Abstract
Study Objectives:
Determine prevalence and consequences of sleepiness and sleep disorders after traumatic brain injury (TBI).
Methods:
Prospective evaluation with polysomnography (PSG), multiple sleep latency test (MSLT), Epworth Sleepiness Scale (ESS) and neuropsychological testing including Psychomotor Vigilance Test (PVT), Profile of Mood States (POMS), and Functional Outcome of Sleep Questionnaire (FOSQ).
Setting:
Three academic medical centers with level I trauma centers, ac credited sleep disorders centers, and rehabilitative medicine programs.
Participants:
Eighty-seven (87) adults at least 3 months post TBI.
Measurements And Results:
Abnormal sleep studies were found in 40 subjects (46%), including 20 (23%) with obstructive sleep apnea (OSA), 10 (11%) with posttraumatic hypersomnia (PTH), 5 (6%) with narcolepsy, and 6 (7%) with periodic limb movements in sleep (PLMS). Among all subjects, 22 (25%) were found to have objective excessive daytime sleepiness with MSLT score <10 minutes. There was no correlation between ESS score and MSLT (r = 0.10). There were no differences in age, race, sex, or education between the sleepy and non-sleepy subjects. Likewise, there were no differences in severity of injury or time after injury between sleepy and non-sleepy subjects. Sleepy subjects had a greater body mass index (BMI) than those who were not sleepy (p = 0.01). OSA was more common in obese subjects (BMI ≥30, p <0.001). Sleepy subjects demonstrated poorer PVT scores (p <0.05), better self-reported sleep related quality of life (FOSQ scores [p <0.05]), and no differences in POMS.
Conclusions:
There is a high prevalence of sleep disorders (46%) and of excessive daytime sleepiness (25%) in subjects with TBI. Sleepy subjects may be more impaired than comparable non-sleepy TBI subjects, yet be unaware of problems. Given the high prevalence of OSA (23%), PTH (11%), and narcolepsy (7%) in this population, there is a clinical indication for NPSG and MSLT.
Citation:
Castriotta RJ; Wilde MC; Lai JM et al. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med 2007;3(4):349-356.
Keywords: Trauma, brain injury, hypersomnia, sleep apnea, narcolepsy, sleep disorders
INTRODUCTION
The increased incidence of sleep disorders after traumatic brain injury (TBI) relative to the general population has become increasingly well recognized.1–6 Traffic accidents are a common cause of TBI, and there is good evidence that the presence of some sleep disorders is associated with traffic accidents.7–9 Cognitive dysfunction is a common and well-researched deficit after TBI and is a key factor preventing return to independent living, social re-adaptation, and vocational pursuits.10–13 Obstructive sleep apnea (OSA) and narcolepsy are associated with some degree of cognitive dysfunction.14–17 However, there is no literature on cognitive dysfunction in posttraumatic hypersomnia (PTH) or periodic limb movements in sleep (PLMS). The literature is sparse on the relationship of cognitive dysfunction to hypersomnolence in TBI.
The purpose of this study was to: 1) examine the prevalence of sleep disorders in a prospectively sampled group of subjects with TBI; 2) explore the relationship between the presence of sleep disorders, injury characteristics, and subject variables; 3) evaluate the impact of sleep disorders on cognitive functioning, mood state, and quality of life after TBI.
METHODS
Subjects
Subjects over 18 years old who were at least 3 months post TBI were prospectively recruited from rehabilitative services at 3 academic medical centers: Memorial Hermann Hospital (Houston, TX), Transitional Learning Center (Galveston, TX) and Philadelphia Veterans Administration Medical Center (Philadelphia, PA). The study was approved by the Committee for the Protection of Human Subjects/Institutional Review Board of all participating institutions. Each subject underwent a history and physical examination, and review of medical records. Exclusion criteria were: 1) presence of circadian rhythm disorder, 2) inability to give informed consent, and 3) use of sedating medications. Each consented subject was scheduled to undergo nocturnal and daytime sleep studies along with neuropsychological testing.
TBI severity was classified by considering both emergency room Glasgow Coma Scale (GCS) and CT scan findings according to traditional criteria.14 A subject was classified as having a severe injury if his or her GCS score was less than 9 irrespective of CT scan findings. A subject was classified as having had a moderate injury with a GCS of 9–12 irrespective of CT findings, or with a GCS of 13–15 and a positive CT scan.18,19 A subject was classified as having a moderate/severe injury with a positive CT scan but without available GCS data to make a finer characterization. A subject was classified as having a mild traumatic brain injury with a GCS score of 13–15 and a negative CT scan.
Sleep Studies
An Epworth Sleepiness Scale (ESS) questionnaire20 was completed by each subject on the night of polysomnography. Nocturnal polysomnograms (NPSG) were performed at least 3 months post injury in sleep laboratories in each center. Using standard techniques,21,22 a computer data acquisition and analysis system recorded the following signals: electroencephalogram (C3A2, C4A1, O1A2, and O2A1), bilateral electroculogram, electrocardiogram, submental and bilateral anterior tibialis electromylogram, thoracic and abdominal excursion by piezocrystals, oral and nasal airflow by thermistor and breath sounds, body position, and oxygen saturation by pulse oximeter. Throughout the studies, subjects were monitored with an infrared video camera and a one-way intercom which connects the bedroom with the monitoring room. All studies were attended by polysomnographic technologists who also scored the studies using 30-second epochs with the Rechtschaffen and Kales criteria,23 and each study was interpreted by a physician certified by the American Board of Sleep Medicine.
