Abstract
Objective:
To evaluate efficacy and safety of ramelteon (MT1/MT2-receptor agonist) in subjects with chronic primary insomnia.
Methods:
Randomized, multicenter, double-blind, placebo-controlled trial of nightly ramelteon treatment (8 mg or 16 mg) in adults (N=405) with primary chronic insomnia (DSM-IV-TR). Latency to persistent sleep (LPS), TST, sleep efficiency, wake time after sleep onset, and number of awakenings were measured by polysomnography. Subject-reported measures were also assessed.
Results:
LPS at Week 1 (primary measure) was significantly shorter with ramelteon 8 mg (32.2 min) or 16 mg (28.9 min) vs placebo (47.9 min; p <0.001). Significant improvements in LPS were maintained at Weeks 3 and 5. TST was significantly longer with both doses of ramelteon at Week 1 (p <0.001) vs placebo. Subject-reported sleep latency was significantly shorter with ramelteon 8 mg at Weeks 1, 3, and 5 (p <0.001) and ramelteon 16 mg at Weeks 1 and 3 (p ≤0.050) vs placebo. Wake time after sleep onset and number of awakenings were not significantly different with ramelteon 8 mg or 16 mg treatment vs placebo. Subjective TST was significantly longer with ramelteon 8 mg at Weeks 1, 3, and 5 (p ≤0.050) and ramelteon 16 mg at Week 1 (p = 0.003) vs placebo. Ramelteon had no clinically meaningful effect on sleep architecture, next-morning psychomotor tasks, alertness, or ability to concentrate. No withdrawal or rebound effects were observed.
Conclusions:
Ramelteon reduced LPS over 5 weeks of treatment in subjects with chronic insomnia, with no clinically meaningful sleep architecture alterations, next-morning residual pharmacologic effects, and no evidence of rebound insomnia or withdrawal. No numerical differences were observed between the 2 doses of ramelteon.
Citation:
Zammit G; Erman M; Wang-Weigand S; Sainati S; Zhang J; Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med 2007;3(5):495-504.
Keywords: MT1/MT2-receptor agonist, melatonin, sleep latency
INTRODUCTION
Insomnia is a common condition that negatively affects day-time functioning and quality of life. Chronic insomnia, lasting months to years, is estimated to occur in about 10% of the general population1 and 19% of clinical populations.2
Available sedative-hypnotics indicated for insomnia include the benzodiazepine receptor agonists. These agents act at gamma-aminobutyric acid (GABA)-A–benzodiazepine receptor complexes, which are widely distributed throughout the brain. GABAergic activation results in hypnotic effects, and increases the potential for secondary side effects, including memory and psychomotor impairment.3–7 Benzodiazepine receptor agonists also have the potential for abuse and dependency,8,9 which has led to their classification by the Drug Enforcement Administration as Schedule IV controlled substances. The availability of alternative therapies for the treatment of chronic insomnia would be beneficial for patients and physicians.
Melatonin, an important component of the sleep-wake cycle, has been considered for the therapy of sleep disorders.10,11 Melatonin MT1-receptor mRNA has been detected in the suprachiasmatic nucleus (SCN), and studies indicate that this receptor mediates the acute inhibition of SCN firing by melatonin.12 Melatonin MT2-receptor mRNA has also been detected in the SCN, and activity at this receptor has been associated with the phase-shifting effects of melatonin on circadian rhythms.13–15 However, the clinical efficacy of exogenous melatonin for the treatment of insomnia is still controversial, due to the lack of standardized preparations and controlled clinical trials.16–19
Ramelteon is a melatonin receptor agonist currently marketed in the United States for the treatment of insomnia. Ramelteon's mechanism of action is based on a high selectivity for MT1 and MT2 receptors. Compared with melatonin, ramelteon has approximately 3- to 5-fold greater affinity for human MT1 and MT2receptors and is up to 17 times more potent at these receptors, according to in vitro studies that measured binding affinities for individual melatonin receptor subtypes and the relative functional activities on forskolin-induced cAMP production.20
Ramelteon showed negligible affinity for the MT3 binding site, according to in vitro studies of hamster brain.20 This melatonin binding site has recently been characterized as a melatonin-sensitive form of quinone reductase 2,21,22 and it is not likely to be involved in the sleep-wake cycle. Ramelteon also showed no significant ligand binding (>50% inhibition) to various CNS receptors or transporters tested at 10 μM and had no impact on the activity of various enzymes tested at 10–1000 μM.20 Ramelteon's negligible affinity to GABA, serotonin, acetylcholine, glutamate, noradrenaline, opioid, histamine, and dopamine receptors is noteworthy, as ancillary activity at these receptors may result in unwanted secondary or residual effects. Ramelteon has a half-life of 1 to 2.6 hours, undergoes a rapid, high first-pass metabolism with peak serum concentrations at less than 1 hour, and shows no evidence of accumulation after multiple dosing.23,24 The unique properties of ramelteon suggest that it may be a promising alternative to currently available sedative-hypnotics as a treatment for insomnia.
In a previous trial of subjects with chronic insomnia, 2-night treatment with ramelteon (4 mg to 32 mg) significantly reduced latency to persistent sleep (LPS) (defined as the first epoch of the first consecutive 30-s epochs not scored as awake) and improved total sleep time (TST) and sleep efficiency, as measured by polysomnography (PSG), with no evidence of next-morning residual effects on psychomotor or memory function.25 The present study was designed to assess these effects over a 5-week period in a larger number of subjects with chronic primary insomnia.
METHODS
Experimental Design
This randomized, double-blind, placebo-controlled, parallel-group study was conducted at 29 sleep laboratories. The study involved medical and sleep screening, a 7-day single-blind screening period, a 5-week double-blind treatment period, and a 2-day single-blind run-out period. During double-blind treatment, subjects and all investigators involved with patient contact or data analysis were blinded to treatment assignment. During single-blind periods, subjects received placebo in a blinded manner, but investigators were aware of drug characteristics. The randomization schedule was generated and kept secure by the study sponsor, Takeda Global Research and Development Center. The study protocol, informed consent forms, and all recruitment materials were approved by the institutional review board for each site. The study was conducted according to the protocol, applicable Food and Drug Administration laws and regulations, the World Medical Association Declaration of Helsinki (1989), and the International Conference for Harmonisation (ICH) Harmonised Tripartite Guideline for Good Clinical Practice.
