Abstract
Study Objectives:
Periodic leg movements in sleep (PLMS) are frequently accompanied by arousals and autonomic activation, but the pathophysiologic significance of these manifestations is unclear.
Design:
Changes in heart rate variability (HRV), HRV spectra, and electroencephalogram (EEG) spectra associated with idiopathic PLMS were compared with changes associated with isolated leg movements and respiratory-related leg movements during sleep. Furthermore, correlations between electromyographic activity, HRV changes, and EEG changes were assessed.
Setting:
Sleep laboratory.
Patients:
Whole-night polysomnographic studies of 24 subjects fulfilling the criteria of either periodic leg movements disorder (n = 8), obstructive sleep apnea syndrome (n = 7), or normal polysomnography (n = 9) were used.
Measurements and Results:
Spectral HRV changes started before all EEG changes and up to 6 seconds before the onset of all types of leg movements. An initial weak autonomic activation was followed by a sympathetic activation, an increase of EEG delta activity, and finally a progression to increased higher-frequency EEG rhythms. After movement onset, HRV indicated a vagal activation, and, the EEG, a decrease in spindle activity. Sympathetic activation, as measured by HRV spectra, was greater for PLMS than for all other movement types. In EEG, gamma synchronization began 1 to 2 seconds earlier for isolated leg movements and respiratory-related leg movements than for PLMS. Significant correlations were found between autonomic activations and electromyographic activity, as well as between autonomic activations and EEG delta activity, but not between higher-frequency EEG rhythms and EMG activity or HRV changes.
Conclusions:
These results suggest a primary role of the sympathetic nervous system in the generation of PLMS.
Citation:
Guggisberg AG; Hess CW; Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. SLEEP 2007;30(6):755-766.
Keywords: Periodic limb movements, heart rate variability, electroencephalogram, sympathetic nervous system, wavelet transform
PERIODIC LEG MOVEMENTS (PLM) ARE REPEATED DORSIFLEXIONS OF THE BIG TOE AND ANKLE,1 OCCURRING DURING WAKEFULNESS AS WELL AS during sleep (PLMS). Periodic limb movements during wakefulness appear mainly in patients with restless legs syndrome (RLS), whereas PLMS can be observed in a wide variety of sleep-wake disorders, including RLS and narcolepsy, as well as in healthy elderly subjects.2 Furthermore, PLMS have been blamed as the cause of disturbed sleep in some patients, inducing either insomnia or daytime sleepiness,2 in which case a periodic limb movement disorder (PLMD) is diagnosed.
However, the impact of PLMS on sleep and general health remains unclear. Whereas the movements have been linked to sleep fragmentation3 and correlated with impaired subjective sleep quality in some studies,4 most groups have found no correlation between the quantity of PLMS and daytime sleepiness5–8 or subjective sleep-quality scores.9 A better understanding of the underlying mechanisms of PLMS might help to elucidate the effect of this common behavior on sleep.
Numerous polysomnographic studies indicate that PLMS are often associated with electroencephalographic (EEG) arousals10–13 or with phase A of the cyclic alternating pattern,14 thus suggesting a disruption of the sleep continuity. Furthermore, changes in heart-rate variability (HRV)10,12,15 and blood pressure16 accompany PLMS, indicating a concomitant oscillation of autonomic activity. However, the significance of these changes for the pathophysiology of PLMS remains unclear. In particular, it is not known whether autonomic activation and arousals are specific for and causally related to PLMS or just coexisting manifestations.4,7,9,17 If a causal relationship is assumed, it is still unknown which polysomnographic phenomenon is the cause and which the consequence.
On the other hand, the origin of other types of leg movements in sleep may be more easily explained. Patients with obstructive sleep apnea (OSA) suffer from repetitive airway obstructions and oxygen desaturations terminated by an arousal reaction, which often exhibits electromyographic (EMG) bursts. These respiratory-related leg movements in sleep (RRLMS) may show a periodicity similar to that of genuine PLMS.18 Also, physiologic spontaneous arousals often include isolated leg movements in sleep (ILMS).
We aimed at investigating the pathogenesis of PLMS by comparing PLMS-associated EEG and HRV changes with changes occurring in association with these other types of movement in sleep. Activations that are specific to, and therefore more directly related with, PLMS were expected to be greater or different during PLMS than during control movements. Furthermore, we looked for correlations between EEG and HRV changes on one hand and electromyogram (EMG) activity induced during leg movements on the other hand. Significant positive correlations would point to a direct functional relationship among PLMS, arousals, and autonomic activation. Lastly, since we intended to analyze causality, the temporal order of occurrence of the 2 phenomena was of special interest. We therefore applied signal analysis methods allowing a sufficiently high time resolution.
METHODS
Patients and Setting
Whole-night polysomnographic studies (PSG) of 24 patients fulfilling the criteria of PLMD (n = 8), OSA (n = 7), or normal PSG (n = 9) were selected from the clinic's database containing all patients having been treated between 2001 and 2005. Patients had been informed that their data may be used for scientific purposes and had given their written informed consent, and the study was approved by the local ethic committee.
Criteria for a Diagnosis of PLMD
Inclusion criteria were a PLM-index (PLMI) of at least 20 per hour and the presence of either excessive daytime sleepiness or a subjective feeling of nonrestorative or disturbed sleep. Furthermore, a concomitant sleep-related breathing disorder was excluded, ie, the clinical impression did not suggest a sleep-related breathing disorder, the apnea-hypopnea-index (AHI) had to be lower than 8 per hour, and no signs of an upper airway resistance syndrome were visible in the respiratory traces. No evidence for narcolepsy was found. All 8 included subjects were in good general condition, 4 had a body mass index in excess of 25 kg/m2. Four of the patients suffered from concomitant diseases: mild depression (no medication), dyslipidemia and ischemic heart disease (treated with a statin and low-dose acetylsalicylic acid), arterial hypertension and ischemic heart disease (treated with an ACE-inhibitor, diuretics, and a β-blocker), and chronic tensiontype headaches (treated with occasional analgesics).
Criteria for OSA
Inclusion criteria were the occurrence of 40 or more obstructive apneas and hypopneas per hour associated with RRLMS. Patients with significant PLMS independent of respiratory events (PLMI > 5/h) were excluded. The 7 included patients had typical OSA. They were all in a good general condition, and 6 of them had a body mass index in excess of 25 kg/m2. Five patients had additional cardiovascular risk factors, 4 of whom had hypertensive/ischemic heart disease, and both were treated with 1 or a combination of the following medications: angiotensin II receptor blockers, ACE-inhibitors, calcium channel blockers, diuretics, statins, β-blockers, and platelet inhibitors.
Criteria for Normal PSG
Patients included in this group needed to have an AHI of less than 8 per hour, a PLMI of less than 5 per hour, and a sleep efficiency of at least 80% for the total recording time. No complaints pointing to a possible diagnosis of narcolepsy were recorded in the patient's history, and 5 out of 9 subjects had an additional Multiple Sleep Latency Test without any sleep-onset rapid eye movement (REM) periods. This group consisted of 9 patients who were assigned to sleep studies for various reasons, with their PSG showing normal results. All of them were in good general condition, 5 had a body mass index in excess of 25 kg/m2. Three of the 9 included subjects complained of excessive daytime sleepiness and their mean sleep latency in the Multiple Sleep Latency Test was less than 8 minutes with no sleep-onset REM periods. All 3 patients also suffered from mild to moderate depressions, and 2 were treated with either a serotonin reuptake inhibitor or a monoamine oxidase inhibitor. Two patients experienced nonrestorative or disturbed sleep due to a mild insomnia with dissociation between subjective and objective sleep time. Three further patients snored but did not have subjective daytime sleepiness, and no or only very mild respiratory disturbances were found (AHI < 8) that, considering the clinical context, did not justify a treatment. One of these patients received antihypertensive treatment consisting of an angiotensin II receptor blocker and a diuretic. Lastly, 1 patient without subjective sleep disturbances was assessed for potential causes of an unexplained loss of consciousness that led to a car accident with cerebral concussion.