During the day subsequent to the sleep study, a multiple sleep latency test (MSLT) was used to assess objective physiologic sleepiness. The test was performed using standard techniques, and sleep onset was defined as the first epoch with any stage of sleep for >50% of the 30-second epoch.24 Each subject took 5 naps of 20 minutes duration at 2-hour intervals. The following signals were recorded during the naps: EEG (C3A2, C4A1, O1A2, and O2A1), bilateral electrooculograms, submental electromylogram, and electrocardiogram. The average sleep latency over these 5 naps was the MSLT score. Those with an MSLT score <10 minutes were termed sleepy and those with an MSLT score ≥10 minutes were non-sleepy. A urine sample was collected after the NPSG and during the MSLT with analysis for possible opiates, benzodiazepines, cannabinoids, amphetamines, or adrenergic drugs.
Respiratory events were scored as previously described.5 Obstructive apnea was defined by cessation of breathing ≥10 seconds with ≥4% fall in oxygen saturation and/or EEG arousal accompanied by continuous respiratory effort. Central apnea was defined by a cessation of breathing ≥10 seconds with ≥4% fall in oxygen saturation and/or EEG arousal without respiratory effort. Hypopnea was defined as >50% reduction in airflow for ≥10 seconds accompanied by ≥4% fall in oxygen saturation and/or EEG arousal. The diagnosis of obstructive sleep apnea (OSA) was made with ≥ 5 apneas/hour of sleep and/or ≥ 10 apneas+hypopneas/hour of sleep. Narcolepsy was defined as an MSLT score (average sleep latency) <5 minutes with ≥2 sleep onset REM periods (SOREMPs) after an unremarkable NPSG with adequate total sleep and REM sleep and negative urine drug screen. Posttraumatic hypersomnia (PTH) was defined as an MSLT score ≤10 minutes with <2 SOREMPs after an unremarkable NPSG and no history of hypersomnolence prior to TBI. Periodic limb movements in sleep (PLMS) were defined as >5 periodic limb movements (PLMs)/hour of sleep and PLMs were scored according to standard criteria.25,26
Neuropsychological Evaluation
Each subject underwent a brief neuropsychological evaluation and completed several self report measures. All subjects were evaluated on 2 occasions. To control for diurnal variations, all evaluations took place beginning at 10:30 between the second and third MSLT nap. The measures used are described below.
Psychomotor Vigilance Test (PVT)
Sustained attention was evaluated with the Psychomotor Vigilance Test (PVT). The PVT was chosen because it is sensitive to the effects of sleepiness on cognitive functioning as well as cognitive problems associated with OSA and its treatment.27–29 The PVT is administered via a small hand-held computerized device with a 3-digit millisecond LED counter and display window (PVT-192: Ambulatory Monitoring Inc, Ardsley, NY). During the PVT, subjects are presented with a 10-minute trial in which they press a response button as soon as a number counting up from 0 is seen. Once the response button is pressed, the counter stops and feedback is given on their reaction time. The amount of time between stimulus presentations varies between minimum and maximum interstimulus intervals of 2000 and 10,000 ms. Performances are recorded in the PVT device and downloaded into a database after the testing bout.
For the purposes of this study, the average of the fastest 10% of reaction times, the average of the slowest 10% reaction times, and the number of lapses (reaction times ≥ 500 ms) from the Psychomotor Vigilance Test (PVT) were selected for analysis. These variable were selected because they have been shown in prior research to be sensitive to sustained attention under conditions of sleep deprivation and in sleep disorders.27–29 Normally, the PVT is given in several testing bouts across time. Owing to the time constraints involved in this study, each subject was exposed to the PVT once.
Profile of Mood States (POMS)
The Profile Of Mood States (POMS)31 is a self-report measure in which subjects rate themselves on each of 65 adjectives using a 1–5 scale. These 65 responses yield 6 mood state scales: Anger-Hostility; Vigor-Activity, Depression-Dejection, Fatigue-Inertia, Tension-Anxiety, and Confusion-Bewilderment. This measure enjoys wide use in sleep research and has been shown to be sensitive to mood problems related to sleep disorders.31,32
Functional Outcome of Sleep Questionnaire (FOSQ)
The Functional Outcome of Sleep Questionnaire (FOSQ) is a self-report measure designed to assess the impact of sleep disorders on daily functioning.33 It has enjoyed use in sleep research and appears to be sensitive to treatment-related change.34–36 There are 30 items which are divided into 5 scales: Activity, Vigilance, General Productivity, Social Outcome, and Intimacy and Sexual Relationships. These scales are summed to make a total score. Higher scores on the FOSQ indicate better daily functioning. The total score was used for this analysis.
Statistical Analysis
Comparability of demographic and baseline characteristics were summarized by subgroups using means and standard deviations (quantitative data) or frequency of counts (qualitative/categorical data).
Parametric t tests for independent samples were used to evaluate group differences when distributions were normal. Because many of the distributions were not normal, nonparametric statistical techniques were employed in the majority of group comparisons. Categorical data was analyzed using chi square tests. Where small cell sizes precluded the use of chi square, Fisher's exact test was employed. For nonparametric independent between group comparisons, the data were subjected to Mann-Whitney U.
