July 31, 2006]; Available at: http://products.sanofi-aventis.us/ambien_cr/am-bienCR.html.
- 26.Botteman M, Ozminkowski R, Wang S, Pashos CL, Foley DJ, Schae-fer K. Cost-effectiveness of longer-term treatment with eszopiclone 3mg in adults with primary insomnia. Value Health. 2005;8:323. [Google Scholar]
- 27.Ozminkowski R, Lenhart G, Wang S, Barry N, Schaefer K, Mu-cha L, Rubens R. Forecasting the impact of adding a new drug on formulary using medical claims data and clinical literature: a case study of insomnia treatment. Value Health. 2005;8:323. [Google Scholar]
- 28.Prescribing information for eszopiclone tablets. [Accessed on: July 31, 2006]; Available at: http://www.lunesta.com/PostedApprovedLabelingText.pdf.
- 29.Martin SA, Aikens JE, Chervin RD. Toward cost-effectiveness analysis in the diagnosis and treatment of insomnia. Sleep Med Rev. 2004;8:63–72. doi: 10.1016/j.smrv.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18:297–328. doi: 10.2165/00023210-200418050-00003. [DOI] [PubMed] [Google Scholar]
- 31.IMS monthly Rx Audit. March 2003-February 2006.
- 32.Drug Topics Red Book. 109th ed. Thomson Medical Economics; 2005. [Google Scholar]
- 33.2006. Market Scan Report, IMS America.
- 34.Carter CA, Brookfield RB. Consequences of prior authorization programs-A case example: DDAVP in pediatric nocturnal enuresis. Am J Manage Care. 1996;2:715–8. [Google Scholar]
- 35.Balkrishnan R, Rasu RS, Rajagopalan R. Physician and patient determinants of pharmacologic treatment of sleep difficulties in out-patient settings in the United States. Sleep. 2005;28:715–9. doi: 10.1093/sleep/28.6.715. [DOI] [PubMed] [Google Scholar]
- 36.Rasu RS. Houston, Texas: University of Texas, Houston School of Public Health; 2005. Physician and patient determinants of the treatment of sleep difficulties in outpatient settings in the United States. Dissertation. [DOI] [PubMed] [Google Scholar]
- 37.Rasu RS, Shenolikar RA, Nahata MC, Balkrishnan R. Physician and patient factors associated with the prescribing of medications for sleep difficulties that are associated with high abuse potential or are expensive: an analysis of data from the National Ambulatory Medical Care Survey for 1996–2001. Clin Ther. 2005;27:1970–9. doi: 10.1016/j.clinthera.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Berger ML, Bingefors K, Hedblom EC, et al. ISPOR Book of Terms. Lawrenceville, NJ: International Society of Pharmacoeconomics and Outcomes Research; 2003. Health Care Cost, Quality, and Outcomes; pp. 227–9. [Google Scholar]