Abstract
Study Objectives:
To evaluate the prevalence and natural history of sleepiness following traumatic brain injury.
Methods:
This prospective cohort study used the Sickness Impact Profile to evaluate sleepiness in 514 consecutive subjects with traumatic brain injury (TBI), 132 non-cranial trauma controls, and 102 trauma-free controls 1 month and 1 year after injury.
Results:
Fifty-five percent of TBI subjects, 41% of non-cranial trauma controls, and 3% of trauma-free controls endorsed 1 or more sleepiness items 1 month following injury (p < .001). One year following injury, 27% of TBI subjects, 23% of non-cranial trauma controls, and 1% of trauma-free controls endorsed 1 or more sleepiness items (p < .001). Patients with TBI were sleepier than non-cranial trauma controls at 1 month (p < .02) but not 1 year after injury. Brain-injured subjects were divided into injury-severity groups based on time to follow commands (TFC). At 1 month, the non-cranial trauma controls were less sleepy than the 1- to 6-day (p < .05), 7- to 13-day (p < .01), and 14-day or longer (p < .01) TFC groups. In addition, the ≤ 24-hour group was less sleepy then the 7- to 13-day and 14-day or longer groups (each p < .05). At 1 year, the non-cranial trauma control group (p < .05) and the ≤ 24-hour TFC group (p < .01) were less sleepy than the 14-day or longer TFC group. Sleepiness improved in 84% to 100% of subjects in the TBI TFC groups, as compared with 78% of the non-cranial trauma control group (p < .01).
Conclusions:
Sleepiness is common following traumatic injury, particularly TBI, with more severe injuries resulting in greater sleepiness. Sleepiness improves in many patients, particularly those with TBI. However, about a quarter of TBI subjects and non-cranial trauma control subjects remained sleepy 1 year after injury.
Citation:
Watson NF; Dikmen S; Machamer J et al. Hypersomnia following traumatic brain injury. J Clin Sleep Med 2007;3(4):363-368.
Keywords: Brain, injury, trauma, sleepiness, hypersomnia
Every year in the United States, 50,000 people die and more than 1.4 million seek medical care for traumatic brain injuries (TBI).1 Approximately 5.3 million Americans live with brain injury-associated long-term disabilities, such as seizure disorders and cognitive and psychosocial impairments.1–3 Sleepiness and fatigue are common sequelae of TBI, although this relationship is complex and poorly understood. Sleepiness following TBI may result from preexisting sleep disorders, as a side-effect of medications, or from the effects of the brain injury itself.4,5 A better understanding of this phenomenon is needed because it interferes with the rehabilitation process, depriving patients of the vitality to regain lost function. It also disengages patients from reinforcing activities, further reducing quality of life.
Available research on posttraumatic sleepiness has focused on small samples of highly selected patients recruited from rehabilitation centers or sleep clinics.4–9 Longitudinal data are often not reported. We, therefore, conducted a longitudinal study of consecutive patients admitted with TBI to a level 1 trauma center. Our objectives were to assess (1) if TBI predisposes to sleepiness more than does non-cranial trauma or trauma-free controls; (2) if brain-injury severity relates to sleepiness; and (3) the prevalence and natural history of TBI-related sleepiness.
METHODS
Subjects
Persons with TBI
Subjects included participants in 3 longitudinal investigations of outcome following TBI (Behavioral Outcome of Head-Injury, Patient Characteristics and Head-Injury Outcome, Phenytoin Prophylaxis of Posttraumatic Seizures) described in detail elsewhere.10–14 Fifty percent of our subjects came from the Patient Characteristics Study, 21% came from the Behavioral Outcome Study, and 29% came from the Phenytoin Prophylaxis Study. Participants were English-speaking adolescents or adults identified upon admission to Harborview Medical Center, a Level 1 trauma hospital, and studied prospectively to 1 year. The subjects were consecutive admissions with a broad spectrum of TBI that met the following minimum brain injury severity criteria: any period of loss of consciousness, posttraumatic amnesia for at least 1 hour, or other objective evidence of head trauma (eg, hematoma). Additional selection criteria included hospitalization, survival 1 month after injury, and consent to participate in the study. These studies were approved by University of Washington Institutional Review Board.