The primary measure of efficacy was mean LPS assessed by PSG during Week 1 (Nights 1 and 2) of double-blind treatment. LPS was also assessed on Week 3 (Nights 15 and 16) and Week 5 (Nights 29 and 30). LPS was defined as the elapsed time from the beginning of PSG recording to the onset of the first 10 minutes of continuous sleep (i.e., total number of epochs before the first 20 consecutive nonwake epochs, divided by 2). Secondary measures assessed by PSG included TST, sleep efficiency (TST divided by time spent in bed multiplied by 100), wake time after sleep onset (WASO), and number of awakenings after persistent sleep. Subject-reported measures of sleep, including sleep latency (sSL), total sleep time (sTST), awake time, and sleep quality, were assessed by post-sleep questionnaire and sleep diary.
Subject Eligibility and Screening
Subjects eligible for study inclusion were men or nonpregnant, nonlactating women aged 18 to 64 years with a diagnosis of primary insomnia (as defined by the Diagnostic and Statistical Manual for Mental Disorders-IV [DSM-IV-TR]) present at the time of evaluation for at least 3 months. Eligible subjects reported an sSL of at least 30 minutes, an sTST of less than 6.5 hours, and daytime complaints associated with their disturbed sleep.
Subjects were excluded from the study if they had participated in any previous studies of ramelteon, had taken any other investigational drug within 30 days, or if they had sleep schedule changes associated with shift work or had taken a flight across more than 3 time zones in the 7 days preceding the initial screening. Medications or supplements known to affect sleep-wake function must not have been taken within 5 days or 5 half-lives of the start of the study. Subjects with a history of sleep apnea, chronic obstructive pulmonary disease, seizures, anxiety, depression, schizophrenia, bipolar disorder, mental retardation, a cognitive disorder, or significant neurological, hepatic, renal, endocrine, cardiovascular, gastrointestinal, pulmonary, hematologic, or metabolic diseases (unless controlled with protocol-allowed medications) were also excluded. Subjects were excluded if they had a history of drug addiction or abuse within 12 months of the study. At screening, subjects were excluded if they had an apnea-hypopnea index >10 or a periodic leg movement arousal index >10. Other exclusion criteria were applied to ensure that treatment did not present undue safety concerns, that a subject's condition would not interfere with drug absorption or metabolism, and that a subject's condition would not confound the analysis or interpretation of the data.
After initial screening, eligible subjects entered a PSG screening period during which they received single-blind placebo treatment. For the first 2 consecutive nights of the screening period, subjects were monitored by PSG in the sleep center, commencing at bedtime and lasting for 8 hours. Subjects were eligible to continue in the double-blind treatment phase if they had a mean LPS ≥20 minutes on the 2 nights of PSG monitoring, with an LPS of no less than 15 minutes on either night. They were also required to exhibit a mean wake time ≥60 minutes per night during the 2 nights of monitoring, with no less than 45 minutes of wake time on either night.
Study Procedures
During the double-blind treatment phase, subjects were instructed to arrive at the sleep center 2 hours prior to their habitual bedtime. They received either ramelteon tablets or an identical-looking placebo 30 minutes before bedtime and start of PSG recording. After 8 hours of continuous PSG recording, subjects were awakened and completed the post-sleep questionnaire to assess subjective level of alertness and ability to concentrate. Upon waking (within 30 to 60 minutes), residual pharmacologic effects were assessed using the Digit Symbol Substitution Test (DSST) and memory recall tests, and patient mood and feeling were evaluated using visual analog scales (VAS) for mood and feelings. In the sleep center, vital signs, clinical laboratory tests, physical examination (Night 15 only), breathalyzer test, and urine drug screen were performed.
On the nights spent at home, subjects were instructed to take 1 tablet each evening at bedtime and to complete a sleep diary the next day. Subjects were instructed to record in their sleep diary each dose of study medication that was taken and were required to bring their medication bottles to each center visit for monitoring of treatment compliance.
The possible occurrence of rebound insomnia and withdrawal effects was assessed during a single-blind placebo run-out period. Rebound insomnia was assessed by the change from baseline in LPS measured by PSG on each of the 2 nights of the placebo run-out period (Nights 36 and 37). Withdrawal effects were assessed by the change in Tyrer Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ) score from the Week 5 visit and the mean of the 2 nights of the placebo run-out period. The BWSQ is a self-reported questionnaire designed to evaluate the symptoms experienced during benzodiazepine withdrawal. The questionnaire consists of 20 items, of which 10 are related to disturbances of perception and sensation, 7 are related to somatic symptoms, and 3 are independent items: depressed mood, loss of control of voluntary movement, and memory loss.26
Residual pharmacologic effects were assessed by VAS for mood and feelings, DSST, memory tests, and items on the post-sleep questionnaire. The VAS for mood consisted of 12 items: drowsy, slowed down, sleepy, sedated, tired, worn out, listless, fatigued, exhausted, sluggish, weary, and bushed. For each mood item, subjects graded their subjective states using a scale of 0 (a little) to 100 (a lot). The VAS for feelings included 8 items: calm/anxious, energetic/fatigued, thinking slowed down/thinking speeded up, peaceful/tense, normal/spacey, at ease/nervous, relaxed/excited, and normal/easily irritated. For each feeling item, subjects graded their feelings using the same scale of 0 mm to 100 mm (e.g., for calm/anxious, a lower score indicated “more calm” and a higher score indicated “more anxious”). The VAS scales are validated tests used to assess subjective mood and feelings and have been used in many sleep studies.27–30
A standard DSST was used, adapted from the Wechsler Adult Intelligence Scale.31 For this test, subjects were given a set of symbols with corresponding single-digit numbers and blank boxes with corresponding digits. They were asked to complete as many symbol-for-digit substitutions as possible in 90 seconds.
For the memory recall test, subjects were read a list of several words to remember and write down (immediate recall). The next morning, they were asked to recall as many words as possible (delayed recall) from the day before. On subsequent tests, they were read a new list of words and asked to write down as many as possible.
The post-sleep questionnaire was designed to assess subjective level of alertness and ability to concentrate using a 7-point Likert scale with 1=excellent and 7=extremely poor. This questionnaire was also used to estimate their sleep the night before.
Statistical Analyses
Statistical analyses were performed using SAS version 8.2. All subjects who received at least 1 dose of study medication were included in the intent-to-treat analysis. Analyses of efficacy (including LPS, TST, sSL, sTST), sleep architecture (including latency to REM sleep and percentages of TST in REM, stage 1, stage 2, stage 3/4 sleep), and measurements of alertness and ability to concentrate were based on last observation carried forward data. Analyses of DSST, memory recall tests, VAS, rebound insomnia, and withdrawal effects were based on observed data only.
