Abstract
Obstructive sleep apnea (OSA) is a common disorder and is associated with adverse cardiovascular consequences, including hypertension and coronary artery disease. While the mechanisms responsible for increased risk of cardiovascular events in OSA have not yet been fully elucidated, hypoxia, inflammation, obesity, metabolic dysregulation, and sympathetic activation, may contribute to these consequences. Endothelial dysfunction may be another link between OSA and cardiovascular disease. Dysfunctional endothelium is characterized by an imbalance in production of vasoactive hormones, increased adherence of inflammatory mediators to endothelial cells and hypercoagulability, and is a known risk factor for cardiovascular events. Studies have directly measured vascular endothelial function in patients with OSA and found a muted response compared to controls. Other studies have evaluated biochemical markers of endothelial function including circulating levels of vasoactive and thrombosis mediators and provide further proof of endothelial dysfunction in this disorder. A better appreciation of the role of the dysfunctional endothelium in OSA will help shed light on the pathogenesis of cardiovascular disease in this disorder and may lead to development of novel therapies aimed at preventing untoward outcomes.
Citation:
Budhiraja R; Parthasarathy S; Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med 2007;3(4):409-415.
Keywords: Endothelial dysfunction, OSA, cardiovascular disease, hypertension, endothelium
INTRODUCTION
Obstructive sleep apnea (OSA) is a widely prevalent disorder characterized by recurrent partial or complete obstruction of upper airway during sleep. Compelling data from several large cross-sectional and longitudinal studies strongly suggest a role for OSA in the development of cardiovascular disorders, including hypertension, coronary artery disease, and stroke.1,2 However, the mechanistic paradigm whereby OSA may lead to cardiovascular pathology is yet to be fully elucidated. One mechanism may involve OSA initiating and/ or propagating vascular endothelial dysfunction through diverse pathways such as hypoxemia, reactive oxygen species (ROS) production, and sympathetic activation. Endothelial dysfunction may lead to vasoconstriction, vascular smooth muscle proliferation, hypercoagulability, thrombosis, and eventually, to adverse cardiovascular events.3 The data continue to accrue suggesting an improvement in endothelial dysfunction with therapy for OSA.
THE NORMAL VASCULAR ENDOTHELIUM
Endothelium is a dynamic tissue layer which constitutes a source and/or target of multiple growth factors and vasoactive mediators involved in regulating the physical and biochemical properties of the systemic vessels, as well as vascular contractility and cell growth.4 The endothelium is a not a homogeneous tissue. Its diversity includes anatomic variability in shape, size, and thickness, and functional heterogeneity, such as magnitude of nitric oxide-dependent dilation in different vascular sites. This diversity partially accounts for site-specific manifestations of different disorders such as retinopathy in diabetes and hepatic sinusoid involvement in veno-occlusive disease.5 However, control of vascular tone, maintenance of homeostasis, and angiogenesis, apart from provision of a selectively permeable barrier between blood and tissues, are the predominant actions of the endothelial layer at most sites.5
ENDOTHELIAL DYSFUNCTION IN OSA
Several studies have suggested impaired endothelial function in patients with OSA.6,7 The sources of the endothelial injury are still not clear, but potential etiologies include hypoxemia with ROS generation and systemic inflammation. OSA is also associated with obesity, hypertension, and metabolic dysregulation, which themselves may contribute to adverse effects on endothelium. Endothelial injury results in alteration of endothelial hormones that are responsible for maintaining vascular tone and preventing abnormal cell proliferation, increased coagulability and altered leukocyte trafficking; and exposes subendothelial structures to diverse growth factors in the blood.4 The resultant vasoconstriction, vascular smooth muscle proliferation, and hypercoagulability may lead to adverse cardiovascular consequences associated with OSA, such as hypertension, coronary artery disease, and cerebrovascular disease.1,8 Treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) therapy has been suggested to improve endothelial function in the systemic circulation.9
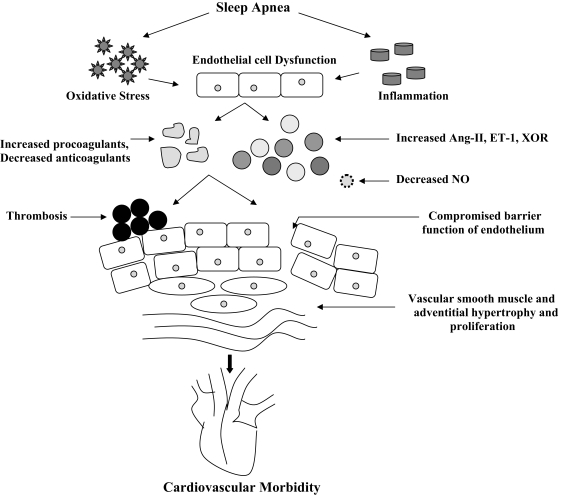
Figure 1.
