Abstract
Study Objectives:
We have previously shown that healthy older volunteers react with an attenuated frontal predominance of sleep electroencephalogram (EEG) delta activity in response to high sleep pressure. Here, we investigated age-related changes in homeostatic sleep regulation under low sleep pressure conditions, with respect to regional EEG differences and their dynamics.
Design:
Analysis of the sleep EEG during an 8-hour baseline night, during a 40-hour multiple nap protocol (150 minutes of wakefulness and 75 minutes of sleep) and during the following 8-hour recovery night under constant posture conditions.
Setting:
Centre for Chronobiology, Psychiatric University Clinics, Basel, Switzerland
Participants:
Sixteen young (20–31 years) and 15 older (57–74 years) healthy volunteers
Interventions:
N/A.
Measurements and Results:
All-night EEG spectra revealed an increase in spindle activity (13–15.25 Hz) for both age groups, but only in the young did we find a significant decrease of delta activity (0.5–1.25 Hz) in response to low sleep pressure conditions, predominantly in occipital brain regions. However, delta activity during the first non-rapid eye movement (NREM) sleep episode was equally reduced in both age groups. This response lasted significantly longer in the young (across the first 2 NREM sleep episodes) than in the older participants (only the first NREM sleep episode).
Conclusion:
The initial EEG delta response to low sleep pressure was similar in healthy older and young participants. Therefore, age-related sleep deteriorations cannot solely be attributed to alterations in the homeostatic sleep-regulatory system. It is, rather, the interplay of circadian and homeostatic factors of sleep regulation, which is changed with aging.
Citation:
Munch M; Knoblauch V; Blatter K et al. Is homeostatic sleep regulation under low sleep pressure modified by age? SLEEP 2007;30(6):781-792.
Keywords: Low sleep pressure, nap protocol, EEG slow-wave activity, EEG spectral analysis, constant routine
INTRODUCTION
Human sleep undergoes homeostatic regulation that is functionally expressed by sleep need and sleep propensity.1 With elapsed time awake, the power of low-frequency components (< 8 Hz) in the human electroencephalogram (EEG) accumulates exponentially and decreases during the following sleep episode.1,2 These reliable physiologic hallmarks reflect a high degree of synchronization in thalamocortical and cortical neuronal firing patterns3 and may be functionally linked with restoration processes, whose underlying mechanisms remain to be elucidated.4,5 There is mounting evidence that genetic factors6,7 and processes related to synaptic plasticity5 are involved in the regulation of slow wave sleep (SWS) and slow-wave activity (SWA; EEG frequency range between 0.75 and 4.5 Hz) and thus in sleep homeostasis.
Challenging sleep-homeostatic processes in humans by prolonged wakefulness (sleep deprivation) has revealed an increase of both EEG theta activity (4.5–8 Hz) during wakefulness8—10 and SWA at the beginning of the recovery sleep episode, predominantly in frontal brain areas.11 In contrast, lowering homeostatic sleep pressure by naps lead to a decrease in SWA12 and EEG theta activity13 during the postnap night.13–15 In contrast to a frontal predominance of the relative SWA increase after sleep deprivation, we have evidence for an “occipital predominance” of relative reduction in SWA in response to low sleep pressure in young participants.14 Taken together, the timing as well as the duration of the scheduled sleep or nap episodes in the above-mentioned studies determines the level of SWA during the following sleep episodes.12–16
Healthy aging is known to be accompanied by less consolidated sleep,17 more daytime napping, and higher sleepiness during the day.18 Additionally, most studies have reported advanced bed- and wake-up times in healthy elderly participants,17,19 and some of them an altered circadian phase angle between habitual wake time and circadian phase markers such as melatonin or core body temperature.20 Besides an attenuation of circadian amplitude with age, an impairment of the homeostatic sleep/wake regulatory system in the elderly has been suggested to contribute to the above mentioned age-related changes.17 The homeostatic SWA response to sustained wakefulness in older participants is comparable with, but in several details not congruent, to that of younger ones. Older participants very consistently show reduced absolute SWA (and SWS) levels during both baseline and recovery nights compared to the young.21,22 It remains controversial whether this general decrease in SWA in the elderly reflects an age effect per se (eg, generally altered cortical functions; frontal aging hypothesis) or whether it reflects an additional age-related dysfunction of the sleep-wake homeostatic system.
Results from studies on homeostatic response to high sleep pressure in older and young participants have not been consistent. After 25 hours of sleep deprivation, elderly have been shown to have a significantly lower rebound of SWA during the recovery night.23 However, since the sleep-deprivation episode in this study ended at the habitual wake time, the circadian change imposed by this protocol precludes direct interpretation of the sleep homeostatic response per se. We recently found a significantly attenuated frontal predominance of EEG delta-power rebound during the recovery night in older volunteers in response to 40 hours of sleep deprivation ending at habitual bedtime.24 Moreover, the dissipation of SWA in the course of the recovery night after sleep deprivation exhibited a shallower decline in the elderly.24
So far, rather few experiments under low sleep pressure conditions and with older volunteers have been conducted.16, 25–29 To our knowledge, only one group has compared the postnap sleep episode of young and older participants.15 Here, a similar homeostatic sleep response to a daytime nap (repeated at 4 different times of day) was found in younger and older volunteers, as indexed by equally reduced relative EEG delta (0.3–3 Hz) power during the following recovery nights.15
Our first analyses of data from the 40-hour nap protocol indicated that our older participants were sleepier in the late afternoon and evening (wake maintenance zone28) than the young, which corroborates the findings of Haimov and Lavie.25 Day-night differences in the lower alpha and spindle range were less pronounced than in younger participants, even though the total amount of sleep was not different, nor did the duration of wakefulness during sleep episodes differ across naps between the age groups (for more results see28, 30). Based on our findings, we interpreted the higher sleepiness levels and the higher amount of total sleep time during the wake maintenance zone in the elderly as an age-related decline of the circadian arousal signal opposing homeostatic sleep pressure in the evening, which could have led to a higher sleep propensity in the older participants at this time of day.28 Since the total duration of prior sleep and wakefulness across the nap protocol was equal for both age groups,28,30 and if the circadian arousal signal in the evening undergoes an age-related weakening (as we earlier stated 28, 30), we assume that sleep propensity and thus sleep pressure before the recovery might be higher in older participants. This would result in higher relative SWA levels in the older than in the young participants, particularly at the beginning of the recovery period. Therefore, we focused on the question whether homeostatic sleep regulation shows age-related alterations under low sleep pressure conditions with respect to its dynamics and regional differences during the recovery night. To test the influence of low sleep pressure conditions in both age groups, EEG sleep stages and EEG power spectra in baseline and recovery nights were compared under constant-posture conditions. In order to assess the achieved level of (low) sleep pressure in both age groups, sleep stages and SWA during the naps were compared with those during the baseline and the recovery night.