None of the subjects took dopaminergic medication, antiepileptic drugs, opiates or benzodiazepines.
Recordings
EMGs of the submental muscle, of forearm muscles, and of the anterior tibial muscles of both sides, as well as the electrocardiogram and the left and right electrooculogram, were recorded with a sampling rate of 200 Hz. Four EEG leads (C4-M1, C3-M2, O2-M1, O1-M2) were recorded, digitized, and sampled at 100 Hz. Impedances were kept below 5 kOhm. Respiration was assessed by recording nasal and oral airflows with thermistors, nasal pressure with a cannula, thoracic and abdominal respiratory movements with strain gauges, and oxygen saturation with a finger oximeter.
Sleep stages were scored according to the standard criteria19 using 30-second epochs.
Apneas and hypopneas were automatically prescored from changes in airflow, respiratory movements, and oxygen desaturations using the software Somnologica® (Medcare, Reykjavik Iceland) and visually corrected where necessary, according to international standards.20
Analysis of Motor Activity and Creation of Movement Segments
Classification of Movements
PLMS were scored in the raw EMG signals using Coleman criteria5: a PLMS sequence required at least 4 consecutive movements each, with a duration of 0.5 to 5 seconds and an intermovement interval of 4 to 90 seconds. Periodic movements with a close temporal relation to apneas or hypopneas were not interpreted as PLMS. Instead, leg movements in patients with OSA having a close temporal relation to apneas or hypopneas were scored as RRLMS. Physiologic isolated leg movements lasting between 0.5 and 5 seconds were analyzed in subjects with normal PSG. Thus, 3 different movement types in sleep were distinguished: (1) PLMS of patients with PLMD, (2) ILMS of subjects with normal PSG, and (3) RRLMS of patients with OSA.
Determination of Movement Onset
Since accurate determination of movement onset was critical for our study, a technique was used that was described as being most accurate for this purpose21: EMG channels of both anterior tibial muscles were high-pass filtered at 10 Hz, rectified, and combined by summing left and right recordings. Smoothing was performed by averaging a moving time window of 50-milliseconds length. From this, leg-movement onset was automatically determined by a moving window with a width of 25 milliseconds and a threshold of 3 standard deviations of the baseline activity and visually corrected where necessary.
Movement Segments
Movement segments covering a time range from 10 seconds before to 10 seconds after movement onset were defined. Segments having an interval of 10 seconds or less relative to the previous movement or showing significant submental or arm EMG activity before leg-movement onset were excluded from further analysis.
Analysis of Spectral EEG Changes
Time-Frequency Decomposition
In order to assess spectral EEG changes in temporal relation to movements in sleep, we calculated the event-related spectral perturbation,22 which measures the mean changes in power spectrum over time related to a certain event (in our case the EMG onset of the movements). For this, a time window of 2-second length was moved in overlapping time steps of 0.1 seconds, and at each step, the power spectrum was computed and averaged across trials. Rather than conventional Fourier transforms, a sinusoidal wavelet transform, in which the number of wavelet cycles increased with frequency, was applied, as implemented in the toolbox EEGLAB22 (http://www.sccn.ucsd.edu/eeglab/). This had the advantage of offering a better frequency resolution at higher frequencies.22 Power changes across the frequency range of 0.5 to 45 Hz were obtained by subtracting the log-transformed mean baseline spectrum (−10 to −7 seconds) from the log-transformed spectral estimate at each time window. The grand average was obtained by averaging across mean values of each subject.
Frequency Bands
An inspection of the time-frequency transforms revealed that adjacent frequency bins within the commonly used EEG frequency bands showed similar power changes over time (see Results). In order to more easily visualize differences between movement types, we therefore also calculated EEG power changes in these broader frequency bands. In addition to the traditional bands—delta (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–12.5 Hz), sigma (12.5–15 Hz), and beta (15–24 Hz)—the gamma frequency band (26–45 Hz) was also analyzed, since previous studies found that important movement-induced EEG power changes occur in this band.23 Rather than using the data from the time-frequency transforms and averaging across the frequency bins belonging to a given band, we decided to calculate the event-related synchronization and desynchronization23 based on Hilbert transforms,24 since this method has been reported to yield an optimal time resolution for all frequency bands without depending on an optimal selection of the analysis time windows.24 Single trials (ie, EEG segments time locked to movement onset) of EEG channel C3-M2 were processed with a bandpass filter and Hilbert transformed in order to obtain the instantaneous amplitude at each sample point within the given frequency band. After averaging across movements of each individual subject, power changes were normalized by dividing the differences between the active and the baseline period (first 3 seconds) by the baseline values and averaged across subjects.
Analysis of HRV
Heart-rate Variability
Movement periods with arrhythmic heart beats or movement artifacts were excluded by visual inspection. The heart rate was calculated from inverse R-R intervals in the electrocardiogram, which were down sampled and interpolated to a sampling frequency of 1 Hz. The HRV was obtained by calculating the difference between the actual heart rate and the heart rate of the previous second.
Time-frequency Decomposition
Furthermore, a spectral analysis of HRV was performed. According to the concept of sympathovagal balance,25 low-frequency oscillations (LF power) of the HRV centered around 0.1 Hz are mediated by sympathetic activity, whereas higher-frequency oscillations (HF power) depend on vagal-nerve activity. The ratio LF/HF is therefore used as an indicator of the so-called sympathovagal balance, with high values indicating an autonomic shift toward sympathetic activity. More recently, these assumptions have been criticized as being too simplistic, since LF power is also largely influenced by vagal activity and HF power depends also on the breathing frequency of the examined subject.26 However, spectral analysis of HRV continues to be a helpful tool to study autonomic activity, which has already been successfully employed for PLMS.15
LF and HF powers are usually calculated from large single HRV segments covering several minutes.15,27 However, here we were particularly interested in the time course of autonomic changes related to PLMS, for which reason a time resolution of minutes was insufficient. To determine the temporal relation of the sympathovagal balance to movement onset, we developed an eventrelated spectral analysis (a time-frequency decomposition) of HRV, based on similar procedures used in EEG analysis (see above). Instead of calculating a power spectral estimate for a long single HRV segment by averaging over time, we calculated a spectral estimate for each sampling point, ie, for each second of multiple HRV segments, by averaging over repeated movements. A continuous wavelet transform was used to derive the spectra rather than a classical Fourier transform, since earlier work had suggested that wavelet transforms are superior for nonstationary signals.27 Furthermore, since we were interested in movement-related changes of LF and HF powers rather than in absolute power values, we normalized absolute power spectra with the mean baseline spectra recorded several seconds before movement onset.
The step-by-step analysis procedure was as follows: a Daubechies 4-wavelet form was used to transform the HRV of each single movement period.15,27 The squared and log-transformed mean baseline wavelet coefficients (first 3 seconds) were then subtracted from the log-squared coefficients of each time point, and the resulting spectral perturbation was averaged across movements at each time-frequency point.
