Abstract
Study Objectives:
To evaluate the influence of chronotype on sleep stages and quantitative sleep EEG when sleep pressure is increased and sleep schedule remains constant.
Design:
A 5-day session comprising an adaptation night, a baseline night, two nights of sleep fragmentation, and a recovery night.
Setting:
Chronobiology laboratory.
Participants:
Twenty-four healthy subjects aged 19–34 years: 12 morning types and 12 evening types selected by questionnaire. Each group included 6 men and 6 women with a habitual sleep duration of 7 to 9 hours.
Interventions:
Two nights of behavioral sleep fragmentation induced by forced 5-min awakenings every half-hour.
Measurements and Results:
Each night of polysomnography recording lasted 8 hours and was based on each subject's preferred sleep schedule. On both nights of sleep fragmentation, stage 1 sleep increased, while both total sleep time and minutes of slow wave sleep decreased. No difference was observed in sleep architecture between morning types and evening types during sleep fragmentation nights or during recovery night. Spectral analysis of all-night NREM sleep EEG showed that during the recovery night, morning types had a larger fronto-central increase in low frequency activities and a larger centro-parietal decrease in 14–15 Hz activity than evening types. The largest group difference was for slow wave activity in the fronto-central area during the first part of the sleep episode.
Conclusions:
These results add further support to a postulated difference in homeostatic sleep regulation between morning types and evening types, with morning types showing indications of a higher homeostatic response to sleep disruption.
Citation:
Mongrain V; Dumont M. Increased homeostatic response to behavioral sleep fragmentation in morning types compared to evening types. SLEEP 2007;30(6):773-780.
Keywords: Morningness-eveningness, sleep fragmentation, sleep, EEG spectral analysis, circadian rhythms, human, sleep regulation
INTRODUCTION
CIRCADIAN TYPOLOGY REFERS TO AN INDIVIDUAL'S PREFERRED TIMING FOR ACTIVITY AND SLEEP. THIS PREFERENCE, IDENTIFIABLE THROUGH questionnaire responses, allows for the classification of people into chronotypes: morning types, evening types, and “neither types.” This interindividual difference has been found to be associated with a difference in endogenous circadian phase as indicated by core body temperature and melatonin secretion rhythms.1–4 In general, the sleep episode and the circadian phase position occur about 2 hours earlier in morning types than in evening types.
The homeostatic process of sleep regulation adjusts sleep intensity as a function of the duration of prior waking and sleep.5,6 During NREM sleep, the dynamics of homeostatic sleep pressure is reflected by the decreasing activity in low EEG frequencies (<10 Hz) and by the increasing spindle frequency activity (12–16 Hz).7–9 In wakefulness, the increase in sleep pressure is associated with increased theta-alpha activity (5–9 Hz).10,11 We recently reported in morning types a faster decay of slow wave activity (SWA; 1–5 Hz) in the frontal brain area and a steeper increase in 13–14 Hz activity in the parieto-occipital area during normal sleep.12 Other researchers reported that morning types have a faster increase of theta-alpha activity during wakefulness than evening types.13These observations suggest that chronotypes differ not only in the position of their circadian phase but also in the dynamics of homeostatic sleep regulation: morning types show indications of faster buildup and dissipation of sleep pressure than evening types.
The response to manipulation of the duration of time awake can be used to evaluate homeostatic sleep regulation.14,15 An increase in the duration of time awake produces a proportional increase in NREM sleep low frequency activities, especially at the beginning of the sleep episode, and a decrease in spindle frequency activities during subsequent sleep.7–9,16–19 Therefore, for a given augmentation of wakefulness, a different response in these sleep EEG activities could reveal a difference in the dynamics of sleep homeostasis.
