Abstract
Adequate sleep is essential for general healthy functioning. This paper reviews recent research on the effects of chronic sleep restriction on neurobehavioral and physiological functioning and discusses implications for health and lifestyle. Restricting sleep below an individual's optimal time in bed (TIB) can cause a range of neurobehavioral deficits, including lapses of attention, slowed working memory, reduced cognitive throughput, depressed mood, and perseveration of thought. Neurobehavioral deficits accumulate across days of partial sleep loss to levels equivalent to those found after 1 to 3 nights of total sleep loss. Recent experiments reveal that following days of chronic restriction of sleep duration below 7 hours per night, significant daytime cognitive dysfunction accumulates to levels comparable to that found after severe acute total sleep deprivation. Additionally, individual variability in neurobehavioral responses to sleep restriction appears to be stable, suggesting a traitlike (possibly genetic) differential vulnerability or compensatory changes in the neurobiological systems involved in cognition. A causal role for reduced sleep duration in adverse health outcomes remains unclear, but laboratory studies of healthy adults subjected to sleep restriction have found adverse effects on endocrine functions, metabolic and inflammatory responses, suggesting that sleep restriction produces physiological consequences that may be unhealthy.
Citation:
Banks S; Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med 2007;3(5):519-528.
Keywords: Sleep restriction, neurobehavioral functions, physiology
There is ample scientific evidence to support the conclusion that sleep is an essential physiological need state that must be satisfied to ensure survival.1–3 Experimental work on sleep restriction has now begun to focus on the basic question of how much sleep people need each day to be healthy and safe. Chronic sleep restriction is frequently experienced due to medical conditions, sleep disorders, work demands, social and domestic responsibilities, and life style. This paper reviews recent research on the effects of chronic sleep restriction on neurobehavioral and physiological functioning relative to implications for health and safety.
SLEEP DURATION
Population-Based Estimates of Sleep Duration
Habitual sleep duration among adults shows considerable variance within and between individuals.4 The largest available database to date on self-reported sleep duration involved 1.116 million Americans (age ≥30 years; mean = 57 years for women and 58 years for men)5 who were queried about their sleep duration in 1982 as part of an American Cancer Society study. Sleep duration was distributed approximately normally, with 52.4% of subjects reporting <7.5 hours of sleep per night. In this sample, 19.7% of subjects reported sleeping <6.5 hours, and 4.0% reported sleeping <5.5 hours per night. At the other end of the spectrum, 9.2% of probands slept ≥8.5 hours, and 3.3% reported sleeping ≥9.5 hours per night. There were only very small differences in sleep duration between men and women in this study. It is not known to what extent these self-reported sleep durations accurately reflected physiologic sleep obtained, but this uncertainty plagues all epidemiological and survey studies of sleep duration. Since the data were acquired more than 24 years ago,5 it is uncertain whether these sleep duration estimates can be interpreted as being consistent with more recent population trends of declining sleep duration.
A 2005 Gallup poll in the USA found that among 1,500 adults (age ≥18 years; mean = 49 years) the average self-reported sleep duration was 6.8 h on weekdays and 7.4 h on weekends.6 However, there was considerable variation in reported sleep duration—16% of those interviewed reported sleeping <6 h per day on weekdays, while 10% did so on weekends.6 The proportion of U.S. adults reporting that they slept ≥8 h on weekdays decreased by 9% from a 1998 poll to a 2005 poll, while those reporting <6 h of sleep on weekdays increased by 4% over the same time period.6 Table 1 displays the results, which suggest that sleep duration as reported by American adults decreased over the past 8 years. There is considerable debate as to whether or not sleep duration has been decreasing among adults, and, if so, whether this is resulting in higher rates of chronic sleep restriction or sleep debt.7,8
Table 1.
Percentage of Participants that Reported Sleep Times in 4 Categories on Weekdays and Weekends from the 1998 and 2005 National Sleep Foundation Gallop Polls.
| Hours of sleep | 1998 weeknight | 2005 weeknight | diff. | 1998 weekend | 2005 weekend | diff. |
|---|---|---|---|---|---|---|
| ≥8 | 35 | 26 | −9 | 53 | 49 | −4 |
| 7–7.9 | 28 | 31 | +3 | 23 | 24 | +1 |
| 6–6.9 | 23 | 24 | +1 | 14 | 15 | +1 |
| <6 | 12 | 16 | +4 | 8 | 10 | +2 |
Data collected from N = 1506 participants (mean age 40.9 yr; 51% female) randomly selected based on U.S. Census household data (e.g., household has individuals over 18 yr).6 Telephone interviews were conducted between September and November 2004. Values in the table are expressed as percentages. Over the years, respondents who reported sleeping ≥7 h on weeknights decreased from 63% in 1998 to 57% in 2005. Additionally, the percentage of people who reported sleeping >7 h on weekend nights has dropped from 76% in 1998 to 73% in 2005. Overall, there appears to be an increase in the percentage of people sleeping <6 h/night and a decrease in those sleeping >7 h/night both during the week and on weekends.
NEUROBEHAVIORAL CONSEQUENCES OF SLEEP RESTRICTION
Unlike total sleep deprivation, which has been extensively investigated experimentally, the effects of partial sleep deprivation have received less scientific attention, even though sleep restriction is more prevalent as a result of medical conditions and sleep disorders, as well as lifestyle (e.g., shiftwork, jet lag, prolonged work hours).
Partial sleep deprivation can occur in 3 ways. The first involves preventing sleep from being physiologically consolidated and is referred to as sleep fragmentation, which can occur in certain sleep disorders (e.g., untreated obstructive sleep apnea). During sleep fragmentation, the normal progression and sequencing of sleep stages is typically disrupted to varying degrees, resulting in less time in consolidated physiological sleep, relative to time in bed. The second type of partial sleep deprivation involves loss of specific physiological sleep stages, and is, therefore, referred to as selective sleep stage deprivation. This is presumed to be less common than the other types, but prevalence estimates do not exist for any type of sleep restriction. Selective sleep stage deprivation can occur if sleep fragmentation is isolated to a specific sleep stage (e.g., when apneic episodes disrupt primarily one stage of sleep such as REM sleep, or when medications suppress a specific sleep stage). The third type of partial sleep deprivation is sleep restriction, which is also referred to as sleep debt,9 which is characterized by reduced sleep duration. Sleep restriction is the focus of this review because it is common, it relates to the fundamental question of how much sleep people need, and there is considerable experimental evidence of its neurobehavioral and physiological effects. Of particular interest are the questions of what changes when sleep is steadily reduced from 8 hours' to 4 hours' duration each day (i.e., the range many people experience sleep restriction), and whether there are cumulative dose response effects of this reduction on sleep physiology and waking functions.