Possible mechanistic paradigm whereby endothelial dysfunction may constitute an etiological link between obstructive sleep apnea and its cardiovascular consequences. Ang-II= Angiotensin II, ET-1= Endothelin-1, XOR= Xanthine Oxidoreducatse, NO= Nitric Oxide
Measurement of Endothelial Dysfunction in OSA
The assessment of endothelial function in OSA has included functional evaluation of vascular responses by assessing changes in blood flow in response to endothelium-dependent vasodilators or hypoxemia, quantification of levels of circulating apoptotic endothelial cells, and plasma indices of diverse endothelial biomarkers including myriad vasoactive, inflammatory, and homeostatic mediators.
Some studies have utilized intra-arterial infusion of endothelium-dependent vasodilators such as acetylcholine and sodium nitroprusside to assess microvascular arterial endothelial function at baseline in OSA patients, as well as in response to CPAP therapy.9,10 Acetylcholine induces endothelium-dependent dilation via endothelial muscarinic membrane receptor-mediated stimulation of nitric oxide synthase. In the presence of endothelial damage, however, acetylcholine may promote smooth muscle-mediated vasoconstriction.11 Measurement of brachial artery pressure, flow, and resistance responses to intra-arterial infusions can thus provide a measure of endothelial integrity. Plethysmography was used to measure forearm blood flow in these studies.9,10
Other studies aimed at detecting endothelial dysfunction have utilized high-resolution ultrasonographic measurements of flow-mediated dilation of the brachial artery.6,12 Flow-mediated dilation refers to nitric oxide-mediated vasodilatation resulting from shear-mediated activation of endothelial nitric oxide synthase in response an acute increase in blood flow.13
One study utilized cerebral blood flow response to hypoxia to assess endothelial function in OSA patients. In healthy humans, the cerebral vasculature responds to hypoxia by vasodilatation, a process mediated by endothelium-dependent release of nitric oxide. A recent study found a muted cerebrovascular blood flow response to hypoxia in patients with OSA compared to controls, and normalization of the response with 4–6 weeks of CPAP therapy.14
Determination of levels of circulating endothelial cells (CECs) is a relatively novel technique which provides a direct marker of endothelial damage. Circulating endothelial cells are increased in myocardial injury15 and atherosclerotic peripheral vascular disease.16 An in-vitro study suggested that in acute coronary syndromes, the extent of endothelial apoptosis correlates with the extent of coronary disease.17 Based on the above data, a recent study investigated the levels of circulating apoptotic endothelial cells in subjects with OSA and found that the levels were increased compared to non-OSA subjects, correlated with abnormal vasorelaxation, and attenuated with CPAP therapy.18
Finally, a multitude of studies have evaluated the levels of inflammatory mediators, markers of oxidative stress, vasoactive mediators, and markers of coagulability as the cause or consequence of endothelial dysfunction. These are described in detail below.