METHODS
Study Participants
Potential study participants were recruited via advertisements in newspapers and at different Swiss Universities. Sixteen young (8 women, 8 men; age range 20–31 years; 25.3 ± 3.3 years; mean ± SD) and 15 older (7 women, 8 men; age range 57–74 years; 65.1 ± 5.6 years; mean ± SD) volunteers were selected for the study. The screening procedure included detailed questionnaires; a medical examination; and, for the older group, a neuropsychological assessment (CANTAB® test battery and the Stroop Test) to exclude motor, attention, or memory impairments. A screening night was recorded to exclude the presence of sleep disorders. The inclusion criteria were a sleep efficiency of at least 80%, fewer than 10 periodic leg movements per hour, and an apnea-hypopnoea index less than 10. Only participants without any medication (with the exception of 4 younger women using oral contraceptives) were included in the study. The drug-free status was verified by urinary toxicologic analysis (Drug-Screen Card Multi-6®; von Minden, Moers, Germany) for the young participants. All study participants were free from medical, psychiatric, and sleep disorders; were nonsmokers; and had no shift work or transmeridian flights during the last 3 months before the study began. Extreme chronotypes, as assessed by the morningness-eveningness type questionnaire31 were excluded. Older volunteers had slightly but significantly higher ratings (earlier chronotypes) than the younger group (18.8 ± 3.0 vs 16.3 ± 3.3; mean ± SD; t-test, P < 0.05). The Pittsburgh Sleep Quality Index (inclusion criterion score ≤ 5 was also slightly higher in the older than in the younger participants (3.4 ± 1.7 vs 2.1 ± 1.3; mean ± SD; t-test, P < 0.05). Younger female participants were studied during the follicular phase of their menstrual cycle. All participants were paid to participate and gave their signed informed consent. The study protocol, screening questionnaires, and consent form were approved by the local Ethics Committee and conformed with the Declaration of Helsinki.
Protocol
One week before the study began (baseline week) participants were asked to abstain from excessive caffeine and alcohol consumption (at most one caffeine-containing beverage per day and fewer than five alcoholic beverages per week were allowed). They were instructed to keep a regular sleep-wake schedule during the baseline week at home (ie, bedtimes and wake times within ± 30 minutes of a self-selected target time between 10:00 pm and 2:00 am) prior to admission to the laboratory. Compliance was verified by sleep logs and ambulatory activity measurements (wrist activity monitor, Cambridge Neurotechnology Ltd®, UK). The timing of the sleep-wake schedule during the protocol was adjusted to individual habitual bedtimes. For each participant, habitual bedtime was calculated by centering the approximately 8-hour sleep episodes during the baseline week at their midpoint. Habitual bedtimes did not significantly differ between the age groups: 11:39 pm ± 52 minutes (younger) vs 11:11 pm ± 40 minutes (older; mean ± SD; t-test: P= 0.1). In the nights preceding the study, the young participants slept on average 8 hours 3 minutes ± 25 minutes (n = 16) and, the older participants, 8 hours 15 minutes ± 32 minutes (n = 15; mean ± SD; P > 0.2, 1-way analysis of variance [ANOVA]).
The protocol comprised two baseline sleep episodes in the chronobiology laboratory, followed by a 40-hour multiple nap protocol with 10 alternating sleep-wake cycles of 75/150 minutes' duration and one recovery sleep episode (Figure 1). Baseline and recovery nights were scheduled at individual habitual bedtimes. Polysomnographic recordings and constant posture started in the afternoon after the first baseline night. Henceforth, participants remained in dim-light conditions (< 8 lux during wakefulness, 0 lux during sleep episodes) under constant semirecumbent posture position in bed with regular meals (for details of the protocol see9). The older participants received a daily low-dose heparin injection (Fragmin®, 0.2 mL, 2500 IE/UI, Pfizer AG, Switzerland) in order to prevent any venous thrombosis. All participants lived under conditions in which they received no time cues throughout the study, nor did they know how long any of the sleep and wake episodes lasted.
Figure 1.
Schematic illustration of the study protocol with two 8-hour baseline (BL) sleep episodes, ten 75-minute naps and one 8-hour recovery sleep episode (REC). Black bars (0 lux) indicate scheduled sleep episodes and white bars episodes of scheduled wakefulness (< 8 lux). Hatched bars delineate the time of controlled posture position (semirecumbent during wakefulness and supine during sleep).
Sleep Recordings and Data Analysis
Sleep episodes were polysomnographically recorded using the Vitaport Ambulatory system (Vitaport-3 digital recorder TEMEC Instruments BV, Kerkrade, the Netherlands). Twelve EEG derivations (F3, F4, Fz, C3, C4, Cz, P3, P4, Pz, O1, O2, Oz referenced against linked mastoids), two electrooculograms, one submental electromyogram, and one electrocardiogram were recorded. All EEG signals were filtered at 30 Hz (fourth-order Bessel-type antialiasing low-pass filter, total 24 dB/Oct), and a time constant of 1.0 second was used prior to online digitization (range 610 μ V, 12 bit AD converter, 0.15 μV/bit; storage sampling rate at 128 Hz). The raw signals were stored online on a Flash RAM Card (Viking, Rancho Santa Margarita, Calif) and downloaded offline to a PC hard drive. All sleep episodes were visually scored on a 20-second epoch basis according to standard criteria.32 EEGs were subjected to spectral analysis using a fast Fourier transform (10% cosine 4-second window), which resulted in a 0.25-Hz resolution. EEG artifacts were detected by automated artifact-detection software (CASA, 2000 Phy Vision BV Kerkrade, The Netherlands). Artifact-free 4-second epochs were averaged over 20-second epochs and matched with the 20-second epochs of the visual sleep scoring.
Sleep stages (1–4), rapid eye movement (REM) sleep, wakefulness, and movement time (MT) were expressed as percentage of total sleep time (TST) during the respective night for all participants (Σ stages 1–4, REM sleep). TST and sleep latencies and REM sleep latency (RL) were indicated in minutes. All sleep latencies (latency to stage 1[SL1], latency to stage 2 [SL2], RL) were logtransformed before statistical analysis. Sleep efficiency (SE) was defined as follows: SE = TST/time between lights off and lights on × 100. Wakefulness after lights off (WALO; % of TST) and wakefulness after sleep onset (WASO; % of TST) were also measured. Non-rapid eye movement (NREM) sleep was defined as stages 2 to 4 (% of TST). In order to compare sleep stages during the 10 naps with those in the baseline night, all sleep stages were additionally expressed as percentage of TST during the baseline night.