Frequency Bands
LF (frequency range 0.05 – 0.15 Hz) and HF powers (frequency range 0.15 – 0.4 Hz) of HRV27 were calculated by summing the squared coefficients of the corresponding frequency bins/wavelet scales, using the traditional and well-established border frequency of 0.15 Hz between HF and LF powers.25–27 From this, a ratio LF/ HF was calculated for each time point. Normalization was done by expression in percentage of change of baseline values (first 3 seconds). The grand averages were obtained by averaging the means of each subject.
Statistical Analyses
Sleep Characteristics and Clinical Data
A comparison of polysomnographic and demographic data between patient groups was performed using a nonparametric Kruskal-Wallis test, since small samples with partly nonnormal distributions, as well as ordinal data, were analyzed.
Time-frequency Decompositions
The procedures used for assessing the statistical significance of time-frequency decompositions of EEG and HRV are described in detail elsewhere.28,29 In short, the statistical significance of power changes at each time-frequency point was tested for each subject using 20,000 bootstrap permutations with pseudo-t statistics, which compared baseline with active time points28. Correction for multiple testing was achieved by using a false discovery rate of 5%.28 Overall significance across subjects was assessed by performing binomial statistics with P values of each subject.30
Comparison Between Patient Groups
In order to test for differences between the 3 movement types in EEG and HRV power changes within the frequency bands defined above, overall one-way analysis of variance tests were done at each time point (the EEG data was downsampled to 2 data points per second for statistical testing). In addition, 2 a-priori comparisons were performed using the contrasts PLMS versus ILMS and PLMS versus RRLMS. Since the number of comparisons did not exceed the degrees of freedom of the movement types, no correction for multiple contrasts was done. However, in order to adjust for testing at multiple time points and frequency bands, the resulting P values were corrected with a false discovery rate of 5%.28 Only values of non-rapid eye movement (NREM) sleep stage 2 were used, since this sleep stage displayed the greatest absolute number of leg movements.
Correlations
The functional relation between leg movements, autonomic activation, and arousals was further analyzed by calculating correlations between spectral EEG and HRV power changes and motor activity values in NREM sleep stage 2. Motor activity was quantified with the area under the curve of the 20-second segments of combined, smoothed, rectified, EMG of both anterior tibial muscles, as well as with the duration of EMG activity, with the PLMI and with the interval between consecutive movements. EEG and HRV changes were quantified with the area under the curve of the corresponding 20-second segments in the different frequency bands. For variables yielding significant correlations, correlation coefficients were also calculated using the sum of the values at time points with significant differences between subjects. Correlations across subjects were analyzed to detect factors functionally related with the condition PLMD by calculating Spearman coefficients for all patients from their mean value. Correlations within subjects, on the other hand, were used to detect processes that are directly related to the generation of PLMS. They were obtained by calculating Spearman correlation coefficients for each patient with PLMD using values of single PLMS. These coefficients were Z transformed and tested for differences to 0 by using a t-test for one sample with 0 as the test value.
General Reporting
All P values needed to be smaller than .05 after corrections to be considered significant.
RESULTS
Clinical Data
Demographic data and sleep indexes for each patient group are shown in Table 1. PLMI (P < 0.001), AHI (P = 0.001), and oxygen saturations (P < 0.001) diverged significantly between groups, as expected. In addition, significant differences in the proportion of REM sleep (P = 0.045), as well as nonsignificant trends for differences in age, body mass index, and the proportion of slow-wave sleep could be found. In contrast, the values of the other parameters were similar for all assessed groups (see Table 1).
Table 1.
Demographic Data and Polysomnography Indexes of the Examined Patient Groups
| PLMD | OSA | Normal PSG | P Value | |
|---|---|---|---|---|
| Demographic Data | ||||
| Subjects, no. | 8.0 | 7.0 | 9.0 | |
| Women, no. | 3.0 | 0.0 | 5.0 | |
| Age, y | 51.0 ± 4.6 | 57.4 ± 4.3 | 39.9 ± 4.6 | 0.06 |
| ESS, score | 9.9 ± 2.2 | 13.0 ± 2.6 | 8.4 ± 1.7 | 0.38 |
| BMI, kg/m2 | 25.1 ± 2.0 | 35.2 ± 3.3 | 26.5 ± 1.8 | 0.07 |
| Polysomnographic Findings | ||||
| TST, min | 364.5 ± 20.2 | 336.0 ± 26.0 | 375.0 ± 15.1 | 0.42 |
| Sleep latency, min | 11.9 ± 3.2 | 13.6 ± 10.1 | 9.8 ± 1.5 | 0.76 |
| REM latency, min | 112.8 ± 27.3 | 174.1 ± 41.8 | 125.0 ± 18.7 | 0.33 |
| Sleep efficiency, % | 84.3 ± 3.1 | 82.0 ± 6.9 | 91.1 ± 1.9 | 0.44 |
| Sleep stage, % of TST | ||||
| REM | 12.9 ± 1.5 | 8.7 ± 2.2 | 16.6 ± 1.6 | 0.045 |
| 1 | 17.1 ± 4.2 | 20.9 ± 2.7 | 12.5 ± 1.7 | 0.10 |
| 2 | 46.0 ± 1.9 | 50.6 ± 7.7 | 49.5 ± 3.3 | 0.72 |
| 3/4 | 10.3 ± 3.0 | 3.6 ± 1.8 | 13.6 ± 2.0 | 0.05 |
| PLMI, no./h | 64.0 ± 17.3 | 1.4 ± 0.9 | 1.4 ± 0.6 | < 0.001 |
| AHI, no./h | 4.5 ± 0.8 | 83.9 ± 7.4 | 4.2 ± 0.8 | 0.001 |
| SaO2, % | ||||
| Mean | 93.2 ± 0.6 | 88.9 ± 1.0 | 94.9 ± 0.6 | < 0.001 |
| Minimum | 85.5 ± 1.3 | 68.6 ± 2.9 | 87.9 ± 1.6 | < 0.001 |
| Mean heart rate, bpm | 63.6 ± 4.2 | 67.3 ± 5.3 | 62.9 ± 2.7 | 0.91 |
Data are presented as mean ± SD, except subjects and women, which are shown as total numbers; P values of statistical comparisons between the patient groups (Kruskal-Wallis tests) are shown. PLMD refers to periodic limb movement disorder; OSA, obstructive sleep apnea; PSG, polysomnography; ESS, Epworth Sleepiness Scale; BMI, body mass index; TST, total sleep time; REM, rapid eye movement sleep; PLMI, periodic limb movement index; AHI, apnea-hypopnea index; SaO2, oxygen saturation.
Time Course of HRV and EEG Changes
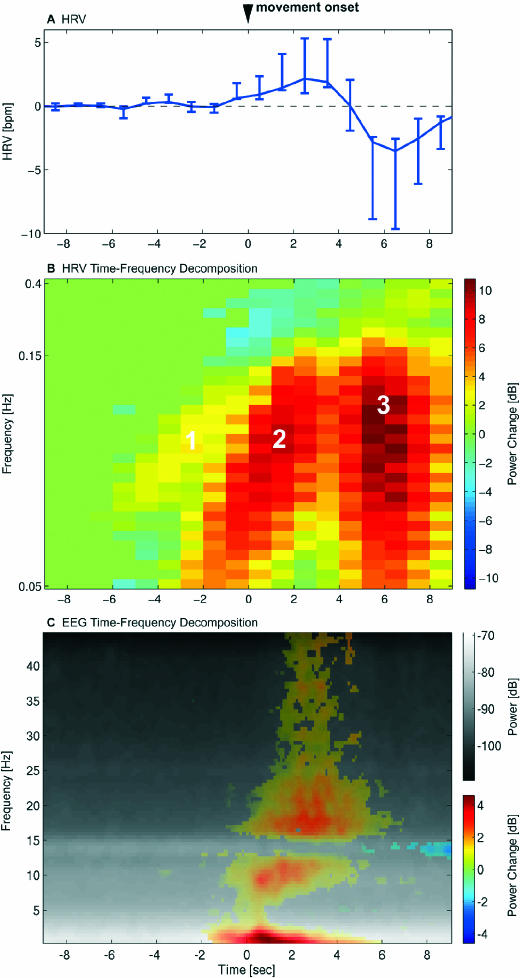
Figure 1A shows the time course of the HRV changes in association with PLMS. An acceleration of the heart rate starting 1 second before movement onset is followed by a deceleration 5 seconds after movement onset.