In chronotypes, differences in daytime sleep have been evaluated following a night of sleep deprivation. Some researchers have found that daytime sleep was more affected in morning types than in evening types,20,21 while another study found larger changes between nighttime and daytime sleep cycles and EEG activity in evening types than in morning types.22 In all these studies, the relationship between circadian phase and the timing of the sleep episode was modified; such modification is known to alter both sleep structure and expression of SWA.23,24 To assess differences in homeostatic markers between subjects having different circadian phases, it is important to maintain the same relationship between the sleep episode and the circadian phase (i.e., the phase angle) for the entire protocol. Behavioral sleep fragmentation25,26 is a useful procedure as it attains this goal since bedtime, wake time, and duration of the sleep episode can remain constant before, during, and after the controlled increase of time awake.
The purpose of this study was to evaluate the difference between morning and evening circadian types in their response to an increase in sleep pressure caused by behavioral sleep fragmentation. This response would represent the effect of sleep homeostasis alone since the relationship between sleep episode and circadian phase remained constant. Since morning types were previously found to experience faster dynamics of buildup and dissipation of sleep pressure, we predicted that they would show an increased response to sleep fragmentation compared to evening types, as assessed by EEG markers of homeostatic sleep pressure during sleep.
METHODS
Subjects
Morning types (M-types) and evening types (E-types) aged 19 to 34 years were recruited using a French version of the Morningness-Eveningness Questionnaire (MEQ) of Horne and Östberg (1976).27 Twenty-four subjects completed the study: 12 M-types (MEQ scores 59 to 71) and 12 E-types (MEQ scores 27 to 40). There were 6 women and 6 men in each group. Age was similar in the 2 groups (M-types: 24.7 y ± 1.5; E-types: 23.4 y ± 0.7). All subjects were in good physical and psychological health and had no sleep complaints. Selected subjects had a regular sleep schedule with a habitual sleep duration of 7 to 9 hours. A 24-hour laboratory screening confirmed, on the basis of a night of polysomnography and daytime sleep latency tests, the absence of sleep and vigilance disorders. Inclusion criteria were sleep efficiency >85%, night sleep latency <30 minutes, indexes of apneas/hypopneas and periodic leg movements <5, and averaged daytime sleep latency >7 min. Subjects had no night work experience in the past year and no transmeridian travel in the past 3 months. They were all nonsmokers and reported no use of recreational drugs or medications, except oral contraceptives. Women not using hormonal contraception (3 M-types and 4 E-types) were studied during the follicular phase of their menstrual cycle. Each subject signed an informed consent form approved by the hospital ethics committee and received financial compensation.
It should be noted that the results presented in this paper derive from a larger study on sleep regulation in morningness-eveningness conducted with the same subjects. Detailed information on sleep-wake cycle and circadian phase assessments can be found in Mongrain et al.4 Briefly, circadian phases of salivary melatonin and rectal temperature rhythms were respectively 2.5 h and 2 h earlier in M-types than in E-types. Averaged phase angles between circadian phases and sleep schedule were similar in the 2 groups of subjects.
Procedures
For the study, individual sleep schedules were determined according to each subject's preferred bedtime and wake time as noted in screening sleep diaries during free days and as reported in the MEQ. The final decision on the study sleep schedule was made after discussion with the subject to ensure that it was close to the schedule that he/she would spontaneously adopt. Bedtime and wake time were determined for a sleep duration of 8 hours. On average, selected laboratory sleep schedules were more than 2.5 h earlier in M-types than in E-types (bedtime: 23:08 ± 11 min vs. 01:45 ± 17 min). Subjects were requested to follow the same sleep schedule (± 30 min) for 7 days before laboratory admission. Compliance was verified by sleep diaries and by ambulatory measures of activity and light exposure (Actiwatch-L, Mini-Mitter Co, Bend, OR).
Subjects were admitted to the laboratory 4 h before their scheduled bedtime and slept 5 consecutive nights according to their individual sleep schedule: an adaptation night (AD), a baseline night (BL), 2 nights of behavioral sleep fragmentation (FR1 and FR2), and a recovery night (REC). During the nights of sleep fragmentation, subjects were awakened for 5 min every half-hour (15 times a night).26 For each awakening, a technician knocked on the door and entered the room with a small flashlight. Subjects had to interact verbally with the technician for the entire 5 minutes. Room light was not turned on and subjects were not required to open their eyes. Another technician stayed in the control room to keep track of the time and to confirm wakefulness according to on-line EEG recording. During daytime, subjects stayed in the laboratory and were continuously monitored. Napping and caffeine intake were forbidden, and only quiet activities were allowed.