Oxidative Stress and Endothelium
OSA is a condition of increased oxidative stress.19 Free radical production from neutrophils and monocytes is augmented in OSA patients and attenuates with CPAP therapy.20,21 Enhanced free radical production may also result from hypoxia-reoxygenation or from sympathetic activation.22 Furthermore, lipid peroxidation is greater in patients with OSA resulting in production of reactive oxygen species (ROS).23 Oxidant stress can, in turn, lead to endothelial injury, and consequently, atherosclerosis.24,25 Additionally, reactive oxygen species can upregulate the production of adhesion molecules in the endothelium, diminish nitric oxide synthase activity, and promote nitric oxide breakdown.26
Endothelium and Inflammation
OSA is associated with increased levels of inflammatory mediators as well as upregulation of the expression of adhesion molecules in the vascular endothelium. The circulating levels of intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin are elevated in patients with OSA.27,28 A central role for cell adhesion molecules has been suggested in the development of disorders such as atherosclerosis.29–31 Adhesion of circulating leukocytes to the endothelial cells is an important step in this process.32 Monocytes derived from OSA patients demonstrate increased adherence to vascular endothelial cells in vitro in comparison to those derived from control subjects.21,33 Migration of leukocytes beneath the endothelium following adherence can lead to formation of early atherosclerotic lesions.31 CPAP therapy for OSA attenuates expression of adhesion molecules on monocytes and ROS production by CD11+ monocytes and decreases monocyte adherence to human endothelial cells.21
Another inflammatory cytokine, interleukin 6 (IL-6), is known to play a pivotal role in atherogenesis.34 A recent study revealed increase in the levels of soluble IL-6 receptors in persons with sleep apnea.35 The IL-6 and its receptor can induce chemokine and leukocyte recruitment36 and contribute to atherogenesis.
C-reactive protein (CRP), a marker and a contributor to the vascular inflammatory process, is also increased in patients with OSA,37,38 and the levels decrease with CPAP therapy.39 Studies suggest an inverse association between CRP levels and endothelial function.40
While the sentinel event that triggers the inflammatory cascade is not clear, hypoxia may provide the initial insult, mediated by the production of ROS derived from hypoxia-reoxygenation. Moreover, local inflammation resulting from the recurrent collapse of upper airway can lead to systemic inflammation. This is suggested by a recent study that showed overexpression of IL-8 in human bronchial epithelial cells in response to a vibratory stimulus.41
Endothelial Dysfunction and Vasoactive Mediators
The endothelium is a source of several vasoactive mediators. A balance between these mediators, including vasoconstrictive factors such as the renin-angiotensin-aldosterone system, endothelin-1, and thromboxane; and vasorelaxant factors such as nitric oxide and prostacyclin; is thought to mediate normal vascular tone, homeostasis, and vascular injury repair and growth.42 An alteration in this balance can change vascular milieu and the architectural and tensile properties of the vasculature, promoting vasoconstriction and impeding endothelium-dependent vasorelaxation.
Nitric Oxide.
Flow-mediated dilation of peripheral arteries depends on nitric oxide release from endothelial cells and is a widely accepted marker of vascular endothelial function, including that in coronary arteries.43,44 Data from a large population-based epidemiologic study, the Sleep Heart Health Study, have demonstrated impaired brachial artery flow-mediated dilation in persons with OSA.7 There was a stronger association between brachial reactivity and hypoxemia rather than AHI, suggesting that hypoxemic stress may be a pivotal factor contributing to endothelial dysfunction. Further evidence for this hypothesis is provided by a recent study that demonstrated an improvement in flow mediated dilation in patients with OSA after intravenous injection of Vitamin C, an antioxidant and free radical scavenger.12 Notably, endothelium dependent vasodilatation is impaired in these patients, even in the absence of hypertension or other illnesses including overt cardiovascular disease, suggesting that OSA is an independent risk factor for endothelial dysfunction.10 Furthermore, the levels of circulating nitric oxide, determined by measuring serum nitrites and nitrates, derivatives of nitric oxide, are decreased in OSA subjects compared with controls, and revert promptly to normal levels with CPAP therapy.33,45 Conversely, plasma concentrations of asymmetric NG, NG-dimethylarginine, an inhibitor of endothelial nitric oxide synthase, are increased in OSA and decrease with CPAP therapy.46
Endothelin-1.