Figure 1.
The time courses of heart-rate variability (HRV) and electroencephalogram (EEG) changes are shown relative to onset of periodic limb movements of sleep (PLMS) at 0 seconds. A. Mean HRV ± 95% confidence interval of all subjects with periodic limb movement disorder (PLMD). B. Time-frequency decomposition of HRV with a continuous wavelet transform event related to leg-movement onset. The grand average of power changes from baseline (−10 to −7 s) of all PLMS is shown with nonsignificant changes set to 0. The frequencies are plotted with a pseudologarithmic scale. Three different processes could be distinguished, as indicated in the graph. As explained in the text, process 1 was interpreted as a general weak autonomic activation, process 2 as sympathetic activation, and process 3 as vagal activation. C. Time-frequency decomposition of EEG channel C3-M2 with a sinusoidal wavelet transform. The mean power spectrum over time is shown in black and white. Significant power changes to baseline (−10 to −7 s) are overlaid in color. EEG changes began clearly after the first spectral HRV changes shown in B.
A continuous wavelet transform of the HRV time locked to the onset of PLMS revealed that the HRV was composed of 3 clearly distinct oscillatory processes, ie, 3 clusters of statistically significant power increases in the time-frequency plane (Figure 1B).
Process 1 consisted of a rather weak but significant increase in LF oscillations (0.05 to 0.15 Hz) in the time range between 6 to 2 seconds before movement onset. The oscillation frequency exponentially increased over time (Figure 1B). In the same time range, a weak increase in the HF power band could be observed (Figure 2, lower left panel, 0.15 to 0.4 Hz), which did not, however, reach statistical significance (Figure 1B).
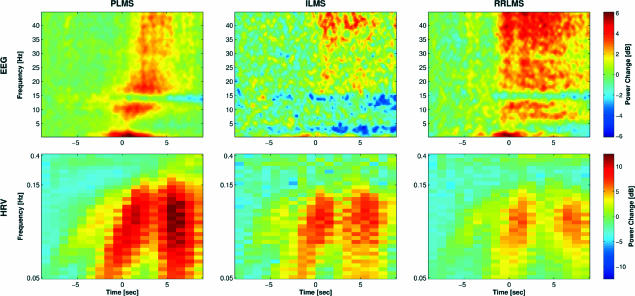
Figure 2.
Comparison of spectral heart-rate variability (HRV) and electroencephalogram (EEG) changes of all 3 leg-movement types in sleep. In contrast to Figure 1, nonsignificant power changes are not masked to 0. Note the stronger intensities of HRV processes in association with periodic limb movements of sleep than during other types of leg movements in sleep and the different time course of EEG changes for different leg-movement types. PLMS refers to periodic limb movements of sleep; ILMS, isolated leg movements in sleep; RRLMS, respiratory-related leg movements in sleep.
Process 2 consisted of a strong increase in LF oscillations in the time range between 3 seconds before to 3 seconds after movement onset. As process 1, it showed exponentially increasing oscillation frequencies over time. However, in contrast to process 1, a significant decrease in HF power can be observed during process 2 (Figure 1B). This process corresponds to the acceleration of the HRV specified above (Figure 1A).
Process 3 was characterized by high-amplitude oscillations in a broad frequency band including both LF and HF ranges (see Figure 1B), beginning 4 seconds after movement onset. During this process, a transient decrease of the HRV can be observed (Figure 1A).
The earliest EEG change associated with PLMS was a prominent delta synchronization, which started 2 seconds before movement onset (see Figure 1C, significant power changes plotted in color). Therefore, spectral EEG changes began clearly after the first spectral changes in HRV (Figure 1B, processes 1 and 2, significant power changes in yellow/red or blue). Subsequently, a gradual progression to faster EEG activity could be observed with beta and gamma activity beginning only after EMG onset of the anterior tibial muscles. Lastly, 5 seconds after movement onset, EEG activity in the sigma (spindle) frequency range started to decrease significantly.
Comparison of HRV and EEG Between Movement Types
Figure 2 illustrates similarities and qualitative differences in spectral EEG and HRV changes during sleep stage 2 between the 3 examined movement types. The bottom row demonstrates that the 3 HRV oscillatory processes described above were also effective during ILMS and RRLMS, although to a lesser extent. The upper row shows that all 3 movement types were accompanied by similar changes in the EEG spectrum. However, whereas the power increase associated with PLMS showed a gradual progression from low to high EEG frequencies, it occurred suddenly and simultaneously over almost the entire frequency range for ILMS and RRLMS.
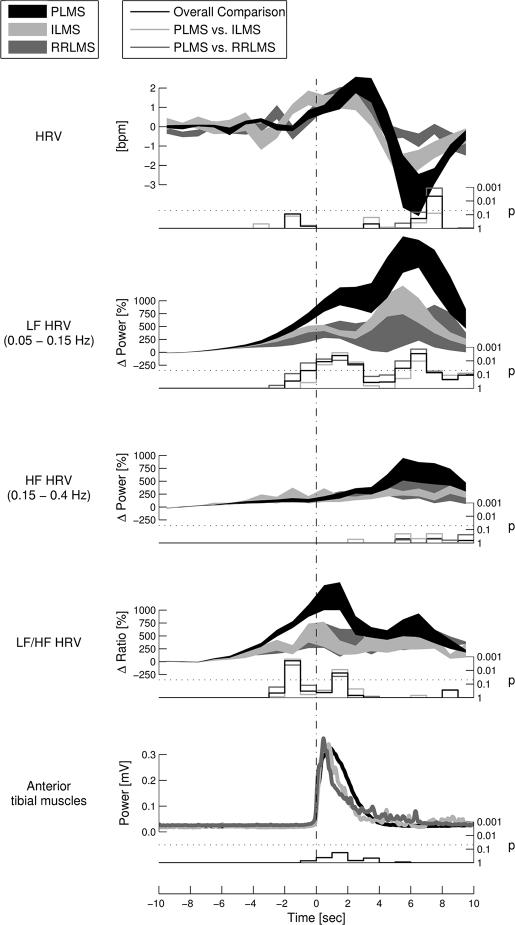
Figure 3 displays a comparison of HRV and LF/HF power changes between the 3 different types of leg movement in sleep. The deceleration of HRV was significantly larger after the onset of PLMS than after RRLMS and tended to be larger after PLMS than after ILMS. During process 2, the increase in LF power as well as the increase in the LF/HF ratio was significantly larger for PLMS than for the other movement types in sleep. During process 3, PLMS were associated with a significantly greater increase of LF power than were the other movement types. No difference between the movement types could be found during process 1.
Figure 3.
The time course of heart-rate variability (HRV) changes are shown in relation to the onset of the 3 different movement types analyzed. Analysis was performed in the time domain (HRV) as well as in the time-frequency plane using a continuous wavelet transform (low frequency [LF], high frequency [HF], and LF/HF). Thick lines show mean ± SEM values. Stair plots below each graph indicate the statistical probability that HRV changes are equal for all 3 movement types at the given time point.
