Abstract
Objectives:
To evaluate the efficacy and safety of indiplon in primary insomnia.
Design:
Randomized, double-blind, placebo-controlled, 3-month study.
Setting:
Multi-center outpatient setting.
Patients:
N=702 (61% female; mean age 46 years) who met DSM-IV criteria for primary insomnia of at least 3 months' duration.
Interventions:
Indiplon 10 mg (n=236), indiplon 20 mg (n=233), or placebo (n=233).
Measurements:
Subjective assessment of each of the following: latency to sleep onset (sLSO), total sleep time (sTST), number of awakenings after sleep onset (sNAASO), wake time after sleep onset (sWASO), sleep quality, Insomnia Severity Index (ISI), and global improvement.
Results:
Treatment with indiplon resulted in significant improvement relative to placebo at all time points for the primary endpoint, sLSO. Mean sLSO at Month 1 for each treatment group was: 10 mg (34.0 ± 1.3 mins), 20 mg (33.0 ± 1.3 mins), and placebo (48.7 ± 1.9 mins; P <0.0001 for both comparisons); efficacy was sustained through Month 3. Both doses of indiplon resulted in significant improvement in sleep maintenance and duration endpoints, sTST and sWASO, as well as sleep quality, ISI, and global improvement at all assessment time points.
Conclusions:
In patients with chronic insomnia, long-term nightly treatment with 10 mg and 20 mg doses of indiplon resulted in significant and sustained efficacy in sleep onset, maintenance, and duration, and significant associated improvement in both daytime functioning and quality of life.
Citation:
Scharf MB; Black J; Hull S et al. Long-term nightly treatment with indiplon in adults with primary insomnia: Results of a double-blind, placebo-controlled, 3-month study. SLEEP 2007;30(6):743-752.
Keywords: Insomnia, indiplon, hypnotics, sleep latency, sleep maintenance, quality of life, functioning
INTRODUCTION
PRIMARY INSOMNIA, MEETING DSM-IV CRITERIA, IS A COMMON ILLNESS WITH AN ESTIMATED PREVALENCE OF 10%–15%, AND A MEDIAN DURATION OF 4 YEARS, with more than one-third of patients reporting chronicity in excess of 10 years.1–3
Cross-sectional studies, utilizing varying definitions, have found the complaint of insomnia to be associated with impaired quality of life and reduced productivity and functioning,4 increased healthcare utilization,4,5 increased risk of accidental injury,6,7 and substantial overall economic cost estimated at well over $20 billion in the United States alone.8
Chronic symptoms of insomnia appear to be associated with an increased risk of coronary events,9,10,11 reduced immune function, 12,13 as well as a significantly increased risk of developing major depression.14 Whether effective treatment reduces these health risks has not yet been tested.
The etiology of chronic insomnia is uncertain, though it is likely to be multifactorial. Transient insomnia may be initially triggered by medical stressors (e.g., pain or illness), psychological stressors (e.g., marital or work stress), or disruptive environmental conditions (e.g., shift work, noisy or uncomfortable sleep setting).15,16,17 In some individuals (the precise proportion is uncertain due to lack of longitudinal studies), transient insomnia persists and may evolve into a chronic illness.18
Treatment recommendations for chronic insomnia typically include sleep hygiene training and pharmacologic or nonpharmacologic interventions,19 though a review recently noted that the benefits of sleep hygiene in chronic insomnia are not yet supported by data from controlled treatment research.20
Recognition of the extent to which insomnia is a chronic and/or recurring illness has led some investigators to call for a systematic program of longitudinal clinical trials research, modeled on the past 20 years of treatment research in depression.21 Only a small handful of double-blind, placebo-controlled trials have been reported which evaluate the efficacy of hypnotics beyond 2 months of nightly treatment.22,23 Additionally, a few double-blind, placebo-controlled trials have studied the long-term (>2 months) efficacy of non-nightly (as needed) treatment for insomnia.24 Overall, the results of these studies have been positive; but given the extensive long-term use of hypnotics, a recent NIH Consensus Conference noted the need for additional long-term treatment studies.25
Indiplon is a pyrazolopyrimidine compound which is a benzodiazepine receptor agonist with high affinity and selectivity for the α1-subtype of the GABA-A receptor complex. The selectivity ratio of indiplon ranges from approximately 10-fold for α1- subtype vs. α2-subtype, and up to 350-fold for the α1-subtype relative to the α4- and α6-subtypes.2,3 The immediate-release capsule formulation of indiplon utilized in the current study has a Tmax of approximately 1 hour and an elimination half-life of 1.5–2 hours.26 It is metabolized by CYP3A4 and by carboxyesterase to inactive metabolites.
The objective of the current study was to evaluate the efficacy and safety of 3 months of nightly treatment with indiplon in adult patients with primary insomnia.
METHODS
Study Design
This was a double-blind, placebo-controlled, parallel-group trial designed to assess the efficacy and safety of 3 months of nightly treatment with 10 mg capsules and 20 mg capsules of indiplon in outpatients meeting DSM-IV criteria for primary insomnia.27 The study was conducted at 64 sites in the United States, Canada, and the United Kingdom. The protocol was approved by institutional review boards (ethics committees) at each site, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice regulations and guidance. The benefits and risks of study participation were explained to prospective study participants, and written informed consent was obtained prior to study entry or any procedures being conducted.
After completing a drug-free screening period of 7 to 14 days, eligible patients were entered into a 3-week, single-blind placebo lead-in. No assessments were obtained during the drug-free screening period. Daily sleep diary assessments were obtained during the single-blind, placebo lead-in period. At the third (baseline) visit, patients who continued to meet study entry criteria after the placebo lead-in period were randomized (1:1:1) to receive 3 months of double-blind treatment with indiplon 10 mg, indiplon 20 mg, or placebo. The randomization schedule was computer generated by the study sponsor, Neurocrine Biosciences. Patients were instructed to take their assigned study medication at bedtime and to return to the study center for monthly visits. At the end of 3 months of nightly treatment, indiplon was abruptly discontinued by substitution of single-blind placebo for one week. A subgroup of 147 patients (21% of the originally randomized sample) received an additional 3 months of study treatment prior to a Sponsor communication with the US Food and Drug Administration (FDA), which resulted in a protocol amendment reducing the duration of study treatment from 6 months to 3 months. Data from Months 4 to 6 for this subgroup are not summarized in this report.