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide that is ubiquitous in human vascular endothelial cells and has mitogenic properties.47 One study found an increase in both plasma ET-1 and blood pressure in rats exposed to intermittent hypoxia/hypercapnia, as might be seen with sleep apnea.48 The human studies assessing ET-1 levels in OSA have yielded conflicting results. Several studies report that patients with OSA have higher systemic levels of the ET-1 than their healthy counterparts49–51 and the levels decrease with CPAP therapy.51 Such an increase in levels of this peptide may play a role in the genesis of hypertension in OSA. However, some other studies have failed to find an association between OSA and ET-1 elevation.52,53 Notably, a majority of patients and controls in one of these studies had history of hypertension and cardiovascular disorders, suggesting the possibility of endothelial dysfunction in both groups, and hence, no significant difference in the ET-1 levels between the 2 groups.52 Yet another study found elevations of plasma big ET-1 (a precursor of ET-1) levels in untreated OSA patients, which attenuated with long-term CPAP therapy.54 In contrast, plasma ET-1 concentrations were within the physiological range in these patients. Finally, a recent study found elevated endothelin-1 levels in moderate or severe OSA, but not in mild OSA.55
Renin-Angiotensin System.
The renin-angiotensin system causes vasoconstriction, endothelial damage, and cell growth, especially via the angiotensin I (AT1) receptor. Endothelium-derived nitric oxide regulates renin release in vivo.56 The endothelium also mediates vascular angiotensin formation by taking up renin.57 Angiotensin II induces expression of ET-1 in endothelial cells.58 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II antagonists improve endothelial function in patients with hypertension.59 Activation of renin-angiotensin by recurrent hypoxia may contribute to elevation in blood pressure in OSA patients. Indeed, Fletcher et al demonstrated an increase in mean arterial pressure in rats exposed to intermittent hypoxia akin to that seen in OSA and attenuation of this response by using an AT1 receptor inhibitor.60 Higher plasma levels of aldosterone and angiotensin II have been reported in patients with OSA.53
Leptin.
While obesity is an important risk factor for OSA, OSA itself may be associated with an increased propensity to gain weight.61 Altered circulating leptin levels or leptin insensitivity have been proposed to underlie this phenomenon.61 Indeed, leptin levels are higher in patients with OSA and decrease with CPAP therapy.62,63 Leptin, in turn, is associated with endothelial dysfunction and cardiovascular disorders.64,65 In support of the link between leptin and atherogenesis is the finding that the ob/ob homozygote mouse, which lacks the functioning leptin ob gene, is resistant to atherosclerosis despite being grossly obese.66 Furthermore, higher leptin levels have been demonstrated to be associated with impaired arterial distensibility in humans.67 Leptin receptors are present on endothelial cells,68 suggesting that the endothelium may be a target for leptin. These receptors may play a role in leptin actions such as angiogenesis69 and vascular smooth muscle proliferation.70 Leptin also induces ROS production in human endothelial cells,71 which again can be important in atherogenesis.
Xanthine Oxidoreductase.