Subjective sleep quality was assessed each morning using a 5-point scale. Sleep episodes were recorded with 20 EEG electrodes, 2 EOG electrodes, and 3 chin EMG electrodes, using a referential montage with linked ears. Signals were recorded using a polygraph Grass Model 15A54 amplifier system (Astro-Med Inc., West Warwick, RI, USA; gain 10000, bandpass 0.3–100 Hz) and digitized at a sampling rate of 256 Hz with commercial software (Harmonie 5.1, Stellate Systems, Montreal, Canada).
Sleep Data Analysis
Sleep stages were visually scored from the C3 monopolar derivation, on 20-sec epochs, according to standard procedures.28 Sleep latency was defined as the time from lights off to the first minute of stage 1 sleep (or to the first epoch of any other sleep stage). Sleep period was the time from sleep onset to final awakening. Sleep efficiency was the time spent asleep divided by the sleep period, multiplied by 100. NREM/REM sleep cycles were determined according to Feinberg and Floyd criteria.29,30 EEG spectral analysis was performed on FZ, CZ, and PZ monopolar derivations with a commercial software package (Sensa, Stellate Systems, Montreal, Canada). Artifacts were automatically detected,31 and further artifacts were identified by visual inspection. Spectral power was obtained by fast Fourier transforms (FFT) performed on 4-second artifact-free sections using a cosine window tapering resulting in a 0.25 Hz spectral resolution. All-night spectral power was calculated over NREM sleep stages (excluding stage 1) for 24 one-Hz frequency bins identified by their lower boundary value. Spectral power was averaged for 6 frequency bands (SWA [1–5 Hz], theta [4–8 Hz], alpha [8–12 Hz], low sigma [12–14 Hz], high sigma [14–16 Hz], and beta [16–24 Hz]) and then averaged in 2-hour periods for the sleep episode of each night. Two-hour periods were calculated according to real time (clock time) and are also identified by their lower boundary value. Spectral data for the PZ derivation of an M-type woman have been excluded because of technical difficulties during FR2 and REC recordings.
Statistical Analysis
Group-by-Night analyses of variance (ANOVAs) were used to assess differences in sleep architecture variables and in all-night EEG power spectra. Because between-group differences were expected to prevail at the beginning of the nights, changes in power spectra for each EEG frequency band were also evaluated over the four 2-h periods of the nights using Group-by-Night-by-Period ANOVAs. Huynh/Feldt corrections were used for repeated measures, but the original degrees of freedom are reported. Significant effects were decomposed using Tukey's HSD test for Night effects and simple effect analysis (contrasts) for significant interactions with the Group factor. The results of these decompositions are reported under 2 subheadings: 1) effects of sleep fragmentation, and 2) recovery from increased sleep pressure. Overall significant effects are reported only once, in the first results section. ANOVAs were computed with Statistica 6 software (StatSoft, Inc., Tulsa, OK, USA).
Nocturnal decay of SWA in NREM sleep during the REC night for the FZ derivation was also investigated with a nonlinear regression analysis. The time of the cycle midpoint was calculated for each cycle in each subject and used as the independent variable. The mean SWA value within each sleep cycle was expressed as the percentage of all-night NREM sleep SWA during BL and used as the dependent variable. An exponential decay function was fitted to the data: SWAt = SWA∞ + SWA0 * e−rt, with t = time of cycle midpoint, SWAt = SWA averaged per cycle, SWA∞ = horizontal asymptote for t = ∞, SWA0 = intercept of the y axis minus asymptote, and r = slope of the decay. Group estimates of SWA∞, SWA0 and r were compared with F-tests. Non-linear regression analysis was computed with GraphPad Prism 4 software (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was set at 0.05, and results are reported as mean ± sem.