Subjects
Men and non-pregnant women (those using medically acceptable contraception unless surgically sterilized or ≥2 years postmenopausal) aged 21–64 years, inclusive, were enrolled if they met DSM-IV criteria for primary insomnia by history for at least 3 months. Prospective subjects were required to meet the following additional criteria: (1) subjective latency to sleep onset (sLSO) ≥45 minutes on ≥3 nights per week; (2) subjective total sleep time (sTST) <6.5 hours ≥3 nights per week; and (3) in the clinical judgment of the investigator, the patient was likely to benefit from nightly use of a hypnotic agent during the study period. The nights on which patients met sLSO and sTST entry criteria were considered “qualifying nights.” Usual bedtime was required to be between 21:00 and 01:00, and could not vary by more than 2 hours on at least 5 nights per week. Key exclusion criteria included the following: (1) presence of any clinically significant or unstable medical disorder in the past 30 days; (2) history of epilepsy or serious head injury; (3) presence of any clinically significant abnormal finding on physical examination, laboratory testing, or electrocardiogram (ECG); (4) past history of sensitivity to benzodiazepines or other drugs acting at the GABA receptor; (5) history in the past 2 years of alcohol or substance dependence or abuse as defined by DSM-IV criteria, or a positive urine drug screen; (6) consumption of ≥5 alcoholic beverages per day or ≥14 alcoholic beverages per week; (7) use (in the 2 weeks prior to single-blind placebo) of any anxiolytics, antidepressants, anticonvulsants, histamine-1 receptor antagonists (except loratidine and fexofenadine), narcotic analgesics, or potent P450 3A4 inhibitors and inducers; (8) <6.5 hours or >9 hours of time in bed on ≥3 nights per week; (9) comorbid history (in past 3 years) of chronic pain, uncontrolled benign prostatic hypertrophy, major depressive disorder, schizophrenia, panic disorder, generalized anxiety disorder, or dementia.
Evaluations
All insomnia and sleep quality parameters were recorded daily in a sleep diary, which was completed each morning upon awakening. The primary outcome measure was sLSO. Additional patient-rated sleep parameters consisted of subjective wake-time after sleep onset (sWASO), subjective number of awakenings after sleep onset (sNAASO), and subjective total sleep time (sTST). Sleep quality was rated using a 7-point global scale (1=extremely good to 7=extremely poor).
In addition to collection of daily morning ratings of sleep quantity and sleep quality, monthly ratings were also obtained at each patient assessment visit using the following investigator- and patient-rated instruments: (1) The Medical Outcome Study (MOS) sleep scale is a 12-item patient-rated scale which contains a comprehensive set of questions assessing the domains of sleep disturbance (initiation and maintenance), awakening short of breath or with a headache, quantity of sleep, optimal sleep, and sleep adequacy. Two sleep index scores can also be computed using a subset of these items. The scale uses Likert or Likert-type response options, and has been shown to have good psychometric properties.28,29 Two items were omitted (snoring during sleep and taking naps during the day), as it was hypothesized they would not be affected by treatment, and their omission does not affect the scoring of the sleep problems indices. (2) The Insomnia Severity Index (ISI)30,31 is a patient-rated instrument which consists of 7 items which quantify, on a 5-point scale, the perceived severity of insomnia and insomnia-related interference with daytime functioning. A composite score is obtained by summing the 7 rated dimensions, with a score of 15–21 indicating a moderate level of insomnia and a score of 22–28 indicates severe insomnia. An ISI total score ≤10 indicates that subjectively rated insomnia symptoms and daytime impairment and quality of life have improved to the minimal-to-none range.31 (3) A 7-point Investigator Global Rating of Severity scale32 (IGR-S), with scores ranging from 1 (“normal, not at all ill”) to 7 (“extremely severe”). (4) A 7-point Investigator Global Rating of Change (IGR-C), with scores ranging from 1 (“marked improvement”) to 7 (“marked worsening”). The IGR-C scale was the only scale not completed at the pre-randomization visit. IGR-C responders were defined as patients who achieved an IGR-C score ≤ 2 (i.e., marked or moderate improvement).32
The effect of insomnia treatment on daytime functioning was evaluated using the 3-item “impact factor” from the Insomnia Severity Index. The ISI impact factor is a validated subscale of the ISI which rates daytime levels of insomnia-related distress and impairment in quality of life and functioning.31
Safety
During the screening period, safety evaluations to assess patient eligibility included physical and neurological examination, vital signs, routine laboratory tests (hematology, serum chemistry, and urinalysis), Hepatitis B surface antigen and Hepatitis C antibody test, pregnancy test for fertile females, 12-lead ECG, and urine drug screen. Vital signs (blood pressure, heart rate, respiratory rate, and oral temperature) were repeated at each visit; weight was measured at screen and end of study. The physical and neurological examination, routine labs, and ECG were repeated at the end of the study. All observed or reported adverse events, irrespective of suspected causality by study drug, were recorded and rated as to severity.
Symptoms of discontinuation were assessed with the Benzodiazepine Withdrawal Symptom Questionnaire33 (BWSQ), a 20-item questionnaire designed to assess, on a 3-point severity scale, symptoms experienced by patients discontinuing drugs that are active at benzodiazepine-GABAergic receptors. The BWSQ (maximum score of 40) was administered at pretreatment baseline, at the end of double-blind treatment (Month 3), on Days 1 and 2 of the discontinuation period, and at the final visit after the discontinuation period (or at the time of early termination).
Statistical Methods
Demographic and clinical data obtained during the placebo lead-in period were summarized with descriptive statistics, and the comparability of the 3 treatment groups were evaluated via analysis of variance (ANOVA) model with treatment and (pooled) center as fixed effects for continuous variables, and via the generalized Cochran-Mantel-Haenszel (CMH) procedure for categorical variables.
The primary efficacy endpoint was the log-transformed sLSO value for each month of the double-blind treatment period. The log transformation was applied because previous studies indicated that sLSO values tended to have a log-normal distribution. The primary efficacy comparisons were made using a mixed effects repeated measures (MERM) model performed on the intent to treat analysis set, defined as all patients who were randomized and received at least one dose of study drug. Terms in the model included baseline log [sLSO], treatment, center, month (as a categorical variable), month by treatment interaction, and month by baseline log [sLSO] interaction. The within-subject covariance structure for the repeated measures analyses was unstructured, and the restricted maximum likelihood method was used to estimate the covariance matrix. Satterthwaite's adjustment for degrees of freedom was used.