Xanthine oxidoreductase (XOR) is a complex molybdoflavoenzyme that catalyzes the hydroxylation of hypoxanthine to xanthine and of xanthine to uric acid.72 Reduction of molecular oxygen by XOR yields superoxide and hydrogen peroxide, which can contribute to endothelial injury.73 Circulating plasma xanthine oxidase causes vascular dysfunction and has been implicated in ischemia-reperfusion injury.74 Furthermore, increased serum levels of uric acid constitute a risk factor for cardiovascular disease.75 Hypoxia, a common feature of OSA, increases XOR levels in endothelial cells.76 While XOR levels have not been studied in patients with OSA, the levels of uric acid, final product of the purine metabolism, are increased in OSA and decrease with CPAP.77
Endothelial Dysfunction and Hypercoagulability
The diverse factors secreted by the normal endothelium—including nitric oxide and prostacyclin which decrease platelet aggregation, thrombomodulin which promotes activated protein C generation, and heparin sulfates which serve as cofactors of antithrombin III—help maintain normal fluidity of the blood.78,79 Endothelial dysfunction may lead to homeostasis alterations resulting in a procoagulant and atherogenic state. The loss of the endothelial “barrier” function exposes the subendothelial structures of the vessel wall to circulating growth factors and mediators of cell proliferation. The vascular collagen can bind to von Willebrand factor, leading to platelet activation and aggregation, and thence, thrombus formation.80,81 Indeed, patients with OSA have been reported to have increased platelet aggregation.82 The blood levels of procoagulant tissue factor, constitutively released by adventitial layer of blood vessels, increase with endothelial damage, and further propagate plasma coagulation.83
Other studies confirm the presence of a hypercoagulable state in OSA.84 Levels of coagulation factors XIIa, VIIa, and thrombinantithrombin complex are elevated in OSA.85 Plasma fibrinogen levels and type I plasminogen activator inhibitor (PAI-1) activity are also increased.86–89 CPAP treatment is associated with a decrease in fibrinogen levels and PAI-1 activity.89,90
P-selectin is stored in platelets and the Weibel-Palade bodies of endothelial cells and mediates the interaction of endothelium with leukocytes and platelets. P-selectin glycoprotein ligand-1 expressed on leukocytes and platelets binds to P-selectin present on endothelial cells and promotes attachment and rolling, a primary step in initiation of atherosclerosis.91 Elevated levels of soluble P-selectin are associated with increased risk of future cardiovascular events.92 A recent study found P-selectin upregulation in a rat model of OSA.93 P-selectin is also increased in patients with OSA,85 and the levels correlate with severity of OSA.94
Genetics
A genetic predisposition may confer an increased risk for development of endothelial dysfunction, or the consequences thereof, in some patients with OSA. The TNF-α (−308A) allele, a polymorphism in TNF-α gene responsible for overproduction of TNF-α, is more prevalent in subjects with OSA than normal controls.95 Overproduction of TNF-α can result in endothelial dysfunction.96,97 An insertion/deletion (I/D, intron 16) polymorphism of the angiotensin-converting enzyme (ACE) gene modulates the circulating ACE levels, D allele being associated with higher plasma ACE activity.98 One study suggested that ACE-D may interact with OSA and increase the risk of hypertension in patients with mild to moderate sleep apnea.99 ACE is a primary enzyme responsible for conversion of angiotensin I to angiotensin II. Angiotensin II is increased in OSA53 and can jeopardize endothelial function, as described earlier. Angiotensin II may also lead to overexpression of VEGF mRNA through AT-1 receptors, resulting in overproduction of vascular endothelial growth factor.100 Vascular endothelial growth factor is a potent angiogenic cytokine that can contribute to progression of atherosclerosis.101
CONCLUSION
Recent research provides strong evidence for endothelial dysfunction in obstructive sleep apnea. The resultant vasoconstriction, abnormal cell proliferation and hypercoagulability may contribute to the genesis or progression of atherosclerotic cardiovascular and cerebrovascular disorders, which are frequently encountered in OSA patients. While the currently available therapies for OSA, such as CPAP therapy, ameliorate endothelial dysfunction, they are cumbersome and have suboptimal patient acceptance or adherence. An improved understanding of the role of dysfunctional endothelium in promoting the adverse consequences of OSA has the potential to stimulate development of adjunct therapies, medical or genetic, that specifically improve endothelial function, in an effort to alleviate the associated cardiovascular morbidity.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Quan has received research support from Respironics and honoraria from Takeda. Drs. Budhiraja and Parthasarathy have indicated no financial conflicts of interest.