The primary measure of efficacy was the mean Week 1 LPS. LPS was defined as the elapsed time from the beginning of the PSG recording to the onset of the first 10 minutes of continuous sleep. The planned sample size was 130 subjects per group (total 390) calculated to provide 90% power to detect an average difference of 12 minutes in LPS between the ramelteon and placebo groups. The calculation assumed a standard deviation in LPS of 25 minutes, 15% missing observations, use of a 2-sided paired t-test with a Bonferroni correction for 2 comparisons, and a significance level of 0.05. Comparisons between the ramelteon groups and placebo were performed using t-tests with least-squares (LS) means and standard errors (SE) obtained from an analysis of covariance (ANCOVA) model: parameter = baseline + treatment + center. The Mixed Model Procedure was applied, with treatment and pooled center as fixed effects and the baseline value of the variable as a covariate. Type III sum of squares was used to generate the ANCOVA results.
The efficacy of ramelteon was assessed using a stepwise procedure, Fisher's protected least significant difference (LSD), to control the Type I error. The primary time point for the efficacy analysis was Week 1. The average of non missing observations from Week 1 was analyzed. Maintenance of efficacy was assessed at Week 3 and Week 5 with a closed sequential testing procedure. That is, testing a time point was contingent on the significance of the F test for overall treatment effect at the previous time point at the 0.050 level.
Important secondary efficacy variables were analyzed with a continuation of the stepwise testing procedure defined for the primary efficacy variable, LPS, and application of Fisher's LSD testing procedure. Analysis of TST at Weeks 1, 3, and 5 was contingent upon observing significance from the F test of treatment comparisons with at least 2 of the 3 time points in the analysis of LPS at Week 1. If the overall F test of TST at Week 1 was significant, the analysis of TST was performed by sequentially performing the treatment comparisons at Weeks 1, 3, and 5. If treatment comparisons were significant with at least 2 of these time points, then the analysis of subjective sleep quality was performed at Weeks 1, 3, and 5.
Residual pharmacologic effects were analyzed using results from the DSST, memory recall tests, VAS for mood, VAS for feelings, and subjective levels of alertness and ability to concentrate. The VAS scores were analyzed as individual items. The ANCOVA model used for the analysis was: mean morning score = treatment + center + Day 1 evening score. Note that the Day 1 evening score was the last measurement before the first dose of double-blind study medication.
Rebound insomnia was assessed by analyzing the change from baseline in LPS on each of the first 2 nights of the single-blind placebo run-out period. Subjective level of alertness, subjective ability to concentrate, rebound insomnia, and withdrawal effects were analyzed using an ANCOVA with their baseline value as the covariate. Results from tests of residual effects are presented using observed values only (i.e., values were not carried forward to replace missing data).
RESULTS
A total of 1078 subjects entered the screening period. Of these, 405 subjects met eligibility criteria and were randomized to a treatment sequence, 371 subjects completed the double-blind treatment, and 367 subjects completed the study. Overall, the reasons for discontinuation during the double-blind treatment period were adverse event (n=6), lack of efficacy (n=2), protocol deviation (n=6), withdrawal of consent (n=18), lost to follow-up (n=1), and other (n=1; subject was withdrawn because of noncompliance). During the single-blind placebo run-out period, 4 subjects discontinued treatment due to adverse event (n=1), withdrawal of consent (n=l), protocol deviation (n=1), and other (n=1; subject missed Visit 5 due to work-related travel). Demographic characteristics of randomized subjects are presented in Table 1. Significant differences were found among the groups for weight, height, and gender. There were no significant differences among groups in baseline sleep characteristics, as measured by PSG during the single-blind screening period.
Table 1.
Demographic Characteristics
| Placebo | Ramelteon 8 mg | Ramelteon 16 mg | P Value | |
|---|---|---|---|---|
| Sex | ||||
| (men/women) | 30/101 | 57/82 | 46/89 | 0.007 |
| Age (yrs) | 39.7 (12.0) | 38.0 (11.5) | 40.2 (12.4) | 0.226 |
| Race | 0.971 | |||
| Caucasian | 79 | 87 | 82 | |
| Hispanic | 27 | 27 | 27 | |
| Black | 21 | 19 | 23 | |
| Other | 4 | 6 | 3 | |
| Weight (kg) | 71.2 (14.9) | 75.9 (15.0) | 72.1 (12.4) | 0.006 |
| Height (cm) | 166.4 (9.1) | 170.2 (10.3) | 168.1 (9.2) | 0.005 |
| BMI (kg/m2) | 25.6 (4.4) | 26.1 (3.6) | 25.5 (3.8) | 0.309 |
Note: Values represent means and standard deviations.
Efficacy
Objective Assessment
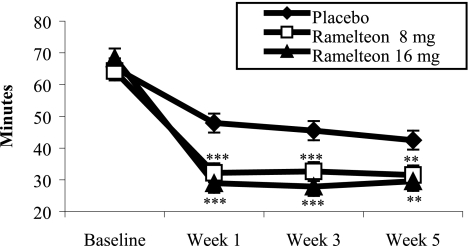
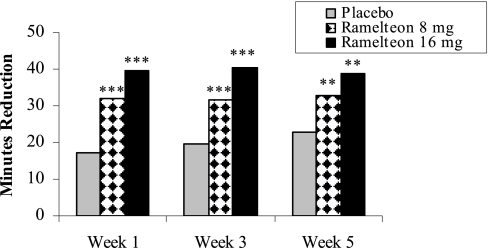
Table 2 summarizes the sleep results measured with PSG. Subjects who received either dose of ramelteon (8 mg or 16 mg) experienced statistically significant improvements in LPS compared to subjects receiving placebo at Week 1 (p ≤0.001). Both doses of ramelteon sustained shorter LPS at Week 3 (p ≤0.001) and Week 5 (p ≤0.01) (Figure 1). Change from baseline analyses revealed that LPS was reduced by approximately 32 to 40 minutes with ramelteon administration and 17 to 23 minutes with placebo (Figure 2).
Table 2.