RESULTS
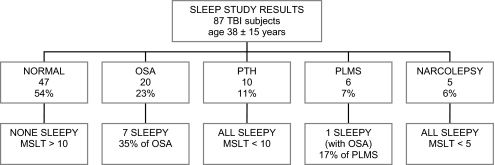
There were a total of 87 TBI subjects who underwent sleep studies. The distributions of demographic and severity variables are listed in Table 1. Note that there were 31 subjects who had insufficient clinical data upon which to make severity determinations. Polysomnographic data from these studies are in Table 2. Forty-seven subjects (54%) had a normal NPSG and MSLT. Twenty (23%) were diagnosed with obstructive sleep apnea (OSA), 10 subjects (11%) were diagnosed with posttraumatic hypersomnia (PTH), 5 (6%) were diagnosed with periodic limb movements in sleep (PLMS), and 5 (6%) were diagnosed with narcolepsy. One subject was diagnosed with both OSA and PLMS. For the purpose of group comparisons, this subject was grouped with the OSA subjects, since this was her primary diagnosis. These distributions are depicted in Table 1 and Figure 1. Those diagnosed with OSA had a mean apnea-hypopnea index (AHI) of 26.1 ± 19. Those diagnosed with PLMS had a mean PLM index of 17 ± 7 and a mean PLM-arousal index of 3.9 ±4.4. Of these PLMS subjects, only one was sleepy (Epworth score of 15 and MSLT score of 5 minutes), and she also had OSA, with an AHI of 13. Although she had 31 PLM-arousals/hour, many of these may have been respiratory-related arousals. The remaining 5 PLMS subjects were asymptomatic, objectively not sleepy, and would not have met criteria for PLMD.
Table 1.
Demographic Data for the Sleepy and Non-sleepy TBI Subjects and the Total Sample.
| MSLT > 10 N (%) | MSLT < 10 N (%) | TOTAL SAMPLE N (%) | |
|---|---|---|---|
| N | 65(74) | 22(25) | 87 |
| Sex | |||
| Male | 47(72) | 16(73) | 63(72) |
| Female | 18(28) | 6(27) | 24(28) |
| Race | |||
| Caucasian | 47(72) | 14(64) | 61(70) |
| African American | 9(14) | 4(18) | 13(15) |
| Hispanic | 9(14) | 3(14) | 12(14) |
| Asian/Pacific Islander | 0(0) | 1(4) | 1(1) |
| Diagnosis | |||
| Normal | 47(72) | 0 | 47(54) |
| OSA | 13(20) | 7(32) | 20(23) |
| PTH | 0(0) | 10(46) | 10(11) |
| PLMS | 5(8) | 1(8)a | 6(7) |
| Narcolepsy | 0 | 5(23) | 5(6) |
| Cause of Injury | |||
| Auto/Vehicle | 49(75) | 15(68) | 64(74) |
| Fall | 7(11) | 1(4) | 8(9) |
| Assault | 4(6) | 2(9) | 6(7) |
| Hit by Falling Object | 4(6) | 2(9) | 6(7) |
| Construction | 1(2) | 2(9) | 3(3) |
| CT Scan Findings | |||
| Positive | 35(54) | 11(50) | 46(53) |
| Not Available | 23(35) | 9(41) | 32(37) |
| Negative | 7(11) | 2(9) | 9(10) |
| Brain Injury Severity | |||
| Unknown | 22(34) | 9(41) | 31(36) |
| Mild | 5(8) | 2(9) | 7(8) |
| Moderate | 13(20) | 2(9) | 15(17) |
| Moderate/Severe | 5(8) | 0 | 5(6) |
| Severe | 20(31) | 9(41) | 29(33) |
| Months Post Injury | |||
| 3 | 10(16) | 3(14) | 13(15) |
| 4–6 | 11(17) | 1(5) | 12(14) |
| 7–12 | 12(18) | 3(14) | 15(17) |
| 13–24 | 8(12) | 4(18) | 12(14) |
| 25–36 | 2(3) | 1(4) | 3(3) |
| >36 | 22(34) | 10(45) | 32(37) |
PLMS + OSA
Table 2.
Sleep Study Data for the Baseline Studies.
| Normal |
OSAa |
PTH |
PLMSa |
NARCOLEPSY |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Total Sleep (h) | 5.87 | 1.41 | 5.56 | 1.40 | 6.77 | .96 | 5.53 | 1.55 | 6.42 | 1.37 |
| Sleep Efficiency | 76.07 | 15.05 | 69.27 | 18.56 | 83.40 | 9.49 | 70.42 | 16.96 | 86.00 | 9.00 |
| Sleep Latency | 36.25 | 37.98 | 47.16 | 102.80 | 11.51 | 25.01 | 37.10 | 43.77 | 17.80 | 18.57 |
| Percent Stage 1 | 8.39 | 6.15 | 17.84 | 13.38 | 11.85 | 6.12 | 9.22 | 2.57 | 9.40 | 5.37 |
| Percent Stage 2 | 68.13 | 13.11 | 62.03 | 14.23 | 58.46 | 15.84 | 71.08 | 11.60 | 68.74 | 16.18 |
| Percent Stage 3 & 4 | 6.38 | 9.38 | 3.66 | 7.45 | 11.21 | 15.22 | 6.22 | 7.33 | 9.24 | 9.29 |
| Percent REM | 16.10 | 7.35 | 17.26 | 9.98 | 16.81 | 8.23 | 13.28 | 8.80 | 16.80 | 2.86 |
| REM Latency | 128.28 | 92.97 | 141.55 | 112.19 | 130.90 | 64.06 | 135.63 | 31.90 | 62.80 | 54.04 |
| Total Arousal Index | 13.06 | 8.98 | 31.63 | 19.67 | 12.83 | 7.67 | 23.84 | 12.54 | 11.24 | 4.67 |
| MSLT | 14.78 | 2.97 | 10.86 | 5.30 | 5.18 | 2.38 | 14.16 | 3.76 | 3.44 | 0.88 |
| PLM Index | 2.54 | 7.94 | 7.21 | 14.50 | 0.80 | 1.62 | 16.64 | 6.62 | 1.60 | 3.58 |
| Apnea-Hypopnea Index | 2.21 | 3.80 | 26.11 | 19.05 | 1.77 | 2.66 | 1.26 | 1.48 | 1.00 | 1.22 |
For the purpose of this analysis, the subject with both OSA and PLMS was placed in the OSA group.