EEG spectra during the baseline and recovery nights were calculated in the frequency range from 0.5 to 25 Hz for the midline derivations (Fz, Cz, Pz, and Oz) on log-transformed data. The recovery night values were expressed as percentage of baseline mean. For graphical illustration, the log-transformed mean values per participant were averaged for each age group separately and then retransformed. Sex differences in EEG power density were assessed by repeated-measures ANOVAs (rANOVA), which yielded a significant 3-way interaction (sex × age group × derivation) for relative EEG power density in the frequency range between 0.5 and 1 Hz (3-way rANOVA; F3,81 > 2.8, P < 0.05). However, posthoc comparisons (Duncan's multiple range test) between men and women for the different EEG derivations and age groups revealed no significant sex differences in this frequency range (0.5–1 Hz; P >0.1).
NREM/REM sleep cycles were defined according to the criteria of Feinberg and Floyd,33 with the exception that, for the last sleep cycle, no minimum REM-sleep duration was required. Thereafter, each sleep cycle was divided into 10 equal time intervals during NREM sleep and 4 equal time intervals during REM sleep.
Accumulated SWA was binned into 15-minute intervals during 11 naps (the first 75 minutes of the recovery night were considered as an additional nap) and into 30-minute intervals for baseline and recovery nights, respectively. Accumulated SWA was expressed as percentage of baseline means for all participants (during NREM sleep; for Fz). The rANOVA for SWA accumulation during baseline and recovery nights was done for the first 7 hours (in order to include all participants in the analysis).
To investigate the decay of EEG delta-power density (0.5–1.25 Hz) across the baseline and the recovery night in young and older volunteers, an exponential decay function was fitted to the data of all participants and NREM cycles: delta = delta0 × e(−rt); with delta0 = intercept on the y axis, delta = mean EEG delta power per sleep cycle, r = slope of the decay, t = average timing of the cycle midpoint.
To evaluate the lowering of sleep pressure in both age groups, we simulated the homeostatic build-up of sleep pressure across the baseline night, 10 naps, and the recovery night with an exponential rising component of process S during wakefulness and an exponential decay rate during sleep 1,8 (data not shown). The simulation included all sleep stages and REM sleep, and was performed based on the formula: Asim,t = Smax −(Smax − Asim,0)× exp(−t/τ). Asim,t is the simulated value of Process S at time t, Asim,0 the initial value at time 0, and Smax represents the asymptote of the exponential function. The time constant was 18.8 hours during wakefulness and 4.2 hours during sleep, according to 1,8. The simulations were based on the 20-second epoch visual-scoring data. For the simulated data, we found no differences between young and older participants in the lowering of sleep pressure after the 10 naps (2-way rANOVA: age × elapsed time; P >0.3).
Statistics
The statistical packages SAS ® (SAS Institute, Inc., Cary, NC; Version 6.12) and Statistica ® (StatSoft Inc., STATISTICA for windows, Tulsa, Okla, Version 6.1) were used. EEG power density was averaged during NREM sleep and expressed as log ratio (recovery night/baseline night) per participant. 1-, 2- and 3-way rANOVAs were used with the categorical factor age group (young vs older) and the repetitive factors derivation, time interval, or sleep cycle. All P values derived from rANOVAs were based on Huynh-Feldt corrected degrees of freedom, but the original degrees of freedom are reported. For posthoc comparisons, Duncan's multiple range test and t-tests were performed. If normal distribution was lacking, a non-parametric test was applied (Mann-Whitney U). All posthoc comparisons were corrected for multiple comparisons according to Curran-Everett.34
RESULTS
Sleep Stages
Recovery Versus Baseline Sleep Episode
Visually scored sleep stages during the baseline and recovery (expressed as percentage of TST during the respective night) of the young (n = 16) and older (n = 15) participants are summarized in Table 1A. The older participants had significantly less TST; less stage 3, 4, and SWS; and a lower SE than the young in both the baseline and recovery night (P < 0.05; 2-way rANOVA: main effect of age group). Furthermore, older participants were significantly longer awake (WALO, WASO) and had more stage 2 sleep (P < 0.05; main effect of age group) during both nights. The interaction between the factors age group and night yielded significance for stage 2, 4, and SWS (P < 0.05; 2-way rANOVA). This interaction came about via a reduction of stage 2 during the recovery in comparison to the baseline night in the older participants, whereas the amount of stage 2 sleep in the recovery night remained unchanged in the young (P < 0.05; Duncan's multiple range test). For SWS, the young participants responded to the multiple nap protocol with less SWS in the recovery night, while the older participants showed no changes (P < 0.05; Duncan's multiple range test).
Table 1A.