RESULTS
1. Effects of sleep fragmentation (BL vs. FR1, FR2)
Sleep Quality and Sleep Architecture
Subjective sleep quality and parameters of sleep architecture during recording nights are shown in Table 1. Total sleep time decreased by more than 2 hours between BL and FR1, then increased by approximately 0.75 h between FR1 and FR2 (F3,66 = 154.7, P <0.001). Therefore, compared to BL, time awake increased by about 2 hours in FR1 and by about 1.3 h in FR2. Significant Night effects were found for most sleep parameters (F3,66≥17.1, P <0.001). For subjective sleep quality, sleep efficiency, duration of stage 2 sleep, and duration and percent of slow wave sleep and REM sleep, there was a decrease from BL to FR1 (P <0.001), followed by an increase from FR1 to FR2 (P ≤0.06). All these parameters, except percentage of slow wave sleep, were still significantly lower in FR2 than in BL. A reverse pattern was found for REM sleep latency, duration and percentage of stage 1 sleep, and percentage of stage 2 sleep, which increased in FR1 compared to BL (P <0.001), and decreased subsequently from FR1 to FR2 (P ≤0.1). During FR2, there was still a significant increase in percentage of stage 1 and stage 2 sleep (P <0.01) compared to BL. No significant difference was found between M-types and E-types on any night, and there was no significant Group-by-Night interaction.
Table 1.
Sleep architecture parameters (mean ± SEM) for morning-types (M-types) and evening-types (E-types) during baseline night, first and second nights of sleep fragmentation, and recovery night.
| Baseline |
Fragmentation 1 |
Fragmentation 2 |
Recovery |
|||||
|---|---|---|---|---|---|---|---|---|
| M-types | E-types | M-types | E-types | M-types | E-types | M-types | E-types | |
| Subjective sleep quality | 4.8 ± 0.1 | 4.2 ± 0.2 | 2.7 ± 0.3 | 2.0 ± 0.2 | 2.9 ± 0.3 | 3.2 ± 0.3 | 4.5 ± 0.3 | 4.4 ± 0.1 |
| Sleep latency (min) | 6.7 ± 0.8 | 7.4 ± 1.5 | 14.7 ± 6.2 | 10.3 ± 1.2 | 6.9 ± 1.5 | 6.2 ± 1.2 | 9.3 ± 1.9 | 11.9 ± 2.5 |
| REM latency (min) | 71.0 ± 10.0 | 67.0 ± 3.0 | 179.0 ± 20.0 | 164.0 ± 21.0 | 106.0 ± 13.0 | 86.0 ± 9.0 | 58.0 ± 5.0 | 61.0 ± 5.0 |