Polysomnographic Results
| Placebo | Ramelteon 8 mg* | Ramelteon 16 mg* | |
|---|---|---|---|
| LPS (min) | |||
| Baseline | 65.3 (3.54) | 64.3 (3.46) | 68.4 (3.54) |
| Week 1 | 47.9 (2.72) | 32.2 (2.67) | 28.9 (2.71) |
| (p <0.001) | (p <0.001) | ||
| Week 3 | 45.5 (2.93) | 32.6 (2.87) | 27.9 (2.92) |
| (p = 0.001) | (p <0.001) | ||
| Week 5 | 42.5 (2.97) | 31.5 (2.91) | 29.5 (2.96) |
| (p = 0.007) | (p = 0.002) | ||
| Run-out | 43.6 (3.39) | 38.9 (3.35) | 39.3 (3.31) |
| TST (min) | |||
| Baseline | 344.1 (4.60) | 350.1 (4.50) | 349.0 (4.59) |
| Week 1 | 375.2 (4.02) | 394.2 (3.94) | 397.6 (4.01) |
| (p <0.001) | (p <0.001) | ||
| Week 3 | 382.0 (4.30) | 387.3 (4.22) | 393.8 (4.29) |
| (p = 0.370) | (p = 0.047) | ||
| Week 5 | 385.9 (4.12) | 391.5 (4.04) | 393.3 (4.11) |
| Run-out | 384.1 (4.43) | 386.5 (4.37) | 387.5 (4.34) |
| Sleep efficiency (%) | |||
| Baseline | 71.7 (0.96) | 73.0 (0.94) | 72.7 (0.96) |
| Week 1 | 78.3 (0.83) | 82.3 (0.81) | 83.4 (0.83) |
| (p <0.001) | (p <0.001) | ||
| Week 3 | 79.7 (0.89) | 80.9 (0.87) | 82.1 (0.89) |
| Week 5 | 80.4 (0.86) | 81.8 (0.84) | 82.0 (0.86) |
| Run-out | 80.3 (0.91) | 80.5 (0.90) | 80.7 (0.89) |
| WASO (min) | |||
| Baseline | 75.8 (3.42) | 71.7 (3.35) | 69.4 (3.42) |
| Week 1 | 60.4 (2.94) | 58.0 (2.88) | 55.4 (2.93) |
| Week 3 | 56.8 (3.27) | 62.6 (3.21) | 61.6 (3.26) |
| Week 5 | 56.4 (3.11) | 59.9 (3.04) | 61.1 (3.10) |
| Run-out | 53.3 (3.30) | 58.0 (3.25) | 57.2 (3.23) |
Note: Values represent least-squares mean (with standard error). A statistically significant overall treatment effect was found for LPS at Week 1 (p <0.001), Week 3 (p <0.001), Week 5 (p = 0.003), and for TST at Week 1 (p <0.001).
p values for ramelteon vs placebo.
Figure 1.
PSG-determined latency to persistent sleep: ramelteon vs placebo treatments. Least-squares mean LPS at Weeks 1, 3, and 5 of double-blind treatment. For comparisons of ramelteon dose and placebo: ***p ≤0.001; **p ≤0.010.
Figure 2.
Change from baseline latency to persistent sleep. Least-squares mean change from baseline for PSG LPS at Weeks 1, 3, and 5 of double-blind treatment. For comparisons of ramelteon dose and placebo: ***p ≤0.001; **p ≤0.010.
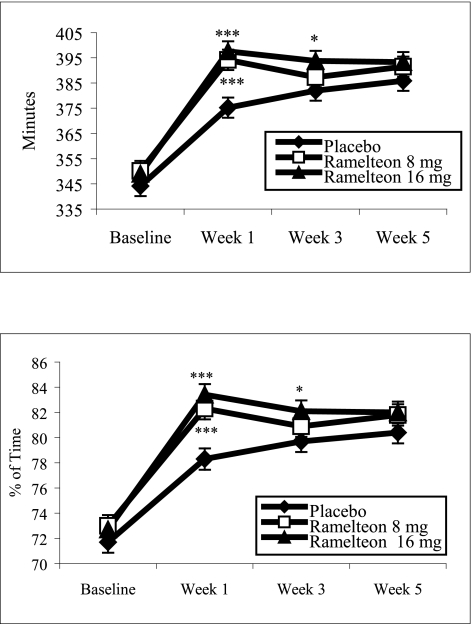
TST and sleep efficiency showed statistically significant increases with both doses of ramelteon compared with placebo at Week 1 (p ≤0.001). Subsequent nights studied by PSG also showed improvements in TST and sleep efficiency following ramelteon administration. For both TST and sleep efficiency at Week 3, the overall treatment effect p values were >0.050; however, comparison of the 16 mg dose vs placebo revealed p values ≤0.050 (Figure 3). WASO and number of awakenings after persistent sleep yielded comparable results among the treatment groups.
Figure 3.
PSG-determined total sleep time and sleep efficiency: comparison of ramelteon and placebo treatments. Least-squares mean TST and sleep efficiency at Weeks 1, 3, and 5 of double-blind treatment. Sleep efficiency was defined as the TST divided by time-in-bed, multiplied by 100. For comparisons of ramelteon dose and placebo: ***p ≤0.001; *p ≤0.050.
Subjective Assessment
Table 3 shows results from the post-sleep questionnaires completed in the sleep center. Subjects reported significantly shorter sSL with ramelteon 8 mg for Weeks 1, 3, and 5 (all with p ≤0.001) and with ramelteon 16 mg for Weeks 1 and 3 (both p ≤0.050) compared with placebo. Significantly longer sTSTs were reported with ramelteon 8 mg at Weeks 1, 3, and 5 (p ≤0.050) and with ramelteon 16 mg at Week 1 (p = 0.003) compared with placebo. Significantly lower subjective awake times were observed at Week 1 in the ramelteon 8 mg (p = 0.026) and 16 mg (p= 0.004) groups compared to placebo. No statistically significant treatment effects were observed in subjective sleep quality compared with the placebo group.
Table 3.