Sleep Stages Derived From Visual Scoring for both Age Groups, Averaged Across Baseline and Recovery Night
| Parameter | Young |
Older |
2-way rANOVA |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Recovery | Baseline | Recovery | Age F1,29 | P | Night F1,29 | P | Age × Night F1,29 | P | |
| TST, min | 441.4 ± 25.1 | 413.7 ± 47.0 | 408.9 ± 46.1 | 368.7 ± 54.1 | 8.1 | * | 16.9 | ** | 0.6 | |
| SE, % | 92.0 ± 5.1 | 86.2 ± 9.8 | 85.2 ± 9.6 | 76.9 ± 11.3 | 8.1 | * | 16.9 | ** | 0.5 | |
| WALO, % | 6.1 ± 5.4 | 13.7 ± 13.6 | 16.8 ± 13.7 | 29.2 ± 21.7 | 9.35 | * | 10.7 | * | 0.7 | |
| WASO, % | 4.0 ± 4.8 | 8.3 ± 9.3 | 14.5 ± 12.2 | 22.9 ± 20.3 | 11.1 | * | 5.7 | * | 0.6 | |
| MT, % | 2.9 ± 1.7 | 4.0 ± 3.1 | 2.2 ± 1.2 | 3.9 ± 2.8 | 0.27 | 11.1 | * | 0.6 | ||
| Sleep Stage, % of TST | ||||||||||
| 1 | 12.3 ± 4.6 | 14.4 ± 4.9 | 14.1 ± 4.9 | 15.6 ± 5.6 | 0.9 | 4.8 | * | 0.1 | ||
| 2 | 49.7 ± 5.0 | 51.3 ± 5.9 | 60.8 ± 9.4 | 57.4 ± 10.2 | 10.9 | * | 0.7 | 5.3 | * | |
| 3 | 10.2 ± 3.9 | 9.4 ± 2.8 | 6.7 ± 4.8 | 7.0 ± 4.3 | 4.6 | * | 0.3 | 2.0 | ||
| 4 | 7.5 ± 5.4 | 5.5 ± 4.2 | 1.5 ± 2.1 | 1.6 ± 2.5 | 14.9 | ** | 4.6 | * | 5.3 | * |
| SWS | 17.7 ± 6.5 | 14.8 ± 5.6 | 8.2 ± 6.6 | 8.6 ± 6.3 | 13.3 | * | 3.7 | ° | 7.0 | * |
| NREM | 79.7 ± 4.5 | 80.4 ± 5.0 | 83.1 ± 5.1 | 81.6 ± 5.3 | 2.2 | 0.2 | 1.3 | |||
| REM | 20.3 ± 4.5 | 19.6 ± 5.0 | 16.9 ± 5.1 | 18.4 ± 5.3 | 2.2 | 0.2 | 1.3 | |||
| SL1, min | 9.3 ± 5.1 | 20.3 ± 20.1 | 8.6 ± 6.2 | 22.5 ± 13.9 | 0.3 | 27.9 | ** | 2.7 | ||
| SL2, min | 14.7 ± 7.4 | 29.7 ± 20.0 | 12.1 ± 8.2 | 33.3 ± 24.8 | 0.1 | 57.5 | ** | 2.4 | ||
| RL, min | 68.9 ± 18.1 | 80.8 ± 42.1 | 80.1 ± 38.5 | 94.4 ± 33.0 | 1.7 | 1.5 | 0.6 | |||
Sleep stages derived from visual scoring for both age groups, averaged across baseline and recovery nights. N = 16 for young and n = 15 for older participants (mean ± SD). TST refers to total sleep time; SE, sleep efficiency; WALO, wakefulness after lights off; WASO, wake after sleep onset; SWS, slow wave sleep; non-rapid eye movement (NREM) sleep; SL1, latency to stage 1 sleep; SL2, latency to stage 2 sleep; RL, latency to rapid eye movement (REM) sleep. The last 6 columns indicate the F and P values of the respective 2-way rANOVA; *P < 0.05, **P ≤ 0.001, °P < 0.1.
Nap Versus Baseline Sleep Episode
Sleep stages during the naps (expressed as percentage of baseline night) are indicated in Table 1B. The older participants (n = 15) had more NREM sleep (more stage 2 but less stage 3, 4) and less REM sleep during 10 naps than the young (n = 16; 1-way ANOVA; F1,29 = at least 6.6; P < 0.05; main effect of age group). TST, SE, and percentage of stage 1 sleep did not differ between age groups (P > 0.1). There was no significant difference within the young and within the older volunteers for stage 4 sleep (P > 0.1; t-test for dependent samples). Both age groups showed significantly less stage 1 sleep and concomitantly more NREM and REM sleep and a higher SE (P < 0.05) during the baseline night in comparison to nap sleep. Only the young group had more stage 3 and SWS during the nap sleep than during the baseline night (P < 0.05), whereas the older slept more during the naps than the baseline night (young: 460.1 ± 75.6 minutes [nap] vs 441.4 ± 25.1 [baseline], P > 0.1; older: 448.3 ± 85.4 [nap] vs 408.9 ± 46 minutes [baseline]; mean ± SD, P < 0.05 t-test for dependent samples).
Table 1B.
Sleep During 10 Naps
| Sleep Parameter | Young | Older | Age F1,29 | P |
|---|---|---|---|---|
| TST (% of TST during baseline night) | 104.2 ± 17.3 | 109.6 ± 12.2 | 0.7 | |
| SE (% of TST during baseline night) | 67.1 ± 11.1 | 69.7 ± 7.9 | 0.4 | |
| Sleep Stages, % of TST during Baseline | ||||
| 1 | 21.7 ± 8.6 | 23.2 ± 9.7 | 0.2 | |
| 2 | 44.6 ± 11.3 | 68.6 ± 12.3 | 32.2 | * |
| 3 | 13.5 ± 4.1 | 7.1 ± 4.9 | 15.7 | * |
| 4 | 9.0 ± 5.9 | 1.3 ± 2.4 | 21.6 | * |
| SWS | 22.4 ± 7.0 | 8.4 ± 6.7 | 32.4 | * |
| NREM | 67.1 ± 12.6 | 77.0 ± 8.6 | 6.5 | * |
| REM | 15.6 ± 7.5 | 8.7 ± 5.8 | 8.0 | * |
TST refers to total sleep time; SE, sleep efficiency; SWS, slow wave sleep; NREM, non-rapid eye movement sleep; REM, rapid eye movement sleep. N = 16 for young and n = 15 for older participants (mean ± SD). F and P values of the respective 1-way ANOVA are indicated.
P < 0.05.
Relative EEG Spectra
Baseline vs Recovery Sleep Episode
Young participants showed significantly less relative EEG power (percentage of baseline night) during the recovery night than the older in some of the frequency bins in the delta (0.5–1.25 Hz) and significantly more relative EEG power in some of the frequency bins in the theta (7.25–7.75 Hz) range (Figure 2; 2-way rANOVA, main effect of age group; P < 0.05). The interaction for the factors age group × derivation yielded significance in the delta-frequency bins (0.5–1 Hz, 1.75–2 Hz, 3–3.5 Hz) and in the alpha-frequency bins (10–10.5 Hz, 10.75–11.25 Hz; P < 0.05). Posthoc comparisons resulted in significantly lower values for younger participants during the recovery night in the delta bins for Cz (0.5–1 Hz), Pz (0.75–1 Hz), and Oz (0.5–1 Hz, 1.75–2 Hz, 3–3.5 Hz; black squares in Figure 2 represent these posthoc comparisons) and concomitantly higher values in the alpha range for Oz (10–10.5 Hz) than for older participants (P < 0.05; t-test and corrected for multiple comparisons34). Finally, the main factor derivation was significant in some frequency bins in the delta (0.5–1 Hz, 1.75–2 Hz), theta (4.25–7.75 Hz), alpha (8–8.5 Hz, 11.25–12 Hz), and sigma range (12–12.75 Hz, 14.75–15 Hz; P < 0.05). A 1-way rANOVA for the averaged SWA over four derivations (Fz, Cz, Pz, Oz) revealed significantly higher SWA (percentage of baseline; on log-ratios) in the older participants during the recovery night than in the young (1-way ANOVA; F1,29 = 6.8; P < 0.05). Both age groups revealed an increased EEG power density during the recovery night in the EEG spindle range between 13.0 and 15.25 Hz when compared to baseline levels (for Fz, Cz, Pz and Oz;P<0.05).
