Abstract
Study Objectives:
We hypothesized that appropriate changes in thermal environment would enhance the quality of sleep.
Design/Setting:
Controlled laboratory study.
Participants:
Healthy young men (n = 7, mean age 26 years).
Interventions:
Nocturnal sleep structures in i-nude subjects were compared between a condition where an ambient temperature (Ta) of 29.5°C was maintained throughout the night (constant Ta), and a second condition (dynamic Ta) where Ta changed slowly within the thermoneutral range (from 27.5°C to 29.5°C).
Measurements and Results:
Statistically significant (P < 0.05) results included a lower and a later occurrence of minimum core body temperature (Tc), and a longer duration of slow-wave (stages 3+4) sleep in dynamic versus constant Ta. However, total sleep time, sleep efficiency, the total durations of light (stages 1+2) and rapid eye movement sleep, and the latencies to sleep onset, slow-wave sleep, and rapid eye movement sleep did not differ between conditions.
Conclusions:
Lowering the minimum and delaying the nadir of nocturnal Tc increases slow-wave sleep (probably by an increase of dry heat loss); use of this tactic might improve the overall quality of sleep.
Citation:
Togo F; Aizawa S; Arai J et al. Influence on Human Sleep Patterns of Lowering and Delaying the Minimum Core Body Temperature by Slow Changes in the Thermal Environment. SLEEP 2007;30(6):797-802.
Keywords: Sleep structure, slow-wave sleep, thermoneutrality, thermoregulation, ambient temperature change
CORE BODY TEMPERATURE (TC) HAS BEEN CONSIDERED A “GOLD-STANDARD” MARKER OF HUMAN CIRCADIAN RHYTHM, AND IT IS STRONGLY RELATED TO sleep regulation. The human sleep-wake cycle is generally synchronized with the circadian rhythm of Tc, partly because the propensity for sleep reaches its maximum during the declining phase of Tc and partly because arousal is promoted after Tc has passed its nadir.1,2 Tc decreases rapidly about 60 minutes prior to the onset of sleep,3 and it decreases further during slow-wave (stages 3+4) sleep (SWS), due to a combination of decreases in metabolism, vasodilation, and an increase in sweating.4,5
Sleep structure is also related to changes in Tc. SWS is decreased if the usual decrease of Tc is restricted by an ambient temperature (Ta) above the thermoneutral zone6 (here defined as the range of Ta over which temperature regulation is achieved by control of sensible heat loss, without regulatory changes in either metabolic heat production or evaporative heat loss7). The thermoneutral zone itself shows a circadian variation, reflecting a corresponding circadian rhythm in the body temperature set point.8 In contrast, stage 4 sleep is increased when the normal nocturnal decrease of Tc is augmented by a constant, mild cold stress, although sleep efficiency (the percentage of time asleep relative to the time spent in bed) is decreased.9 Thus, during the customary hours of sleep Ta is an important determinant of both the quality and the quantity of sleep achieved,10 and the appearance of SWS seems to be facilitated by a greater nocturnal decrease of Tc.
Gradual changes of Ta within the thermoneutral range while a person is sleeping can affect Tc and sleep structure without inducing arousal.11 In the experiments of Dewasmes and associates,11Ta was maintained at 29°C for 60 minutes after lights-out; it was then decreased linearly from 29°C to 26°C over the period 60 to 180 minutes, increased from 26°C to 32°C between 180 and 420 minutes, and decreased back to the initial value of 29°C from 420 to 480 minutes after lights-out. This pattern of change advanced the nadir of Tc (rectal temperature, Tre) and also increased the amplitude of change in Tc during nocturnal sleep. Dewasmes et al11noted further that, under these conditions, the peak propensity for rapid eye movement (REM) sleep was advanced and the amount of REM sleep tended to increase.
Based on these observations, we hypothesized that, if, in contrast, the nadir of Ta was delayed by a slower change in Ta within the thermoneutral zone, then Tc would reach a lower value after a longer delay and the amount of SWS would be increased, without any loss of sleep efficiency. We decided to test this hypothesis, in order to increase our understanding of how externally induced changes in Tc could enhance sleep structure.