The 3 studies differed on 2 selection criteria—preexisting conditions and injury severity. Preexisting conditions included hospitalization for prior TBI, treatment for alcohol abuse, cerebral disease (eg, encephalitis), mental retardation, or significant psychiatric disorder (eg, schizophrenia, manic-depressive illness). The Patient Characteristics Study did not exclude subjects on the basis of preexisting conditions, whereas the other 2 studies considered these conditions exclusionary.10–14 The Behavioral Outcome Study also excluded people over the age of 60. The Phenytoin Prophylaxis Study enrolled patients with more severe brain injuries than did the other 2 studies. These subjects had a Glasgow Coma Scale score of 10 or below at admission or other evidence of increased risk of seizures, that is, a cortical contusion documented by computed tomography scan, a depressed skull fracture, a hematoma (subdural, epidural, or intracerebral), a penetrating head injury, or a seizure within the first 24 hours after the injury.12 The subjects of all 3 studies were consecutive admissions recruited from the same hospital.
Of the 514 brain-injured subjects fulfilling their respective study requirements, 166 were not included in the 1-month postinjury analyses because they were unable to follow simple commands or were too impaired to participate. At 1 year after injury, 104 of TBI subjects did not participate due to death (n = 17), persistent cognitive impairments precluding valid assessments (n = 38), miscellaneous reasons (n = 10), or lost to follow-up (n = 39).
Comparison Groups
Trauma Controls
General trauma patients (n = 132) without TBI were enrolled in the Patient Characteristics Study.10 Of these, 131 subjects completed the 1-month evaluation, with 1 subject not completing the Sickness Impact Profile (SIP). One-hundred and twenty-four completed the 1-year follow-up evaluation. Eight subjects were lost to follow-up. For the purposes of this study, the term “trauma controls” refers to these general-trauma patients who did not have cranial trauma.
Trauma-free Controls
A cohort of noninjured controls (n = 102) were enrolled in the Behavioral Outcome Study.11 These subjects were selected from the friends of the TBI patients, using methods from Pocock and Simon15 and Taves.16 There is evidence to suggest that TBI subjects differ from the general population, not only in basic demographic characteristics (eg, age, sex) but also in psychosocial characteristics, such as willingness to take risks, personal relationships, and vocational and psychological background.17–20 These latter characteristics are often either too poorly defined or too difficult to ascertain for matching purposes. Therefore, preinjury friends were used as trauma-free controls based on the assumption that one usually chooses friends similar to oneself. In this manner, we sought to match these groups both demographically and psychosocially. These trauma-free controls were recruited around the time of the 1-month examination for the TBI subjects, rather than during the course of the 1-year follow-up, to ensure a sample as comparable as possible with the pretrauma status of the TBI subjects.
One-hundred and one subjects completed the 1-month evaluation, with 1 subject not completing the SIP. Eighty-eight completed the 1-year follow-up evaluation and 14 subjects were lost to follow-up.
Measures
Independent variables included age, sex, educational status, and TBI severity identified by time to follow simple commands (TFC), operationally defined as the time from injury to a consistent score of 6 on the motor scale of the Glasgow Coma Scale.21 The major dependent variable included items from the SIP—a detailed measure of heath-related quality of life.22 The SIP, a self-administered questionnaire, evaluates dysfunction as a result of health or injury in 12 areas of living. Sleepiness was ascertained by extracting the following 4 items from the “Sleep and Rest” scale of this instrument: (1) I am sleeping or dozing most of the time—day or night, (2) I sit around half-asleep, (3) I sleep or nap more during the day, and (4) I sleep longer during the night. These items were completed 1 month and 1 year after injury. Subjects were instructed to endorse these items only if they considered them to be related to their current health or injury. We defined sleepiness severity by number of endorsed items, giving a range of 0 to 4. Change in sleepiness was evaluated by difference scores (1 month – 1 year) for trauma controls and TBI subjects. Change in sleepiness was only considered in subjects filling out the SIP at both time points who endorsed at least 1 sleepiness item at 1 month.
Data Analysis
The first set of analyses compared sleepiness at 1 month and 1 year after injury among TBI subjects, trauma controls, and trauma-free controls to determine whether TBI subjects differed from controls. The second set of analyses compared TBI-severity groups with trauma controls on sleepiness at 1 month and 1 year after injury. Brain injury severity groups were formed by dividing the TBI subjects into the following 4 groups based on how long it took them to consistently follow simple commands after injury, as defined by the motor component of the Glasgow Coma Scale23: 24 hours or less, 1 to 6 days, 7 to 13 days, 14 days or longer. The final set of analyses examined change in sleepiness from 1 month to 1 year between TBI severity groups and trauma controls for subjects who had 1 or more sleepiness items endorsed at 1 month. Analyses were performed with analysis of variance. Because the outcomes were not normally distributed, we also performed Kruskal-Wallis distribution-free analyses of variance, which yielded almost identical results. Significant results were subjected to posthoc subgroup comparisons using Tukey's Honestly Significant Difference test. All analyses were carried out using SPSS version 11.5 (SPSS, Inc. Chicago, Ill).