Figure 2.
Relative EEG spectra during recovery night (% of baseline) between 0.5–25 Hz for Fz, Cz, Pz, and Oz for young (open circles; n = 16) and older participants (black circles; n = 15). Black squares illustrate significant posthoc comparisons between the young and older age group (P < 0.05; t-test corrected for multiple comparisons).
In a next step, relative EEG spectra per NREM-REM sleep cycle (percentage of baseline night cycle) were calculated for both age groups for the midline derivations (Figure 3). Because not all participants completed 4 sleep cycles, the analyses were restricted to the first 3. A 3-way rANOVA with the factors age group, derivation, and sleep cycle reached significant interaction only for three 0.25-Hz frequency bins: 1.25 to 1.5 Hz, 9.5 to 9.75 Hz, and 10 to 10.25 Hz (P < 0.05). Therefore, each sleep cycle was analyzed separately (Figure 3). The main effect of age group was significant during NREM sleep episode 1 for frequency bins in the sigma range (15.25–15.5 Hz,16.5–16.75 Hz) and for NREM sleep episode 2 in the delta range (0.5–1.25 Hz), alpha range (8.25–9.75 Hz, 10.5–10.75 Hz), and sigma range (11–13.25 Hz, 14.25–15.5 Hz). For NREM sleep episode 3, some frequency bins in the theta (4.25–4.5 Hz, 4.75–5 Hz, 5.25–5.75 Hz, 6–7.75 Hz), alpha (9–9.25 Hz), and sigma frequency range yielded significance (15.5–15.75 Hz; P < 0.05; 2-way rANOVA). The interaction with the factors age group × derivation yielded significance for some frequency bins in the alpha range (9.75–11 Hz, 10.25–10.5 Hz) for cycle 2 and 3 (P < 0.05), and posthoc comparisons revealed significantly higher values for the younger volunteers in Pz and Oz during the second and for Oz during the third sleep cycle (P < 0.05; t-test for independent samples).
Figure 3.
EEG power spectra (% of baseline [BL] night cycle) per NREM/REM cycle 1–3 between 0.5–25 Hz for Fz, Cz, Pz and Oz in young (open circles; n = 16) and older participants (black circles; n = 15). Black circles at the bottom indicate significant frequency bins for which the factor age yielded significance; white circles indicate those frequency bins for which the interaction age × derivation yielded significance (2-way rANOVA per sleep cycle; P < 0.05).
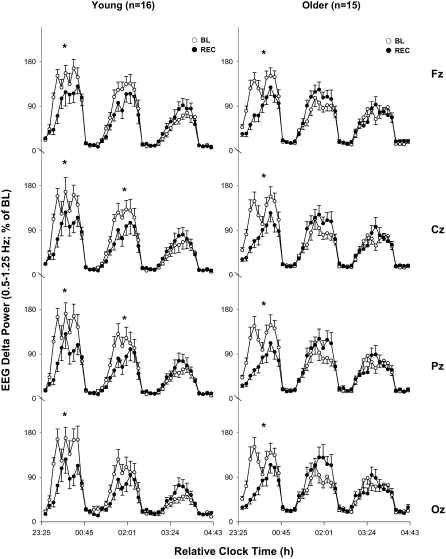
To investigate the time course of EEG delta activity in more detail, percentiles (see Methods) in the frequency range between 0.5 and 1.25 Hz were expressed as a percentage of the baseline mean and calculated for NREM-REM sleep cycle 1 to 3 in Fz, Cz, Pz, and Oz (Figure 4). These EEG bands were chosen based on the significant main effect of age group across the relative recovery (percentage of baseline) night in this frequency range. A 4-way rANOVA (age group, cycle, night and derivation) was not significant (P = 0.2), and, therefore, each derivation was analyzed separately. A 3-way rANOVA (age group × cycle × night) per derivation revealed significant interaction for Fz, Cz, Pz, and Oz (P < 0.05; F at least 3.3). These interactions most likely reflect the age-related difference in the time course of the 0.5- to 1.25-Hz band across the first 3 NREM-REM sleep cycles. Posthoc comparisons within each age group separately (Figure 4) indicated for both young and older participants a significant decrease in EEG delta power during the first NREM sleep episode in the recovery night compared to the baseline for all derivations along the midline. This significant decrease in EEG delta power continued into the second NREM sleep episode in the young (Cz, Pz; P < 0.05; tendency for Fz and Oz P < 0.1; Duncan's multiple range test) but not in the older participants. The lengths of the first three sleep cycles during the baseline and recovery nights did not significantly differ between both age groups in any cycle (2-way rANOVA; P > 0.1, data not shown).
Figure 4.
EEG delta activity (0.5–1.25 Hz) per sleep cycle (1–3) for midline derivations (Fz, Cz, Pz and Oz). The left hand panels shows mean values per NREM/REM sleep cycle for the young (n = 16) and the right hand panels those for the older age group (n = 15; mean + or − SEM). White circles indicate the baseline (BL) and filled circles the recovery (REC) night. Asterisks indicate significant differences between mean values per cycle between baseline and recovery night within each group (* P < 0.05; Duncan's multiple range test).
In order to analyze the decay function of EEG delta power for young and older participants during both nights, the mean value of all sleep cycles in the relative EEG delta range (0.5–1.25 Hz; percentage of baseline night) of all sleep cycles was fitted with a nonlinear regression function (see Methods). Figure 5 shows the fitted decline of both age groups during the baseline and recovery night for Fz (1 data point is missing in Figure 5 because it differed more than 2 SD from the next.) The missing data point was higher and came from one young participant in sleep cycle 2 (eg, 2.84 hours after sleep onset). Table 2 depicts the values of the decay rates for both groups, Fz. The mean values of the baseline slopes did not overlap with the 95% confidence interval of recovery nights within young and older participants; neither did the mean decay of the baseline night of one group reach the 95% confidence interval of the baseline night of the other age group. A slight overlap occurred between the mean recovery night values of the older with the 95% confidence interval of the recovery night in the young group (Figure 5, Table 2). The goodness of fit is indexed by R-values for the respective regression curve. The estimation of the delta decay rates during the recovery night performed for Fz, Cz, Pz and Oz revealed R-values ≤ 0.5 for both age groups (for Fz see Table 2).