| Total sleep time (min) | 450.0 ± 4.0 | 452.0 ± 3.0 | 318.0 ± 18.0 | 330.0 ± 11.0 | 369.0 ± 7.0 | 369.0 ± 7.0 | 460.0 ± 2.0 | 455.0 ± 3.0 |
| Sleep efficiency (%) | 95.9 ± 0.6 | 96.3 ± 0.6 | 68.3 ± 3.6 | 70.2 ± 2.3 | 78.2 ± 1.4 | 77.8 ± 1.5 | 97.6 ± 0.2 | 97.2 ± 0.5 |
| Stage 1 | ||||||||
| min | 27.1 ± 4.8 | 22.5 ± 2.4 | 38.4 ± 3.4 | 32.3 ± 2.5 | 31.9 ± 2.1 | 27.4 ± 1.4 | 21.1 ± 3.7 | 17.2 ± 2.2 |
| % | 6.1 ± 1.1 | 5.0 ± 0.6 | 12.8 ± 1.5 | 10.1 ± 1.0 | 8.7 ± 0.6 | 7.5 ± 0.4 | 4.6 ± 0.8 | 3.8 ± 0.5 |
| Stage 2 | ||||||||
| min | 264.0 ± 7.0 | 273.0 ± 8.0 | 216.0 ± 15.0 | 227.0 ± 6.0 | 241.0 ± 10.0 | 248.0 ± 5.0 | 256.0 ± 8.0 | 262.0 ± 8.0 |
| % | 58.7 ± 1.4 | 60.4 ± 1.8 | 67.6 ± 1.6 | 69.1 ± 1.3 | 65.2 ± 2.1 | 67.3 ± 1.5 | 55.6 ± 1.6 | 57.7 ± 2.0 |
| Slow-wave sleep (stages 3 and 4) | ||||||||
| min | 42.4 ± 9.8 | 38.2 ± 8.0 | 18.4 ± 5.7 | 16.2 ± 4.5 | 32.8 ± 6.7 | 25.6 ± 5.4 | 60.8 ± 10.5 | 52.6 ± 9.3 |
| % | 9.4 ± 2.1 | 8.4 ± 1.7 | 5.8 ± 1.8 | 4.7 ± 1.2 | 9.0 ± 1.8 | 6.8 ± 1.3 | 13.2 ± 2.3 | 11.4 ± 2.0 |
| REM sleep | ||||||||
| min | 116.1 ± 4.7 | 118.1 ± 5.8 | 45.2 ± 5.8 | 54.3 ± 5.8 | 63.5 ± 3.2 | 68.5 ± 5.4 | 122.1 ± 3.5 | 123.1 ± 8.0 |
| % | 25.9 ± 1.1 | 26.1 ± 1.3 | 13.8 ± 1.2 | 16.1 ± 1.4 | 17.2 ± 0.7 | 18.4 ± 1.2 | 26.6 ± 0.8 | 27.1 ± 1.7 |
All-night 1-Hz EEG Power Spectra
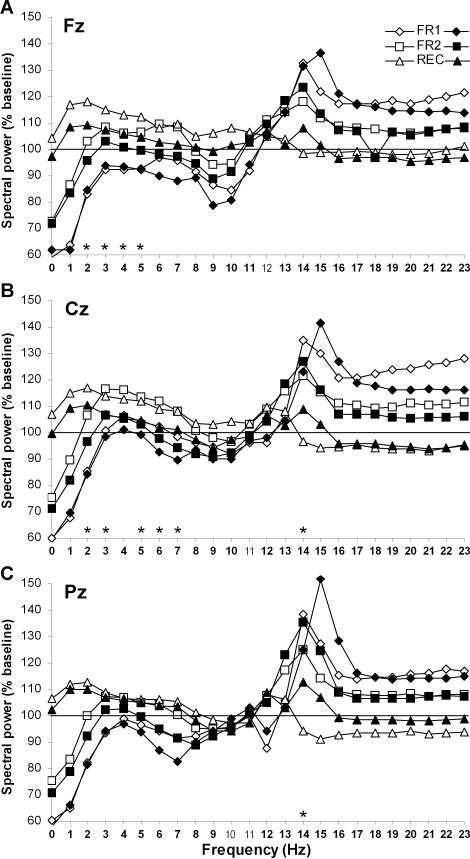
All-night power spectra, computed by one-Hz bins in experimental nights relative to the baseline night, are shown in Figure 1 for M-types and E-types. In the Fz derivation (Figure 1A), no main Group effect was found but Night effects were significant for all Hz bins except 12 Hz (F3,66 ≥4.2, P <0.05). In general, activity below 12 Hz decreased significantly between BL and FR1, while activity above 12 Hz increased. Between FR1 and FR2, activity below 8 Hz increased whereas activity above 14 Hz decreased. Compared to BL, spectral activity in FR2 was higher for most Hz bins >12 Hz but was equivalent for Hz bins <12 Hz. Significant Group-by-Night interactions were found in Hz bins 2 to 5 (F3,66 ≥3.2, P <0.05), showing that M-types had a larger activity increase between FR1 and FR2 than E-types (P <0.05), whereas no between-group difference appeared between BL and FR1 or BL and FR2.