Changes in Sleep Architecture During Sleep Restriction
Sleep restriction alters sleep architecture, but it does not affect all sleep stages equally. Depending on the timing and duration of sleep, and the number of days it is reduced, some aspects of sleep are conserved, occur sooner, or intensify, while other aspects of sleep time are diminished. For example, healthy adults fell asleep more quickly and had decreased time in NREM stage 2 sleep and REM sleep when restricted to 4 h of nocturnal sleep for multiple nights, but they had no decrease in NREM slow wave sleep (SWS) relative to a typical 8-h nocturnal sleep period10–13 (see Figure 1). While visually scored NREM SWS was conserved, slow wave sleep activity (SWA) derived from power spectral analysis of delta wave activity (0.5–4.0 Hz) in the EEG during NREM stages 2, 3, and 4 sleep showed some dynamic increases as restriction of sleep to 4 h continued for more than a day.11,12 The conservation of SWS and intensification of SWA during sleep restricted to 4 h/night in healthy adults, has suggested the hypothesis that NREM EEG slow waves are essential and perhaps protected aspects of the physiological recovery afforded by sleep to waking brain functions. It remains to be determined whether the lack of SWS and SWA response to sustained (chronic) restriction of sleep to 4 h a night, relative to steady increases in physiological and neurobehavioral measures of sleepiness,12 can account for the latter deficits. Neither SWS nor NREM SWA show the magnitude of increases following chronic sleep restriction observed following total sleep deprivation.12 Consequently, while SWS and NREM SWA may be largely conserved in chronic sleep restriction to 4–7 hours per night, they do not appear to either reflect the severity of daytime cognitive deficits or prevent these deficits, raising serious doubts about SWS and NREM SWA as the only aspects of sleep critical to waking functions.
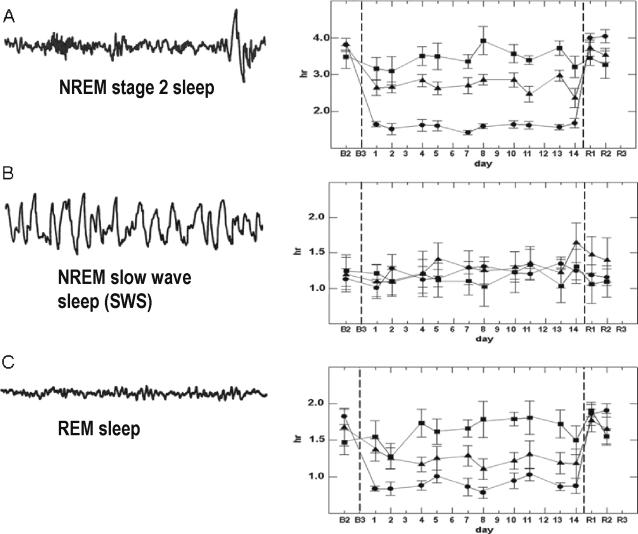
Figure 1.
The effects of sleep restriction on NREM stage 2 sleep in Panel A; on NREM slow wave sleep (SWS) in Panel B; and on REM sleep in Panel C. Data are adapted from Van Dongen et al.12 Following 8 hours of time in bed on baseline nights (B1, B2, B3), sleep was restricted for 14 consecutive nights to either 4 hours of time in bed (•, n = 13 healthy adults), 6 hours of time in bed (▲, n = 13), or 8 hours of time in bed (■, n = 9). Restriction was implemented by delaying bed time and holding sleep offset time constant (07:30). Sleep restriction nights were followed by 3 nights of 10 hours of time in bed for recovery sleep (R1, R2, R3). Sleep stages were scored polysomnographically for 2 out of every 3 nights during the experiment. Panel A: During the 14 nights of restriction to 4 h of time in bed, NREM stage 2 sleep was decreased an average of more than 2 h per night relative to the 8-h control condition (p < 0.001). Stage 2 sleep was decreased approximately 1 h per night in the 6-h condition relative to the control condition (p < 0.001). Panel B: In contrast to NREM stage 2 sleep, NREM slow wave sleep (SWS) showed no significant reduction in either the 4-h or 6-h sleep restriction conditions relative to the 8-h control condition. Panel C: Relative to the 8-h control condition, REM sleep was reduced by approximately 47 minutes a night during the 14 nights of restriction to 4 h time in bed (p < 0.01), and by 24 minutes a night during the 14 nights of restriction to 6 h time in bed (p < 0.05).
Experimental Control of Wakefulness in Sleep Restriction Experiments
Experimental protocols that restrict healthy adult sleep duration across consecutive days provide the most appropriate paradigms for addressing the question of whether waking neurobehavioral deficits accumulate, and, if so, the rate of accumulation as the reduced sleep duration is maintained for multiple days. However, the cost and logistical complexities of maintaining tight experimental control over the sleep and waking activities of a large number of subjects, 24-hours a day for 1–3 weeks have resulted in only a few experiments on chronic sleep restriction being done in a scientifically sound manner. Most early experimental reports (before 1965) on the waking neurobehavioral effects of prolonged sleep restriction to durations people commonly experience (i.e., 4–6 h sleep per day) bordered on the anecdotal and lacked adequate sample sizes and control groups.9 Subsequent experimental reports (1970–1995) on the cognitive and subjective effects of sleep restricted to 4–6 hours a night often failed to ensure that subjects maintained the assigned sleep-wake schedules; used infrequent, confounded and/or insensitive measures of sleep and waking; lacked sophisticated time series analyses; and generally drew conclusions not substantiated by the quantitative results (for reviews, see 9,14,15). These methodological inadequacies and small sample sizes resulted in conflict as to whether or not sleep restriction resulted in cumulative waking cognitive and subjective changes, which prompted 3 widely repeated conclusions: (1) that reducing nightly sleep duration to between 4 and 6 h had little adverse effects on daily functions16–19; (2) that only a “core sleep” duration of 4–6 h was physiologically essential, and any additional sleep beyond that core duration was optional sleep that reflected residual capacity9,20; and (3) that an individual could adapt to a reduced amount of sleep with few neurobehavioral consequences.20 These conclusions were subsequently shown to be incorrect, as lightly controlled experiments on chronic partial sleep restriction failed to support them.10,12,15 The results of these more recent, scientifically controlled studies will be discussed in following sections.