Figure 5.
Fitted exponential decay function [delta = delta0 × e(−rt)] to relative EEG delta power (0.5–1.25 Hz; percentage of baseline night) NREM sleep across all NREM sleep episodes for Fz. Left-hand panel: young (n = 16), right-hand panel: older participants (n = 15). Open circles and dashed lines indicate baseline (BL) night values, and, filled circles together with solid lines, the values for the nonlinear regression during the recovery (REC) night (one data point of a younger participant during the baseline night is not shown, was denoted as an outlier, see results).
Table 2.
Nonlinear Regression Analysis for the Decay of EEG Delta Power During Baseline and Recovery Nights in Young and Older Participants
| Young |
Older |
|||
|---|---|---|---|---|
| Baseline | Recovery | Baseline | Recovery | |
| Estimated Decay Rate, /h | 0.209 ± 0.018 | 0.119 ± 0.024 | 0.144 ± 0.017 | 0.074 ± 0.02 |
| 95% Confidence Interval | 0.174–0.244 | 0.072–0.166 | 0.111–0.177 | 0.035–0.113 |
| R value | 0.85 | 0.53 | 0.75 | 0.44 |
| P < 0.05 | * ** | * ** | * ** | * |
Parameters of the nonlinear regression analysis for the decay of EEG delta power during baseline and recovery nights for both age groups are indicated for Fz during all NREM sleep episodes (mean of all cycles ± SEM). The 95% confidence intervals are shown for both age groups and nights; *indicates the lack of overlap of mean values with the 95% confidence intervals between baseline and recovery night within the respective age groups and **between young and older volunteers for the baseline and recovery night (P < 0.05).
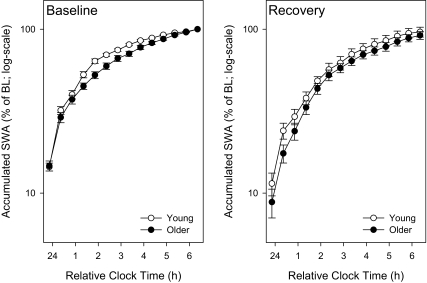
Figure 6A shows the accumulated relative SWA (percentage of baseline mean; on a log-scale) during the first 7 hours of the baseline and recovery sleep episode (30-minute intervals; n = 16 for the young and n = 15 for the older group). There was no significant interaction between the factors age × night × time interval (3-way rANOVA; P > 0.2), nor any other interaction with the factor age.
Figure 6A.
Accumulated relative SWA (% of baseline night; on log-scales; for Fz) during the baseline (BL) and the recovery (REC) night for young (open circles; n = 16) and older participants (filled circles; n = 15) (binned into 30-min intervals; mean ± SEM).
Baseline Night vs Naps
The accumulation of relative SWA (percentage of baseline mean) within 11 naps (nap 11 = first 75 minutes of recovery night) is shown for both age groups across 15-minute intervals in Figure 6B. In the older group, significantly higher SWA values were found in three time intervals during the first nap and in two and three time intervals, respectively, during nap 4 and 10 (coincided with the wake maintenance zone in the evening), as well as during nap 6 and 7. Lower relative SWA occurred in the older participants during nap 5 (P < 0.05; all Mann-Whitney U test and corrected for multiple comparisons). Young participants had, on average, significantly more accumulated SWA across the nap sleep episode than the older group (P < 0.05; 1-way ANOVA with mean values of accumulated SWA during 10 naps for Fz; F1,29 = 11.8, on log-ratios; n = 16 for the young and n = 15 for the older).
Figure 6B.
Accumulated SWA (% of baseline [BL] mean) during 10 naps and the first 75 minutes of the recovery night for young (open circles; n = 16) and older participants (filled circles, n = 15), binned into 15-minute intervals for Fz (mean ± SEM; * P < 0.05).
DISCUSSION
We have investigated the impact of low sleep pressure on sleep structure and spectral components of the sleep EEG in young and older volunteers. The main differences in the spectral EEG components between the age groups across the entire recovery night occurred in EEG frequency bins of the delta and alpha range: the young participants responded with a significant relative decline of EEG delta power, predominantly in more occipital brain regions. This occipital predominance of the SWA response to low sleep pressure found in the younger participants was not present in the older group. This came about an altered time course of EEG delta activity during the recovery night, such that during the first NREM sleep cycle EEG delta power declined equally in both age groups but increased significantly earlier (eg, during the second sleep cycle) in the older participants. Thus, our results turned out to be more complex than originally hypothesized and did not unambiguously show a weaker homeostatic EEG delta response during the recovery in the elderly when compared to the young. This suggests that low sleep pressure elicits age-related changes in the sleep EEG during the recovery night different from those after high sleep pressure,24 which is at least true for regional differences (attenuated frontal vs occipital predominance of EEG delta activity) and the time course of EEG delta activity across the night.
The build up of relative SWA during the individual 75-minute naps showed age differences, such that the older participants accumulated more SWA during the first nap and naps coinciding with the wake maintenance zone (nap 4 and 10). Transient changes in the SWA accumulation curve (Figure 6b) during naps occurring in the second half of the biological night and early morning (naps 1, 6, 7) could be explained by the fact that the young participants had more REM sleep at that time of day (eg, greater circadian amplitude in REM sleep propensity).24,30 As the older participants slept more during the first nap after the baseline night might be a consequence that they slept less during the baseline than the young, even though they felt subjectively more alert (please see our previous reports30). The young participants accumulated more SWA in the nap following the first wake maintenance zone (nap 5). We interpret these findings as a weaker evening circadian arousal signal in the older participants, allowing more SWA to build up in the evening compared with in the young.
Indexes for an Age-Related Weakening of the Homeostatic Process
Based on three results in this study, one could argue for a weaker homeostatic sleep regulation with increasing age: first, the less-pronounced EEG delta response to low sleep pressure as observed in the all-night EEG spectra in the older group, second, the earlier increase of EEG delta power in the course of the recovery and, third, the higher subjective sleepiness ratings prior the recovery. Since the amount of prior wakefulness is the most important predictor for sleep EEG delta activity, it could be that the older participants were longer awake during the multiple nap protocol and hence showed a different delta response than the young. This argument can be rejected because we did not observe any significant difference in TST or SE between both age groups across the preceding 10 nap episodes (with all participants included), although the distribution of TST and SE across the scheduled naps was different between the age groups.28 When we only included those participants who could sleep during 8 naps (eg, without nap 4 and 10, because only few of the young were able to sleep during these two naps during the wake maintenance zone14), then the young (n = 8) slept longer than the elderly (n = 11). But this again is a circular argument, as the distribution over time and not the total amount of sleep differed between age groups. Nevertheless, the older participants had less SWS (and stage 3 and 4) during the 10 naps (percentage of TST during baseline) but more NREM and stage 2 than the young, which points to a less deep sleep during the naps with a concomitant higher homeostatic increase of sleep pressure. In line with this is also less accumulated relative SWA across 10 naps in the older participants when compared with the young.