Note the 2 distinctive peaks of LF and HF power changes as well as of LF/HF ratio changes, occurring similarly for all examined movement types. LF-power- and LF/HF-ratio changes before and immediately after movement onset (indicating sympathetic activations, process 2) were significantly different between movement types. Furthermore, LF-power changes 4 seconds after movements (indicating vagal activations, process 3) were significantly different. PLMS refers to periodic limb movements of sleep; ILMS, isolated leg movements in sleep; RRLMS, respiratory-related leg movements in sleep.
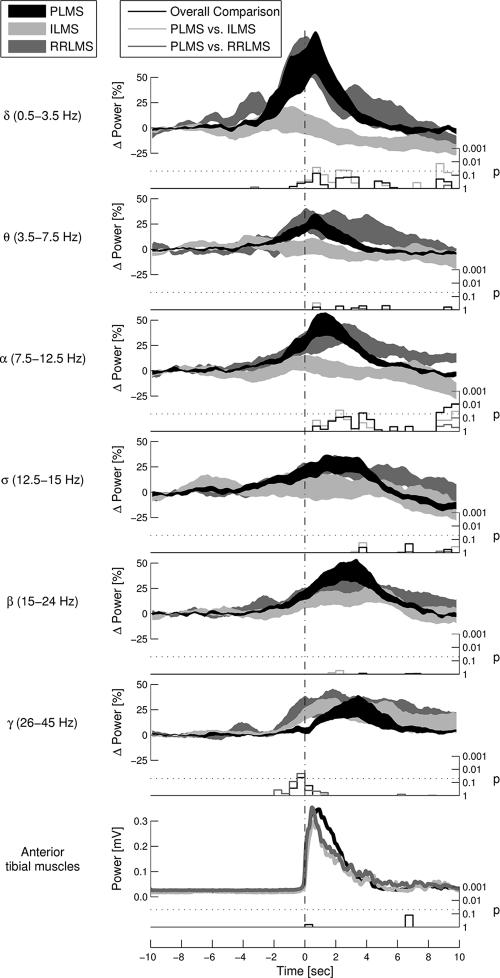
In Figure 4, EEG changes occurring in the 6 assessed frequency bands during sleep stage 2 are compared between the movement types. Significant differences could be observed in the delta and alpha frequency bands, the synchronization peaks being smaller for ILMS than for PLMS. Furthermore, the onset of gamma synchronization differed significantly between movement types. Whereas gamma rhythms started to increase already before movement onset in the case of RRLMS and ILMS, they increased only after movement onset in the case of PLMS (Figure 2 and Figure 4).
Figure 4.
Comparison of the time course of electroencephalogram (EEG) changes during sleep stage 2 of electrode C3-M2 between the different leg-movement types. Thick lines show mean ± SEM values. Stair plots below each graph indicate the statistical probability that EEG changes are equal for all 3 movement types at the given time point.
Note that the spectral EEG changes were similar for all examined movement types. However, delta and alpha synchronization were significantly smaller for isolated leg movements in sleep (ILMS) than for periodic limb movements of sleep (PLMS). Furthermore, an increase in gamma activity occurred later for PLMS than for the other movement types. RRLMS refers to respiratory-related leg movements in sleep.
Correlations Between HRV and EMG
Significant correlations across subjects were found between markers of sympathetic activity and EMG activity: the LF/HF ratio correlated with longer-lasting movements (r = 0.47, P = 0.02), shorter intermovement intervals (r = −0.60, P = 0.002), and a greater PLMI (r = 0.49, P = 0.015). When only the time points corresponding to HRV oscillatory process 2 were used to correlate the LF/HF ratio with EMG activity, these correlations were even stronger (r ≥ 0.53, P ≤ 0.008), as is also illustrated in the scatter plots of Figure 5. In addition, the LF power increase of process 2 was correlated with a greater PLMI (r = 0.54, P = 0.006). LF power changes during process 3 were correlated with shorter intermovement intervals (r = −0.42, P = 0.042) and a greater PLMI (r = 0.63, P = 0.001).
Figure 5.
Scatter plots illustrating the correlations between sympathetic activation during process 2 and motor activity in sleep stage 2. Significant correlations were found between LF/HF change during process 2 and movement duration (r=0.53, P=0.008), inter-movement interval (r=−0.70, P<0.001), and PLMS-index (r=0.58, P=0.003).”
A weak but significant positive correlation within subjects was found between LF oscillations of HRV and EMG magnitude of PLMS (r = 0.2, P = 0.002). This correlation was significant during both process 2 (r = 0.19, P < 0.001) and process 3 (r = 0.18, P = 0.017). Furthermore, HF power changes during process 3 correlated with greater PLMS magnitude (r = 0.13, P = 0.004).
Correlations Between EEG and EMG
Among all EEG frequency bands, only the sleep-spindle band showed any significant correlations across subjects with EMG activity, showing greater power increases with shorter intermovement intervals (r = −0.46, P = 0.025) and greater PLMI (r = 0.50, P = 0.012). None of the EEG-frequency bands showed significant within-subjects correlations with periodic leg motor activity (|r| ≤ 0.05, P ≥ 0.33).
Correlations Between HRV and EEG
No significant correlation across subjects could be detected between EEG and HRV activity (|r| ≤ 0.29, P ≥ 0.11). Also the correlation coefficients within subjects were generally quite small, but a significant positive correlation could be observed between the LF/HF ratio increase during process 2 and delta-power changes (r = 0.09, P = 0.048), as well as between the LF/ HF ratio during process 2 and spindle-power changes (r = 0.07, P = 0.049). In contrast, the HF-power increase during process 3 was significantly negatively correlated with delta synchronization (r = −0.08, P = 0.002).
DISCUSSION
In an attempt to characterize the underlying mechanisms of PLMS, this study analyzed the accompanying spectral HRV and EEG changes and contrasted PLMS with other movements in sleep, namely isolated (ie, nonperiodic) leg movements in sleep (ILMS) and RRLMS. Our finding that HRV and EEG changes occur uniformly before and during all types of leg movements indicates that PLMS are not mediated by a specific process. Instead, physiologic processes being effective also during other movements of sleep, particularly ILMS, seem to be involved. More importantly, we were able to show significant differences in the intensity of HRV changes between PLMS and other leg movements of sleep, which points to sympathetic activity as the physiologic process most probably causally related to PLMS.
Timing and Interpretation of HRV and EEG Changes
In accordance with other authors,10–15 we construe the observed changes in HRV and EEG as signs of an autonomic activation, sleep instability, and arousal reactions. These manifestations exhibit a stereotyped time course, beginning with a sympathetic activation and then progressing to a thalamocortical activation, which is initiated by K-complexes and delta bursts. Thereafter, a vagal activation and a decrease of spindle activity can be observed.
Our results demonstrate that autonomic activation starts several seconds before movement onset and before EEG changes become evident (Figure 1) and that it occurs in 3 clearly distinct processes (Figures 1–3):
Process 1 indicates a general weak autonomic activation. Since HRV does not show any clear changes during this first process, both the vagal and the sympathetic system seem to be activated to a similar degree, and their effects on HRV largely cancel each other out. Indeed, an increase in LF power, as observed during this process, can be due to both sympathetic and vagal activations.25,26 Figure 2 also shows a nonsignificant increase in HF power during process 1, which suggests a weak vagal activation.
Process 2 represents a sympathetic activation, since the HRV transiently increases (Figure 1A) and the wavelet transform discloses a strong increase in LF power (Figure 1B, process 2) and the LF/HF ratio (Figure 3).