RESULTS
Demographics
Demographics appear in Table 1. Most subjects were young high-school-educated men. No differences existed between the TBI subjects and controls in regards to sex or education level. Age was significantly different (p < .001) among the groups, with trauma-free controls being younger than trauma controls and TBI subjects. However, there was no difference in age between the trauma controls and the TBI group. Most TBI subjects (78%) participating at 1 month were in the ≤ 24-hour TFC group, suggesting milder injuries; of those participating at 1 year, 66% followed commands within a day.
Table 1.
Demographic Characteristics of Traumatic Brain Injured Subjects, Trauma Controls, and Trauma-Free Controls
| Characteristic | Traumatic Brain Injury | Trauma Controls | Trauma-Free Controls |
|---|---|---|---|
| Number | 514 | 132 | 102 |
| Age, ya,b | 30.0 ± 14.0 | 31.4 ± 13.5 | 24.5 ± 8.1 |
| Men, % | 73 | 72 | 64 |
| Education, yb | 12.1 ± 2.4 | 11.9 ± 2.5 | 12.4 ± 1.7 |
p < .001
Data are presented as mean ± SD.
Sleepiness 1 Month and 1 Year Following Traumatic Injury
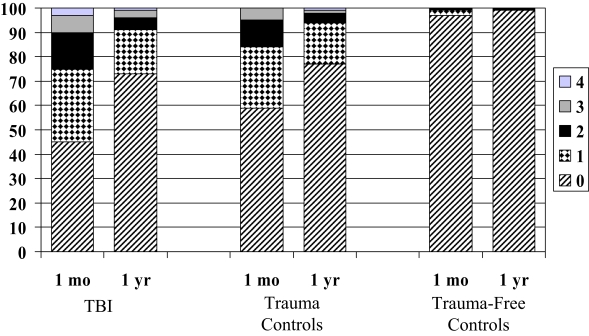
One month following traumatic injury, 55% of TBI subjects endorsed 1 or more sleepiness items, as opposed to 41% of trauma controls and 3% of trauma-free controls (p < .001; Figure 1). Posthoc analysis revealed that the TBI subjects were sleepier than both the trauma controls (p < .02) and trauma-free controls (p < .001). Trauma controls were also significantly sleepier than trauma-free controls (p < .001). A greater percentage of subjects with TBI endorsed each of the 4 sleepiness items than did both the trauma controls and trauma-free controls (Table 2).
Figure 1.
Percentage of traumatic brain injury (TBI) subjects, trauma controls, and trauma-free controls endorsing between 0 and 4 of the following sleepiness items (I am sleeping or dozing most of the time —day or night, I sit around half-asleep, I sleep or nap more during the day, and I sleep longer during the night.) at 1 month and 1 year following injury (p < .001 for comparison among all 3 groups at 1 month and 1 year).
Table 2.
Endorsement of Sleepiness at 1 Month and 1 Year by Group.