In order to control for the multiplicity associated with conducting 6 statistical comparisons (2 doses versus placebo across 3 time points), a sequential or closed testing procedure was applied to the Hochberg method.34 More specifically, if the maximum of the two P values was <0.05, significance was claimed for both doses, and testing at subsequent visits proceeded in the same manner. If the maximum P value was >0.05 and the minimum P value was <0.025, significance was claimed for the dose with the minimum P value. If the maximum P value was >0.05 and the minimum P value was >0.025, statistical significance was not claimed, and no subsequent significance was claimed. All P values were reported on the table regardless of the closed testing procedure, but significance was only claimed as described. Claims of significance at Months 2 and 3 were based on the Hochberg method outlined above and a sequential or closed testing procedure. The closed testing procedure requires that testing at later months be contingent on claims of significance from previous months.
Analyses of the secondary variables sTST, sWASO, sNAASO, and sleep quality were conducted via the MERM model described for the primary efficacy analysis. The IGR-S data, IGR-C data, and Patient Reported Outcome (PRO) variables were analyzed using the observed cases (OC) at each month with an ANOVA model including effects of treatment and center, or with an ANCOVA model when a baseline covariate was measured.
Discontinuation effects were examined by analyzing the proportion of patients meeting rebound criteria on Nights 1 and 2 post-discontinuation and by calculating the mean BWSQ total score for the first week of discontinuation. Rebound was defined a priori as an increase in sLSO of >15 minutes from the pretreatment baseline, calculated as the average of the qualifying nights (i.e., nights which met sLSO criteria for study entry). Discontinuation effects were also examined by analyzing the mean change from the qualifying nights baseline in sLSO and sTST on Night 1 and Night 2.
Finally, to evaluate the effect of attrition on efficacy, sLSO was analyzed using observed case data, completer data, and LOCF data, in addition to MERM models. Adverse event and safety data were summarized using descriptive statistics.
RESULTS
Patient Characteristics
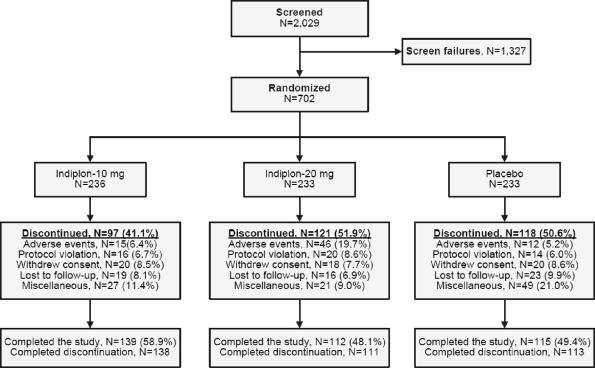
A total of 2,029 patients were screened, of whom 702 met eligibility criteria at the end of the 3-week, single-blind, placebo lead-in period. These 702 subjects were randomized to study treatment (Figure 1). At intake, the mean ± SD age of the study sample in years was 45.9 ± 11.7 in the placebo group, 44.9 ± 11.4 in the indiplon 10 mg group, and 46.0 ± 10.6 in the indiplon 20 mg group. In the placebo, indiplon 10 mg, and indiplon 20 mg groups, respectively, the proportion of females was 65.7% vs. 60.2% vs. 56.7% (P = 0.0153 for indiplon 20 mg vs. placebo). The proportion of patients who were white was 86.7% vs. 80.1% vs. 82.0%. No between-group baseline comparisons were significant for any sleep-related or investigator-rated measure (Table 1).
Figure 1.
Flow Diagram
Table 1.
Efficacy of Indiplon on Patient-rated Sleep Measures and Investigator-rated Global Measures, ls-mean ± se
| Placebo (N=214) | Indiplon |
Placebo comparison P value |
|||||
|---|---|---|---|---|---|---|---|
| 10 mg (N=228) | 20 mg(N=221) | Indiplon 10 mg | Indiplon 20 mg | ||||
| t | P | t | P | ||||
| sLSO, mins | |||||||
| Baseline | 56.4 ± 2.4 | 56.9 ± 2.4 | 58.6 ± 2.5 | 0.8734 | 0.5151 | ||
| Month 1 | 48.7 ± 1.9 | 34.0 ± 1.3 | 33.0 ± 1.3 | 6.72 | <0.0001 | 7.25 | <0.0001 |
| Month 2 | 42.3 ± 2.1 | 31.6 ± 1.5 | 31.0 ± 1.5 | 4.22 | <0.0001 | 4.43 | <0.0001 |
| Month 3 | 41.9 ± 2.3 | 29.7 ± 1.6 | 31.6 ± 1.7 | 4.50 | <0.0001 | 3.60 | 0.0003 |
| sTST, mins | |||||||
| Baseline | 315.4 ± 5.2 | 318.1 ± 5.0 | 304.9 ± 5.1 | 0.7057 | 0.1421 | ||
| Month 1 | 327.5 ± 4.0 | 362.8 ± 3.9 | 372.1 ± 4.0 | 6.36 | <0.0001 | 7.98 | <0.0001 |
| Month 2 | 339.5 ± 4.5 | 366.6 ± 4.3 | 375.8 ± 4.4 | 4.43 | <0.0001 | 5.84 | <0.0001 |
| Month 3 | 338.2 ± 4.9 | 364.8 ± 4.6 | 372.8 ± 4.8 | 3.96 | <0.0001 | 5.06 | <0.0001 |
| sWASO, mins | |||||||
| Baseline | 73.9 ± 4.1 | 73.8 ± 3.9 | 78.3 ± 4.1 | 0.9866 | 0.4244 | ||
| Month 1 | 61.1 ± 2.5 | 49.7 ± 2.4 | 42.2 ± 2.5 | 3.32 | 0.0009 | 5.46 | <0.0001 |
| Month 2 | 56.1 ± 2.8 | 44.0 ± 2.6 | 43.1 ± 2.7 | 3.20 | 0.0014 | 3.39 | 0.0008 |
| Month 3 | 52.8 ± 2.8 | 42.5 ± 2.6 | 43.4 ± 2.7 | 2.75 | 0.0061 | 2.46 | 0.0141 |
| sNAASO | |||||||
| Baseline | 1.7 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.2101 | 0.0861 | ||
| Month 1 | 1.6 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 4.80 | <0.0001 | 6.58 | <0.0001 |
| Month 2 | 1.6 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 3.28 | 0.0011 | 4.56 | <0.0001 |