METHODS
Subjects
Our subjects were 7 young healthy men, living in central Japan, a region in which the ambient outdoor temperature throughout the year is 15°C ± 8°C (mean ± SD). The mean (range) of values for age, height, and body mass were 26 (22–28) years, 1.74 (1.71–1.78) meters, and 70.8 (64.5–76.0) kilogram, respectively. Participants did not usually sleep in a prone position and had no history of sleep problems. They were nonsmokers and were not taking any medications. All gave written informed consent to participate in this institutionally approved study after the protocol, stresses, and possible risks had been fully explained to them.
Experimental Procedures
Subjects kept to their regular personal schedules for sleeping, waking, meals, and physical activity for at least 1 week prior to each polysomnographic session. Compliance was verified by the use of daily sleep diaries and physical-activity monitors (modified Kenz Lifecorder, Suzuken, Nagoya, Japan; for details of this apparatus and its use, see Togo et al12). Subjects refrained from ingestion of alcohol or caffeine and avoided either napping or prolonged and/or strenuous exercise throughout the experimental period.
Two nights of habituation were followed by 1 baseline and 1 experimental nighttime polysomnography session, administered in random order (baseline-experimental [n = 4] or experimental-baseline [n = 3]). The interval between 2 consecutive polysomnography sessions was at least 1 week. All night-time polysomnography sessions were carried out in a 3.0×3.0×2.5-m soundproof, air-conditioned, and shaded sleeping chamber. Ta was monitored at 2 locations (near the subject's head and feet); values were maintained within ± 0.1°C of the target figures, using proportionally controlled cooling and heating elements.
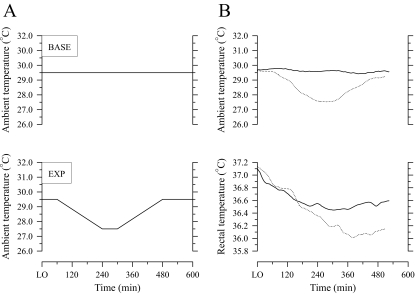
During each session, the subjects went to bed at their normal time and got up when they wished. They slept on a bed without a quilt and remained without covering or clothing except for their underpants. They ingested a normal amount of food and avoided hot soup and drinks from 300 minutes before lights out. From 240 minutes before lights out, the subjects sat awake in what was for them a thermoneutral condition (Ta 29.5°C, relative humidity 60%). After lights out, a constant Ta of 29.5°C was maintained throughout habituation and baseline sessions (Figure 1A). During the experimental night (Figure 1A), Ta was maintained at 29.5°C for 60 minutes after lights out; it was then decreased linearly from 29.5°C to 27.5°C over the period 60 to 240 minutes, maintained at 27.5°C from 240 to 300 minutes, and increased linearly back to the initial value of 29.5°C from 300 to 480 minutes after lights out. Previous studies have considered this range of Ta as within the thermoneutral zone for naked humans during sleep.7,13–15
Figure 1.
Schematic changes in ambient temperature (A) and observed changes in ambient and rectal temperatures of a subject (B) during baseline (BASE, solid line) and experimental (EXP, dotted line) nights. LO refers to lights out.
Measurements
Our indicator of Tc (Tre) was monitored by a thermistor probe (LT-ST08-11, Gram Corporation, Saitama, Japan) inserted 10 cm past the anal sphincter. The local skin temperature on the left anterior surface of the mid-lower leg (Tl) was also monitored continuously, using a thermistor probe (LT-ST08-12, Gram Corporation) attached to the skin surface. Tre and Tl were recorded every minute by a logging instrument (LT-8A, Gram Corporation) and laptop computer (ThinkPad 380A, IBM Japan, Tokyo, Japan).