| Sickness Impact Profile Sleepiness Items | FC (102) |
TC (132) |
TBI (514) |
≤ 24 hours TFC |
1–6 days TFC |
7–13 days TFC |
≥ 14 days TFC |
|---|---|---|---|---|---|---|---|
| 1 Month | |||||||
| (n) | (101) | (131) | (346) | (272) | (45) | (19) | (9) |
| Number of sleepiness items endorsed [mean (SD)] | 0.04 (0.24) | 0.63 (0.90)ABC | 0.91 (1.05) | 0.80 (0.96)D | 1.2 (1.2)A | 1.5 (1.4)BD | 1.8 (1.6)CD |
| I am sleeping or dozing most of the time-day or night (%) | 0 | 17 | 19 | 16 | 20 | 40 | 56 |
| I sit around half asleep (%) | 1 | 10 | 13 | 12 | 13 | 25 | 33 |
| I sleep or nap more during the day (%) | 2 | 25 | 36 | 34 | 49 | 40 | 33 |
| I sleep longer during the night (%) | 1 | 12 | 22 | 18 | 33 | 37 | 56 |
| 1 Year | |||||||
| (n) | (88) | (124) | (410) | (269) | (50) | (33) | (58) |
| Number of sleepiness items endorsed [mean (SD)] | 0.02 (0.21) | 0.31 (0.66)A | 0.40 (0.77) | 0.30 (0.68)B | 0.44 (0.93) | 0.55 (0.90) | 0.69 (0.84)AB |
| I am sleeping or dozing most of the time-day or night (%) | 0 | 6 | 6 | 4 | 10 | 15 | 5 |
| I sit around half asleep (%) | 0 | 2 | 6 | 4 | 14 | 9 | 9 |
| I sleep or nap more during the day (%) | 1 | 14 | 14 | 10 | 14 | 21 | 28 |
| I sleep longer during the night. (%) | 1 | 10 | 13 | 12 | 6 | 9 | 28 |
FC=trauma-free controls, TC= trauma controls, TBI=traumatic brain injury. Time to follow commands (TFC) groups are subgroups of TBI subjects. Significant post-hoc comparisons between TC and TFC groups and between individual TFC groups are indicated by the same letter (p <0.05). For the number of sleepiness items endorsed, groups with means marked by the same letter at each time point are significantly different using Tukey's post hoc test.
One year following traumatic injury, 27% of TBI subjects endorsed 1 or more sleepiness items, as opposed to 23% of trauma controls and 1% of trauma-free controls (p < .001; Figure 1). The posthoc analysis revealed that the TBI subjects remained sleepier than the trauma-free controls (p < .001) but not the trauma controls. The trauma controls were also sleepier than the trauma-free controls (p < .05).
Sleepiness as a Function of Brain-Injury Severity
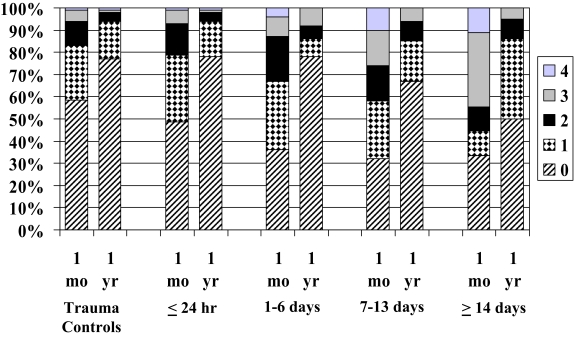
Patients with TBI, when divided into groups based on TFC, were sleepier than trauma-control patients 1 month after injury (p < .001; Figure 2). Posthoc analysis revealed that the 1- to 6-day (p < .05), 7- to 13-day (p < .01) and 14-day or longer (p < .01) TFC groups were sleepier than the trauma-control group. In addition, the ≤ 24-hour group was significantly less sleepy then the 7- to 13-day and the 14-day or longer groups (each p < .05). Brain-injured patients, when divided into TFC groups, were sleepier than trauma-control patients 1 year after injury as well (p < .01; Figure 2). Posthoc analysis revealed that the 14-day or longer group was sleepier than both the trauma controls (p < .05) and the ≤ 24-hour TFC group (p < .01).
Figure 2.
Percentage of subjects endorsing between 0 and 4 of the following sleepiness items (I am sleeping or dozing most of the time—day or night, I sit around half-asleep, I sleep or nap more during the day, and I sleep longer during the night.) at 1 month and 1 year following injury. Trauma controls are compared with subjects with traumatic brain injury divided into injury-severity subgroups (≤ 24 hour, 1–6 days, 7–13 days, ≥ 14 days), based on time from injury until commands were consistently followed (p < .001 at 1 month; p < .01 at 1 year).
Recovery of Sleepiness From 1 Month to 1 Year
Sleepiness decreased in every subject in the 14-day or longer TFC group. Sleepiness reductions were vigorous in the other TFC groups as well. Reductions were observed in 92% of the 7- to 13-day group, 91% of the 1- to 6-day group, and 84% of the ≤ 24-hour group. Posthoc tests did not identify any differences in individual subgroups. Although trauma controls also demonstrated a robust 78% reduction in sleepiness, this was significantly less then in the brain-injured TFC groups (p < .01).