Physiological Sleep Propensity During Sleep Restriction
The tendency to fall asleep is among the most well validated measures of sleepiness. It is based on the assumption that sleepiness is a physiologic need state that leads to an increased tendency to fall asleep, and it is operationalized as the speed of falling asleep in both sleep-conducive and nonconducive conditions.21
The effects of chronic sleep restriction on daytime physiological sleep propensity has been evaluated using the multiple sleep latency test (MSLT)22 and the maintenance of wakefulness test (MWT).23 During the MSLT, the subject is instructed to close the eyes and try to fall asleep, while lying supine for 20-min periods, two hours apart, four to five times throughout the day, while polysomnography (PSG) recordings are made (these include EEG, EOG, and EMG). The MWT uses a similar protocol to the MSLT, but subjects are seated upright and instructed to try to stay awake. The time taken to fall asleep on both tests is a measure of sleep propensity.
The MSLT has been shown to vary linearly following a single night of sleep restricted to between 1 and 5 h of time in bed.24 In addition, the MSLT showed progressive shortening (i.e., more sleep propensity) when healthy young adults were restricted to 5 h of sleep a night for 7 consecutive nights.24 This seminal finding of sleep propensity increasing across days of sleep restriction was confirmed in a later study using the psychomotor vigilance task as a measure of daytime behavioral alertness.15
Dose-response effects of chronic sleep restriction on daytime sleep propensity have also recently been found in an experiment on the effects of reduced nocturnal sleep dosages on daytime sleep latencies of commercial truck drivers.10 A significant increase in sleep propensity across 7 days of sleep restricted to either 3 or 5 h per night was observed, with no differences found when sleep was restricted to 7 or 9 h per night.10 Sleep propensity, as measured by the MWT, has also been found to increase in experiments in which adults were restricted to 4 h for sleep for 7 nights13,25 and for 5 nights.13,25
In an epidemiological study of predictors of objective sleep tendency in the general population,26 a dose-response relationship was found between self-reported nighttime sleep duration and objective sleep tendency as measured by MSLT. Persons reporting >7.5 hours of sleep had significantly less probability of falling asleep on the MSLT than those reporting to between 6.75 to 7.5 h per night (27% risk of falling asleep) and than those reporting sleep durations less than 6.75 h per night (73% risk of falling asleep).26 Consequently, to date, studies consistently suggest that chronic curtailment of nocturnal sleep increases daytime sleep propensity.
Sleep loss has also been found to affect oculomotor responses. Eyelid closure and slow rolling eye movements are part of the initial transition from wake to drowsiness and light sleep (i.e., stage 1 sleep). Eye movements and eye closures have been studied during sleep loss protocols, under the premise that increases in the number and duration of slow eye movements and slow eyelid closures are reflections of increased sleep tendency. It has been demonstrated experimentally that slow eyelid closures during performance demands reliably track lapses of attention on a vigilance task27,28 and during simulated driving.29,30 Chronic sleep restriction has been reported to lead to a decrease in saccadic velocity in subjects allowed only 3 h or 5 h of time in bed for sleep over 7 nights, and an increase in the latency to pupil constriction.10 These changes in oculomotor activity were positively correlated with sleep latency, subjective sleepiness measures, and accidents on a simulated driving task.31
Effects of Sleep Reduction on Behavioral Alertness and Cognitive Performance
Restricted sleep time affects many different aspects of waking cognitive performance, but especially behavioral alertness.32 Performance on psychomotor vigilance tasks requiring vigilant attention is very sensitive to sleep loss in general and sleep restriction in particular.33,34 Many experiments have demonstrated that sleep deprivation increases behavioral lapses during performance,33,34 which are assumed to reflect microsleeps.35,36 As sleep loss continues, lapses can range in duration from 0.5 seconds to well over 10 sec, and they can progress to full blown sleep attacks (i.e., lapses from which subjects will not spontaneously arise without additional stimulation).35,36 It has been hypothesized37,38 that the lapses produced by sleep loss may originate in sleep-initiating subcortical systems (e.g., hypothalamus, thalamus, and brainstem).39 This has been conceptualized as “wake state instability,”33,34,37 which refers to moment-to-moment shifts in the relationship between neurobiological systems mediating wake maintenance and those mediating sleep initiation.39,40 Behavioral alertness as measured by psychomotor vigilance tasks—or other sustained attention tasks—has proven to be very sensitive to sleep restriction.35,37,38
The 2 most extensively controlled experiments on chronic sleep restriction in healthy adults have found systematic evidence that behavioral alertness—as measured by psychomotor vigilance testing35,36—deteriorated steadily across days when nightly sleep duration was between 3 and 7 h,10 with deterioration being more rapid as time allowed for sleep was reduced. In the experiment by Belenky and colleagues,10 commercial truck drivers were kept in the laboratory for 14 d and randomized to seven nights of 3, 5, 7, or 9 h in bed for sleep per night. Those in the 3- and 5-h conditions had growing daytime deficits over the week in response to speed and number of lapses on the psychomotor vigilance task (PVT).10 Subjects allowed 7 h/night had a significant decrease in PVT response speed. In contrast, performance in the group allowed 9 h time in bed was stable over the week. A similar experiment completed in our laboratory12 kept healthy adults (mean age 28 y) in the laboratory for 20 days, randomizing them to either 4, 6, or 8 h time in bed per night for 14 consecutive nights. Psychomotor vigilance test performance and working memory performance were tested every 2 hours throughout each day. Cumulative daytime deficits in both PVT and cognitive throughput were observed for the 4- and 6-h sleep restriction conditions, but not the 8-h condition. In order to quantify the magnitude of cognitive deficits experienced during 14 days of restricted sleep, the effects of sleep restriction were compared to 1, 2, and 3 nights of total sleep deprivation.12 This comparison revealed that both 4- and 6-h sleep periods resulted in the development of impairments of behavioral alertness that increased to levels found after 1, 2, and even 3 nights of total sleep deprivation.12
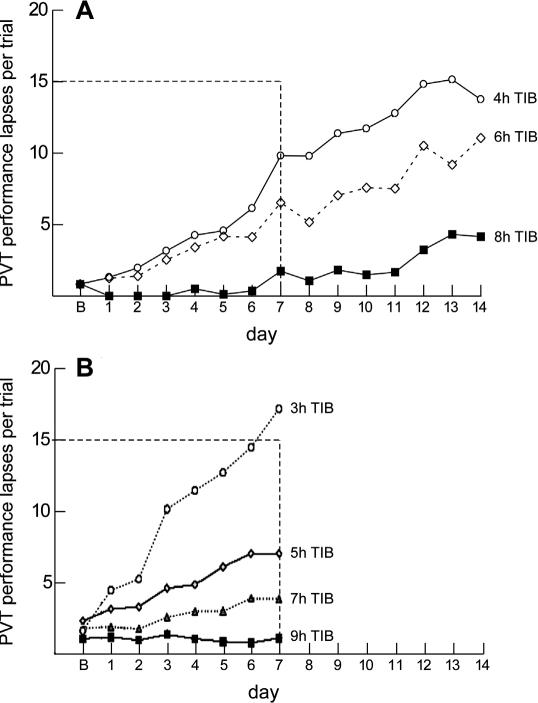
Figure 2 shows the number of PVT lapses per test bout each day from both of these controlled large-scale dose-response sleep-restriction experiments.10,12 The remarkable similarity and internal consistency of the dependence of severity of PVT lapsing on the chronic sleep dose suggests that when the nightly sleep period is restricted to ≤7 h, healthy adults have increasing numbers of lapses of attention in proportion to the dose of sleep allowed (between subjects) and the number of days of sleep restriction (within subjects). A similar finding was observed for cognitive throughput performance on a working memory task,12 which is shown in Figure 3.