Figure 1.
Study subjects by diagnosis and sleepiness status
There were 24 females (28%) and 63 males (72%) in the total sample. The racial make up of the sample is included in Table 1. The average age of the entire sample was 38.3 (± SD 15.1) years. The average education for the sample was 12.7 (± 2.2) years. The average time post injury was 64.3 (± 117.7) months.
Subgroup Analysis by MSLT
When objective sleepiness was measured by MSLT, 10 (11%) had MSLT scores <5 minutes, and 12 (14%) had MSLT scores 5-10 minutes. The subjects were divided into 2 groups based on the results of their initial MSLT. Those subjects with an MSLT score ≥10 minutes were classified as not sleepy. Those with an MSLT score <10 minutes were classified as sleepy. These included all of the narcolepsy and PTH subjects, along with 7 (35%) of the 20 OSA subjects. As noted above, the subject with both OSA and PLMS was in the sleepy group. The descriptive data for these two groups are depicted in Tables 1 and 3 and Figure 1.
Table 3.
Demographic and Performance Data for the Sleepy and Non-sleepy TBI Subjects.
| MSLT > 10 |
MSLT < 10 |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | p | |
| Age (y) | 38.03 | 15.43 | 39.23 | 14.46 | 0.88d |
| Education (y) | 12.74 | 2.29 | 12.68 | 2.10 | 0.59e |
| Months Post Injury | 56.32 | 100.84 | 87.73 | 155.98 | 0.32e |
| GCS | 8.63 | 4.84 | 6.69 | 4.42 | 0.15e |
| Epworth Sleepiness Scale | 8.28 | 5.31 | 8.91 | 5.00 | 0.94d |
| MSLT | 14.57 | 3.12 | 4.76 | 2.06 | < 0.01d |
| BMI | 26.46 | 4.29 | 31.02 | 8.10 | 0.01d |
| PVT Number of Lapsesa | 6.55 | 11.14 | 10.45 | 14.64 | 0.045 |
| PVT Fastest 10% RTa,f | 221.90 | 45.61 | 197.43 | 262.29 | 0.03e |
| PVT Slowest 10% RTa,f | 881.06 | 1576.76 | 1515.76 | 2491.62 | 0.07e |
| POMS Fatigueb | 9.35 | 7.24 | 8.86 | 6.62 | 0.54e |
| POMS Confusionb | 9.25 | 5.54 | 8.68 | 7.31 | 0.74d |
| POMS Tensionb | 10.82 | 7.29 | 8.55 | 7.77 | 0.12d |
| POMS Vigorb | 12.18 | 6.35 | 15.59 | 7.46 | 0.07d |
| POMS Depressionb | 12.20 | 12.16 | 13.09 | 15.12 | 0.72e |
| POMS Angerb | 9.22 | 10.66 | 10.95 | 12.76 | 0.94e |
| FOSQ Total Scorec | 9.08 | 6.47 | 14.35 | 6.44 | 0.01e |
N = 79 (Not Sleepy = 59 and Sleepy = 20).
N = 82 (Not Sleepy = 59 and Sleepy = 23).
N = 64 (Not Sleepy = 48 and Sleepy = 16).
Analyses conducted using the parametric t tests.
Analyses conducted using the nonparametric Mann-Whitney U.
Reaction Times (RT) in milliseconds
There were 22 sleepy subjects and 65 non-sleepy subjects. There were no significant differences (p >0.05) between the 2 groups in terms of age, education, GCS scores, or Epworth scores. There were equivalent numbers of males and females in each group χ2 (N = 87) = 0.01, p >0.05. Race, (Fisher's exact p = 0.40), cause of injury (Fisher's exact p = 0.36), positive CT scan findings (Fisher's exact p = 1.00), and injury severity (Fisher's exact p >0.53), frequencies were similar between the groups. The frequencies of different sleep diagnoses were different between the groups largely due to the fact that the normal subjects were not sleepy (Fisher's exact p <0.01). The time post injury was classified as 3 months, 4–6 months, 7–12 months, 13–24 months, 25–36 months, and greater than 36 months, and the distributions of subjects in each group were compared. Fisher's exact test disclosed no significant difference between the groups (p = 0.64). There was a significant difference between the groups on body mass index (BMI), with the sleepy subjects being heavier (p = 0.01). These distributions are depicted in Table 3.
We next evaluated the relationship between sleepiness, cognitive functioning, mood state, and quality of life. The Functional Outcome of Sleep Questionnaire (FOSQ) total score, the 6 scales from the Profile of Mood States (POMS), the average of the fastest 10% of reaction times, the average of the slowest 10% reaction times, and the number of lapses (reaction times ≥ 500 ms) from the Psychomotor Vigilance Test (PVT) were selected for this analysis. The distributions are depicted in Table 3. Note that there were missing data from some of these neuropsychological tests. PVT analysis was based on 60 non-sleepy subjects and 20 sleepy subjects. The results of these group comparisons disclosed: 1) that the sleepy subjects' fastest reaction times were significantly slower than the non-sleepy subjects (p <0.05); 2) that the sleepy subjects made more lapses (p < 0.05) than the non-sleepy group; 3) that there was a trend toward the sleepy subjects having a slower average slow reaction times (p = 0.05).