Electroencephalograms (C3-A2, P4-A2), bilateral electrooculograms (left, right), the mental electromyogram, and electrocardiogram (standard bipolar leads) were monitored continuously throughout each night. Analog signals for electroencephalogram, electrooculogram, electromyogram, and electrocardiogram were processed on a real-time basis at a sampling frequency of 200 Hz (Digital Bio-Amplifier System, NF Electronic Instruments, Yokohama, Japan), using a program (5100 Series Data Acquisition Software, NF Electronic Instruments) adapted for a personal computer (ThinkPad A30, IBM Japan, Tokyo, Japan).
Visual Electroencephalographic Scoring
Two investigators used standard criteria16 to score sleep stages manually, using the blind polysomnography recordings taken every 30 seconds. Each recording was scored independently by both observers, and epochs in which the scorers disagreed were reviewed jointly. Sleep onset was defined as the first three consecutive epochs of sleep stage 1 or the first epoch of other stages of sleep.
Probabilities for the appearance of SWS and REM sleep (rated from 0–1, by increments of ≈ 0.143) were calculated as the number of subjects who had SWS and REM at any given instant, divided by the total number of subjects (n = 7). Such values were calculated for each 30-second estimate of sleep structure. Sleep efficiency was calculated as the total sleep time divided by the time spent in bed.
Electroencephalogram Spectral Analyses
Fast Fourier transformations were performed on C3-A2–lead whole-night sleep data for all subjects, in order to obtain electroencephalogram power spectrum density for 30-second nonoverlapping windows. All spectra were estimated by averaging spectra obtained from 10 time-shifted subsets of 4096 data points. The power observed in the 0.75- to 4.5-Hz frequency band was regarded as slow-wave activity, a quantitative measure of slow-wave dynamics and a physiologic marker of the intensity of non-REM sleep. Any 30-second epoch containing an artifact caused by factors such as body movement or muscle contraction was arbitrarily replaced with an estimate obtained by linear interpolation from data obtained immediately before and after the artifact-containing epoch.
RR Interval Data Correction
RR intervals were measured from the electrocardiogram. All RR intervals were scanned for extra or missing beats that could affect the time-domain analysis. Abnormal intervals were corrected by either the insertion (for missing beats) or the omission of beats (for doubled or tripled beats). The number of beats thus corrected was <0.5%.
Statistical Analyses
Differences in sleep structure, Tre, Tl, RR interval, and slow-wave activity between nights were assessed using 3-way analyses of variance or 2-factor repeated measures analyses of variance, as appropriate. Posthoc analyses used Tukey studentized range tests to compare means between pairs of conditions. Differences were considered significant when P was <0.05.
RESULTS
Representative changes in Ta and Tre for baseline and experimental nights are shown in Figure 1B. Statistically significant decreases in Tre occurred during all sessions. The Tre at lights out did not differ significantly between nights, but the nadir of Tre seen on the experimental night was 0.2°C lower than that observed on the baseline night (P < 0.05; Table 1). Furthermore, the nadir of Tre on the experimental night was delayed by 86 minutes relative to the baseline session (P < 0.05; Table 1). Tl at lights out and minimum Tre did not differ significantly between nights (Table 1). The RR interval at lights out also did not significantly differ between nights, but the RR interval at the nadir of Tre was significantly increased on the experimental night relative to the baseline night (Table 1).
Table 1.
Effects of Slow Changes in the Ambient Thermal Environment on Rectal and Leg Skin Temperatures and RR Interval
| Parameter | Baseline | Experimental |
|---|---|---|
| Rectal temperature | ||
| @ lights-out, °C | 37.0 ± 0.2 | 37.0 ± 0.2 |
| Minimum, °C | 36.4 ± 0.1 | 36.2 ± 0.1* |
| Time to minimum, min | 251.0 ± 62 | 337.0 ± 69* |
| Leg skin temperature | ||
| @ lights-out, °C | 33.5 ± 1.0 | 33.3 ± 1.0 |
| @ minimum rectal temperature, °C | 33.6 ± 0.4 | 33.3 ± 1.0 |
| RR interval | ||
| @ lights-out, ms | 1105.0 ± 163 | 1093.0 ± 134 |
| @ minimum rectal temperature, ms | 1183.0 ± 110 | 1249.0 ± 162* |
Values are mean ± SD (n = 7) during baseline and experimental nights.