REFERENCES
- 1.Budhiraja R, Quan SF. Sleep-disordered breathing and cardiovascular health. Curr Opin Pulm Med. 2005;11:501–506. doi: 10.1097/01.mcp.0000183058.52924.70. [DOI] [PubMed] [Google Scholar]
- 2.Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109:951–957. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 3.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 4.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 5.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 6.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–360. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 8.Budhiraja R, Quan SF. Sleep-disordered breathing and Hypertension. J Clin Sleep Med. 2005;1:401–404. [PubMed] [Google Scholar]
- 9.Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 11.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 12.Grebe M, Eisele HJ, Weissmann N, et al. Antioxidant Vitamin C Improves Endothelial Function in Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 13.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 14.Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Effects of CPAP on Cerebral Vascular Response to Hypoxia in Obstructive Sleep Apnea Patients. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200609-1271OC. [DOI] [PubMed] [Google Scholar]
- 15.Mutin M, Canavy I, Blann A, Bory M, Sampol J, Dignat-George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood. 1999;93:2951–2958. [PubMed] [Google Scholar]
- 16.Makin AJ, Blann AD, Chung NA, Silverman SH, Lip GY. Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells. Relationship with von Willebrand factor and tissue factor. Eur Heart J. 2004;25:371–376. doi: 10.1016/j.ehj.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Valgimigli M, Agnoletti L, Curello S, et al. Serum from patients with acute coronary syndromes displays a proapoptotic effect on human endothelial cells: a possible link to pan-coronary syndromes. Circulation. 2003;107:264–270. doi: 10.1161/01.cir.0000045665.57256.86. [DOI] [PubMed] [Google Scholar]
- 18.El Solh AA, Akinnusi ME, Baddoura FH, Mankowski CR. Endothelial Cell Apoptosis in Obstructive Sleep Apnea: A Link to Endothelial Dysfunction. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200611-1598OC. [DOI] [PubMed] [Google Scholar]
- 19.Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 20.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 21.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 22.Zhang GX, Kimura S, Nishiyama A, et al. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–238. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–128. [PubMed] [Google Scholar]
- 24.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 25.Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003;79:195–199. doi: 10.1136/pmj.79.930.195. quiz 198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ohga E, Nagase T, Tomita T, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87:10–14. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- 28.El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121:1541–1547. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 29.Jang Y, Lincoff AM, Plow EF, Topol EJ. Cell adhesion molecules in coronary artery disease. J Am Coll Cardiol. 1994;24:1591–1601. doi: 10.1016/0735-1097(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 30.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 32.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 33.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is sup-pressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 34.Luc G, Bard JM, Juhan-Vague I, et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 35.Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166:1725–1731. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- 36.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 37.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 38.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–1984. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 39.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 40.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 41.Puig F, Rico F, Almendros I, Montserrat JM, Navajas D, Farre R. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammation. Sleep. 2005;28:1312–1316. doi: 10.1093/sleep/28.10.1312. [DOI] [PubMed] [Google Scholar]