The POMS analysis was based on 59 non-sleepy subjects and 23 sleepy subjects and disclosed no significant differences between sleepy and non-sleepy subjects. The FOSQ analysis was based on 48 non-sleepy subjects and 16 sleepy subjects. The sleepy subjects reported significantly higher FOSQ scores than did the non-sleepy group (p <0.05), indicating better self-rated quality of life in the sleepy subjects.
Relation Between Sleepiness and Diagnosed Sleep Disorders
All of the PTH and narcolepsy subjects were objectively sleepy by definition. Of the 20 OSA subjects, 7 (35%) had MSLT scores <10 minutes and there was no significant correlation between apnea-hypopnea index (AHI) and MSLT score (r = −0.18, p >0.05). Only one of the PLMS subjects had an MSLT <10 minutes. However, this subject was found to have both OSA and PLMS. There was no significant correlation between PLM index and MSLT score (r = 0.11, p >0.05).
Data Analysis by Diagnosis
In this analysis TBI subjects who were diagnosed with sleep disorders were compared to non-sleep-disordered subjects on the same variables described in the prior analyses. The descriptive data for these 2 groups are depicted in tables 4 and 5. There were 40 sleep-disordered subjects and 47 non-sleep-disordered subjects. There were no significant differences (p >0.05) between the 2 groups in terms of education or GCS scores. However, the sleep-disordered subjects were significantly older than their non-sleep-disordered peers (43.5 ± 13 vs 34.3 ± 14.8 years, p = 0.01). There were equivalent numbers of males and females in each group χ2 (N = 87) = 0.96, p >0.05. Race, (Fisher's exact p = 0.86), cause of injury (Fisher's exact p = 0.33), positive CT scan findings (Fisher's exact p = 0.28), and injury severity (Fisher's exact p = 0.53) frequencies were similar between the groups. The time post injury data was classified as 3, 4–6, 7–12, 13–24, 25–36 and greater than 36 months, and the distributions of subjects in each group were compared. Fisher's exact test disclosed no significant difference between the groups (p = 0.62). There was a significant difference between the groups on body mass index (BMI), with the sleep disordered subjects being heavier (29.2 ± 7 vs 26.3 ± 4.1 kg/m2, p < 0.05).
Table 4.
Demographic Data for the Sleep-disordered and Non-sleep-disordered TBI Subjects.
| N | Non-Sleep-Disordered N (%) |
Sleep-Disordered N (%) |
|---|---|---|
| 47 | 40 | |
| Sex | ||
| Male | 32(68) | 31(78) |
| Female | 15(32) | 9(22) |
| Race | ||
| Caucasian | 34(72) | 27(67) |
| African American | 7(15) | 6(15) |
| Hispanic | 6(13) | 6(15) |
| Asian/Pacific Islander | 0(0) | 1(2) |
| Diagnosis | ||
| Normal | 47(100) | 0(0) |
| Narcolepsy | n/a | 5(13) |
| OSA | n/a | 20(50) |
| PLMS | n/a | 5(13) |
| PTH | n/a | 10(25) |
| Cause of Injury | ||
| Assault | 3(6) | 3(8) |
| Auto/Vehicle | 38(81) | 26(65) |
| Construction | 1(2) | 2(5) |
| Fall | 4(9) | 4(10) |
| Hit by Falling Object | 1(2) | 5(12) |
| CT Scan Findings | ||
| Not Available | 15(32) | 17(42) |
| Negative | 6(15) | 2(5) |
| Positive | 25(53) | 21(53) |
| Brain Injury Severity | ||
| Unknown | 14(30) | 17(42) |
| Mild | 5(11) | 2 (5) |
| Moderate | 9(19) | 6(15) |
| Moderate/Severe | 4(8) | 1 (3) |
| Severe | 15(32) | 14(35) |
| Months Post Injury | ||
| 3 | 8(17) | 5(12) |
| 4–6 | 9(19) | 3(7) |
| 7–12 | 8(17) | 7(18) |
| 13–24 | 6(13) | 6(15) |
| 25–36 | 1(2) | 2(5) |
| >36 | 15(32) | 17(43) |
Table 5.
Demographic and Performance Data
| Non-Sleep-Disordered |
Sleep-Disordered |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | p | |
| Age (y) | 34.32 | 13.40 | 43.05 | 15.80 | 0.01c |
| Education (y) | 12.53 | 1.79 | 12.95 | 2.67 | 0.39d |
| Months Post Injury | 45.47 | 81.41 | 87.56 | 146.79 | 0.09d |
| GCS | 8.66 | 4.89 | 7.45 | 4.64 | 0.38d |
| MSLT Score | 14.78 | 2.97 | 8.93 | 5.42 | <.001 |
| Epworth Sleepiness Scale | 7.34 | 4.87 | 9.58 | 5.34 | 0.04c |
| BMI | 26.32 | 4.08 | 29.18 | 7.08 | 0.03c |
| PVT Number of Lapsesa | 5.84 | 11.31 | 9.58 | 12.92 | 0.01d |
| PVT Fastest 10% RTa | 218.53 | 47.43 | 213.42 | 195.77 | 0.04d |
| PVT Slowest 10% RTa | 910.73 | 1812.90 | 1197.41 | 1910.35 | 0.03d |
| POMS Fatigueb | 8.77 | 6.80 | 9.72 | 7.36 | 0.54d |
| POMS Confusionb | 8.58 | 4.99 | 9.67 | 7.01 | 0.42c |
| POMS Tensionb | 9.98 | 6.75 | 10.46 | 8.22 | 0.77c |
| POMS Vigorb | 11.86 | 6.38 | 14.46 | 7.04 | 0.08c |
| POMS Depressionb | 11.28 | 11.34 | 13.72 | 14.53 | 0.40d |
| POMS Angerb | 8.12 | 9.14 | 11.41 | 13.02 | 0.19d |
| FOSQ Total Scorec | 8.94 | 6.37 | 12.27 | 7.01 | 0.05d |
Data analysed using nonparametric Mann-Whitney U.