Significantly (P < 0.05) different from baseline night.
The order of presentation of baseline and experimental nights had no significant effects on the selected characteristics of Tre, Tl, and RR interval (Table 1). All subjects reported that the Ta of 29.5°C was comfortable.
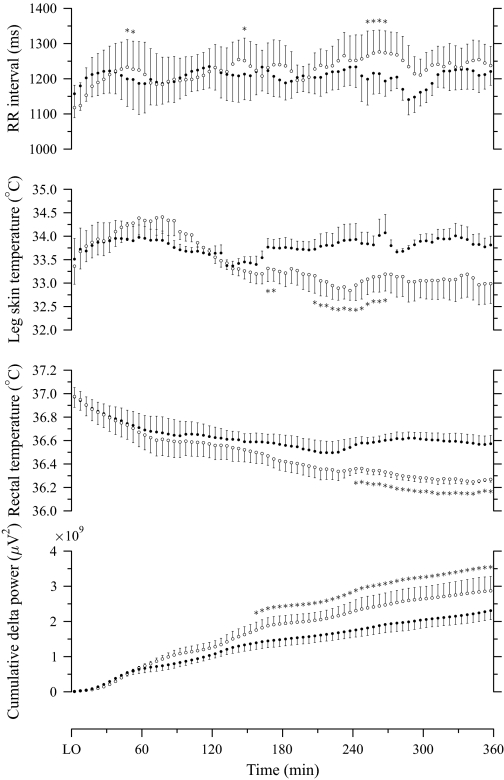
Figure 2 depicts group-averaged patterns for the 5-minute mean RR interval, Tl, Tre, and cumulative slow-wave activity during baseline and experimental nights, from lights out to the shortest total sleep time among all polysomnography sessions. The RR interval from 205 to 360 minutes was slightly longer on the experimental night than on the baseline night (Figure 2). On the experimental night, Tl values tended to be lower than on the baseline night, beginning at 135 minutes, and this difference became statistically significant from 165 to 175 minutes and from 205 to 270 minutes (Figure 2). Tre on the experimental night was significantly lower than on the baseline night from 240 to 360 minutes (Figure 2). Slow-wave activity on the experimental night tended to exceed that on the baseline night from 60 minutes, and this difference became statistically significant from 155 to 360 minutes (Figure 2).
Figure 2.
Mean (± SEM; n = 7) curves of RR interval, skin temperature at left anterior mid-lower leg, rectal temperature, and cumulative slow-wave activity during baseline (filled circle) and experimental (unfilled circle) nights, from lights out (LO) to the shortest total sleep time among all polysomnographic sessions. *Significantly (P < 0.05) different from baseline night.
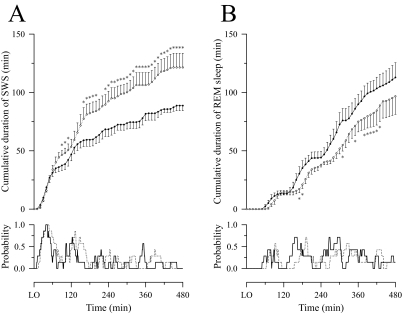
On both nights, the greatest portion of SWS occurred from 0 to 180 minutes after lights out. The cumulative duration of SWS was similar for the 2 sessions until 60 minutes after lights out, but thereafter the probability of SWS was greater and SWS accumulated more rapidly on the experimental than on the baseline night (Figure 3A). On the other hand, the probability of REM sleep within each sleep cycle was delayed on the experimental night relative to the baseline night, and, from 150 minutes after lights out onward, the cumulative duration of REM sleep tended to increase more slowly on the experimental night than on the baseline night (Figure 3B).
Figure 3.