Subjective Results from Post-Sleep Questionnaire
| Placebo | Ramelteon 8 mg* | Ramelteon 16 mg* | |
|---|---|---|---|
| sSL (min) | |||
| Baseline | 77.0 (3.7) | 78.5 (3.7) | 75.2 (3.8) |
| Week 1 | 70.2 (3.8) | 52.9 (3.7) | 56.3 (3.9) |
| (p <0.001) | (p = 0.009) | ||
| Week 3 | 65.7 (3.9) | 47.2 (3.8) | 54.3 (3.9) |
| (p <0.001) | (p = 0.034) | ||
| Week 5 | 61.5 (3.7) | 44.8 (3.6) | 53.8 (3.8) |
| (p = 0.001) | (p = 0.134) | ||
| Run-out | 54.9 (4.7) | 53.6 (4.6) | 55.1 (4.6) |
| sTST (min) | |||
| Baseline | 311.6 (5.5) | 325.1 (5.4) | 313.2 (5.5) |
| Week 1 | 329.6 (5.5) | 353.8 (5.4) | 352.0 (5.6) |
| (p = 0.001) | (p = 0.003) | ||
| Week 3 | 340.1 (5.4) | 360.3 (5.4) | 349.7 (5.5) |
| (p = 0.006) | (p = 0.197) | ||
| Week 5 | 347.1 (5.7) | 365.4 (5.6) | 358.9 (5.7) |
| (p = 0.018) | (p = 0.132) | ||
| Run-out | 359.7 (6.4) | 363.8 (6.4) | 365.0 (6.4) |
| Sleep quality | |||
| Baseline | 4.2 (0.07) | 4.1 (0.07) | 4.3 (0.07) |
| Week 1 | 3.9 (0.05) | 3.8 (0.05) | 3.8 (0.05) |
| Week 3 | 3.7 (0.06) | 3.7 (0.06) | 3.7 (0.06) |
| Week 5 | 3.7 (0.06) | 3.6 (0.06) | 3.6 (0.06) |
| Run-out | 3.6 (0.08) | 3.6 (0.08) | 3.5 (0.08) |
| Awake time (min) | |||
| Baseline | 99.9 (5.65) | 86.3 (5.52) | 93.4 (5.69) |
| Week 1 | 86.1 (4.54) | 72.3 (4.46) | 67.8 (4.63) |
| (p = 0.026) | (p = 0.004) | ||
| Week 3 | 69.2 (4.68) | 72.2 (4.58) | 74.1 (4.70) |
| Week 5 | 71.2 (4.80) | 70.3 (4.70) | 68.0 (4.82) |
| Run-out | 64.8 (4.78) | 60.8 (4.72) | 58.6 (4.73) |
sSL = subjective sleep onset, sTST = subjective total sleep time
Values represent least-squares mean (with standard error).
p values for ramelteon vs placebo.
Sleep Architecture
Table 4 shows the percentage of time spent in the sleep stages. At Week 3, the 16 mg ramelteon group had a small, but statistically significant, difference in percentage of time spent in REM sleep compared to placebo (p = 0.010). Compared to placebo at Weeks 1, 3, and 5, both ramelteon groups demonstrated a slightly higher percentage in stage 1 sleep and a slightly higher or similar percentage in stage 2 sleep (p >0.05). Both ramelteon groups at Weeks 1, 3, and 5 experienced a statistically significant decrease in stage 3/4 sleep except at Week 5, where the comparison between ramelteon 16 mg and placebo was not statistically significant.
Table 4.
Sleep Architecture
| Placebo | Ramelteon 8 mg* | Ramelteon 16 mg* | |
|---|---|---|---|
| Percentage of time spent in stage 1 (%) | |||
| Baseline | 10.8 (0.45) | 10.4 (0.44) | 11.0 (0.45) |
| Week 1 | 10.0 (0.36) | 10.8 (0.36) | 10.4 (0.36) |
| Week 3 | 10.1 (0.33) | 10.6 (0.32) | 10.1 (0.33) |
| Week 5 | 9.6 (0.34) | 10.5 (0.34) | 10.2 (0.34) |
| Percentage of time spent in stage 2 (%) | |||
| Baseline | 59.3 (0.71) | 58.1 (0.69) | 59.2 (0.70) |
| Week 1 | 60.1 (0.47) | 60.8 (0.46) | 60.6 (0.47) |
| Week 3 | 60.2 (0.51) | 60.2 (0.50) | 60.1 (0.51) |
| Week 5 | 59.6 (0.50) | 59.9 (0.49) | 60.1 (0.50) |
| Percentage of time spent in stage 3/4 (%) | |||
| Baseline | 10.1 (0.68) | 10.1 (0.66) | 9.6 (0.68) |
| Week 1 | 9.2 (0.38) | 7.8 (0.37) | 7.6 (0.38) |
| (p = 0.005) | (p = 0.002) | ||
| Week 3 | 9.5 (0.39) | 8.3 (0.39) | 8.1 (0.39) |
| (p = 0.025) | (p = 0.012) | ||
| Week 5 | 9.5 (0.39) | 8.3 (0.38) | 8.1 (0.39) |
| (p = 0.024) | (p = 0.067) | ||
| Percentage of time spent in REM (%) | |||
| Baseline | 19.9 (0.48) | 21.4 (0.47) | 20.2 (0.48) |
| Week 1 | 20.6 (0.39) | 20.7 (0.38) | 21.4 (0.39) |
| Week 3 | 20.2 (0.40) | 20.9 (0.40) | 21.6 (0.40) |
| (p = 0.181) | (p = 0.010) | ||
| Week 5 | 21.1 (0.42) | 21.4 (0.41) | 21.3 (0.42) |
Note: Values represent least-squares mean (with standard error). For sleep quality, 1=excellent and 7=extremely poor.
p values for ramelteon vs placebo.
Next-Morning Pharmacologic Residual Effects
Next-morning residual effects of treatment were assessed using objective neurocognitive tests and subjective scales. Results for the DSST, memory recall tests, and post-sleep questionnaire assessments of alertness and ability to concentrate are shown in Table 5. Ramelteon showed no evidence of next-day psychomotor impairment, with no statistically significant differences between ramelteon and placebo on DSST performance (p >0.05 for Weeks 1, 3, and 5). Subjects in the 8 mg ramelteon group demonstrated a lower mean score compared with placebo on the immediate memory recall test at Week 3 (7.5 vs 8.2, p = 0.005) and on the delayed memory recall test at Week 1 (3.6 vs 4.2, p = 0.004). At other time points, no significant differences between ramelteon and placebo were found on the memory function tests. Subjective levels of alertness and ability to concentrate were similar between ramelteon groups and placebo, except at Week 1, where the ramelteon 8 mg group reported an improved ability to concentrate compared to the placebo group (3.7 vs 3.9, p = 0.031) and at Week 5, where the ramelteon 8 mg group reported an improved level of alertness compared to the placebo group (3.5 vs 3.7, p = 0.034).
Table 5.