N = 82 (Not Sleepy = 59 and Sleepy = 23).
Analyses conducted using the parametric t tests.
Analyses conducted using the nonparametric Mann-Whitney U.
The 2 groups were compared on the same measures as in previous analyses. The distributions are depicted in Table 5. Note that there was incomplete data for some of these analyses. The PVT data analysis was based on 44 non-sleep-disordered subjects and 36 sleep-disordered subjects. The results of these group comparisons disclosed: 1) that the sleep-disordered subjects' fastest reaction times were significantly slower than the non-sleep-disordered subjects (p <0.05); 2) that the sleep-disordered subjects demonstrated significantly slower slow reaction times (p <0.05); and 3) that the sleep-disordered subjects made more lapses (p <0.05) than the non-sleep-disordered group.
The POMS analysis was based on 43 non-sleep-disordered subjects and 39 sleep-disordered subjects. POMS scores did not differ significantly between the groups. The FOSQ analysis was based on 36 non-sleep-disordered subjects and 28 sleep-disordered subjects. There was a trend toward the sleep disordered subjects reporting significantly higher FOSQ scores than the non-sleep-disordered subjects (p = 0.08). In order to determine if there was an association between self-reported sleepiness and objectively verified sleepiness, a bivariate correlation was calculated between the MSLT and Epworth Sleepiness Scale (ESS) using all of the subjects with complete data. The resulting correlation was not significant r (80) = 0.10, p >0.05.
DISCUSSION
In the present study, we evaluated the presence and impact of sleep disorders in a cohort of prospectively recruited TBI subjects. Forty-seven percent of our sample was found to have a sleep disorder: OSA (23%), PTH (11%), narcolepsy (6%), or PLMS (7%). Twenty-six percent of the sample had EDS as measured by the MSLT score <10. Injury severity, the presence of a positive CT scan, and GCS scores were not associated with the presence of EDS. Subjects with an MSLT <10 objectively demonstrated more problems with vigilance but actually reported better sleep-related quality of life than non-sleepy subjects. There were no significant differences in self-reported mood state between the 2 groups. Comparisons of sleep-disordered versus non-sleep-disordered subjects disclosed no relationship between the presence of a sleep disorder and injury severity, cause of injury, or the presence of positive CT scan findings. Sleep-disordered subjects were more likely to have a higher BMI and demonstrated difficulties with psychomotor vigilance; they showed no differences in mood state and showed a trend toward better self-reported sleep related quality of life.
Prior studies4,5 have shown that in symptomatic (sleepy) TBI subjects, 32%–70% have SDB, 1%–3% have narcolepsy, and 1%–4% have PTH. Lankford et al.37 reported 8 of 9 sleepy TBI subjects to have posttraumatic narcolepsy, while the 9th met criteria for PTH. Only 2 prior studies have been done on unselected TBI subjects, but these patients were recruited from inpatient rehabilitation facilities, had severe injury, and were often less than 3 months post injury. These studies found that 12%–36% of their samples had SDB. Only one of these studies included MSLTs. This study included only inpatients and found that 47% were objectively sleepy (MSLT score <10 minutes), 3% had narcolepsy, and 28% had PTH. The current study found fewer subjects with objective sleepiness (26%), as would be expected from a prospective study that included predominantly outpatients who were more than 3 months post injury. The prevalence of demonstrable sleep disorders in our study (46%) may be more representative than studies composed of acute or referred samples. The presence of PLMS as a diagnostic finding presents some problems, since most of these subjects were asymptomatic and had less than 5 PLM-arousals/hour. Thus the periodic limb movements (PLMs) noted may constitute an incidental finding of little or no clinical significance in most subjects. There was only one subject with PLMS and an MSLT score <10 minutes, and that subject also had OSA. If we exclude PLMS, then 40% of our TBI subjects had significant sleep disorders. PTH was the second most common sleep disorder (11%) after OSA (23%) in TBI subjects, while 6% had narcolepsy. The MSLT is a necessary part of the sleep evaluation in TBI subjects, since approximately 17% of our TBI subjects had either PTH or narcolepsy, both of which require MSLT for diagnosis.
The fact that narcolepsy is so frequent (6%) in our study sample and in other studies compared to the general population (0.05%) suggests that either some of the subjects had pre-existing narcolepsy, or that TBI may precipitate the onset of narcolepsy symptoms.2,4,5,37 Since both those subjects with OSA8 and narcolepsy9 have a greater chance of MVAs and hence TBI, it is not surprising that both conditions have a higher prevalence in post-TBI subjects. It is very possible that some of our subjects may have had a preexisting undiagnosed sleep disorder, and that the presence of a sleep disorder may actually have contributed to the occurrence of the accident that caused traumatic brain injury. We cannot determine whether TBI or sleep disorder came first, since the purpose of our study was to examine the prevalence and consequences of sleep disorders in patients with TBI, and there was no way to determine how many of these subjects actually had a sleep disorder prior to injury. Pre-TBI symptoms of hypersomnia were not found by Guilleminault et al2 in any of the 59 TBI patients with SDB, while 3 of 10 subjects in another study5 had preexisting symptoms. Two of these had SDB and one had narcolepsy. The reliability of the history of preexisting symptoms is very questionable in most cases, given both the medical-legal implications and the dubious reliability of cognitively impaired post-TBI subjects as historians.