The cumulative duration (mean ± SEM; n = 7) and the probability of slow-wave sleep (SWS) (A) and rapid eye movement (REM) sleep (B) during baseline (filled circle and/or solid line) and experimental (unfilled circle and/or dotted line) nights. LO refers to lights out. *Significantly (P < 0.05) different from baseline night.
When sleep structures were compared between nights, the total duration of SWS was significantly greater during the experimental night than during the baseline night (Table 2). However, total sleep time, sleep efficiency, the total durations of light (stages 1+2) and REM sleep, and the latencies to sleep onset, SWS, and REM sleep did not differ significantly between sessions (Table 2). Changes in the respective durations of REM and SWS bore no significant relationship to each other within the limits imposed by our small sample size (r = −0.57, P = 0.18).
Table 2.
Effects of Slow Changes in the Ambient Thermal Environment on Sleep Structure
| Parameter | Second habituation | Baseline | Experimental |
|---|---|---|---|
| Total sleep time, min | 430.0 ± 60 | 423.0 ± 71 | 422.0 ± 77 |
| Sleep efficiency, % | 96.9 ± 4.2 | 97.0 ± 3.4 | 97.1 ± 2.3 |
| Latency, min | |||
| Sleep onset | 7.4 ± 7.1 | 8.0 ± 5.6 | 9.6 ± 6.1 |
| SWS | 22.0 ± 13 | 22.0 ± 11 | 25.0 ± 8 |
| REM sleep | 80.0 ± 15 | 71.0 ± 15 | 83.0 ± 13 |
| Total duration, min | |||
| Light sleep | 229.0 ± 28 | 213.0 ± 30 | 202.0 ± 40 |
| SWS | 94.0 ± 17 | 89.0 ± 11 | 121.0 ± 32* |
| REM sleep | 107.0 ± 27 | 121.0 ± 42 | 99.0 ± 44 |
Values are mean ± SD (n = 7) during second habituation, baseline, and experimental nights. Sleep phases are categorized as light (stages 1+2) sleep, slow-wave (stages 3+4) sleep (SWS), and rapid eye movement (REM) sleep.
Significantly (P < 0.05) different from second habituation and baseline nights.
There were no significant differences in any selected sleep structure between the baseline night and the second night of habituation (Table 2). Further, the order of polysomnography sessions had no significant effects on any selected sleep structure (Table 2).
DISCUSSION
SWS is related to brain or whole-body cooling, and such heat loss is thought to be associated with increases in brain and body temperatures during the preceding period of wakefulness. If brain and body temperatures are increased by either exercise or passive heating while a person is awake, then the amount of SWS is increased during the ensuing night;17,18 the amount of SWS is positively correlated with both Tc at sleep onset19 and the magnitude of the decrease in Tc during sleep.17 Environmental temperatures influence sleep structure through both direct thermal stimulation of the centers controlling sleep and waking20,21 and indirect thermoregulatory responses.22,23 If the environmental temperature exceeds the thermoneutral value, Tc rises to a relatively constant level of 37°C, and SWS is decreased.24 In contrast, if Ta is 21°C or 24°C (below the thermoneutral value of 29°C for a naked sleeping human), Tre drops, and the duration of SWS is decreased, with an increased frequency of arousals.7,13 Thus, the amount of SWS is maximal in a thermoneutral environment; this allows adequate heat loss without augmenting the number of arousals.
If Ta is changed gradually within the thermoneutral range while subjects are asleep, this alters the minimum Tre and can increase the amplitude of change in Tre without augmenting arousals.11 In this situation, the main factors that influence the dynamics and nadir of Tre are likely to be changes in convective and/or radiant heat exchange, rather than changes in metabolic heat production,14 and we would anticipate an increase of dry heat loss from the body.