DISCUSSION
This prospective cohort study demonstrated that sleepiness is more common following traumatic brain injury 1 month after injury than following non-cranial traumatic injuries and in traumafree controls. Sleepiness was present in about half of TBI subjects at this time. Injury severity also affected sleepiness endorsement, with more severely injured TBI patients endorsing greater sleepiness than trauma controls and the ≤ 24-hour TFC group at 1 month and at 1 year. The TBI cohort demonstrated the greatest improvement over the course of a year, suggesting increased resiliency in central nervous system factors involved in postinjury sleepiness. Despite improvement, sleepiness continued to be reported in about a quarter of TBI and trauma-control cases a year following their injury.
At 1 year, the TBI group continued to be sleepier than the trauma-free controls, but no overall difference could be demonstrated between the TBI group and the trauma controls. There are 2 explanations for this finding. First, differences emerged when the TBI subjects were divided into TFC groups. As TFC increased, especially beyond 1 or 2 weeks, endorsement of sleepiness increased. However, the majority of cases in this study suffered mild brain injuries, following commands within 24 hours of their trauma. Second, postinjury cognitive impairment prevented more than 90% of subjects in the most severe TBI subgroup from taking the SIP at 1 month. Almost half could not take it at 1 year. Especially at 1 year, those with more severe brain injuries endorsed greater sleepiness. These factors may have mitigated differences in sleepiness at 1 year between the TBI group and trauma controls causing an underestimation of the true extent of sleepiness in the TBI group.
Comparing posttraumatic sleepiness rates between studies is difficult for many reasons. The definition of sleepiness, which may be subjective or objective, is often inconsistent among studies. The time from injury until sleepiness assessment can vary, and measures of TBI severity are often unique. Also, different studies contain patients with variable TBI severity. Despite these limitations, our results are similar to those of other reports of subjective and objective sleepiness following TBI. Parcell et al reported subjective sleepiness, defined as an Epworth Sleepiness Scale score greater than 9, in 19% of TBI subjects.9 Masel et al reported a 47% sleepiness rate, as indicated by a mean sleep latency of 10 minutes or less on multiple sleep latency testing.6 When defined by subjective endorsement of 1 or more sleepiness items, we found evidence of sleepiness in 55% of TBI subjects at 1 month and 27% of subjects at 1 year. Our study also expands knowledge of post-TBI sleepiness in 3 important ways. First, our study population was community based, which makes it more applicable to posttraumatic sleepiness in the general population. Second, we followed patients prospectively for 1 year, allowing us to comment on improvement rates in sleepiness following TBI. Third, we divided TBI subjects by injury severity, allowing observation of the effect of injury severity on sleepiness.
Our study evaluated the presence of sleepiness following TBI but did not reveal causation. Post-TBI pain and sleep-onset and sleep-maintenance insomnia can disrupt sleep, causing sleepiness in this population.24 Injury to the posterolateral hypothalamus provides a potential physiologic explanation for postinjury sleepiness. Hypocretin-1 (orexin A) is an excitatory hypothalamic neuropeptide associated with sleep-wake cycle regulation known to be reduced in patients with narcolepsy.25 Compared with controls, hypocretin-1 levels have been shown to be abnormally lower in 95% of patients with acute moderate to severe TBI.26 Thus, trauma-induced reductions in hypocretin-1 levels could have caused the sleepiness observed in our TBI subjects, an issue deserving of further study in our population.
Previous studies have demonstrated increased rates of sleep disordered breathing and periodic limb movement disorder in TBI patients, as compared with the general population.4,6 Other studies have described narcolepsy following brain injury.8,27 This association suggests that these sleep disorders are either an epiphenomenon of TBI or they were present prior to the trauma, resulting in sleepiness and putting subjects at risk for being involved in a traumatic injury.28 Both scenarios likely contribute to sleepiness following trauma.