Process 3 is an indicator of a vagal activation, since the HRV rapidly decreases (Figure 1A) and the continuous wavelet transform displays high-amplitude HRV oscillations in a broad frequency band including both HF and LF powers (Figure 1B, process 3; Figure 3). It is well known that vagal activity can be manifested in the LF range as well as in the HF range.26 In case of process 3, it is reasonable to assume that both the LF and HF power changes are mainly induced by vagal activity because of the rapidly decreasing heart rate during this phase. A great concomitant sympathetic activation would be difficult to bring in line with the observed slowing of the heart rate.
HF power is known to depend also on respiration frequency.26 This is of importance in case of RRLMS, in which apneas and hypopneas may lead to biased HF power changes. However, since HF power changes of RRLMS were similar to those of ILMS (Figure 3), in which no apneas occurred, the influence of respiration is probably negligible in our setting.
The spectral increase in EEG delta activity observed up to 2 seconds before movement onset is due to the slow component of K-complexes and to delta bursts occurring mainly during NREM sleep stage 2 and slow-wave sleep. K-complexes are thought to be cortically generated elementary forms of arousals, which have, at the same time, a sleep-structuring function by triggering and grouping other sleep oscillations, such as spindles and delta bursts.31
The frequency shift toward alpha, beta, and gamma rhythms observed after delta synchronization represents typical arousals, as defined by the American Association of Sleep Disorders.32 They depend on an intact corticothalamic function and can be induced by stimulation of the brain stem reticular formation.33,34
Gamma activity has also been observed in relation to voluntary movements and has been interpreted as an indicator of cortical movement preparation and execution.23
The decrease of spindle frequencies 5 seconds after movement onset points to an attenuation of the “sleep-protecting” processes occurring after leg movements in sleep.35
It is noteworthy that other studies13,36 have also observed increased alpha and beta rhythms, to a lesser extent, after onset of periodic limb movements during wakefulness. This has been attributed to inhibitory processes terminating the motor activity and to sensory afferences.13,23 The same processes may also be effective during PLMS and contribute to the associated EEG changes.
PLMS are Triggered Subcortically, Whereas ILMS and RRLMS Depend on a Cortical Activation
When comparing the HRV associated with different types of leg movements in sleep, we found significantly greater sympathetic activation for PLMS than for the other leg-movement types (Figure 3, LF and LF/HF changes). This finding is in accordance with a recent study reporting that sleep periods with PLMS are associated with significantly greater sympathetic activation than are sleep periods without PLMS of the same subject suffering from RLS.15 Thus, sympathetic activity appears to be functionally related with PLMS.a
A comparison of EEG changes between different movement types further revealed that, whereas gamma rhythms as markers of cortical arousal and cortical movement preparation started to increase before the onset of ILMS and RRLMS, they only started after onset of PLMS (Figure 4, sixth graph). We may thus conclude that PLMS occur at an earlier stage of cortical activation than do other movement types. In contrast, the time course of gamma-frequency changes observed in association with ILMS and RRLMS resembles that of voluntary movements in wakefulness.23 This premovement gamma synchronization was attributed to synchronous activity in the primary and supplementary motor areas controlling voluntary movements. We therefore suggest that ILMS and RRLMS depend on cortical activation and share similarities with voluntary movements, whereas PLMS are mediated by subcortical processes.
Alternatively, one may argue that the difference in onset of gamma activity is due to a delayed detection of movement onset in the case of RRLMS and ILMS, since other leg muscles might have been activated before the anterior tibial muscles. However, all movements with prior arm or submental muscle activity were excluded from this analysis, and the potential onset delay of maximally some 100 milliseconds is negligible in relation to the observed difference of 1 to 2 seconds. As another potential source of inaccuracy, contamination of high frequency EEG rhythms by EMG artifacts was avoided by taking great care to preclude all movements with EMG artifacts.
PLMS Are Closely and Directly Related With Autonomic Activity
This claim is substantiated by the highly significant differences in autonomic activation between PLMS and control movements, as well as by the significant correlations across subjects shown in Figure 5: great LF/HF ratio values during process 2, indicating a strong sympathetic activation, were significantly correlated with a greater PLMI, a shorter intermovement interval, and longer movement duration. These findings confirm an earlier study, which found a positive correlation between sympathetic activation and the PLMI in patients with RLS.15 The high temporal resolution of the event-related continuous wavelet transform introduced in this study allows us to demonstrate that the sympathetic activation clearly starts prior to movement onset (Figures 1–3). We therefore argue that the increased sympathetic activation facilitates PLMS rather than the PLMS being responsible for an increased sympathetic activation. The significant within-subjects correlations between sympathetic activity (process 2) and movement magnitude further suggest that sympathetic structures are directly involved in the generation of each individual movement rather than being merely generally associated with them.
Besides sympathetic activity (process 2), vagal activity occurring after movements (process 3) also showed a significant positive correlation with PLMS magnitude, which points to a functional relationship between networks generating PLMS and the vagal centers.
PLMS Cooccur With Cortical Arousals but Are Not Directly Related to Them
The observation that PLMS are associated with EEG arousals had initially led to the concept of PLMS causing sleep disturbance, in analogy to the situation in sleep apnea syndrome. However, in this and former studies,10–13 the first EEG signs of arousals were found to start clearly before PLMS onset, which is difficult to bring in line with the concept of PLMS being the cause of arousals and sleep disturbance. Instead, it has been hypothesized that PLMS are the consequence of an underlying arousal disorder.11 However, several recent studies have generally questioned that EEG changes are of any significance for the pathophysiology of PLMS. The sleep macrostructure and microstructure, as well as the EEG-spectra, of patients with PLMD have been reported to remain unchanged when PLMS occur, as compared with sleep periods without PLMS.17 Furthermore, EEG power spectra during sleep have been shown to not differ between patients with RLS and healthy subjects.37 These findings suggest that EEG arousals are not specific for or causally related to PLMS but are just coexisting manifestations.
The findings of this study are in accordance with these previous reports. Although ILMS were accompanied by less delta and alpha synchronization than were PLMS, EEG changes of PLMS were generally similar to changes in other types of leg movements of sleep. In addition, we were unable to find any significant correlations within subjects between the magnitude of arousal in EEG and the magnitude, duration, or intermovement interval of PLMS. Considering correlations across subjects, only the movement-related increase in spindle activity—probably reflecting sleep protection—but none of the arousal equivalents, was correlated with EMG activity. These findings cast doubts on a simple functional relationship between arousals and PLMS.
On the other hand, our study confirms the well-known striking temporal relation between leg movements and arousals in the sense that they mostly occur at the same time. In accordance with other authors,12,14 we argue that this discrepancy between apparent causal independence of the 2 phenomena from each other and strong temporal coexistence is best explained by a common underlying mechanism that stimulates both the motor and arousal systems.
Autonomic Activity Is Closely Related With Delta Activity in EEG
From earlier studies in animals38 as well as in human subjects,39–41 it is well known that sympathetic nerve activity and K-complexes in the EEG are closely related. In agreement with this notion, we report here a small but significant positive within-subjects correlation between sympathetic activation on one hand and delta-spindle activity on the other hand. Vagal activation was negatively correlated with delta activity in EEG, which points to an inhibitory effect of vagal centers on thalamocortical networks generating K-complexes. For the remaining EEG frequency bands, no within-subjects correlation with autonomic activity could be observed in this study. However, other studies have reported greater sympathetic activations at higher grades of EEG arousal,42–44 thus suggesting an important role of the sympathetic system for controlling arousals in general.