Next-Day Residual Effects
| Placebo | Ramelteon 8 mg* | Ramelteon 16 mg* | |
|---|---|---|---|
| DSST | |||
| Baseline | 43.8 (1.12) | 45.2 (1.10) | 44.0 (1.12) |
| Week 1 | 43.9 (0.59) | 43.8 (0.58) | 43.5 (0.59) |
| Week 3 | 45.0 (0.67) | 44.9 (0.67) | 44.8 (0.66) |
| Week 5 | 46.1 (0.74) | 46.8 (0.75) | 46.4 (0.73) |
| Memory Recall Test (immediate) | |||
| Baseline | 7.6 (0.19) | 7.5 (0.19) | 7.5 (0.19) |
| Week 1 | 7.7 (0.17) | 7.3 (0.17) | 7.6 (0.17) |
| Week 3 | 8.2 (0.17) | 7.5 (0.17) | 8.1 (0.17) |
| (p = 0.005) | (p = 0.594) | ||
| Week 5 | 8.1 (0.18) | 7.7 (.018) | 8.3 (0.18) |
| Memory Recall Test (delayed) | |||
| Week 1 | 4.2 (0.16) | 3.6 (0.15) | 3.8 (0.16) |
| (p = 0.004) | (p = 0.057) | ||
| Week 3 | 5.5 (0.22) | 5.4 (0.22) | 5.6 (0.21) |
| Week 5 | 4.5 (0.19) | 4.1 (0.19) | 4.4 (0.19) |
| Level of Alertness | |||
| Baseline | 3.8 (0.08) | 3.8 (0.08) | 3.6 (0.08) |
| Week 1 | 3.8 (0.07) | 3.7 (0.07) | 3.9 (0.07) |
| Week 3 | 3.7 (0.07) | 3.6 (0.07) | 3.7 (0.07) |
| Week 5 | 3.7 (0.07) | 3.5 (0.07) | 3.6 (0.07) |
| (p = 0.034) | (p = 0.308) | ||
| Ability to Concentrate | |||
| Baseline | 3.7 (0.08) | 3.8 (0.08) | 3.6 (0.08) |
| Week1 | 3.9 (0.07) | 3.7 (0.07) | 3.9 (0.07) |
| (p = 0.031) | (p = 0.957) | ||
| Week 3 | 3.7 (0.07) | 3.6 (0.07) | 3.6 (0.07) |
| Week 5 | 3.7 (0.07) | 3.6 (0.07) | 3.6 (0.07) |
Note: Values represent least-squares mean (SE). For the DSST and memory recall tests, a higher score is better. For level of alertness and ability to concentrate, a lower score is better.
p values for ramelteon vs placebo.
On the VAS for feelings, there were no statistically significant differences on any item at any time point, except at Week 1, where subjects in the 8 mg ramelteon group reported feeling more “fatigued” than “energetic” (46.2 mm vs 41.5 mm, p = 0.023) compared with placebo and at Week 3, where subjects in the 8 mg ramelteon group reported feeling more “easily irritated” than “normal” (23.1 mm vs 19.7 mm, p = 0.038). On the VAS for mood, there were no statistically significant differences on any item at any time point except at Week 3 where subjects in the 8 mg ramelteon group reported feeling more “sluggish” compared to placebo (26.5 mm vs 22.2 mm; p = 0.042).
Rebound Insomnia and Withdrawal Results
There was no evidence of rebound insomnia following discontinuation of ramelteon treatment. Subjects who had received placebo during double-blind treatment continued to exhibit a reduction in LPS from baseline on Days 1 and 2 of the single-blind run-out period (mean change from baseline: −19.1 min and −29.8 min, respectively). Likewise, subjects in the ramelteon groups exhibited reductions in LPS. In the ramelteon 8 mg group, mean change from baseline on Days 1 and 2 of the run-out period were −34.7 min and −22.8 min, respectively. In the ramelteon 16 mg group, the mean change from baseline values on Days 1 and 2 of the run-out period were −29.1 min and −28.1 min, respectively. When compared with placebo, the ramelteon 8 mg group experienced a significantly greater reduction in LPS (p = 0.007) on Day 1 of the run-out period.
Withdrawal effects of ramelteon were assessed by measuring the change from Week 5 in total score on the BWSQ during the single-blind run-out period. The BWSQ scores between placebo and ramelteon 8 mg or 16 mg were comparable on either day of the run-out period, indicating no evidence of withdrawal effects of ramelteon. The mean changes in BWSQ score for the ramelteon 8 mg, ramelteon 16 mg, and placebo groups were 0.1, −0.1, and −0.2, respectively, on Day 1 of the run-out period; and −0.1, −0.2, and −0.1, respectively, on Day 2 of the run-out period. The improvement in the ramelteon 16 mg group on Day 1 was statistically significant (p = 0.047) when compared with placebo.
Safety
Table 6 lists all adverse events reported in at least 3% of subjects in any treatment group. The most common adverse event was headache, and the incidence was similar in all 3 treatment groups. Only headache, somnolence, and fatigue were reported for 5% or more of subjects in any group. Most adverse events were reported as mild or moderate. Adverse events considered to be severe were reported by 7 subjects in the placebo group, 5 subjects in the ramelteon 8 mg group, and 3 subjects in the ramelteon 16 mg group. One serious adverse event, coronary artery disease, was discovered in a patient who received placebo during the single-blind baseline period, but was unrelated to study drug.
Table 6.
Most Commonly Reported Adverse Events
| Adverse Event | Placebo | Ramelteon (8 mg) | Ramelteon (16 mg) |
|---|---|---|---|
| Any | 63 (48.1%) | 71 (51.1%) | 74 (54.8%) |
| Headache NOS | 24 (18.3%) | 27 (19.4%) | 24 (17.8%) |
| Somnolence | 2 (1.5%) | 11 (7.9%) | 10 (7.4%) |
| Fatigue | 3 (2.3%) | 13 (9.4%) | 6 (4.4%) |
| Nausea | 3 (2.3%) | 6 (4.3%) | 6 (4.4%) |
| Nasopharyngitis | 4 (3.1%) | 4 (2.9%) | 4 (3.0%) |
| Diarrhea NOS | 2 (1.5%) | 2 (1.4%) | 5 (3.7%) |
| Dizziness | 5 (3.8%) | 5 (3.6%) | 2 (1.5%) |
| Upper respiratory tract infection NOS | 4 (3.1%) | 6 (4.3%) | 1 (0.7%) |
| Nasal congestion | 1 (0.8%) | 2 (1.4%) | 4 (3.0%) |
Note: Listed are all adverse events that occurred in ≥3% of subjects in any treatment group (N [%]).
Adverse events are sorted by incidence rate based on total ramelteon (8 mg and 16 mg) events reported.
NOS = not otherwise specified.
Six subjects discontinued participation in the study because of treatment-emergent adverse events: 2 in the placebo group, 3 in the 8 mg ramelteon group, and 1 in the 16 mg ramelteon group. None of the adverse events that occurred following ramelteon treatment were considered related to study drug.
Clinical laboratory values, vital signs, and ECG parameters were monitored throughout the study. Subjects receiving ramelteon exhibited a decrease in serum uric acid levels and small decreases in serum albumin and total protein. However, these changes were small and of questionable clinical significance. Mean changes from baseline for uric acid were −3.2 and −9.0 μmol/L in ramelteon 8 mg and 16 mg groups, respectively. Mean changes from baseline for albumin were −0.5 and −0.7 g/L in the ramelteon 8 mg and 16 mg groups, respectively. Mean changes in total protein were −1.0 and −1.5 g/L in the ramelteon 8 mg and 16 mg groups, respectively. No other laboratory trends were noted. There were no consistent changes in vital signs or ECG parameters observed during the study.