- 42.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 43.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 44.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 45.Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax. 2000;55:1046–1051. doi: 10.1136/thorax.55.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohike Y, Kozaki K, Iijima K, et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure—possible involvement of nitric oxide and asymmetric NG NG-dimethylarginine. Circ J. 2005;69:221–226. doi: 10.1253/circj.69.221. [DOI] [PubMed] [Google Scholar]
- 47.Howard PG, Plumpton C, Davenport AP. Anatomical localization and pharmacological activity of mature endothelins and their precursors in human vascular tissue. J Hypertens. 1992;10:1379–1386. doi: 10.1097/00004872-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–515. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- 49.Zamarron-Sanz C, Ricoy-Galbaldon J, Gude-Sampedro F, Riveiro-Riveiro A. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Arch Med Res. 2006;37:552–555. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Saarelainen S, Seppala E, Laasonen K, Hasan J. Circulating endothelin-1 in obstructive sleep apnea. Endothelium. 1997;5:115–118. doi: 10.3109/10623329709079869. [DOI] [PubMed] [Google Scholar]
- 51.Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–66. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 52.Grimpen F, Kanne P, Schulz E, Hagenah G, Hasenfuss G, Andreas S. Endothelin-1 plasma levels are not elevated in patients with obstructive sleep apnoea. Eur Respir J. 2000;15:320–325. doi: 10.1034/j.1399-3003.2000.15b17.x. [DOI] [PubMed] [Google Scholar]
- 53.Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–280. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 54.Jordan W, Reinbacher A, Cohrs S, et al. Obstructive sleep apnea: Plasma endothelin-1 precursor but not endothelin-1 levels are elevated and decline with nasal continuous positive airway pressure. Peptides. 2005;26:1654–1660. doi: 10.1016/j.peptides.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens. 2007;20:44–52. doi: 10.1016/j.amjhyper.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 56.Sigmon DH, Carretero OA, Beierwaltes WH. Endothelium-derived relaxing factor regulates renin release in vivo. Am J Physiol. 1992;263:F256–261. doi: 10.1152/ajprenal.1992.263.2.F256. [DOI] [PubMed] [Google Scholar]
- 57.Hilgers KF, Veelken R, Muller DN, et al. Renin uptake by the endothelium mediates vascular angiotensin formation. Hypertension. 2001;38:243–248. doi: 10.1161/01.hyp.38.2.243. [DOI] [PubMed] [Google Scholar]
- 58.Imai T, Hirata Y, Emori T, Yanagisawa M, Masaki T, Marumo F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension. 1992;19:753–757. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- 59.Yavuz D, Koc M, Toprak A, et al. Effects of ACE inhibition and AT1-receptor antagonism on endothelial function and insulin sensitivity in essential hypertensive patients. J Renin Angiotensin Aldosterone Syst. 2003;4:197–203. doi: 10.3317/jraas.2003.032. [DOI] [PubMed] [Google Scholar]
- 60.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol. 2002;92:627–633. doi: 10.1152/japplphysiol.00152.2001. [DOI] [PubMed] [Google Scholar]
- 61.Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17:1297–1300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 62.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 63.Harsch IA, Konturek PC, Koebnick C, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 64.Luo JD, Zhang GS, Chen MS. Leptin and cardiovascular diseases. Drug News Perspect. 2005;18:427–431. doi: 10.1358/dnp.2005.18.7.939346. [DOI] [PubMed] [Google Scholar]
- 65.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 66.Schafer K, Halle M, Goeschen C, et al. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler Thromb Vasc Biol. 2004;24:112–117. doi: 10.1161/01.ATV.0000105904.02142.e7. [DOI] [PubMed] [Google Scholar]
- 67.Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 68.Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 69.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 70.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J Med Sci. 2001;47:141–150. [PubMed] [Google Scholar]
- 71.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–1238. [PubMed] [Google Scholar]
- 72.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 73.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 74.White CR, Darley-Usmar V, Berrington WR, et al. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci U S A. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 76.Budhiraja R, Kayyali US, Karamsetty M, et al. Estrogen modulates xanthine dehydrogenase/xanthine oxidase activity by a receptor-independent mechanism. Antioxid Redox Signal. 2003;5:705–711. doi: 10.1089/152308603770380007. [DOI] [PubMed] [Google Scholar]