We did not find any specific relationship between the presence of sleep disorders and the severity of injury. We did not find significantly different distributions of mildly, moderately, or severely injured subjects in the sleepy and non-sleepy groups or the sleep-disordered and non-sleep-disordered TBI subjects. Similarly, there was no relationship between the presence of CT lesions and sleep disorders or sleepiness. Unfortunately, our study is missing severity data and CT data on a number of subjects, which is a weakness of this study. However, the lack of significant relationships between TBI severity or the presence of CT lesions and sleep disorders has been found previously in a large cohort.4 Thus, the weight of the evidence would suggest that TBI severity and the presence of CT lesions are independent of sleep disorders and sleepiness.
This is the first study demonstrating that the presence of a sleep disorder adds an additional cognitive burden in TBI subjects. Sleepy subjects showed slower reaction times and made more lapses on the PVT than non-sleepy subjects. The relationship between sleepiness and decreased neurobehavioral functioning has been reported previously in sleep-deprived nonclinical samples.29 In addition, vigilance problems and EDS appear to commonly co-occur in narcolepsy subjects.39 TBI has long been associated with significant cognitive impairments.40 It would appear from our results that vigilance problems are increased in this population by the presence of a sleep disorder or EDS, and it is possible that these vigilance problems may underlie the cognitive problems of TBI subjects with heretofore unrecognized sleep disorders. Since the sleep-disordered subjects were somewhat older than the non-sleep-disordered subjects, it could be argued that the differences in vigilance might be the result of this age difference. However, correlational analyses disclosed small relationships between PVT variables and age that ranged from r = −0.13 to r = 0.23), which would suggest that age is not responsible for this finding.
In spite of objective evidence of poor vigilance, there was a trend towards sleepy TBI subjects and TBI subjects with a sleep disorder diagnosis to actually report better sleep related quality of life than those that did not carry a diagnosis and/or were not sleepy. TBI is often associated with reduced awareness of problems.41 Thus, it is highly likely that poor awareness resulted in subjects overreporting self-perceived quality of life and perhaps underreporting mood changes and subjective sleepiness. This conclusion is supported by the low correlation between the MSLT (objective sleepiness) and the ESS (subjective sleepiness) and nonsignificant group differences on POMS measures that would be sensitive to sleep related problems such as Fatigue and Vigor. Future research with this population of sleep disorder subjects as well as clinical evaluations should include objective performance measures as well as collateral report for family and significant others in order to establish a reliable symptom picture and history.
Vigilance problems may make these subjects more prone to have problems in daily functioning. Thus, it is possible that the presence of a sleep disorder causes more functional disability in TBI. The impact of vigilance problems on the day to day functioning of TBI subjects has not been established. This paper could not address the first possibility, because of apparently unreliable reports from our subjects and the lack of data on day to day functioning from collateral informants. This is one of the weaknesses of this study and emphasizes the importance of data from collateral informants in studies on functional outcome. A future paper from this project will begin to address whether optimal treatment improves vigilance problems.
The high prevalence of excessive daytime sleepiness, obstructive sleep apnea, posttraumatic hypersomnia, and narcolepsy after traumatic brain injury leaves us with the conclusion that these subjects should undergo complete sleep evaluations, including NPSG and MSLT. Sleepy TBI subjects have more impaired cognitive function and vigilance performance than other TBI subjects but may be unaware of problems. This may also explain the lack of correlation between MSLT and ESS in these subjects. Hence objective testing should be used in order to assess pathology. Since daytime sleepiness and some neuropsychological deficits of TBI subjects may be due to treatable sleep disorders, their diagnosis and treatment may have a favorable impact on care.
ACKNOWLEDGEMENTS
This research was supported by the Moody Foundation and Cephalon, Inc. We would like to express our appreciation to Amal Abuelheiga, RPSGT for her assistance with this project.
Footnotes
Disclosure Statement
This study was conceived and initiated by the investigators and supported by the Moody Foundation with additional support from Cephalon, Inc. Dr. Wilde has also received research support from Northstar Corporation. Dr. Masel has also received research support from and has participated in speaking engagements for Pfizer. Drs. Castriotta, Lai, Atanasov, and Kuna have reported no other financial conflicts of interest.