Because of limited medication data in 2 of the 3 studies from which subjects were enrolled, we were unable to control for sedating-medication exposure in the analysis. However, a subanalysis of 119 phenytoin-free subjects from the Phenytoin Prophylaxis Study revealed similar results to our overall 1-month and 1-year analyses, including our change-in-sleepiness analysis. The TFC subgroup analysis was also unchanged at 1 month, and our 1-year TFC analysis continued to reveal increased sleepiness in the longer-TFC groups, as compared with the trauma controls, although this was no longer significantly different (due to reduced power from decreasing TBI subject number by 77% in the subanalysis). Regarding sedating pain-medication exposure, we believe this issue is minimal, as we are not aware of consistent differences in prescribing practices for pain in TBI versus trauma to other parts of the body. For these reasons, we feel lack of control for sedating medicines did not substantively affect our results. Another study limitation is that, due to selection criteria, participants in the Phenytoin Prophylaxis and Behavioral Outcome Studies were not completely representative of typical hospitalized patients with TBI who could take the SIP at each time point. The Patient Characteristics Study, on the other hand, was representative of hospitalized brain-injured survivors, since it did not exclude subjects based on injury severity or preexisting conditions. Therefore, we performed weighted analyses of the number of sleepiness items endorsed, downweighting overrepresented groups and more heavily weighting those in the Patient Characteristics Study, in order to have the analysis mimic what would be seen if all cases were unselected. The results of the weighted analysis were very similar to those of the unweighted analysis presented, with neither analysis consistently showing more effects of TBI on sleepiness. Lastly, we were concerned that the relationship between traumatic injury and sleepiness between groups might be confounded by injury severity. We performed an adjusted analysis controlling for injury severity using a modified Injury Severity Score.29 Because the adjusted and unadjusted analysis did not differ substantively, we present only the unadjusted analysis.
Another study limitation was the subjective nature of our sleepiness data, necessitated by the large number of subjects which, although a strength of our study, made objective testing with polysomnography and multiple sleep latency testing impractical. In addition, we used sleep items from the SIP to quantify subjective sleepiness. Although the SIP is a validated instrument assessing health-related quality of life, the sleep subsection has not been separately validated. This limits the comparability of our results with those obtained with traditional validated subjective sleepiness instruments such as the Epworth or Stanford Sleepiness Scales. Furthermore, subjective assessments of sleepiness do not necessarily consistently correlate with objective measures.30,31
Posttraumatic hypersomnia (hypersomnia due to medical condition) describes sleepiness following TBI not explained by some other medical or neurologic disorder and remains a diagnosis of exclusion.32 Results of previous studies in TBI subjects suggest that this diagnosis comprises the largest proportion (30%) of sleepy patients following brain injury.6 In addition to sleepiness, patients with posttraumatic hypersomnia may experience fatigue, headaches, and cognitive impairment. Mechanistically, this disorder may result from any trauma to the central nervous system, including direct blows and neurosurgical manipulation. From an anatomic perspective, in addition to the posterolateral hypothalamus, sleepiness is more likely to occur following trauma to specific areas of the brain such as the third ventricle, reticular activating system, midbrain, or pons.4,5,33
In conclusion, sleepiness is common early following even mild trauma, with brain injury conferring greater risk than trauma to other parts of the body. Injury severity plays a role in the level of sleepiness experienced by the TBI patient, with more-severe injuries resulting in greater sleepiness. Although patients can be counseled that most will improve over the course of a year, significant sleepiness may persist in up to a quarter of patients 1 year after injury.
ACKNOWLEDGMENTS
This work was performed at the University of Washington and Harborview Medical Center.
Grant support: Supported by grants R01 NS19463 from NINNESTDS, and HS04146 and HS05304 from AHCPR.
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Watson, Dikmen, Machamer, Doherty, and Temkin have indicated no financial conflicts of interest.