| Month 3 | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 3.55 | 0.0004 | 4.22 | <0.0001 |
| Sleep qualitya | |||||||
| Baseline | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.4 ± 0.1 | 0.9189 | 0.7344 | ||
| Month 1 | 4.1 ± 0.1 | 3.7 ± 0.1 | 3.5 ± 0.1 | 5.91 | <0.0001 | 7.89 | <0.0001 |
| Month 2 | 4.0 ± 0.1 | 3.7 ± 0.1 | 3.5 ± 0.1 | 3.56 | 0.0004 | 5.47 | <0.0001 |
| Month 3 | 3.9 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.1 | 3.65 | 0.0003 | 3.78 | 0.0002 |
| IGR-Severity | |||||||
| Baseline | 4.7 ± 0.1 | 4.8 ± 0.1 | 4.9 ± 0.1 | 0.4486 | 0.1628 | ||
| Month 1 | 4.3 ± 0.1 | 3.6 ± 0.1 | 3.4 ± 0.1 | 5.62 | <0.0001 | 7.41 | <0.0001 |
| Month 2 | 3.8 ± 0.1 | 3.4 ± 0.1 | 3.1 ± 0.1 | 3.81 | 0.0002 | 5.70 | <0.0001 |
| Month 3 | 3.6 ± 0.1 | 3.0 ± 0.1 | 2.9 ± 0.1 | 3.94 | <0.0001 | 4.22 | <0.0001 |
| IGR-Change | |||||||
| Month 1 | 3.3 ± 0.1 | 2.7 ± 0.1 | 2.5 ± 0.1 | 4.52 | <0.0001 | 6.81 | <0.0001 |
| Month 2 | 3.2 ± 0.1 | 2.6 ± 0.1 | 2.2 ± 0.1 | 4.32 | <0.0001 | 7.00 | <0.0001 |
| Month 3 | 3.0 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 5.09 | <0.0001 | 4.58 | <0.0001 |
All between-group comparisons at baseline were nonsignificant
Based on mixed effects repeated measures model on the intent-to-treat sample; sLSO analysis used log-transformed data, untransformed data was used for the other parameters;
Sleep Quality: 1=extremely good to 7=extremely poor
A similar percentage of patients randomized to indiplon 20 mg (48.1%) and placebo (49.4%) completed the study, while a higher proportion were completers in the 10 mg group (58.9%; Figure 1). Discontinuation due to adverse events was comparable for indiplon 10 mg and placebo groups (6.4% vs. 5.2%), but was significantly higher for the 20 mg group (19.7%; chi square, 32.9; P < 0.001 vs. placebo).
Effect of Indiplon on Sleep Onset
Both doses of indiplon produced statistically significant improvement relative to placebo on the primary outcome, latency to sleep onset (Table 1). Improvement was sustained across all 3 months of study treatment with no evidence of change in mean response over time.
Effect of Indiplon on Sleep Maintenance And Duration
Treatment with indiplon produced significant improvement versus placebo in sleep maintenance and duration measures, sTST, sWASO, and sNAASO (Table 1). The least square mean change in sTST was +45 minutes at Month 1 on indiplon 10 mg and ± 67 minutes on indiplon 20 mg. The improvements observed at Month 1 in sTST, sWASO, and sNAASO were sustained throughout the 3 months of study treatment, with no evidence of change in mean response over time. Subjective WASO was reduced by at least 30 minutes in the majority of patients on both indiplon 10 mg and 20 mg.
Effect on First Treatment Night
Use of indiplon resulted in statistically significant improvement on the first night of treatment in both sleep onset and sleep maintenance and duration measures. Latency to sleep onset was significantly lower in the indiplon 10 mg group compared to placebo (37.6 ± 2.3 vs. 51.9 ± 3.2; t=3.81; P = 0.0002). Latency to sleep onset was also significantly shorter compared to placebo in the indiplon 20 mg group (32.8 ± 2.0; t=5.38; P <0.0001). Similarly, sTST was significantly longer in the indiplon 10 mg group compared to placebo (365.3 ± 6.9 vs. 324.4 ± 7.0; t=4.22; P <0.0001). Subjective total sleep time was also significantly longer compared to placebo in the indiplon 20 mg group (371.8 ± 7.1; t=4.83; P <0.0001).
Global Improvement
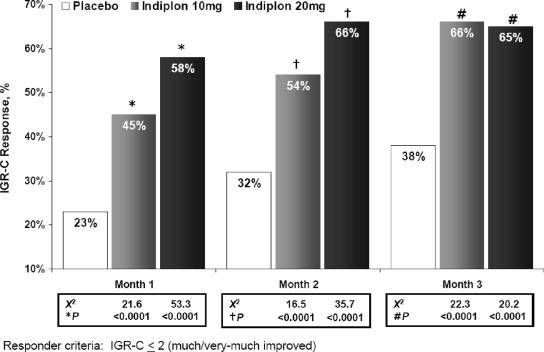
Indiplon demonstrated significantly greater global improvement relative to placebo on both the IGR-S and IGR-C scales throughout the 3 months of study treatment (Table 1). There were significantly more responders (IGR-C ≤2) on both doses of indiplon compared to placebo at each month of study treatment (observed case analysis; Figure 2). On a 3-month LOCF-endpoint analysis, IGR-C responder rates were also significantly higher than placebo (31%) when compared to both indiplon 10 mg (55%; χ2, 24.9; P <0.0001) and indiplon 20 mg (54%; χ2, 23.5; P <0.0001).
Figure 2.
Proportion of patients meeting IGR-C responder criteria during 3 months of treatment: results of an observed case analysis. Responder criteria: IGR-C ≤2 (much/very-much improved).
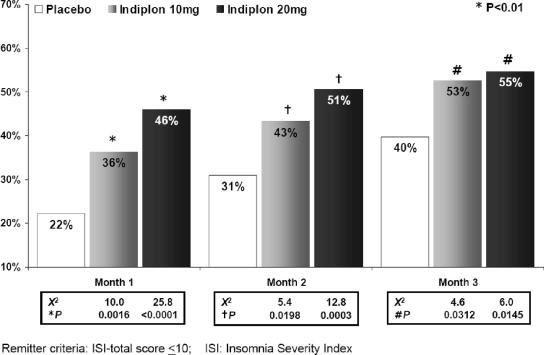
The Insomnia Severity Index (ISI) total score was analyzed to provide an illness-specific measure of improvement in insomnia symptoms and insomnia-related daytime impairment. An ISI total score ≤10 was utilized as a previously validated criterion to indicate symptom-free response (minimal-to-no symptoms or impairment).31 A significantly greater number of patients achieved an ISI total score ≤10 on both doses of indiplon versus to placebo at each month of study treatment (Figure 3).