When looking at a subjective correlate of sleep pressure during wakefulness, we found that the older volunteers were sleepier than the young in the afternoon and early evening on the first and also on the second day of the nap protocol.28 Objective evidence for higher sleep pressure during the preceding wake episodes in the elderly will come from the waking EEG. Preliminary results from the high sleep pressure protocol (40-hour sleep deprivation) revealed more EEG power in the low-frequency range (< 7 Hz) in the older participants during the wake maintenance zone.35 Concomitantly, the elderly performed worse during the biological day (ie, outside the melatonin secretory phase, during the first 16 hours after baseline night), as assessed by the Psychomotor Vigilance Test,36 and were sleepier (unpublished data). Therefore, one could argue that the elderly exhibited a faster wake-dependent homeostatic increase of sleep pressure, at least under “normal” sleep pressure conditions. The elderly may live under higher sleep pressure during wakefulness, as has been reported for short sleepers.37 Relative SWA during the entire recovery night (percentage of baseline) was higher in our older group. In line with this is the earlier increase of EEG delta activity during the second sleep cycle, indicating either a shorter lasting response to low sleep pressure or an intrasleep rebound of sleep pressure. The increase in the EEG spindle range was also shorter lasting in the elderly than in the young. Similar immediate intranight rebounds in SWA have been reported after artificial reduction of SWA levels in the first part of the night via acoustic stimuli.38 Interestingly, an altered time course of homeostatic sleep regulation is also suspected in young narcoleptic patients, with an intrasleep increase of SWS during the third sleep cycle after high sleep-pressure conditions.39 It was assumed that this intrasleep rebound reflects a higher sleep need.
Another explanation could be that the circadian signal is attenuated in the elderly and thus weaker in opposing the homeostatic sleep pressure build up during wakefulness. This eventually leads to a more “linear” increase in sleepiness and a less consolidated 16-hour bout of wakefulness, as is normally seen in young participants. In line with a weaker circadian arousal signal are the less-pronounced day-night differences in the lower EEG alpha and spindle frequency range during the nap sleep episodes with less REM sleep,28 as well as a weaker coupling of the circadian rhythm of EEG spindle frequency and sleep propensity to the circadian rhythm of melatonin secretion in the older volunteers.30 Furthermore, we have evidence for an age-related diminution in the circadian rhythms of salivary melatonin28,30 and core body temperature (unpublished data). Thus, we argue that the interaction of the circadian with the homeostatic process plays an important role in age-related changes of sleep regulation and sleep timing, rather than alterations in the homeostatic process per se.
How alterations in the dynamics of EEG delta activity under low sleep pressure are reflected in cellular and molecular mechanisms is not known. According to Saper et al,40 there is some evidence that at least one mechanism for sleep drive is the accumulation of a sleep-promoting substance that enhances the activity of sleep-promoting cells and reduces the activity of wake-promoting neurons. Potential mechanisms for a weakened homeostatic sleep regulation with age might be found in the connection among sleep drive, SWA, and adenosine concentrations in the forebrain. Recently, a reduced sensitivity for adenosine41 and a decline in adenosine A1 receptors42 were found in aged rat brains, which may, for the first time, indicate a weaker responsiveness of the homeostatic system with age. It remains to be elucidated whether this also plays a role in the human central nervous system.
Evidence for Intact Homeostatic Sleep Regulation in Elderly
There was no age difference between relative TST and SE during the naps, and both age groups responded to low sleep pressure with lower SE, longer sleep latencies, and more wakefulness after lights out during the recovery night. Concerning the EEG spectra, which allow quantification of the sleep homeostatic process, a very similar EEG delta power decline during the first NREM cycle was found in both age groups during the recovery night. If we assume that the SWA level at the beginning of the sleep episode is one of the most reliable physiologic markers for accumulated homeostatic sleep pressure,1 we do not have strong evidence for an age difference in homeostatic delta response to low sleep pressure, similar to the findings of Campbell and Feinberg.15 In that study, neither age-related differences in the mean EEG delta response (0.3–3.0 Hz) during the postnap night nor a change in the period-amplitude incidence of EEG delta power were found.15 The increase in EEG power density in the spindle frequency range in response to low sleep pressure was very similar for both age groups in our study, at least at the beginning of the night. This is a further argument for intact homeostatic sleep regulation in the elderly, since EEG power density in the spindle frequency range is under clear homeostatic control.14
The time constants of the fitted decay function for delta dissipation during baseline were different between the age groups, which is in accordance with results of other studies.21,22The same was true for the recovery night, although the goodness of fit, as indexed by the R-values, was rather low for both age groups, indicating that using exponential decay functions is not a very reliable way to fit the delta dissipation after low sleep pressure conditions, particularly in occipital EEG derivations.
CONCLUSION
Our results confirm and further extend the conclusion that agerelated sleep deterioration cannot unambiguously be attributed to a weakening of the homeostatic sleep-regulatory system. The age-related, diminished occipital decline of EEG delta activity in response to low sleep pressure in the all-night EEG spectra was no longer present when we took a closer look at the time course of the delta dissipation across sleep cycles, in which both age groups showed a similar decrease in EEG delta activity during the first sleep cycle. Subsequently, older participants exhibited an earlier intrasleep increase of EEG delta activity. Whether this reflects a homeostatic intrasleep rebound in the elderly, and a possible relationship to the age-related lower sleep consolidation and weakening of homeostatic sleep regulation, remains to be elucidated. We conclude that it is the interaction of circadian with homeostatic processes that play an important role in age-related changes in sleep regulation and sleep timing, rather than alterations in the homeostatic process per se.
ACKNOWLEDGEMENTS
We thank Kurt Krauchi for statistical advice; our technicians Claudia Renz, Marie-France Dattler, and Giovanni Balestrieri for their excellent help in data acquisition; Drs. Carmen Schroder and Corina Schnitzler-Sack for medical screenings; and the volunteers for participating. This research was supported by Swiss National Science Foundation Grants START 3100-055385.98 and 3130-054991.98 to CC, the Velux Foundation (Switzerland) and Bühlmann Laboratories, Allschwil (Switzerland).