REFERENCES
- 1.Binder S, Corrigan JD, Langlois JA. The public health approach to traumatic brain injury: an overview of CDC's research and programs. J Head Trauma Rehabil. 2005;20:189–95. doi: 10.1097/00001199-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44:11–7. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- 3.Dikmen SS, Ross BL, Machamer JE, Temkin NR. One year psychosocial outcome in head injury. J Int Neuropsychol Soc. 1995;1:67–77. doi: 10.1017/s1355617700000126. [DOI] [PubMed] [Google Scholar]
- 4.Guilleminault C, Faull KF, Miles L, van den Hoed J. Posttraumatic excessive daytime sleepiness: a review of 20 patients. Neurology. 1983;33:1584–9. doi: 10.1212/wnl.33.12.1584. [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C, Yuen KM, Gulevich MG, et al. Hypersomnia after head-neck trauma: a medicolegal dilemma. Neurology. 2000;54:653–9. doi: 10.1212/wnl.54.3.653. [DOI] [PubMed] [Google Scholar]
- 6.Masel BE, Scheibel RS, Kimbark T, Kuna ST. Excessive daytime sleepiness in adults with brain injuries. Arch Phys Med Rehabil. 2001;82:1526–32. doi: 10.1053/apmr.2001.26093. [DOI] [PubMed] [Google Scholar]
- 7.LaChapelle DL, Finlayson MA. An evaluation of subjective and objective measures of fatigue in patients with brain injury and healthy controls. Brain Inj. 1998;12:649–59. doi: 10.1080/026990598122214. [DOI] [PubMed] [Google Scholar]
- 8.Castriotta RJ, Lai JM. Sleep disorders associated with traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1403–6. doi: 10.1053/apmr.2001.26081. [DOI] [PubMed] [Google Scholar]
- 9.Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after traumatic brain injury. Arch Phys Med Rehabil. 2006;87:278–85. doi: 10.1016/j.apmr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Dacey R, Dikmen SS, Temkin NR, et al. Relative effects of brain and non-brain injuries on neuropsychological and psychosocial outcome. J Trauma. 1991;31:217–22. [PubMed] [Google Scholar]
- 11.Dikmen SS, McLean A, Jr, Temkin NR, Wyler AR. Neuropsychologic outcome at one-month postinjury. Arch Phys Med Rehabil. 1986;67:507–13. [PubMed] [Google Scholar]
- 12.Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, doubleblind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 13.Dikmen SS, Temkin NR, Miller B, Machamer J, Winn HR. Neurobehavioral effects of phenytoin prophylaxis of posttraumatic seizures. Jama. 1991;265:1271–7. [PubMed] [Google Scholar]
- 14.Dikmen SS, Temkin NR, Machamer JE, et al. Employment following traumatic head injuries. Arch Neurol. 1994;51:177–86. doi: 10.1001/archneur.1994.00540140087018. [DOI] [PubMed] [Google Scholar]
- 15.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 16.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–5. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 17.Fahy TJ, Irving MH, Millac P. Severe head injuries. A six-year follow-up. Lancet. 1967;2:475–9. doi: 10.1016/s0140-6736(67)91650-9. [DOI] [PubMed] [Google Scholar]
- 18.Weddell R, Oddy M, Jenkins D. Social adjustment after rehabilitation: a two year follow-up of patients with severe head injury. Psychol Med. 1980;10:257–63. doi: 10.1017/s0033291700044019. [DOI] [PubMed] [Google Scholar]
- 19.Jennett B. Prognosis after severe head injury. Clin Neurosurg. 1971:200–7. doi: 10.1093/neurosurgery/19.cn_suppl_1.200. [DOI] [PubMed] [Google Scholar]
- 20.Jameison K. Proc Internat Symp Head Injuries. Edinburgh: Churchill Livingstone; 1971. Prevention of head injury; pp. 12–15. [Google Scholar]
- 21.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 22.Bergner M, Bobbitt RA, Pollard WE, Martin DP, Gilson BS. The sickness impact profile: validation of a health status measure. Med Care. 1976;14:57–67. doi: 10.1097/00005650-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 24.Beetar JT, Guilmette TJ, Sparadeo FR. Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch Phys Med Rehabil. 1996;77:1298–302. doi: 10.1016/s0003-9993(96)90196-3. [DOI] [PubMed] [Google Scholar]
- 25.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 26.Baumann CR, Stocker R, Imhof HG, et al. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–9. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- 27.Lankford DA, Wellman JJ, O'Hara C. Posttraumatic narcolepsy in mild to moderate closed head injury. Sleep. 1994;17:S25–8. doi: 10.1093/sleep/17.suppl_8.s25. [DOI] [PubMed] [Google Scholar]
- 28.Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17:84–93. doi: 10.1093/sleep/17.1.84. [DOI] [PubMed] [Google Scholar]
- 29.Civil ID, Schwab CW. The Abbreviated Injury Scale, 1985 revision: a condensed chart for clinical use. J Trauma. 1988;28:87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Olson LG, Cole MF, Ambrogetti A. Correlations among Epworth Sleepiness Scale scores, multiple sleep latency tests and psychological symptoms. J Sleep Res. 1998;7:248–53. doi: 10.1046/j.1365-2869.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 31.Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology. 1999;52:125–31. doi: 10.1212/wnl.52.1.125. [DOI] [PubMed] [Google Scholar]
- 32.International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 33.Evans BM. What does brain damage tell us about the mechanisms of sleep? J R Soc Med. 2002;95:591–7. doi: 10.1258/jrsm.95.12.591. [DOI] [PMC free article] [PubMed] [Google Scholar]