Figure 3.
Proportion of patients meeting ISI Remission criteria during 3 months of treatment: results of an observed case analysis. Remitter criteria: ISI-total score ≤10; ISI: Insomnia Severity Index.
Sleep Quality
Sleep quality was evaluated using 2 patient-rated measures, a 7-point global sleep quality scale, and the MOS Sleep Scale. Sleep quality was significantly improved on indiplon across all 3 months of study treatment (Table 1). The proportion of patients who reported good-to-extremely-good sleep quality was significantly higher than placebo on indiplon 10 mg and 20 mg, respectively, at Month 1 (9% vs. 28% [P <0.0001] and 20% [P <0.01]), at Month 2 (14% vs. 26% [P <0.01] and 24% [P = 0.025]), and at Month 3 (14% vs. 25% [P = 0.025] and 25% [P = 0.025]). Similar significant improvement was also observed across most of the insomnia-related dimensions of the MOS Sleep Scale (Table 2).
Table 2.
Efficacy of Indiplon on Additional Patient-rated Measures, ls-mean ± se (Except % for ISI-impact Factor)
| Placebo (N=214) | Indiplon |
Placebo comparison P value |
|||||
|---|---|---|---|---|---|---|---|
| 10 mg (N=228) | 20 mg(N=221) | Indiplon 10 mg | Indiplon 20 mg | ||||
| t | P | t | P | ||||
| MOS Sleep Scalea | |||||||
| Sleep problems | |||||||
| Baseline | −0.02 ± 0.04 | −0.01 ± 0.04 | 0.02 ± 0.03 | ||||
| Month 1 | 0.26 ± 0.04 | −0.03 ± 0.04 | −0.09 ± 0.04 | 5.53 | <0.0001 | 6.55 | <0.0001 |
| Month 2 | 0.22 ± 0.05 | −0.05 ± 0.04 | −0.14 ± 0.04 | 4.24 | <0.0001 | 5.61 | <0.0001 |
| Month 3 | 0.24 ± 0.05 | −0.08 ± 0.05 | −0.08 ± 0.05 | 4.82 | <0.0001 | 4.68 | <0.0001 |
| Sleep disturbance | |||||||
| Baseline | −0.04 ± 0.05 | 0.02 ± 0.04 | 0.01 ± 0.04 | ||||
| Month 1 | 0.30 ± 0.05 | −0.04 ± 0.05 | −0.15 ± 0.05 | 5.20 | <0.0001 | 6.78 | <0.0001 |
| Month 2 | 0.25 ± 0.06 | −0.07 ± 0.05 | −0.15 ± 0.06 | 4.22 | lt;0.0001 | 5.15 | <0.0001 |
| Month 3 | 0.33 ± 0.06 | −0.10 ± 0.06 | −0.11 ± 0.06 | 5.18 | <0.0001 | 5.12 | <0.0001 |
| Sleep quantity | |||||||
| Baseline | 68.8 ± 1.1 | 68.6 ± 1.1 | 69.0 ± 1.1 | ||||
| Month 1 | 57.1 ± 1.0 | 62.3 ± 1.0 | 62.8 ± 1.0 | 3.70 | 0.0002 | 4.04 | <0.0001 |
| Month 2 | 50.0 ± 1.0 | 54.9 ± 0.9 | 54.0 ± 0.9 | 3.80 | 0.0002 | 3.06 | 0.0023 |
| Month 3 | 56.0 ± 1.2 | 58.9 ± 1.1 | 60.6 ± 1.1 | 1.80 | 0.0733 | 2.82 | 0.0050 |
| Sleep adequacy | |||||||
| Baseline | 26.5 ± 1.5 | 26.4 ± 1.4 | 24.0 ± 1.3 | ||||
| Month 1 | 32.5 ± 1.7 | 42.2 ± 1.6 | 44.3 ± 1.7 | 4.15 | <0.0001 | 5.06 | <0.0001 |
| Month 2 | 37.6 ± 2.0 | 43.8 ± 1.9 | 49.0 ± 2.0 | 2.30 | 0.0221 | 4.11 | <0.0001 |
| Month 3 | 40.4 ± 2.2 | 46.9 ± 2.1 | 46.4 ± 2.2 | 2.16 | 0.0316 | 1.92 | 0.0557 |
| ISI Total Scoreb | |||||||
| Baseline | 17.4 ± 0.3 | 17.2 ± 0.3 | 17.2 ± 0.3 | ||||
| Month 1 | 14.4 ± 0.4 | 12.1 ± 0.4 | 11.8 ± 0.4 | 4.63 | <0.0001 | 5.03 | <0.0001 |
| Month 2 | 13.6 ± 0.4 | 11.5 ± 0.4 | 10.8 ± 0.4 | 3.95 | <0.0001 | 5.16 | <0.0001 |
| Month 3 | 13.1 ± 0.5 | 11.0 ± 0.4 | 11.1 ± 0.5 | 3.50 | 0.0005 | 3.26 | 0.0012 |
| ISI-impact factor | |||||||
| score < 6 c | X2 | P | X2 | P | |||
| Baseline | 35.2% | 34.8% | 36.2% | ||||
| Month 1 | 51.2% | 62.4% | 63.0% | 5.25 | 0.0220 | 5.79 | 0.0161 |
| Month 2 | 60.1% | 72.5% | 74.6% | 5.72 | 0.0167 | 7.62 | 0.0058 |
| Month 3 | 63.3% | 79.6% | 76.1% | 9.18 | 0.0024 | 5.16 | 0.0231 |
Medical Outcome Study
ISI = Insomnia Severity Index
minimal-to-no impairment in insomnia-related daytime functioning and quality of life
Improvement in Subjective Daytime Functioning
Insomnia-related impairment in daytime functioning, indexed using the 3-item ISI-impact factor, was significantly improved on both doses of indiplon versus placebo (Table 2). The proportion of patients reporting minimal-to-no daytime impairment in functioning and quality of life on the ISI-impact factor (score <6) was also significantly greater for both doses of indiplon versus placebo across all 3 months of study treatment (Table 2).