DISCUSSION
In this study of adult subjects with chronic primary insomnia, both doses of ramelteon promoted significant reductions in LPS compared to placebo at all time points assessed over a 5-week nightly treatment period. The improvement in LPS with ramelteon was observed immediately on Nights 1 and 2, and this was sustained at Week 5. Results of the post-sleep questionnaires also revealed robust, statistically significant differences between ramelteon and placebo in sSL and sTST. The current findings support other studies of ramelteon that showed significant improvements in sleep.25,32–35
Compared to baseline, treatment with ramelteon resulted in approximately 32- to 40-minute (49% to 59%) reductions in LPS. These improvements in LPS are comparable to the results seen with benzodiazepine receptor agonists, such as zolpidem and eszopiclone. In a 5-week study of subjects with chronic insomnia, 10 mg and 15 mg zolpidem decreased LPS from baseline by approximately 28 minutes each at Week 3, and by 29 and 25 minutes, respectively, at Week 5.36 In a 6-week study, subjects treated with 2 mg eszopiclone had approximately 18-minute reductions in LPS from baseline at Night 15 and 23-minute reductions at Night 29; similar reductions were observed with 3 mg eszopiclone.37
In the current trial, the placebo group also exhibited improvements from baseline values in LPS, TST, and sleep efficiency over time. Such placebo effects are common in sleep studies38,39 and may be caused by several factors, particularly those related to increased attention to sleep hygiene. Total sleep time was significantly longer with ramelteon compared with placebo at Week 1; however, the difference from placebo was not statistically significant at Week 5, and this may be due to the consistent improvements seen in the placebo group.
Analysis of sleep architecture following administration of ramelteon revealed few differences compared with placebo. No consistent patterns in latency to, or percentage of time, spent in REM, stage 1, or stage 2 sleep were observed with either dose of ramelteon compared with placebo. A small but statistically significant decrease in stage 3/4 (slow-wave sleep – SWS) was observed with both dosages of ramelteon. Although the clinical implications of any decrease or increase in SWS are currently unknown, the magnitude of the difference was not considered sufficient to constitute a clinically meaningful effect.
An important finding in this study is that ramelteon showed no rebound insomnia and withdrawal effects following discontinuation of the 5-week ramelteon treatment. These results were corroborated by another 5-week study in 829 elderly subjects with chronic insomnia, in which ramelteon showed no rebound insomnia or withdrawal effects during a 7-day placebo run-out period.35
Ramelteon also had no statistically significant effect on next-morning psychomotor performance as measured by the DSST, which has been demonstrated across studies.25,33,34 The treatment differences seen on next-morning memory tasks, VAS scores, level of alertness, and ability to concentrate were not consistent and were not considered clinically meaningful. On the memory tasks, for example, the mean differences observed between ramelteon and placebo accounted for less than 1 word. On the VAS, the differences observed between ramelteon and placebo were approximately 5 mm out of a 100 mm scale. The lack of residual effects with ramelteon overall is consistent with other studies of ramelteon in healthy subjects and subjects with chronic insomnia.25,32–34
With regard to adverse events, the most common event was headache, reported for 18.3% of subjects in the placebo group, 19.4% of subjects in the ramelteon 8 mg group, and 17.8% of subjects in the ramelteon 16 mg group. The incidence of adverse events in ramelteon-treated subjects was similar to that in placebo-treated subjects except for the following adverse events: somnolence 1.5%, 7.9%, and 7.4%; fatigue 2.3%, 9.4%, and 4.4%; and nausea 2.3%, 4.3%, and 4.4% in the placebo, ramelteon 8 mg, and ramelteon 16 mg groups, respectively.
A distinction from standard sedative-hypnotic sleep medications is that ramelteon appears to promote sleep in a dose-independent manner.25,32,33,35 In a previous multiple-dose crossover study in subjects with chronic insomnia, ramelteon at doses ranging from 4 mg to 32 mg produced similar reductions in LPS (reduced 13.4 to 14.8 minutes compared with placebo).25 In the present study, similar or greater effects on sleep were observed at the 8 mg dose compared with the 16 mg dose of ramelteon. Both doses of ramelteon showed a lack of rebound insomnia after treatment was discontinued, unlike benzodiazepine receptor agonists, which can produce rebound insomnia in a dose-dependent manner. Just as importantly, both ramelteon doses resulted in similar incidences of adverse events. Moreover, ramelteon has shown no dose-related effects on adverse event rates across studies.25,32–35 In a previous study, subjects who received ramelteon at doses up to 20 times the recommended therapeutic dose showed no signs of impairment on a variety of behavioral and cognitive tasks.40 (The recommended therapeutic dose of ramelteon is 8 mg.)
A limitation of this study is the lack of data supporting the reliability and validity of the immediate and delayed memory recall tests used; however, these tasks are comparable to memory recall tasks used in other insomnia studies.3
In summary, the ability of ramelteon to promote sleep without significant adverse events and residual effects warrants further study in diverse patient populations. Because of its unique mechanism of action, its efficacy and safety profile, and its nonscheduled status, ramelteon represents a reasonable pharmacologic option for the treatment of insomnia.
ACKNOWLEDGMENTS
This study was funded by Takeda Pharmaceutical Company. Preparation of this manuscript was supported by Takeda Pharmaceuticals North America.
Footnotes
Disclosure Statement
This study was funded by Takeda Pharmaceutical Company. Dr. Zammit has received research support from Ancile Pharmaceuticals, Arean, Aventis, Cephalon, Elan, Epix, Forest, GlaxoSmithKline, H. Lundbeck A/S, King Pharmaceuticals, Merck, National Institutes of Health, Neurim, Neurocrine Biosciences, Neurogen, Organon, Orphan Medical, Pfizer, Respironics, Sanofi-Aventis, Sanofi-Synthelabo, Schering-Plough, Sepracor, Somaxon, Takeda, UCB Pharma, Predix, Vanda, and Wyeth-Ayerst Research; has been a consultant for Aventis, Cephalon, Elan, GlaxoSmithKline, Jazz, King Pharmaceuticals, Merck, Neurocrine Biosciences, Organon, Pfizer, Sanofi-Aventis, Sepracor, Shire, and Takeda; and has participated in speaking engagements for Neurocrine Biosciences, King Pharmaceuticals, McNeil, Sanofi-Aventis, Sanofi-Synthelabo, Sepracor, Takeda, Vela, and Wyeth-Ayerst Research. Dr. Erman has received research support from Cephalon, Takeda, Sanofi-Aventis, Neurocrine, Eli Lilly, and Merck and has been a consultant for Takeda, Sanofi-Aventis, Cephalon, Mallinckrodt and Neurocrine. Drs. Wang-Weingand, Sainati, and Zhang are employed by Takeda Global Research and Development. Dr. Roth has received research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schoering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport; is a consultant for Abbott, Accadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, BMS, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Johnson & Johnson, King, Lundbeck, McNeil, MedicNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Orginer, Prestwick, Proctor and Gamble, Pfizer, Purdue, Restiva, Roche, Sanofi, Shoering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport; and has participated in speaking engagements supported by Sanofi and Takeda.