REFERENCES
- 1.Guilleminault C, Faull KF, Miles L, van den Hoed J. Posttraumatic excessive daytime sleepiness: a review of 20 subjects. Neurology. 1983;33:1584–9. doi: 10.1212/wnl.33.12.1584. [DOI] [PubMed] [Google Scholar]
- 2.Guilleminault C, Yuen KM, Gulevich MG, Karadeniz D, Leger D, Philip P. Hypersomnia after head-neck trauma: a medicolegal dilemma. Neurology. 2000;8(54):653–9. doi: 10.1212/wnl.54.3.653. [DOI] [PubMed] [Google Scholar]
- 3.Webster JB, Bell KR, Hussey JD, Natale TK, Lakshminarayan S. Sleep apnea in adults with traumatic brain injury: a preliminary investigation. Arch Phys Med Rehabil. 2001;82:316–21. doi: 10.1053/apmr.2001.20840. [DOI] [PubMed] [Google Scholar]
- 4.Masel BE, Scheibel RS, Kimbark T, Kuna ST. Excessive daytime sleepiness in adults with brain injuries. Arch Phys Med Rehabil. 2001;82:1526–32. doi: 10.1053/apmr.2001.26093. [DOI] [PubMed] [Google Scholar]
- 5.Castriotta RJ, Lai JM. Sleep disorders associated with traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1403–6. doi: 10.1053/apmr.2001.26081. [DOI] [PubMed] [Google Scholar]
- 6.Mansfield RT. Head injuries in children and adults. Crit Care Clin. 1997;13:611–28. doi: 10.1016/s0749-0704(05)70331-6. [DOI] [PubMed] [Google Scholar]
- 7.Ellen RLB, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-King M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 8.Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17:84–93. doi: 10.1093/sleep/17.1.84. [DOI] [PubMed] [Google Scholar]
- 9.Kotterba S, Mueller N, Leidag M, et al. Comparison of driving simulator performance and neuropsychological testing in narcolepsy. Clin Neurol Neurosurg. 2004;106:275–9. doi: 10.1016/j.clineuro.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Rehabilitation of persons with traumatic brain injury. NIH Consensus Statement. 1998. pp. 1–41. [PubMed]
- 11.Sherer M, Novack TA, Sander AM, Struchen MA, Alderson A, Thompson RN. Neuropsychological assessment and employment outcome after traumatic brain injury: a review. Clin Neuropsychol. 2002;16:157–78. doi: 10.1076/clin.16.2.157.13238. [DOI] [PubMed] [Google Scholar]
- 12.Levin HS. Neurobehavioral recovery. J Neurotrauma. 1992;9(Suppl 1):S359–73. [PubMed] [Google Scholar]
- 13.Levin HS. Head trauma. Curr Opin Neurol. 1993;6:841–6. doi: 10.1097/00019052-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 15.Naumann A, Daum I. Narcolepsy: pathophysiology and neuropsychological changes. Behav Neurol. 2003;14:89–98. doi: 10.1155/2003/323060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5:423–45. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- 17.Schneider C, Fulda S, Schulz H. Daytime variation in performance and tiredness/sleepiness ratings in subjects with insomnia, narcolepsy, sleep apnea and normal controls. J Sleep Res. 2004;13:373–83. doi: 10.1111/j.1365-2869.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–8. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Dikmen S, Machamer J, Temkin N. Mild head injury: facts and artifacts. J Clin Exp Neuropsychol. 2001;23:729–38. doi: 10.1076/jcen.23.6.729.1019. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 21.American Sleep Disorders Association Report Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.Practice parameters for the indications for polysomnography and related procedures Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–22. [PubMed] [Google Scholar]
- 23.Rechtschaffen A., Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- 24.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 25.Coleman R. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo Park: Addison Wesley; 1982. pp. 265–95. [Google Scholar]
- 26.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic and coding manual; p. 185. [Google Scholar]
- 27.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in subjects with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 28.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA subjects with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 29.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267. [PubMed] [Google Scholar]
- 30.McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego, CA: Educational Testing Service; 1971. [Google Scholar]
- 31.Yu BH, Ancoli-Israel S, Dimsdale JE. Effect of CPAP treatment on mood states in subjects with sleep apnea. J Psychiatr Res. 1999;33:427–32. doi: 10.1016/s0022-3956(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 32.Bardwell WA, Ancoli-Israel S, Dimsdale JE. Types of coping strategies are associated with increased depressive symptoms in subjects with obstructive sleep apnea. Sleep. 2001;24:905–9. doi: 10.1093/sleep/24.8.905. [DOI] [PubMed] [Google Scholar]
- 33.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 34.Gooneratne NS, Weaver TE, Cater JR, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51:642–9. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 35.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 36.Weaver TE, Maislin G, Dinges DF, et al. Self-efficacy in sleep apnea: instrument development and subject perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep. 2003;26:727–32. doi: 10.1093/sleep/26.6.727. [DOI] [PubMed] [Google Scholar]
- 37.Lankford DA, Wellman JJ, O'Hara C. Posttraumatic narcolepsy in mild to moderate closed head injury. Sleep. 1994;17(8) Suppl:S25–S28. doi: 10.1093/sleep/17.suppl_8.s25. [DOI] [PubMed] [Google Scholar]
- 38.Rogers NL, Szuba MP, Staab JP, Evans DL, Dinges DF. Neuroimmunologic aspects of sleep and sleep loss. Semin Clin Neuropsychiatry. 2001;6:295–307. doi: 10.1053/scnp.2001.27907. [DOI] [PubMed] [Google Scholar]
- 39.Fronczek R, Middelkoop HA, van Dijk JG, Lammers GJ. Focusing on vigilance instead of sleepiness in the assessment of narcolepsy: high sensitivity of the Sustained Attention to Response Task (SART) Sleep. 2006;29:187–91. [PubMed] [Google Scholar]
- 40.Levin HS, Eisenberg HM. Management of head injury. Neurobehavioral outcome. Neurosurg Clin N Am. 1991;2:457–72. [PubMed] [Google Scholar]
- 41.Prigatano GP. Impaired self-awareness after moderately severe to severe traumatic brain injury. Acta Neurochir Suppl. 2005;93:39–42. doi: 10.1007/3-211-27577-0_5. [DOI] [PubMed] [Google Scholar]