- 77.Braghiroli A, Sacco C, Erbetta M, Ruga V, Dormer CF. Overnight urinary uric acid: creatinine ratio for detection of sleep hypoxemia. Validation study in chronic obstructive pulmonary disease and obstructive sleep apnea before and after treatment with nasal continuous positive airway pressure. Am Rev Respir Dis. 1993;148:173–178. doi: 10.1164/ajrccm/148.1.173. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg RD, Aird WC. Vascular-bed—specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–1564. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]
- 79.Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981;78:2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruf A, Morgenstern E. Ultrastructural aspects of platelet adhesion on subendothelial structures. Semin Thromb Hemost. 1995;21:119–122. doi: 10.1055/s-2007-1000385. [DOI] [PubMed] [Google Scholar]
- 81.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 82.Bokinsky G, Miller M, Ault K, Husband P, Mitchell J. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure. A preliminary investigation. Chest. 1995;108:625–630. doi: 10.1378/chest.108.3.625. [DOI] [PubMed] [Google Scholar]
- 83.Hayashi M, Fujimoto K, Urushibata K, Takamizawa A, Kinoshita O, Kubo K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology. 2006;11:24–31. doi: 10.1111/j.1440-1843.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 84.von Kanel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–1967. doi: 10.1378/chest.124.5.1956. [DOI] [PubMed] [Google Scholar]
- 85.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004;59:777–782. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wessendorf TE, Thilmann AF, Wang YM, Schreiber A, Konietzko N, Teschler H. Fibrinogen levels and obstructive sleep apnea in ischemic stroke. Am J Respir Crit Care Med. 2000;162:2039–2042. doi: 10.1164/ajrccm.162.6.2001048. [DOI] [PubMed] [Google Scholar]
- 87.Nobili L, Schiavi G, Bozano E, De Carli F, Ferrillo F, Nobili F. Morning increase of whole blood viscosity in obstructive sleep apnea syndrome. Clin Hemorheol Microcirc. 2000;22:21–27. [PubMed] [Google Scholar]
- 88.Rangemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18:188–194. doi: 10.1093/sleep/18.3.188. [DOI] [PubMed] [Google Scholar]
- 89.von Kanel R, Loredo JS, Ancoli-Israel S, Dimsdale JE. Association between sleep apnea severity and blood coagulability: Treatment effects of nasal continuous positive airway pressure. Sleep Breath. 2006;10:139–146. doi: 10.1007/s11325-006-0060-3. [DOI] [PubMed] [Google Scholar]
- 90.Chin K, Ohi M, Kita H, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–1976. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- 91.Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–495. doi: 10.1161/01.cir.103.4.491. [DOI] [PubMed] [Google Scholar]
- 93.Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155:93–96. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 94.O'Brien LM, Serpero LD, Tauman R, Gozal D. Plasma adhesion molecules in children with sleep-disordered breathing. Chest. 2006;129:947–953. doi: 10.1378/chest.129.4.947. [DOI] [PubMed] [Google Scholar]
- 95.Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26:673–678. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 96.Gao X, Belmadani S, Picchi A, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 97.Picchi A, Gao X, Belmadani S, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 98.Murphey LJ, Gainer JV, Vaughan DE, Brown NJ. Angiotensin-converting enzyme insertion/deletion polymorphism modulates the human in vivo metabolism of bradykinin. Circulation. 2000;102:829–832. doi: 10.1161/01.cir.102.8.829. [DOI] [PubMed] [Google Scholar]
- 99.Lin L, Finn L, Zhang J, Young T, Mignot E. Angiotensin-converting enzyme, sleep-disordered breathing, and hypertension. Am J Respir Crit Care Med. 2004;170:1349–1353. doi: 10.1164/rccm.200405-616OC. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi S, Nakamura Y, Nishijima T, Sakurai S, Inoue H. Essential roles of angiotensin II in vascular endothelial growth factor expression in sleep apnea syndrome. Respir Med. 2005;99:1125–1131. doi: 10.1016/j.rmed.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 101.Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:2108–2116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]