In our experiment, although Ta decreased gradually from 60 minutes after lights out, there were no significant differences of Tl between baseline and experimental nights from lights out to 135 minutes (Figure 2). The “overall” dry heat loss is proportional to both the body surface temperature and the difference between skin and ambient temperatures.25 Thus, we believe that dry heat loss was increased on the experimental night compared to the baseline night, at least from 60 to 135 minutes after lights out. This additional heat loss in turn induced a decrease in both Tre and Tl, and the RR interval was lengthened slightly. The difference between Tl and Ta on the experimental night remained larger than on the baseline night from 135 minutes onward (Figure 2), so that the dry heat loss on the experimental night likely continued to exceed that on the baseline night. However, the RR interval did not differ significantly between conditions for most of the observation period (Table 1, Figure 2). Changes in sleeping heart rate generally reflect changes in energy expenditure.26 Therefore, although the longer total duration of SWS (the period of the night that includes the lowest metabolic rate27) could have caused a temporary local decrease in heat production on the experimental night, our results do not suggest a decrease in metabolic heat production as the main cause of decreases in Tre and Tl.
Under the experimental condition, the total duration of SWS was increased, even if the length of the waking period, the amount of prior physical activity, and Tre at the onset of sleep remained unchanged. The total duration of REM sleep was also decreased somewhat, due to the time delay within each sleep cycle caused by gradual changes in Ta. However, this does not seem to have had a major impact on the overall sleep pattern, since REM in the experimental condition remained slightly longer than that previously reported for young (20–29 years of age) good sleepers with a total average sleep time of 375 minutes.28 Further, within the limits of our data, the changes in duration of REM and SWS bore no significant relationship to each other. A constant Ta within the thermoneutral range has a relatively limited influence on homeostatic function during sleep when compared to a Ta outside the thermoneutral range. Nevertheless, our data offer a suggestion that a dynamic thermal environment that extends the declining phase and decreases the minimum of Tc without increasing arousal might augment SWS. Further observations are desirable to confirm this outcome, since it seems a simple potential intervention to improve the overall quality of sleep. An important next step in this investigation will be to establish the Ta pattern that maximizes the total duration of SWS.
This is the first study to suggest that increasing passive heat loss during sleep has a positive effect upon sleep structure, especially SWS, without decreasing sleep efficiency. The underlying mechanism as yet remains unclear, but our results seem to support the model of sleep regulation proposed by Van Someren.29 This model proposes that, although an increase in skin temperature has an important role in sleep regulation, optimization of sleep also requires a decrease in Tc. An increase in skin temperature that induces heat loss stimulates thermosensitive peripheral skin neurons that innervate the predominant integrator of thermal information (the preoptic area/anterior hypothalamus).30 Such impulses may in turn stimulate the thermosensitive neurons in the preoptic area/anterior hypothalamus that are responsible for inducing sleep.31 On the other hand, a decrease in Tc reduces the activity of the midbrain reticular formation. If a decrease in Tc is prevented, sleep is impaired despite the increase in skin temperature that is evoked.15,32–34 Taken together with our findings that Tl was lower in the experimental than in the baseline condition, it seems that an increase in skin temperature may not always be necessary to maintain sleep state and/or SWS after the onset of sleep. The changes in Tre could be masked by changes in Ta and sleep states, and, at present, it remains unclear how far the circadian system of our subjects was modified.
We may conclude that, although acute changes in Ta cause frequent arousal,35 gradual changes of Ta within the thermoneutral range do not induce arousal.9 From our data, it appears that an increase of passive heat loss caused by a decrease of Ta during sleep increases the total duration of and/or the propensity to SWS without augmenting arousal. Changes in Ta may thus prove an effective intervention to improve sleep structure in some types of insomnia. Many older individuals have difficulty in sleeping, due to a phase advance in Tc and/or a smaller decrease of Tc during sleep.36,37 In such individuals, heat loss is decreased during sleep. These agerelated physiologic changes and the resulting insomnia might be reduced through gradual nocturnal changes in Ta, which would decrease the nadir of Tc and delay its occurrence. Further research is needed to clarify the responses that we have observed and to test their reproducibility, especially in older individuals. Future studies on a larger sample of subjects should look at the detailed effects of changes in Ta on circadian physiology (particularly heat production and heat loss) during sleep, focusing on the resultant decrease and phase delay in Tc, and examining whether such changes are indeed helpful in the treatment of age-related insomnia.