Figure 2.
The effects of varying doses of nocturnal sleep time on lapses of attention from the psychomotor vigilance test (PVT). Panel A from Van Dongen et al. 12 involved experimental sleep restriction of n = 36 healthy adults for 14 consecutive nights. In this experiment sleep was restricted for 14 consecutive nights. Subjects were randomized to 4 h time in bed (n = 13), 6 h time in bed (n = 13), or 8 h time in bed (n = 9). PVT performance was assessed every 2 h (9 times each day) from 07:30 to 23:30. The graph shows systematic increases in lapses of sustained attention when sleep was restricted to either 4 h (p < 0.001) or 6 h (p < 0.001) per night, but not when sleep was restricted to 8 h per night (p = 0.29). The increase in lapsing was worse in the 4-h sleep condition than in the 6-h sleep condition (p = 0.036), further supporting a dose-response relationship within and between conditions. The horizontal dotted line shows the level of lapsing found in a separate experiment when subjects had been awake continuously for 64–88 h. For example, by day 7, subjects in the 6-h sleep restriction condition averaged 54 lapses (6 lapses × 9 test times) that day, while those in the 4-h sleep condition averaged 70 lapses that day. Panel B shows comparable sleep restriction data from Belenky et al.10 In this study sleep was restricted for 7 consecutive nights in n = 66 healthy adults. They were randomized to 3 h time in bed (n = 13), 5 h time in bed (n = 13), 7 h time in bed (n = 13), or 9 h time in bed (n = 16). Performance was assessed 4 times each day from 09:00 to 21:00. PVT lapses increases steadily across days in the 3-h (p = 0.001) and 5-h (p = 0.001) sleep restriction conditions (PVT response speed, but not lapses, was reduced in the 7-h condition, not shown). As in Panel A, the horizontal dotted line shows the level of lapsing found in a separate experiment when subjects had been awake continuously for 64–88 h.12 Considering data in both Panels A and B, it is clear that restriction of nocturnal sleep time to <7 h per night in healthy adults results in systematic increases in lapses of waking attention that get progressively worse across days, in a dose-response manner.
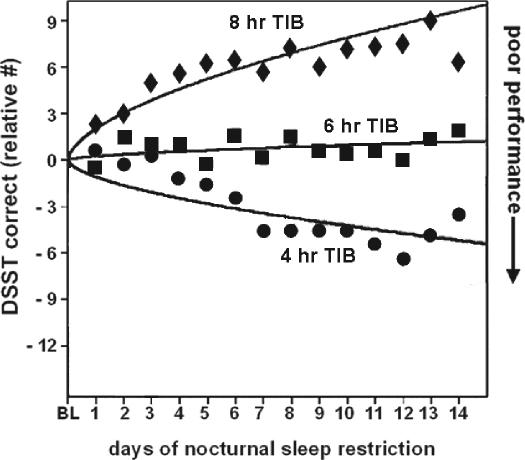
Figure 3.
Digit symbol substitution task (DSST) performance responses to varying doses of daily sleep across 14 days. Data from n = 35 subjects (8h condition n = 9, 6h condition n = 13 and 4h condition n = 13). Mean DSST per day (07:30–23:30), measured at 2-h intervals expressed relative to baseline (BL). The curves represent statistical nonlinear model-based best-fitting profiles of the DSST performance response to sleep loss. Adapted from Van Dongen et al.12
The cognitive performance findings from these 2 major laboratory-based dose-response experiments on the effects of chronic sleep restriction in healthy adults are consistent with those on the effects of sleep restriction on physiological sleep propensity measures (MSLT, MWT) described above.10,13,24,25 Collectively they suggest that there is a neurobiological integrator that either accumulates homeostatic sleep drive or the neurobiological consequences of excess wakefulness.10,12 There has as yet been no definitive evidence of what is accumulating and destabilizing cognitive functions over time when sleep is regularly restricted to less than 7 hours per night, but one intriguing line of evidence suggests that it may involve extracellular adenosine in the basal forebrain.41–43
Although functional neuroimaging of cognitive changes produced by total sleep deprivation have been extensively studied,44,45 there are as yet no experimental reports on the effects of chronic sleep restriction on brain activation. While the neurobehavioral effects of chronic sleep restriction appear similar to those of total sleep deprivation,12 the primary physiologic measure of homeostatic sleep—slow wave activity in the spectrally analyzed NKEM EEG—shows a much more muted response to the former than to the latter, suggesting that there may be a different neurobiological mechanisms sub-serving the adverse effects of chronic sleep restriction.
Sleep Restriction Effect on Subjective Reports of Sleepiness and Mood
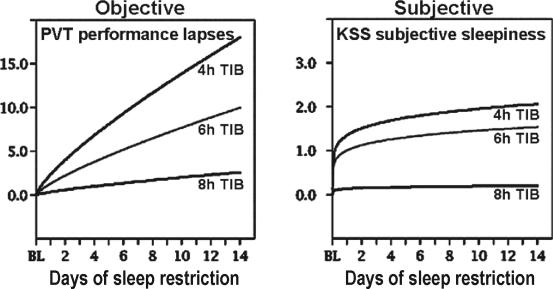
Like NREM SWA, subjective sleepiness responses during chronic sleep restriction show a different dynamic profile than those found for total sleep deprivation. While the latter results in immediate increases in feelings of sleepiness, fatigue and cognitive confusion, with concomitant decreases in vigor and alertness21,35,36,38,46,47 chronic sleep restriction yields much smaller changes in these psychometric ratings of internal state.10,12 Thus, in contrast to the continuing accumulation of cognitive performance deficits associated with nightly restriction of sleep <8 h, ratings of sleepiness repeatedly made by subjects on standardized sleepiness scales did not parallel performance deficits.12 As a consequence, after a week or two of sleep restriction, subjects were markedly impaired and less alert, but rated themselves subjectively as only moderately sleepy (see Figure 4). This suggests that people frequently underestimate the cognitive impact of sleep restriction and overestimate their performance readiness when sleep restricted. Other experiments using driving simulators have found comparable results.48
Figure 4.