Discontinuation Data
Discontinuation effects were examined by 3 methods: (1) as a continuous outcome: change (from pretreatment baseline) in sLSO and sTST on discontinuation Night 1 and Night 2; (2) as a categorical outcome: the proportion of patients meeting a priori rebound criteria; and (3) an analysis of withdrawal symptomatology as measured by the BWSQ. For the first 2 analyses, pretreatment baseline was defined a priori as the average of the qualifying nights (nights during the single-blind placebo lead-in period with sLSO ≥45 minutes and sTST <6.5 hours). Rebound was defined, also a priori, as an increase in the primary outcome measure, sLSO, of >15 minutes from the pretreatment baseline.
Method 1: Change from Baseline
When indiplon was discontinued abruptly by placebo substitution after 3 months of double-blind treatment, sleep onset benefit was largely maintained on post-discontinuation Night 1 (mean improvement in sLSO relative to pre-treatment baseline [qualifying night data]: indiplon 10 mg, −24.7 ± 9.0 mins [within-group significance, t = 2.74; P = 0.0072]; indiplon 20 mg, −20.6 ± 11.2 mins [t = 1.84; P = 0.0689]; and placebo −57.6 ± 7.7 mins [t=7.46; P<0.0001]); and on Night 2 (indiplon 10 mg, −39.2 ± 7.9 mins [t = 4.96; P <0.0001]; indiplon 20 mg, −37.0 ± 11.1 mins [t = 3.32; P = 0.0014]; and placebo, −66.8 ± 7.2 mins [t = 9.34; P <0.0001]). Total sleep time was also largely maintained on post-discontinuation Night 1 (mean improvement in sTST relative to pre-treatment baseline [qualifying night data]: indiplon 10 mg, +41.4 ± 10.5 mins [t = 3.94; P = 0.0001]; indiplon 20 mg, +30.4 ± 13.8 mins [t = 2.20; P = 0.0304]; and placebo +73.1 ± 9.8 mins [t = 7.43; P <0.0001]), and on Night 2 (indiplon 10 mg, +63.3 ± 10.5 mins [t = 6.05; P <0.0001]; indiplon 20 mg, +54.0 ± 13.5 mins [t = 4.00; P = 0.0001]; and placebo, +92.5 ± 10.0 mins [t = 9.21; P <0.0001]).
Method 2: Rebound Analysis
On discontinuation night 1, the proportion of patients who met rebound criteria was lower in the placebo group (N=93; 8.8%) than the indiplon 10 mg group (N = 111; 18.9%) and the indiplon 20 mg group (N = 87; 22.1%). On discontinuation night 2, the proportion of patients who met rebound criteria was also lower in the placebo group (1.1%) compared with the indiplon 10 mg group (11.1%) and the indiplon 20 mg group (11.4%).
Method 3: Withdrawal Analysis
To evaluate whether discontinuation of long-term indiplon treatment was associated with benzodiazepine-like withdrawal symptomatology, the mean BWSQ total scores were analyzed at various times during the study including one week after abruptly stopping indiplon. In the indiplon 10 mg group, the BWSQ score was similarly low on the last night of double-blind treatment compared to week 1 post discontinuation (1.4 ± 0.2 vs. 1.3 ± 0.2; t = 0.88; P = 0.38). Similarly, in the indiplon 20 mg group, the BWSQ score was low compared with the last night of double-blind treatment (2.3 ± 0.4 vs. 2.2 ± 0.4; t = 0.33; P = 0.74).
Safety
The following 5 adverse events occurred with an incidence of ≥5% on one or both doses of indiplon: upper respiratory infection (placebo, 5.2%; indiplon 10 mg, 5.9%; indiplon 20 mg, 6.0%); amnesia (placebo, 0%; indiplon 10 mg, 1.3%; indiplon 20 mg, 6.4%); dizziness (placebo, 3.0%; indiplon 10 mg, 4.7%; indiplon 20 mg, 6.9%), headache (placebo, 6.9%; indiplon 10 mg, 8.5%; indiplon 20 mg, 9.0%); and somnolence (placebo, 1.3%; indiplon 10 mg, 3.0%; indiplon 20 mg, 7.3%).
Among the top 5 adverse events listed above, none were rated as “severe” on placebo, and only headache was rated as severe among patients (1.3%) treated with indiplon 10 mg. Among patients treated with indiplon 20 mg, the following adverse events were rated as “severe”: amnesia (1.3%), dizziness (0.9%), headache (1.7%), and somnolence (1.3%).
Most adverse events were relatively transient across the 3 months of study treatment. Median duration of top 5 adverse events experienced while on indiplon were: headache (3 days), amnesia (5 days), dizziness (9 days), upper respiratory infection (11 days), and somnolence (19 days). The severity of adverse events was mild-to-moderate in 95% of patients in the indiplon 10 mg group, 83% in the indiplon 20 mg group, and in 93% in the placebo group.
No serious adverse events (SAEs) occurred that investigators judged to be related to study drug. Five non–treatment-related SAEs occurred on indiplon 10 mg (uterine fibroids, metastatic breast cancer, a transient ischemic attack, chest pain, and a completed suicide), 5 non–treatment-related SAEs occurred on indiplon 20 mg (a renal calculus, breast cancer, an episode of diabetic ketoacidosis, viral labyrinthitis, and musculoskeletal chest pain), and one non–treatment-related SAE occurred on placebo, during the single-blind lead-in phase (congestive heart failure).
Abnormal liver function tests were reported as adverse events by 4 patients on placebo, 1 patient on indiplon 10 mg, and 2 patients on indiplon 20 mg. The elevations in liver function tests in the 3 patients on indiplon were mild and transient and judged to be unrelated to study drug. A clinically significant laboratory abnormality consisted of an episode of diabetic ketoacidosis, mentioned in the paragraph above. Two patients on the 20 mg dose of indiplon discontinued prematurely; one due to an increase in heart rate and the other due to an abnormal ECG. There were no other clinically significant treatment-emergent abnormalities in ECG, vital signs, or physical examination.