ACKNOWLEDGEMENTS
The authors are grateful to the subjects who participated in this study.
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Togo, Aizawa, Arai, Yoshikawa, Ishiwata, Shephard, and Aoyagi have indicated no financial conflicts of interest.
REFERENCES
- 1.Borbély AA. A two-process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure and electroencephalographic slow waves and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy PJ, Campbell SS. Nighttime drop in body temperature: a physiological trigger for sleep onset? Sleep. 1997;20:505–11. doi: 10.1093/sleep/20.7.505. [DOI] [PubMed] [Google Scholar]
- 4.Glotzbach SF, Heller HC. Central nervous regulation of body temperature during sleep. Science. 1976;194:537–9. doi: 10.1126/science.973138. [DOI] [PubMed] [Google Scholar]
- 5.Sagot JC, Amoros C, Candas V, Libert JP. Sweating responses and body temperature during nocturnal sleep in humans. Am J Physiol Regulatory Integrative Comp Physiol. 1987;252:R462–70. doi: 10.1152/ajpregu.1987.252.3.R462. [DOI] [PubMed] [Google Scholar]
- 6.Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. The effects of high and low ambient temperatures on human sleep stages. Electroencephalogr Clin Neurophysiol. 1981;51:494–501. doi: 10.1016/0013-4694(81)90226-1. [DOI] [PubMed] [Google Scholar]
- 7.The Commission for Thermal Physiology of the International Union of Physiological Sciences (IUPS Thermal Commission) Glossary of terms for thermal physiology: third edition. Jpn J Physiol. 2001;51:i–xxxvi. [Google Scholar]
- 8.Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol Regulatory Integrative Comp Physiol. 1994;267:R819–29. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 9.Sewitch DE, Kittrell EM, Kupfer DJ, Reynolds CF., 3rd Body temperature and sleep architecture in response to a mild cold stress in women. Physiol Behav. 1986;36:951–7. doi: 10.1016/0031-9384(86)90459-2. [DOI] [PubMed] [Google Scholar]
- 10.Glotzbach SF, Heller HC. Temperature regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: WB Saunders; 1999. pp. 289–304. [Google Scholar]
- 11.Dewasmes G, Signoret P, Nicolas A, Ehrhart J, Muzet A. Advances of human core temperature minimum and maximal paradoxical sleep propensity by ambient thermal transients. Neurosci Lett. 1996;215:25–8. doi: 10.1016/s0304-3940(96)12936-0. [DOI] [PubMed] [Google Scholar]
- 12.Togo F, Watanabe E, Park H, Shephard RJ, Aoyagi Y. Meteorology and the physical activity of the elderly: the Nakanojo Study. Int J Biometeorol. 2005;50:83–9. doi: 10.1007/s00484-005-0277-z. [DOI] [PubMed] [Google Scholar]
- 13.Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. Metabolism and thermoregulation during stages of sleep in humans exposed to heat and cold. J Appl Physiol. 1981;51:948–54. doi: 10.1152/jappl.1981.51.4.948. [DOI] [PubMed] [Google Scholar]
- 14.Dewasmes G, Nicolas A, Rodriguez D, et al. Human core temperature minimum can be modified by ambient thermal transients. Neurosci Lett. 1994;173:151–4. doi: 10.1016/0304-3940(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto-Mizuno K, Mizuno K, Michie S, Maeda A, Iizuka S. Effects of humid heat exposure on human sleep stages and body temperature. Sleep. 1999;22:767–73. [PubMed] [Google Scholar]
- 16.Rechtstchaffen A, Kales A. A Manual of standardized terminology, techniques and scoring system for sleep states of human subjects. Washington: US Government Printing Office; 1968. [Google Scholar]
- 17.Horne JA, Staff LHE. Exercise and sleep: body-heating effects. Sleep. 1983;6:36–46. doi: 10.1093/sleep/6.1.36. [DOI] [PubMed] [Google Scholar]