Data from n = 35 subjects (8h condition n = 9, 6h condition n = 13 and 4h condition n = 13). Restriction of nocturnal sleep in healthy adults resulted in near-linear increases in Psychomotor Vigilance Test (PVT) lapses of attention across 14 days (coefficients of change near 1.0), but subjective ratings of sleepiness and fatigue (regardless of the psychometric scale used) showed a nonlinear coefficient below 0.5 for change over days. This meant that as objective performance continued to decline near-linearly, there were only minor further increases in the subjective ratings of sleepiness. By the end of the 14 days of sleep restriction, when performance was at its worst levels, subjects in the 4-h and 6-h sleep period conditions reported feeling only slightly sleepy. Therefore, unlike performance measures, sleepiness ratings appeared to show adaptation to chronic partial sleep deprivation. The lack of reports of intense feelings of sleepiness during chronic sleep restriction may explain why sleep restriction is widely practiced—people have the subjective impression they have adapted to it because they do not feel particularly sleepy. Adapted from Van Dongen et al.12
Driving and Simulated Driving Following Sleep Reduction
One real-world risk associated with sleep restriction is decreased driving ability. Studies have primarily focused on the effects of short-term sleep restriction on driving ability and crash risk.49,50 An epidemiological study found an increased incidence of sleep-related crashes in drivers reporting <7 h of sleep per night on average.51 Additional contributing factors to these crashes included poor sleep quality, dissatisfaction with sleep duration (i.e., undersleeping), daytime sleepiness, previously driving drowsy, amount of time driving and time of day (i.e., driving late at night). Studies have also examined the effects of sleep restriction on performance on various driving simulators. It has been found that driving performance decreased (e.g., more crashes) and subjectively reported sleepiness increased when sleep was restricted to between 4 and 6 h per night.31,48,52–57
Individual Differences in Responses to Sleep Restriction
Interindividual variability in sleep and circadian parameters are substantial, and this is equally the case for neurobehavioral and physiological responses to sleep deprivation.4,21,31,35–38,46,47 Sleep loss not only increases cognitive performance variability within subjects (intrasubject variability that is characterized as state instability),21,35,36,38,46,47 but it also exposes marked neurobehavioral differences between subjects. That is, as sleep loss continues over time, intersubject differences in the degree of cognitive deficits also increase markedly.31,37 This interindividual variability is also seen in responses to experimentally restricted sleep. For example, while sleep duration limited to less than 7 h per day resulted in cumulative cognitive performance deficits in a majority of healthy adults,10,12 not everyone was affected to the same degree.10,12 At opposite ends of the spectrum are those who experience very severe impairments even with modest sleep restriction versus those who show few if any neurobehavioral deficits until sleep restriction is severe (in duration or chronicity). Moreover, there is some data to suggest that the nature of the cognitive impairments can be quite different among subjects for different cognitive tasks,9,58 such that those with increasing problems performing working memory tasks may not have problems with psychomotor vigilance. Recently, and perhaps most importantly for future studies of the possible genetic contributors to differential vulnerability to sleep loss, is the finding that the neurobehavioral responses to sleep deprivation were stable and reliable within subjects,59 suggesting they were trait-like.58,59 The biological bases of differential responses to sleep loss are not known, although recent neuroimaging studies suggest that it may be possible to predict them before subjects are deprived of sleep.45,60,61
In summary, when sleep duration in healthy adults was experimentally reduced <7 h per night, many waking neurobehavioral functions progressively deteriorated. A range of cognitive tasks (e.g., decision making) and normal daily behaviors (e.g., driving) were adversely affected by reduced sleep time.35,38,46,47,50 These adverse neurobehavioral effects of sustained sleep restriction have the potential to lower productivity and increase the risks for errors and accidents.
PHYSIOLOGICAL CONSEQUENCES OF SLEEP RESTRICTION
As noted above, recent epidemiological studies have found that both relatively long sleepers (≥8 h sleep per day) and relatively short sleepers (<7 h sleep per day) had increased risks of all-cause mortality.5,62,63 There is also epidemiological evidence that reduced sleep duration is associated with larger body mass index (BMI).64,65 Laboratory studies of experimental restricted sleep in healthy adults suggest some mechanisms by which sleep duration may influence obesity, morbidity, and mortality.
A range of physiological indices have been found to be altered by reduced sleep time. While the clinical significance of these findings in healthy adults is unknown, the indices affected have been related to health outcomes in patient populations. Several studies have reported an increased incidence and risk of medical disorders and health dysfunction related to shift work schedules, which have been attributed to both circadian disruption and sleep disturbance (for review, see 66). Short-term sleep restriction results in a number of abnormal physiologic changes, including reduced glucose tolerance,67 increased blood pressure,68 activation of the sympathetic nervous system,69 reduced leptin levels,70 and increased inflammatory markers.71 Although the magnitude of the physiologic changes found in these short-term studies was modest, the changes provide a potential mechanism whereby longterm sleep restriction may affect health.