REFERENCES
- 1.Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5:5–15. doi: 10.1016/s1098-3597(03)90031-7. [DOI] [PubMed] [Google Scholar]
- 2.Shochat T, Umphress J, Israel AG, Ancoli-Israel S. Insomnia in primary care patients. Sleep. 1999;22:S359–65. [PubMed] [Google Scholar]
- 3.Allain H, Bentue-Ferrer D, Tarral A, Gandon J. Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo. Eur J Clin Pharmacol. 2003;59:179–88. doi: 10.1007/s00228-003-0591-5. [DOI] [PubMed] [Google Scholar]
- 4.Roehrs T, Merlotti L, Zorick F, Roth T. Sedative, memory, and performance effects of hypnotics. Psychopharmacology (Berl) 1994;116:130–4. doi: 10.1007/BF02245054. [DOI] [PubMed] [Google Scholar]
- 5.Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–74. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 6.O'Hanlon J. Residual effects on memory and psychomotor performance of zaleplon and other hypnotic drugs. Prim Care Companion J Clin Psychiatry. 2002;4(Suppl 1):38–44. [Google Scholar]
- 7.Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–55. [PubMed] [Google Scholar]
- 8.Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction. 2003;98:1371–8. doi: 10.1046/j.1360-0443.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 9.Rush CR, Frey JM, Griffiths RR. Zaleplon and triazolam in humans: acute behavioral effects and abuse potential. Psychopharmacology (Berl) 1999;145:39–51. doi: 10.1007/s002130051030. [DOI] [PubMed] [Google Scholar]
- 10.Shochat T, Haimov I, Lavie P. Melatonin—the key to the gate of sleep. Ann Med. 1998;30:109–14. doi: 10.3109/07853899808999392. [DOI] [PubMed] [Google Scholar]
- 11.Turek FW, Gillette MU. Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists. Sleep Med. 2004;5:523–32. doi: 10.1016/j.sleep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 13.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–20. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 14.Jin X, von Gall C, Pieschl RL, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23:1054–60. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–62. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health. Drugs and insomnia. Natl Inst Health Consens Dev Conf Summ; NIH Consensus Development Conference; 1984. pp. 1–9. [PubMed] [Google Scholar]
- 17.Mendelson WB. A critical evaluation of the hypnotic efficacy of melatonin. Sleep. 1997;20:916–9. doi: 10.1093/sleep/20.10.916. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health State-of-the-Science Conference Statement: Manifestations and Management of Chronic Insomnia in Adults; 2005 Jun 13; 2005. pp. 1–18. [Google Scholar]
- 19.Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301–10. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Nosjean O, Ferro M, Coge F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–7. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 22.Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. BiochemPharmacol. 2001;61:1369–79. doi: 10.1016/s0006-2952(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 23.Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006;46:140–8. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson S, Cornelisson K, Clarke E, Hibberd M. Study of the absorption, metabolism, and excretion of (14C)-ramelteon (TAK-375) Clin Pharmacol Ther. 2004;75:P22. [Google Scholar]
- 25.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Tyrer P, Murphy S, Riley P. The Benzodiazepine Withdrawal Symptom Questionnaire. J Affect Disord. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Luria R. Reliability, validity, and clinical application of the visual analog mood scale. Psychol Med. 1973;3:479–86. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 28.Zisapel N, Nir T. Determination of the minimal clinically significant difference on a patient visual analog sleep quality scale. J Sleep Res. 2003;12:291–8. doi: 10.1046/j.0962-1105.2003.00365.x. [DOI] [PubMed] [Google Scholar]
- 29.Schneider C, Fulda S, Schulz H. Daytime variation in performance and tiredness/sleepiness ratings in patients with insomnia, narcolepsy, sleep apnea and normal controls. J Sleep Res. 2004;13:373–83. doi: 10.1111/j.1365-2869.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- 30.Gift AG. Visual analog scales: measurement of subjective phenomena. Nurs Res. 1989;38:286–8. [PubMed] [Google Scholar]
- 31.Wechsler D. WAIS-R manual. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 32.Roth T, Seiden D, Weigand S, Zhang J, Rieckhoff H, Sainati S. Phase III study to determine the efficacy of ramelteon in elderly patients with chronic insomnia [abstract]. Proceedings of New Clinical Drug Evaluation Unit; June 6-9, 2005; Boca Raton, FL. [Google Scholar]
- 33.Roth T, Stubbs C, Walsh J. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28:303–7. [PubMed] [Google Scholar]
- 34.Zammit G, Schwartz H, Roth T, Wright L, Sainati S, Zhang J. Phase III study of ramelteon in a first-night-effect model of transient insomnia. Sleep Med. 2005;6(Supp 2):S50–S51. doi: 10.1016/j.sleep.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7:312–8. doi: 10.1016/j.sleep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Scharf MB, Roth T, Vogel GW, Walsh JK. A multicenter placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192–9. [PubMed] [Google Scholar]
- 37.Zammit GK, McNabb LJ, Caron J, Amato DA, Roth T. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20:1979–91. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]
- 38.Elie R, Ruther E, Farr I, Emilien G, Salinas E. Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel non-benzodiazepine hypnotic. J Clin Psychiatry. 1999;60:536–44. doi: 10.4088/jcp.v60n0806. [DOI] [PubMed] [Google Scholar]
- 39.McCall WV, D'Agostino R, Jr, Dunn A. A meta-analysis of sleep changes associated with placebo in hypnotic clinical trials. Sleep Med. 2003;4:57–62. doi: 10.1016/s1389-9457(02)00242-3. [DOI] [PubMed] [Google Scholar]
- 40.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative side effects. Arch Gen Psychiatry. 2006;63:1149–57. doi: 10.1001/archpsyc.63.10.1149. [DOI] [PubMed] [Google Scholar]