- 18.Horne JA, Reid AJ. Night-time sleep EEG changes following body heating in a warm bath. Electroencephalogr Clin Neurophysiol. 1985;60:154–7. doi: 10.1016/0013-4694(85)90022-7. [DOI] [PubMed] [Google Scholar]
- 19.Berger RJ, Palca JW, Walker JM, Phillips NH. Correlations between body temperatures, metabolic rate and slow wave sleep in humans. Neurosci Lett. 1988;86:230–4. doi: 10.1016/0304-3940(88)90576-9. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Glotzbach SF, Heller HC. Influence of hypothalamic and ambient temperatures on sleep in kangaroo rats. Am J Physiol. 1979;237:R80–8. doi: 10.1152/ajpregu.1979.237.1.R80. [DOI] [PubMed] [Google Scholar]
- 21.Graf R, Heller HC, Sakaguchi S, Krishna S. Influence of spinal and hypothalamic warming on metabolism and sleep in pigeons. Am J Physiol. 1987;252:R661–7. doi: 10.1152/ajpregu.1987.252.4.R661. [DOI] [PubMed] [Google Scholar]
- 22.Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol. 1995;269:R1240–9. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- 23.Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in wakefulness and non rapid eye movement sleep. Brain Res. 1996;718:76–82. doi: 10.1016/0006-8993(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 24.Karacan I, Thornby JI, Anch AM, Williams RL, Perkins HM. Effects of high ambient temperature on sleep in young men. Aviat Space Environ Med. 1978;49:855–60. [PubMed] [Google Scholar]
- 25.Kräuchi K. How is the circadian rhythm of core body temperature regulated? Clin Auton Res. 2002;12:147–9. doi: 10.1007/s10286-002-0043-9. [DOI] [PubMed] [Google Scholar]
- 26.Mischler I, Vermorel M, Montaurier C, et al. Prolonged daytime exercise repeated over 4 days increases sleeping heart rate and metabolic rate. Can J Appl Physiol. 2003;28:616–29. doi: 10.1139/h03-047. [DOI] [PubMed] [Google Scholar]
- 27.Fontvieille AM, Rising R, Spraul M, Larson DE, Ravussin E. Relationship between sleep stages and metabolic rate in humans. Am J Physiol. 1994;267:E732–7. doi: 10.1152/ajpendo.1994.267.5.E732. [DOI] [PubMed] [Google Scholar]
- 28.Hirshkowitz M. Normal human sleep: an overview. Med Clin North Am. 2004;88:551–65. doi: 10.1016/j.mcna.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Van Someren EJ. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000;17:313–54. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- 30.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31(Suppl 5):S157–61. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 31.Raymann RJ, Swaab DF, Van Someren EJ. Cutaneous warming promotes sleep onset. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1589–97. doi: 10.1152/ajpregu.00492.2004. [DOI] [PubMed] [Google Scholar]
- 32.Bonegio RG, Driver HS, King LM, Laburn HP, Shapiro CM. Circadian temperature rhythm blunting and sleep composition. Acta Physiol Scand Suppl. 1988;574:44–7. [PubMed] [Google Scholar]
- 33.Ohnaka T, Tochihara Y, Kanda K. Body movements of the elderly during sleep and thermal conditions in bedrooms in summer. Appl Human Sci. 1995;14:89–93. doi: 10.2114/ahs.14.89. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher A, van den Heuvel C, Dawson D. Sleeping with an electric blanket: effects on core temperature, sleep, and melatonin in young adults. Sleep. 1999;22:313–8. doi: 10.1093/sleep/22.3.313. [DOI] [PubMed] [Google Scholar]
- 35.Candas V, Libert JP, Muzet A. Heating and cooling stimulations during SWS and REM sleep in man. J Therm Biol. 1982;7:155–8. [Google Scholar]
- 36.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–6. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 37.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regulatory Integrative Comp Physiol. 1998;275:R1478–87. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]