Endocrine Responses
A number of recent studies have focused on endocrine and metabolic consequences of chronic sleep restriction. Comparison of sleep restriction (4 h/night for 6 nights) to sleep extension (12 h/night for 6 nights) in healthy young adults revealed an elevation in evening cortisol, increased sympathetic activation, decreased thyrotropin activity, and decreased glucose tolerance in the restricted versus extended sleep condition.67 Similarly, an elevation in evening cortisol levels, and advance in the timing of the morning peak in cortisol, so that the relationship between sleep termination and cortisol acrophase was maintained, was found following 10 nights of sleep restricted to 4.2 h time in bed for sleep each night compared to baseline measures and a control group allowed 8.2 h time in bed for sleep for 10 nights.72 In the same protocol, a significant delay in melatonin onset73 and in the timing of the peak in growth hormone, equivalent to the delay in sleep onset induced to achieve the restricted sleep period, were found, with no effect on growth hormone levels during the sleep period.74
Changes in the timing of the growth hormone secretory profile associated with sleep restriction to 4 h per night for 6 nights, with a bimodal secretory pattern have also been reported.75 Decreased leptin levels (adipocyte-derived hormone that suppresses appetite) and increased ghrelin (predominantly a stomach-derived peptide that stimulates appetite) have been reported when sleep was restricted to 4 h a night relative to a 12-h control condition.70,76 These effects are similar to what has been found for total sleep deprivation.77 Thus, it is possible that sleep restriction produces alterations in the secretory profiles of appetite-regulating hormones, which in turn alter the signaling of hunger and appetite and promote increased weight gain and obesity.76
The possibility that sleep restriction may be associated causally with obesity by altered regulation of appetite-regulating hormones has also been suggested by findings of a study of 1,024 volunteers from the Wisconsin Sleep Cohort Study—a population-based longitudinal study of sleep disorders.64 In this study, participants underwent nocturnal polysomnography and reported on their sleep habits through questionnaires and sleep diaries. Following polysomnography, morning fasted blood samples were evaluated for serum leptin, ghrelin, adiponectin, insulin, glucose, and lipid profile. Relationships among these measures, BMI, and sleep duration revealed a curvilinear (U-shaped) association between sleep duration and BMI. In persons sleeping <8 hours (74.4% of the sample), increased BMI was proportional to decreased sleep duration. Short sleep was associated with low leptin and high ghrelin independent of BMI. Since reduced leptin and elevated ghrelin are likely to increase appetite, this may explain the increased BMI observed with short sleep duration and how chronic sleep curtailment could contribute to obesity.13
Immune Responses
The potential impact of chronic sleep restriction on immune responses has received little attention, although total sleep deprivation has been shown to activate non-specific host defense mechanisms and to elevate certain inflammatory cytokines (IL-6, TNF) in healthy young adults.78,79 Although the effects of sleep restriction on cellular and humoral immune responses are largely unexplored, antibody production to vaccination has been reported to be decreased by sleep restriction. In one study it was reported that antibody titers were decreased by more than 50% 10 days post-vaccination for influenza.80 Subjects had been vaccinated immediately following 6 nights of sleep restricted to 4 h per night compared to those who were vaccinated following habitual sleep duration. By 3–4 weeks post-vaccination, there was no difference in antibody levels between the 2 groups. In a another study, attenuation of the febrile response to an endotoxin (E. coli) challenge in subjects undergoing chronic sleep restriction to 4 h/night for 10 nights (relative to subjects allowed 8 h for sleep) was observed.81
These two limited studies suggest that sleep restriction alters the acute immune response to vaccination, and decreases the febrile response to an endotoxin signal.
In a third experiment in which healthy young adults had their sleep restricted to 6 h per night, the 24-h secretory profile of IL-6 was increased in both sexes and TNF-alpha was increased in men.82 Both IL-6 and TNF-alpha are markers of systemic inflammation that may lead to insulin resistance, cardiovascular disease and osteoporosis.83
Cardiovascular Responses
An increase in cardiovascular events and cardiovascular morbidity associated with reduced sleep durations has been reported in a number of epidemiological studies5,62,84–87 and in a case-control study examining insufficient sleep due to work demands.88 In the Nurses' Health Study, there was evidence of increased risk of coronary events in female subjects obtaining ≤7 h sleep per night compared to those averaging 8 h per night.62 In another epidemiological study, a 2–3–fold increase in risk of cardiovascular events was found for subjects with an average sleep duration of ≤5 h per night (or chronically having <5 h of sleep per night at least twice per week) was reported.88 Similar findings have also been observed in studies examining cardiovascular health in shift workers, who typically experience chronic reductions in sleep duration, in addition to circadian disruption.89–92
The mechanisms underlying the link between chronic sleep restriction and increased cardiovascular risk are unknown; however, one potential mechanism may be by activation of inflammatory processes during sleep loss, as described above. C-reactive protein (CRP) is an inflammatory marker that is positive predictor of increased risk for cardiovascular disease.93 We have found that high-sensitivity CRP was increased in healthy adults following both total sleep deprivation and chronic sleep restriction.71 Figure 5 illustrates these findings. It remains to be determined how chronic sleep restriction activates mechanisms involved in cardiovascular morbidity and mortality, but elevated CRP may be a link.
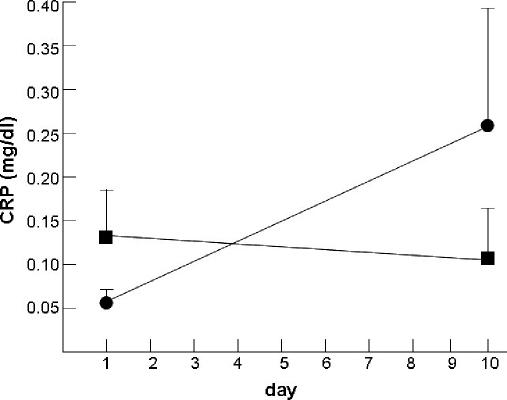
Figure 5.
Mean (SEM) plasma high-sensitivity C-reactive protein (CRP) in n = 4 subjects undergoing 10 consecutive nights of sleep restricted to 4.2 h time in bed, and in n = 5 control subjects who had 10 consecutive nights of sleep restricted to 8.2 h time in bed (closed squares). Significance of difference in change from baseline to day 10 between groups (p = 0.08 for interaction) by mixed-models analysis of variance on log-transformed data: the change from baseline to day 10 for the 4-h sleep restriction group was significant (p = 0.05), whereas the change from baseline to day 10 in the 8-h control group was not (p = 0.72). Figure adapted from Meier-Ewert et al.71
CONCLUSION
Restricted sleep time—particularly when chronic can cause significant and cumulative neurobehavioral deficits and physiological changes, some of which may account for the epidemiological findings that reduced sleep durations are associated with obesity, cardiovascular morbidity, traffic accidents and death. Recent careful controlled experiments in healthy adults reveal that as sleep was repeatedly restricted to less than 7 h per night, significant daytime cognitive dysfunction (i.e., state instability, reduced vigilant attention and working memory) accumulated as restriction continued to levels comparable to that found after severe acute total sleep deprivation. This strongly suggests the existence of a neurobiological integrator in the brain that instantiates either the need for sleep across days or the accumulation of excess wakefulness. These experiments also reveal that individuals differ markedly in their cognitive vulnerabilities to sleep restriction, which suggests a trait-like (possibly genetic) basis for the response. Research also demonstrates that experimentally induced chronic sleep restriction results in several adverse physiologic consequences, including reduced glucose tolerance, increased blood pressure, and increased inflammatory markers in healthy adults. Consistent with these reports are epidemiologic studies that find self-reported short sleep duration is associated with obesity, heart disease, and mortality. Thus, current research findings on the effects of sleep restriction on neurobehavioral and physiological functioning suggest that adequate sleep duration (7–8 hours per night) is vital.
ACKNOWLEDGMENTS
Institution where research conducted: University of Pennsylvania School of Medicine
Support: The substantive evaluation on which this review was based was supported by NIH grants NR04281 and RR00040; and National Space Biomedical Research Institute through NASA NCC 9-58.