Long-term Treatment and Efficacy
On the primary outcome measure, sLSO, efficacy was sustained and significant at 3 months regardless of the analytic strategy, or the method used to account for attrition. On the LOCF analysis, the least square mean sLSO value was significantly higher for indiplon 10 mg vs. placebo (n = 228; 30.9 ± 1.4 vs. n=214; 45.1 ± 2.2; t = 5.74_; P <0.0001), and indiplon 20 mg vs. placebo (n = 221; 32.5 ± 1.5 vs. 45.1 ± 2.2; t = 4.96; P <0.0001). On the completer analysis, the LS-mean sLSO was significantly higher for indiplon 10 mg vs. placebo (n=154; 29.7 ± 1.7 vs. n=130; 38.5 ± 2.4; t = 3.11; P = 0.002), and indiplon 20 mg vs. placebo (n=134; 29.9 ± 1.8 vs. 38.5 ± 2.4; t = 2.91; P = 0.0038). On the observed case analysis, the LS-mean sLSO was significantly higher for indiplon 10 mg vs. placebo (n=158; 29.8 ± 1.7 vs. n=132; 38.3 ± 2.3; t = 0.03; P = 0.0026), and indiplon 20 mg vs. placebo (n = 140; 30.1 ± 1.8 vs. 38.3 ± 2.3; t = 2.81; P = 0.0052). Since significance levels are influenced by sample size, perhaps a more useful method for evaluating the effect of attrition on outcome is to examine the endpoint indiplon vs. placebo effect sizes at Month 3. The effect size was larger for the LOCF sample compared with the observed case and 3-month completer samples, respectively, for both indiplon 10 mg (0.56 vs. 0.37 and 0.38) and indiplon 20 mg (0.50 vs. 0.36 and 0.37).
DISCUSSION
The results of this double-blind, placebo-controlled study demonstrate that indiplon significantly improves subjective measures of sleep onset, sleep maintenance and duration, and overall sleep quality in patients with primary insomnia. Improvement in sleep was immediate, occurring on the first night of treatment, and was sustained across all 3 months of study treatment, with no evidence of change in response with time. Improvement on indiplon in primary insomnia symptoms was associated with significant subjective improvement in daytime functioning and quality of life.
Concurrent with improvement in sleep onset and sleep maintenance, treatment with both doses of indiplon resulted in significantly greater responder rates using the investigator-rated criteria, the IGR-C. Despite a high degree of chronicity in the current treatment sample, a significant proportion of patients on both indiplon 10 mg and 20 mg responded to the point that insomnia symptoms and insomnia-related impairment in daytime functioning and quality of life were rated as mild/a-little-to-none/not-at-all on the Insomnia Severity Index. Again, mean improvement for each treatment group was sustained through Month 3.
The ISI-impact factor, which indexes the effect of insomnia on daytime functioning and quality of life, was significantly improved on both doses of indiplon. While patient self-report assessments such as the ISI provide a useful proxy measure of daytime functioning, much more research is needed to characterize the precise nature of daytime impairments in cognitive, psychomotor, interpersonal, and occupational functioning. Available studies using objective measures have tended (with a few exceptions35,36) to find no daytime impairment secondary to insomnia. But the majority of the research has been conducted using small samples, in individuals not meeting DSM-IV criteria for insomnia, and using measures of daytime functioning with low validity. Furthermore, we are unaware of any adequately powered, double-blind, placebo-controlled clinical trials which report the daytime effects of pharmacologic treatment using valid objective functional measures. A recent NIH conference identified use of such objective functional measures in clinical trials as one of the major research goals in the field of insomnia treatment research.25
Most adverse events were of mild-to-moderate intensity, and were transient in duration. The incidence of adverse events, and discontinuation due to adverse events, were similar for the 10 mg dose of indiplon and placebo. Treatment with the 20 mg dose of indiplon was associated with an incremental increase in the incidence of adverse events. There was no evidence of any clinically significant effect of indiplon on ECG parameters, laboratory tests, physical examination, or vital signs.
In the current study, discontinuation of indiplon was not associated with any increase in benzodiazepine-like withdrawal symptoms. Similarly, improvement in the primary outcome measure, sLSO, was largely maintained on the first 2 nights of discontinuation.
Several limitations of the current study should be noted. First, while subjective data indicate that improvement in sleep parameters for the 2 indiplon dosage groups were sustained through the 3-month endpoint, no polysomnographic data were available in this study to provide objective confirmation of sustained benefit. Subjective sleep data generally, but not always, parallel those of polysomnography. The absence of polysomnography data is also a design limitation of another recent long-term insomnia study.23
Second, the absence of polysomnography in the current study limited the ability to definitively rule out the presence of rebound. Transient rebound has been reported for hypnotics with an affinity for the benzodiazepine binding site on the GABA receptor, though rebound is not always systematically evaluated, even by self-reported measures, in studies lasting >3 months.23
Third, the discontinuation rate of ∼50% raises a concern that attrition may have introduced bias in the endpoint analysis of efficacy. The robustness of treatment significance, using a range of inferential statistical methods including mixed effects repeated measures analysis, and LOCF, observed case, and completer analyses, suggests that the long-term efficacy of indiplon was not secondary to bias introduced by differentially higher attrition in nonresponders.
Fourth, patients with current psychiatric and acute medical illnesses were excluded. These exclusions are standard in placebo-controlled efficacy trials, but nonetheless, they reduce the generalizability of the study results from research to clinical practice settings where psychiatric comorbidity frequently occurs.
A final study limitation was the potential for noncompliance, which may have resulted in an underestimation of treatment effect. Pill counts were done on returned medication, but there was no formal method for assessing adherence to nightly dosing.
In conclusion, this study demonstrates that indiplon, in doses of 10 mg and 20 mg, is a safe treatment which demonstrates efficacy in patients with primary insomnia; it demonstrates reduced tolerability at the 20 mg dose, which is higher than the expected therapeutic dosage range for indiplon. In addition, indiplon significantly improved sleep onset and sleep maintenance and duration, as well as subjectively assessed levels of daytime functioning and quality of sleep. Improvement occurred on the first night of treatment and was sustained for the group as a whole throughout 3 months of treatment.
ACKNOWLEDGEMENTS:
Funding was provided by Neurocrine Biosciences, Inc and Pfizer Inc.