It is noteworthy, however, that none of the EEG frequency bands showed any significant correlation across subjects with autonomic activity. This indicates that the increase in sympathetic tone observed in patients with PLMS does not lead to a change in arousal behavior.
Hypotheses Concerning the Pathophysiology of PLMS
Experimental studies showing an increased spinalcord excitability in patients experiencing PLMS45 suggest a spinal origin of PLMS. However, if the cause of this disinhibition of the spinal cord is obvious in patients with spinal injury,46 it remains less clear in patients with idiopathic PLMS. Furthermore, a spinal disinhibition does not explain the periodicity of PLM and the sensory and sleep disturbances that frequently accompany them.
The observations of this study suggest an important role of the sympathetic system in the generation of idiopathic PLMS that we can try to incorporate in a model of the pathophysiology of PLMS. A sympathetic activation occurs periodically in the setting of the physiologic sleep-wake control and, once surpassing a certain threshold, triggers or facilitates PLMS, the magnitude and intermovement interval of which depend on the magnitude and frequency of the sympathetic oscillation.
Previous pharmacoclinical studies have also suggested an involvement of the sympathetic nervous system, not only in the generation of PLM, but also in the generation of the sensory discomfort that frequently accompanies them. Patients with PLMS have been found to have an increased vascular tone and a decreased blood flow to the extremities, and phenoxybenzamine, a α1-adrenergic receptor blocker, normalizes these changes, decreases PLMS, and improves insomnia.47 Moreover, sympathetic nerve blockade with bupivacaine has also been shown to have beneficial effects on PLMs during wakefulness,48 and a randomized, double-blind, placebo-controlled study of clonidine, an activator of inhibitory presynaptic α2-adrenergic receptors, found significant improvements of subjective leg discomfort and restlessness, as well as of objective sleep latency, but not of PLMS, in patients with RLS.49
Previous work has shown that extrapyramidal centers in the brainstem are active during PLM in patients with RLS.50 The findings of these earlier results may be combined with the present study by speculating that potentially dysfunctional extrapyramidal motor networks are periodically activated by sympathetic projections and, thus, induce PLMS. This sympathoextrapyramidal pathway could be affected differently in idiopathic and symptomatic forms of PLMD and RLS. In patients with spinalcord lesions46 or with a dysfunctional extrapyramidal system due to a Parkinson syndrome,51 normal fluctuations in sympathetic activity might be sufficient to trigger PLMS, the autonomic nervous system being merely responsible for the periodicity of the movements. However, this study showed that, in patients with idiopathic PLMD, sympathetic activation reaches greater intensities than in control patients, whereas positron emission tomography and single photon emission computed tomography studies examining the presynaptic and postsynaptic nigrostriatal dopaminergic function of patients with idiopathic RLS and PLMD showed no or only discrete extrapyramidal dysfunctions,52 thus suggesting that the sympathetic hyperactivity could be the main reason for the occurrence of PLMS in this patient subgroup. An autonomic disequilibrium may also be responsible for the PLMS that are frequently observed in patients with multiple system atrophy53 or diabetes mellitus.54
Because sympathetic activity also precedes EEG arousals and is known to be closely related with K-complexes,39–41 the sympathetic system also seems to be involved in arousal control. This suggests that it might be the common mechanism underlying both PLMS and arousals, which in turn explains that these 2 phenomena cooccur at the same time without being directly related with each other (see above). Alternatively, one might postulate a common mechanism proximal to the autonomic, arousal, and motor systems, which periodically activates all 3 of them, with the sympathetic system having a lower threshold for activation than the arousal and motor systems. However, this alternative does not account for the consistently reported close relationship between autonomic activity and PLMS, as well as between autonomic activity and K-complexes, which contrasts sharply with the consistently reported lack of direct relationship between arousals and PLMS. Instead, the data suggest that, if common proximal mechanisms are effective, they mostly act via autonomous centers on motor and arousal networks. A potential candidate for such a common mechanism is the cyclic alternating pattern, which has been shown to have a gate-control function in the generation of PLMS.14 Future research will have to elucidate the exact relationship between the cyclic alternating pattern and autonomic function.
In conclusion, a model assuming a primary role of the sympathetic nervous system in the pathophysiology of PLM correctly reflects empiric findings and also, potentially, accounts for all clinical features of PLM (ie, for motor and sensory symptoms and for the periodicity, as well for the insomnia or sleep disturbances commonly experienced by patients with PLM). Furthermore, it opens the possibility of treating PLMD and related disorders with medication that modulates the autonomic nervous activity.
ACKNOWLEDGEMENTS
The authors would like to thank Pietro Ballinari, statistician, Institute for Psychology, University of Berne, for his help with statistical analysis of the data.
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Guggisberg, Hess, and Mathis have indicated no financial conflicts of interest.
FOOTNOTE
The β-receptor-blocking medication that some of the analyzed patients took probably did not significantly affect the observed differences in autonomic activation, since patients with RRLMS and ILMS showed very similar event-related autonomic activation patterns, even though 3 of the former and none of the latter took β-blockers.
REFERENCES
- 1.Montplaisir J, Nicolas A, Godbout R, Walters AS. Restless legs syndrome and periodic limb movement disorder. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2000. pp. 742–52. [Google Scholar]
- 2.Coleman RM, Pollak CP, Weitzman ED. Periodic movements in sleep (nocturnal myoclonus): relation to sleep disorders. Ann Neurol. 1980;8:416–21. doi: 10.1002/ana.410080413. [DOI] [PubMed] [Google Scholar]
- 3.Bastuji H, Garcia-Larrea L. Sleep/wake abnormalities in patients with periodic leg movements during sleep: factor analysis on data from 24-h ambulatory polygraphy. J Sleep Res. 1999;8:217–23. doi: 10.1046/j.1365-2869.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 4.Carrier J, Frenette S, Montplaisir J, Paquet J, Drapeau C, Morettini J. Effects of periodic leg movements during sleep in middle-aged subjects without sleep complaints. Mov Disord. 2005;20:1127–32. doi: 10.1002/mds.20506. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RM, Bliwise DL, Sajben N, Boomkamp A, de Bruyn LM, Dement WC. Daytime sleepiness in patients with periodic movements in sleep. Sleep. 1982;5(Suppl 2):S191–S202. doi: 10.1093/sleep/5.s2.s191. [DOI] [PubMed] [Google Scholar]
- 6.Mendelson WB. Are periodic leg movements associated with clinical sleep disturbance? Sleep. 1996;19:219–23. doi: 10.1093/sleep/19.3.219. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas A, Lesperance P, Montplaisir J. Is excessive daytime sleepiness with periodic leg movements during sleep a specific diagnostic category? Eur Neurol. 1998;40:22–6. doi: 10.1159/000007951. [DOI] [PubMed] [Google Scholar]
- 8.Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164:1454–8. doi: 10.1164/ajrccm.164.8.2011062. [DOI] [PubMed] [Google Scholar]