Footnotes
Disclosure Statement
This is not an industry supported study. Dr. Dinges has received research support and honoraria from Cephalon and has consulted for Arena Pharmaceuticals, Cephalon, Merck, Novartis, Pfizer, GlaxoSmithKline, Mars Masterfoods, and Procter & Gamble. Dr. Banks has indicated no financial conflicts of interest.
REFERENCES
- 1.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: I. Conceptual issues. Sleep. 1989;12:1–4. doi: 10.1093/sleep/12.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Dinges DF, Chugh DK. Physiologic correlates of sleep deprivation. In: Kinney JM, Tucker HN, editors. Physiology, stress, and malnutrition: Functional correlates, nutritional intervention. New York: Lippincott-Raven; 1997. pp. 1–27. [Google Scholar]
- 4.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 4.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatr. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 6.National Sleep Foundation. Washington DC: National Sleep Foundation; 2006. „Sleep in America‟ poll. [Google Scholar]
- 7.Dinges DF. Sleep debt and scientific evidence. Sleep. 2004;27:1–3. [PubMed] [Google Scholar]
- 8.Horne J. Is there a sleep debt? Sleep. 2004;27:1047–9. [PubMed] [Google Scholar]
- 9.Van Dongen HPA, Rogers NL, Dinges DF. Understanding sleep debt: theoretical and empirical issues. Sleep Biol Rhythms. 2003;1:4–12. [Google Scholar]
- 10.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 11.Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 12.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Powell NB, Martinez S, et al. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003;4:177–84. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 14.Carskadon MA, Roth T. Sleep restriction. In: Monk TH, editor. Sleep, sleepiness and performance. Chichester: John Wiley & Sons; 1991. pp. 155–67. [Google Scholar]
- 15.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 16.Webb WB, Agnew J, H. W. The effects of a chronic limitation of sleep length. Psychophysiology. 1974;11:265–74. doi: 10.1111/j.1469-8986.1974.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 17.Friedmann J, Globus G, Huntley A, Mullaney D, Naitoh P, Johnson L. Performance and mood during and after gradual sleep reduction. Psychophysiology. 1977;14:245–50. doi: 10.1111/j.1469-8986.1977.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 18.Horne JA, Wilkinson S. Chronic sleep reduction: daytime vigilance performance and EEG measures of sleepiness, with particular reference to “practice” effects. Psychophysiology. 1985;22:69–78. doi: 10.1111/j.1469-8986.1985.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 19.Blagrove M, Alexander C, Horne JA. The effects of chronic sleep reduction on the performance of cognitive tasks sensitive to sleep deprivation. Appl Cogn Psychol. 1995;9:21–40. [Google Scholar]
- 20.Horne J. The functions of sleep in humans and other mammals. Oxford: Oxford University Press; 1988. Why we sleep. [Google Scholar]
- 21.Roehrs T, Carskadon MA, Dement WC, Roth T. Daytime sleepiness and alertness. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. WB. Saunders Company; 2005. [Google Scholar]
- 22.Carskadon MA, Dement WC. Nocturnal determinants of daytime sleepiness. Sleep. 1982;5:S73–81. doi: 10.1093/sleep/5.s2.s73. [DOI] [PubMed] [Google Scholar]
- 23.Mitler MM, Gujavarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluation treatment efficacy in patients with excessive somnolence. Electroencephalogr ClinNeurophysiol. 1982;53:658–61. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–13. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 25.Banks S, Dinges DF. Is the maintenance of wakefulness test sensitive to varying amounts of recovery sleep after chronic sleep restriction? Sleep. 2005;28:A136. [Google Scholar]
- 26.Punjabi NM, Bandeen-Roche K, Young T. Predictors of objective sleep tendency in the general population. Sleep. 2003;26:678–83. doi: 10.1093/sleep/26.6.678. [DOI] [PubMed] [Google Scholar]
- 27.Dinges DF, Mallis M, Maislin G, Powell JW. (U.S. Department of Transportation, National Highway Traffic Safety Administration). Evaluation of techniques for ocular measurement as an index of fatigue and as the basis for alertness management. 1998 [Google Scholar]
- 28.Dinges DF, Price NJ, Maislin G, et al. Prospective laboratory revalidation of ocular-based drowsiness detection technologies and countermeasures. NHTSA Drowsy Driver Detection and Interface Project. 2002 Report No.: Task Order No. 7, DTNH 22-00-D-07007. [Google Scholar]
- 29.Wierville WW, Ellsworth LA, Wreggit SS, Fairbanks RJ, Kirn CL. (National Traffic Safety Administration). Research on vehicle-based driver status/performance monitoring: development, validating, and refinement of algorithms for detection of driver drowsiness. 1994 Report No.: DOT HS 808 247. [Google Scholar]
- 30.Wierwille WW, Ellsworth LA. Evaluation of driver drowsiness by trained raters. Accid Anal Prev. 1994;26:571–581. doi: 10.1016/0001-4575(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 31.Russo A, Thomas A, Thorne D, et al. Oculomotor impairment during chronic partial sleep deprivation. Clin Neurophysiol. 2003;114:723–36. doi: 10.1016/s1388-2457(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 32.Dinges DF, Rogers NL, Baynard MD, editors. WB. Saunders; 2005. Chronic sleep deprivation: [Google Scholar]
- 33.Balkin TJ, Bliese PD, Belenky G, et al. Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J Sleep Res. 2004;13:219–27. doi: 10.1111/j.1365-2869.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 34.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida C, editor. Sleep Deprivation. New York: Marcel Dekker; 2005. [Google Scholar]
- 35.Dinges DF, Kribbs NB, editors. Winchester, England: John Wiley & Sons; 1991. Performing while sleepy: effects of experimentally induced sleepiness. [Google Scholar]
- 36.Kleitman N. Second ed. Chicago: University of Chicago Press; 1963. Sleep and wakefulness. [Google Scholar]
- 37.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 38.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 39.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 40.Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5:1071–5. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- 41.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strecker RE, Morairty S, Thakkar MM, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 43.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 45.Chee MWL, Chuah LYM, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. Neuroimage. In press. [DOI] [PubMed]
- 46.Dorrian J, Dinges DF. Encyclopedia of sleep medicine. Hoboken, NJ: J. Wiley & Sons; 2005. Sleep deprivation and its effects on cognitive performance. [Google Scholar]