The authors wish to acknowledge the individual study investigators for their participation in this trial:
United States: Theodore Amgott, MD; Robert Ballard, MD; Michael Biber, MD; Jed Black, MD; Richard Bogan, MD; Christopher Calder, MD; Nancy Campbell, MD; Clinton Corder, MD; Bruce Corser, MD; G. Dempsey, MD; Helene Emsellem, MD; Gary Erdy, MD; Mildred Farmer, MD; Thomas Fiel, DO; Eugene Fletcher, MD; Suzanne Gazda, MD; Harry Geisberg, MD; Steven Glass, MD; E. Walter Hood, MD; Timothy Howard, MD; John Hudson, MD; Steven Hull, MD; E Christopher Iliades, MD; Rakesh Jain, MD; Arifulla Khan, MD; R. Sanford Kiser, MD; Alan Kivitz, MD; Keith Klatt, MD; Michael Levy, MD; Veena Luthra, MD; Mark Mahowald, MD; F. Timm McCarty, DO; Louis McNabb, MD; Dennis Munjack, MD; John Murphy, MD; Linda Murray, DO; Ralph Pascualy, MD; Jayant Phadke, MD; Bryan Pogue, MD; Raj Rajani, MD; Marc Raphaelson, MD; Michele Reynolds, MD; Ernie Riffer, MD; Leon Rubenfaer, MD; David Sack, MD; Marshall Sack, MD; Martin Scharf, PhD; Donald Schexnayder, MD; Douglas Schumacher, MD; Howard Schwartz, MD; David Seiden, MD; John Stoukides, MD; Stephen Thein, PhD; John Townsend, MD. Canada: Leonid Kayumov, PhD; Adam Moscovitch, MD; Saibal Nandy, MD; and Lee Rasmusen, MD. United Kingdom: Robert Cook, MD; John Ham, MD; David Haworth, MD; J. Langan, MBChB; R. Macleod, MD; and A. Rotheray, MD
The authors wish to acknowledge Phil Jochelson for contributions to the original study design; Richard Zhang, PhD, for assistance with the statistical analysis; and Edward Schweizer, MD, for assistance in the preparation of the manuscript.
Footnotes
Disclosure of Off-Label Use
Indiplon is currently under review by the FDA, and has not been approved for use in insomnia
Disclosure Statement
Funding for this study was provided by Neurocrine Biosciences, Inc. and Pfizer, Inc. Dr. Scharf has received research support from Neurocrine, GlaxoSmithKline, Merck, Somaxon, Takeda, and Cephalon; has participated in speaking engagements for Sepracor, Jazz Pharmaceuticals, and Primed; and has been a consultant for Neurocrine. Dr. Black has received research support from Cephalon, Organon, GlaxoSmithKline, and Jazz Pharmaceuticals and has been a scientific advisor or paid speaker for GlaxoSmithKline, Takeda, and Jazz Pharmaceuticals. Dr. Hull has participated as a principal investigator for Pfizer, Neurocrine Biosciences, Sanofi-Aventis, Merck, Takeda, Somaxon, Sepracor, and Cephalon and is on the speaker's bureau for Sepracor. Dr. Landin is Associate Director of Biostatistics at Neurocrine Biosciences. Dr. Farber is Associate Director, Clinical Development, at Neurocrine Biosciences.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men: 10-year prospective population based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 4.Leger D, Guilleminault C, Bader G, Levy E, Paillard M. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–9. [PubMed] [Google Scholar]
- 5.Hatoum HT, Kong SX, Kania CM, Wong JM, Mendelson WB. Insomnia, health-related quality of life and healthcare resource consumption. A study of managed-care organisation enrollees. Pharmacoeconomics. 1998;14:629–37. doi: 10.2165/00019053-199814060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Balter MB, Uhlenhuth EH. New epidemiologic findings about insomnia and its treatment. J Clin Psychiatry. 1992;53(12) suppl:34–9. [PubMed] [Google Scholar]
- 7.Leger D. Public health and insomnia: economic impact. Sleep. 2000;23:S69–76. [PubMed] [Google Scholar]
- 8.Metlaine A, Leger D, Choudat D. Socioeconomic impact of insomnia in working populations. Ind Health. 2005;43:11–19. doi: 10.2486/indhealth.43.11. [DOI] [PubMed] [Google Scholar]
- 9.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: psychosocial predictors from a 20-year follow-up of women in the Framingham Study. Am J Epidemiol. 1992;135:854–64. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 10.Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomer K. Poor sleep increases the prospective risk for recurrent events in middle-aged women with coronary disease. The Stockholm Female Coronary Risk Study. J Psychosom Res. 2003;54:121–7. doi: 10.1016/s0022-3999(02)00475-0. [DOI] [PubMed] [Google Scholar]
- 11.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 12.Savard J, Laroche L, Simard S, Ivers H, Morin CM. Chronic insomnia and immune functioning. Psychosom Med. 2003;65:211–21. doi: 10.1097/01.psy.0000033126.22740.f3. [DOI] [PubMed] [Google Scholar]
- 13.Irwin M, Clark C, Kennedy B, Gillin JC, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 14.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–18. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 15.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 16.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 18.Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18:163–76. doi: 10.1002/da.10151. [DOI] [PubMed] [Google Scholar]
- 19.Schenck CH, Mahowald MW, Sack RL. Assessment and management of insomnia. JAMA. 2003;289:2475–79. doi: 10.1001/jama.289.19.2475. [DOI] [PubMed] [Google Scholar]
- 20.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7:215–25. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]
- 21.Jindal RD, Buysse DJ, Thase ME. Maintenance treatment of insomnia: what can we learn from the depression literature? Am J Psychiatry. 2004;161:19–24. doi: 10.1176/appi.ajp.161.1.19. [DOI] [PubMed] [Google Scholar]
- 22.Oswald I, French C, Adam K, Gilham J. Benzodiazepine hypnotics remain effective for 24 weeks. Br Med J (Clin Res Ed) 1982;284:860–3. doi: 10.1136/bmj.284.6319.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 24.Perlis ML, McCall WV, Krystal AD, Walsh JK. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128–37. doi: 10.4088/jcp.v65n0816. [DOI] [PubMed] [Google Scholar]
- 25.National Institute of Health State-of-the-Science Conference Statement: Manifestations and management of chronic insomnia in adults. 2005 Jun 15; www.consensus.nih.gov.
- 26.Jocheslson P, Chen TK, Farber R, Campbell B. Lack of pharmacological and pharmacokinetic tolerance following repeat dosing of indiplon. Sleep. 2003;26(suppl):A85. [Google Scholar]
- 27.Washington, DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders: DSM-IV. [Google Scholar]
- 28.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005;6:41–44. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JE, editors. Measuring functioning and well-being: the Medical Outcomes Study approach. Durham, NC: Duke University Press; 1992. pp. 235–259. [Google Scholar]
- 30.Morin CM. New York: Guilford Press; 1993. Insomnia: psychological assessment and management. [Google Scholar]
- 31.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 32.Guy W. Washington, DC: National Institute of Mental Health-US Dept of Health, Education, and Welfare publication (ADM); 1996. ECDEU assessment manual for psychopharmacology; pp. 276–338. [Google Scholar]
- 33.Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. J Affect Disord. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 34.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–2. [Google Scholar]
- 35.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 36.Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Med Rev. 2000;4:277–98. doi: 10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]