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Munch, Knoblauch, Blatter, Wirz-Justice, and Cajochen have indicated no financial conflicts of interest.
REFERENCES
- 1.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 2.Dijk DJ, Beersma DGM, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 4.Benington JH, Heller HG. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 5.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–21. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rétey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci USA. 2005;102:15676–81. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–94. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 9.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol Regulatory Integrative Comp Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 10.Cajochen C, Knoblauch V, Krauchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport. 2001;12:2277–81. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- 11.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–9. [PubMed] [Google Scholar]
- 12.Feinberg I, Maloney T, March JD. Precise conservation of NREM period 1 (NREMP1) delta across naps and nocturnal sleep: implications for REM latency and NREM/REM alternation. Sleep. 1992;15:400–3. doi: 10.1093/sleep/15.5.400. [DOI] [PubMed] [Google Scholar]
- 13.Werth E, Dijk DJ, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol Regulatory Integrative Comp Physiol. 1996;271:501–10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 14.Knoblauch V, Krauchi K, Renz C, Wirz-Justice A, Cajochen C. Homeostatic control of slow-wave and spindle frequency activity during human sleep: effect of differential sleep pressure and brain topography. Cereb Cortex. 2002;12:1092–100. doi: 10.1093/cercor/12.10.1092. [DOI] [PubMed] [Google Scholar]
- 15.Campbell I, Feinberg I. Homeostatic sleep response to naps is similar in normal elderly and young adults. Neurobiol Aging. 2005;26:135–44. doi: 10.1016/j.neurobiolaging.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Feinberg I, Campbell IG. Kinetics of non-rapid eye movement delta production across sleep and waking in young and elderly normal subjects: theoretical implications. Sleep. 2003;26:192–200. doi: 10.1093/sleep/26.2.192. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buysse DJ, Browman KE, Monk TH, Reynolds CF, 3rd, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992;40:779–86. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 19.Carrier J, Monk TH, Reynolds CF, 3rd, Buysse D J, Kupfer DJ. Are age differences in sleep due to phase differences in the output of the circadian timing system? Chronobiol Int. 1999;16:79–91. doi: 10.3109/07420529908998714. [DOI] [PubMed] [Google Scholar]
- 20.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regulatory Integrative Comp Physiol. 1998;275:R1478–87. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 21.Landolt HP, Dijk DJ, Achermann P, Borbèly AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 22.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiol. 2001;38:232–42. [PubMed] [Google Scholar]
- 23.Gaudreau H, Morettini J, Lavoie HB, Carrier J. Effects of a 25-h sleep deprivation on daytime sleep in the middle-aged. Neurobiol Aging. 2001;22:461–68. doi: 10.1016/s0197-4580(00)00251-7. [DOI] [PubMed] [Google Scholar]
- 24.Munch M, Knoblauch V, Blatter K, Schroder C, Schnitzler C, Krauchi K, Wirz-Justice A, Cajochen C. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20:1402–10. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 25.Haimov I, Lavie P. Circadian characteristics of sleep propensity function in healthy elderly: a comparison with young adults. Sleep. 1997;20:294–300. doi: 10.1093/sleep/20.4.294. [DOI] [PubMed] [Google Scholar]
- 26.Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24:680–87. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- 27.Niggemyer KA, Begley A, Monk TH, Buysse DJ. Circadian and homeostatic modulation of sleep in older adults during a 90 minute day study. Sleep. 2004;27:1535–41. doi: 10.1093/sleep/27.8.1535. [DOI] [PubMed] [Google Scholar]
- 28.Munch M, Knoblauch V, Blatter K, Schroder C, Schnitzler C, Krauchi K, Wirz-Justice A, Cajochen C. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26:1307–19. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28:1365–76. doi: 10.1093/sleep/28.11.1365. [DOI] [PubMed] [Google Scholar]
- 30.Knoblauch V, Munch M, Blatter K, Martens W, Krauchi K, Schnitzler C, Schroder C, Cajochen C. Age-related changes in the circadian modulation of sleep-spindle frequency during nap sleep. Sleep. 2005;28:1093–101. doi: 10.1093/sleep/28.9.1093. [DOI] [PubMed] [Google Scholar]
- 31.Torsvall L, Akerstedt T. A diurnal type scale. Construction, consistency and validation in shift work. Scand J Work Environ Health. 1980;6:283–90. doi: 10.5271/sjweh.2608. [DOI] [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda: US Department of Health, Education and Welfare, Public Health Service; 1968. [Google Scholar]
- 33.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiol. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 34.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regulatory Integrative Comp Physiol. 2000;279:R1–8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 35.Cajochen C, Imai M, Munch M, Blatter K, Kobialka S, Knoblauch V, Renz C, Wirz-Justice A. Age-related changes in the dynamics of frontal low-EEG activity during sustained wakefulness. J Sleep Res. 2006;15(Suppl):57–8. [Google Scholar]
- 36.Blatter K, Graw P, Munch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Beh Brain Res. 2006;168:312–17. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Aeschbach D, Postolache TT, Sher L, Matthews JR, Jackson MA, Wehr TA. Evidence from the waking electroencephalogram that short sleepers live under higher homeostatic sleep pressure than long sleepers. Neurosci. 2001;102:493–502. doi: 10.1016/s0306-4522(00)00518-2. [DOI] [PubMed] [Google Scholar]
- 38.Dijk DJ, Beersma D, Daan S, Bloem G, Van Den Hoofdakker RH. Quantitative analysis of the effects of slow wave sleep deprivation during the first 3 h of sleep on subsequent EEG power density. Eur Arch Psychiatry Neurol Sci. 1987;236:323–28. doi: 10.1007/BF00377420. [DOI] [PubMed] [Google Scholar]
- 39.Tafti M, Villemin E, Carlander B, Besset A, Billiard M. Sleep in human narcolepsy revisited with special reference to prior wakefulness duration. Sleep. 1992;15:344–51. doi: 10.1093/sleep/15.4.344. [DOI] [PubMed] [Google Scholar]
- 40.Saper CB, Scammel T, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 41.Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual R J, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–70. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Meerlo P, Roman V, Farkas E, Keijser JN, Nyakas C, Luiten PG. Ageing-related decline in adenosine A1 receptor binding in the rat brain: an autoradiographic study. J Neurosci Res. 2004;78:742–48. doi: 10.1002/jnr.20314. [DOI] [PubMed] [Google Scholar]