- 47.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: A review. J Expl Psychol: Appl. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 48.Banks S, Catcheside P, Lack L, Grunstein RR, McEvoy RD. Low levels of alcohol impair driving simulator performance and reduce perception of crash risk in partially sleep deprived subjects. Sleep. 2004;27:1063–7. doi: 10.1093/sleep/27.6.1063. [DOI] [PubMed] [Google Scholar]
- 49.Philip P, Ghorayeb I, Stoohs R, et al. Determinants of sleepiness in automobile drivers. J Psychosom Res. 1996;41:279–88. doi: 10.1016/0022-3999(96)00127-4. [DOI] [PubMed] [Google Scholar]
- 50.Philip P, Taillard J, Guilleminault C, Quera Salva MA, Bioulac B, Ohayon M. Long distance driving and self-induced sleep deprivation among automobile drivers. Sleep. 1999;22:475–80. doi: 10.1093/sleep/22.4.475. [DOI] [PubMed] [Google Scholar]
- 51.Stutts JC, Wilkins JW, Osberg JS, Vaughn BV. Driver risk factors for sleep-related crashes. Accid Anal Prev. 2003;35:321–31. doi: 10.1016/s0001-4575(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 52.De Valck E, Cluydts R. Slow-release caffeine as a countermeasure to driver sleepiness induced by partial sleep deprivation. J Sleep Res. 2001;10:203–9. doi: 10.1046/j.1365-2869.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 53.De Valck E, De Groot E, Cluydts R. Effects of slow-release caffeine and a nap on driving simulator performance after partial sleep deprivation. Percept Mot Skills. 2003;96:67–78. doi: 10.2466/pms.2003.96.1.67. [DOI] [PubMed] [Google Scholar]
- 54.Lenne MG, Dwyer F, Triggs TJ, Rajaratnam S, Redman JR. The effects of a nap opportunity in quiet and noisy environments on driving performance. Chronobiol Int. 2004;21:991–1001. doi: 10.1081/cbi-200035956. [DOI] [PubMed] [Google Scholar]
- 55.Macchi MM, Boulos Z, Ranney T, Simmons L, Campbell SS. Effects of an afternoon nap on nighttime alertness and performance in long-haul drivers. Accid Anal Prev. 2002;34:825–34. doi: 10.1016/s0001-4575(01)00089-6. [DOI] [PubMed] [Google Scholar]
- 56.Newcombe JP, Desai A, Joffe D, Engleman HM, Seale JP, Grunstein RR. Modafinil improves alertness and driving simulator performance in sleep-deprived mild obstructive sleep apnoea (OSA) patients. Sleep. 2001;24:A260–1. [Google Scholar]
- 57.Horne JA, Baulk SD. Awareness of sleepiness when driving. Psychophysiology. 2004;41:161–5. doi: 10.1046/j.1469-8986.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 58.Van Dongen HP, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med. 2004;75:A147–54. [PubMed] [Google Scholar]
- 59.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 60.Caldwell JA, Mu Q, Smith JK, et al. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behav Neurosci. 2005;119:694–707. doi: 10.1037/0735-7044.119.3.694. [DOI] [PubMed] [Google Scholar]
- 61.Mu Q, Mishory A, Johnson KA, et al. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–46. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- 62.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. JAMA. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 63.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–4. [PubMed] [Google Scholar]
- 64.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 66.Rogers NL, Szuba MP, Staab JP, Evans DL, Dinges DF. Neuroimmunologic aspects of sleep and sleep loss. Semin Clin Neuropsychiatry. 2001;6:295–307. doi: 10.1053/scnp.2001.27907. [DOI] [PubMed] [Google Scholar]
- 67.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 68.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–24. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 69.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 70.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 71.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 72.Rogers NL, Price NJ, Mullington JM, Szuba MP, Van Dongen HP, Dinges DF. Plasma cortisol changes following chronic sleep restriction. Sleep. 2000;23:A70–1. [Google Scholar]
- 73.Ecker AJ, Schaechter J, Price NJ, et al. Changes in plasma melatonin secretion following chronic sleep restriction. Sleep. 2000;23:A184–5. [Google Scholar]
- 74.Orthmann JL, Rogers NL, Price NJ, et al. Changes in plasma growth hormone levels following chronic sleep restriction. Sleep. 2001;24:A248–9. [Google Scholar]
- 75.Spiegel K, Leproult R, Colecchia EF, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol. 2000;279:R874–83. doi: 10.1152/ajpregu.2000.279.3.R874. [DOI] [PubMed] [Google Scholar]
- 76.Spiegel K, Leproult R, Tasali E, Penev P, Van Cauter E. Sleep curtailment results in decreased leptin levels and increased hunger and appetite. Sleep. 2003;26:A174. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 77.Mullington JM, Chan JL, Van Dongen HPA, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 78.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 80.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–2. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 81.Balachandran DD, Ewing SB, Murray BJ, LeBeau L, Mullington JM. Human host response during chronic partial sleep deprivation. Sleep. 2002;25:A106–7. [Google Scholar]
- 82.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 83.Vgontzas AN, Bixler EO, Papanicolaou DA, Chrousos GP. Chronic systemic inflammation in overweight and obese adults. JAMA. 2000;283:2235. doi: 10.1001/jama.283.17.2235. author reply 2236. [DOI] [PubMed] [Google Scholar]
- 84.Appels A, Devos Y, Vandiest R, Hoppner P, Mulder P, Degroen J. Are sleep complaints predictive of future myocardial-infarction. Activitas Nervosa Superior. 1987;29:147–51. [PubMed] [Google Scholar]
- 85.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: Psychosocial predictors from a 20-year follow-up of women in the Framingham study. Am J Epidemiol. 1992;135:854–64. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 86.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz SW, Cornoni-Huntley J, Cole SR, Hays JC, Blazer DG, Schocken DD. Are sleep complaints an independent risk factor for myocardial infarction? Ann Epidemiol. 1998;8:384–92. doi: 10.1016/s1047-2797(97)00238-x. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Tanaka H, Group TFHS Overtime work, insufficient sleep, and risk of non-fatal acute myocardial infarction in Japanese men. J Occup Environ Med. 2002;59:447–51. doi: 10.1136/oem.59.7.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tenkanen L, Sjoblom T, Harma M. Joint effect of shift work and adverse life-style factors on the risk of coronary heart disease. Scand J Work Environ Health. 1998;24:351–7. doi: 10.5271/sjweh.355. [DOI] [PubMed] [Google Scholar]
- 90.Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 91.Knutsson A, Hallquist J, Reuterwall C, Theorell T, Akerstedt T. Shiftwork and myocardial infarction: a case-control study. Occup Environ Med. 1999;56:46–50. doi: 10.1136/oem.56.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koller M. Health risks related to shift work. Int Arch Occ Environ Health. 1983;53:59–75. doi: 10.1007/BF00406178. [DOI] [PubMed] [Google Scholar]
- 93.Shamsuzzaman ASM, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]