- 9.Hornyak M, Riemann D, Voderholzer U. Do periodic leg movements influence patients' perception of sleep quality? Sleep Med. 2004;5:597–600. doi: 10.1016/j.sleep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 11.Karadeniz D, Ondze B, Besset A, Billiard M. EEG arousals and awakenings in relation with periodic leg movements during sleep. J Sleep Res. 2000;9:273–7. doi: 10.1046/j.1365-2869.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrillo F, Beelke M, Canovaro P, et al. Changes in cerebral and autonomic activity heralding periodic limb movements in sleep. Sleep Med. 2004;5:407–12. doi: 10.1016/j.sleep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Lavoie S, de Bilbao F, Haba-Rubio J, Ibanez V, Sforza E. Influence of sleep stage and wakefulness on spectral EEG activity and heart rate variations around periodic leg movements. Clin Neurophysiol. 2004;115:2236–46. doi: 10.1016/j.clinph.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Parrino L, Boselli M, Buccino GP, Spaggiari MC, Di GG, Terzano MG. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol. 1996;13:314–23. doi: 10.1097/00004691-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Sforza E, Pichot V, Barthelemy JC, Haba-Rubio J, Roche F. Cardiovascular variability during periodic leg movements: a spectral analysis approach. Clin Neurophysiol. 2005;116:1096–104. doi: 10.1016/j.clinph.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–5. [PubMed] [Google Scholar]
- 17.Karadeniz D, Ondze B, Besset A, Billiard M. Are periodic leg movements during sleep (PLMS) responsible for sleep disruption in insomnia patients? Eur J Neurol. 2000;7:331–6. doi: 10.1046/j.1468-1331.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 18.Briellmann RS, Mathis J, Bassetti C, Gugger M, Hess CW. Patterns of muscle activity in legs in sleep apnea patients before and during nCPAP therapy. Eur Neurol. 1997;38:113–8. doi: 10.1159/000113173. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, technique and scoring system for sleep stages of human sleep. UCLA: Los Angeles Brain Information Service, Brain Information Institute; 1968. [Google Scholar]
- 20.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 21.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101:511–9. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 22.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 24.Knosche TR, Bastiaansen MC. On the time resolution of event-related desynchronization: a simulation study. Clin Neurophysiol. 2002;113:754–63. doi: 10.1016/s1388-2457(02)00055-x. [DOI] [PubMed] [Google Scholar]
- 25.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 26.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–32. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 27.Pichot V, Gaspoz JM, Molliex S, et al. Wavelet transform to quantify heart rate variability and to assess its instantaneous changes. J Appl Physiol. 1999;86:1081–91. doi: 10.1152/jappl.1999.86.3.1081. [DOI] [PubMed] [Google Scholar]
- 28.Durka PJ, Zygierewicz J, Klekowicz H, Ginter J, Blinowska KJ. On the statistical significance of event-related EEG desynchronization and synchronization in the time-frequency plane. IEEE Trans Biomed Eng. 2004;51:1167–75. doi: 10.1109/TBME.2004.827341. [DOI] [PubMed] [Google Scholar]
- 29.Zygierewicz J, Durka PJ, Klekowicz H, Franaszczuk PJ, Crone NE. Computationally efficient approaches to calculating significant ERD/ERS changes in the time-frequency plane. J Neurosci Methods. 2005;145:267–76. doi: 10.1016/j.jneumeth.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–56. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Amzica F, Steriade M. The functional significance of K-complexes. Sleep Med Rev. 2002;6:139–49. doi: 10.1053/smrv.2001.0181. [DOI] [PubMed] [Google Scholar]
- 32.American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 33.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 34.Steriade M, Dossi RC, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991;88:4396–400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jankel WR, Niedermeyer E. Sleep spindles. J Clin Neurophysiol. 1985;2:1–35. doi: 10.1097/00004691-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Rau C, Hummel F, Gerloff C. Cortical involvement in the generation of „involuntary“ movements in restless legs syndrome. Neurology. 2004;62:998–1000. doi: 10.1212/01.wnl.0000115389.37555.ca. [DOI] [PubMed] [Google Scholar]
- 37.Hornyak M, Feige B, Voderholzer U, Riemann D. Spectral analysis of sleep EEG in patients with restless legs syndrome. Clin Neurophysiol. 2005;116:1265–72. doi: 10.1016/j.clinph.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Camerer H, Stroh-Werz M, Krienke B, Langhorst P. Postganglionic sympathetic activity with correlation to heart rhythm and central cortical rhythms. Pflugers Arch. 1977;370:221–5. doi: 10.1007/BF00585530. [DOI] [PubMed] [Google Scholar]
- 39.Johnson LC, Karpan WE. Autonomic correlates of the spontaneous K-complex. Psychophysiology. 1968;4:444–52. doi: 10.1111/j.1469-8986.1968.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 40.Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG. Sympathetic muscle nerve activity during sleep in man. Brain. 1991;114:1281–95. doi: 10.1093/brain/114.3.1281. [DOI] [PubMed] [Google Scholar]
- 41.Tank J, Diedrich A, Hale N, et al. Relationship between blood pressure, sleep K-complexes, and muscle sympathetic nerve activity in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285:R208–R214. doi: 10.1152/ajpregu.00013.2003. [DOI] [PubMed] [Google Scholar]
- 42.Davies RJ, Belt PJ, Roberts SJ, Ali NJ, Stradling JR. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74:1123–30. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- 43.Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000;111:1611–9. doi: 10.1016/s1388-2457(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 44.Togo F, Cherniack NS, Natelson BH. Electroencephalogram characteristics of autonomic arousals during sleep in healthy men. Clin Neurophysiol. 2006;117:2597–603. doi: 10.1016/j.clinph.2006.07.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54:1609–16. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- 46.Yokota T, Hirose K, Tanabe H, Tsukagoshi H. Sleep-related periodic leg movements (nocturnal myoclonus) due to spinal cord lesion. J Neurol Sci. 1991;104:13–8. doi: 10.1016/0022-510x(91)90210-x. [DOI] [PubMed] [Google Scholar]
- 47.Ware JC, Blumoff R, Pittard JT. Peripheral vasoconstriction in patients with sleep related periodic leg movements. Sleep. 1988;11:182–6. doi: 10.1093/sleep/11.2.182. [DOI] [PubMed] [Google Scholar]
- 48.Uchihara T, Ichikawa T, Furukawa T, Tsukagoshi H. Myoclonus with burning sensation in legs that remits with sympathetic blockade. J Neurol Sci. 1990;100:161–4. doi: 10.1016/0022-510x(90)90028-l. [DOI] [PubMed] [Google Scholar]
- 49.Wagner ML, Walters AS, Coleman RG, Hening WA, Grasing K, Chokroverty S. Randomized, double-blind, placebo-controlled study of clonidine in restless legs syndrome. Sleep. 1996;19:52–8. doi: 10.1093/sleep/19.1.52. [DOI] [PubMed] [Google Scholar]
- 50.Bucher SF, Seelos KC, Oertel WH, Reiser M, Trenkwalder C. Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann Neurol. 1997;41:639–45. doi: 10.1002/ana.410410513. [DOI] [PubMed] [Google Scholar]
- 51.Happe S, Pirker W, Klosch G, Sauter C, Zeitlhofer J. Periodic leg movements in patients with Parkinson's disease are associated with reduced striatal dopamine transporter binding. J Neurol. 2003;250:83–6. doi: 10.1007/s00415-003-0957-8. [DOI] [PubMed] [Google Scholar]
- 52.Wetter TC, Eisensehr I, Trenkwalder C. Functional neuroimaging studies in restless legs syndrome. Sleep Med. 2004;5:401–6. doi: 10.1016/j.sleep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Vetrugno R, Provini F, Cortelli P, et al. Sleep disorders in multiple system atrophy: a correlative video-polysomnographic study. Sleep Med. 2004;5:21–30. doi: 10.1016/j.sleep.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Schiavi RC, Stimmel BB, Mandeli J, Rayfield EJ. Diabetes, sleep disorders, and male sexual function. Biol Psychiatry. 1993;34:171–7. doi: 10.1016/0006-3223(93)90388-t. [DOI] [PubMed] [Google Scholar]