Figure 1.
All-night spectral EEG power during experimental nights (FR1, FR2 and REC) expressed relative to baseline night values, separately for morning types (open symbols) and evening types (filled symbols). Spectral power was calculated in NREM sleep (stages 2, 3, and 4 sleep) for the Fz (A), Cz (B), and Pz (C) derivations. Hz bins are identified by their lower boundary value. Bold Hz bins indicate significant Night effect and stars indicate significant Group-by-Night interactions.
In the CZ derivation (Figure 1B), a main Group effect was found in the 13-Hz bin with M-types having a higher activity level than E-types (F1,22 = 5.7, P <0.05). Main Night effects were significant for all Hz bins except 11 Hz (F3, 66 ≥4.0, P <0.05). In general, activity <11 Hz decreased significantly between BL and FR1, while activity >13 Hz increased. Between FR1 and FR2, activity <14 Hz increased whereas activity for ≥14 Hz decreased. Spectral activity was higher in FR2 than in BL for Hz-bins 3 to 6 and 13 to 15. Significant Group-by-Night interactions were found in Hz bins 2, 3, 5, 6, and 7 (F3,66 >3.1, P <0.05), and showed that M-types had a larger activity increase between BL and FR2 (P <0.05 except 3 Hz), and between FR1 and FR2 (P <0.05 except 6 and 7 Hz), than E-types. A significant Group-by-Night interaction for the 14-Hz bin (F3,66 = 4.1, P <0.01) showed that M-types had a larger activity decrease between FR1 and FR2 than E-types (P <0.05).
In the PZ derivation (Figure 1C), main Group effects were found in the 12 and 13 Hz bins (F1,21 >4.4, P <0.05) with M-types having a higher activity level than E-types. Main Night effects were significant for all Hz bins except 10 and 11 Hz (F3, 63 ≥3.9, P <0.03). Overall, activity <10 Hz decreased significantly between BL and FR1, while activity >13 Hz increased. Between FR1 and FR2, activity <14 Hz increased whereas activity of ≥14 Hz decreased. Spectral activity was lower in FR2 than in BL for Hz bins 0, 1, 8, and 9, but higher in FR2 than in BL for Hz bins 13, 14, and 19 to 23. The only significant Group-by-Night interaction was found in the 14-Hz bin (F3,63 = 4.8, P <0.01) and showed that M-types had a larger activity decrease between FR1 and FR2 (P = 0.05) than E-types.
Time Course of Spectral Activity in EEG Frequency Bands
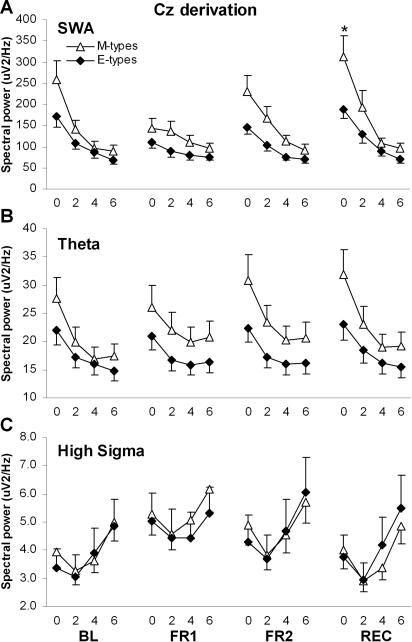
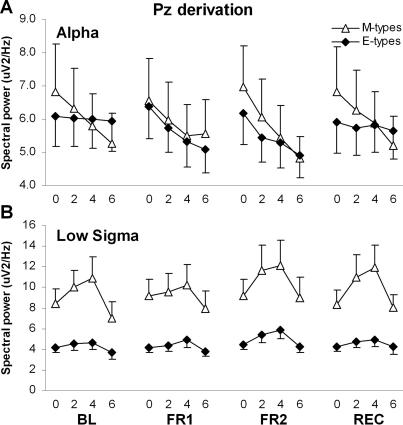
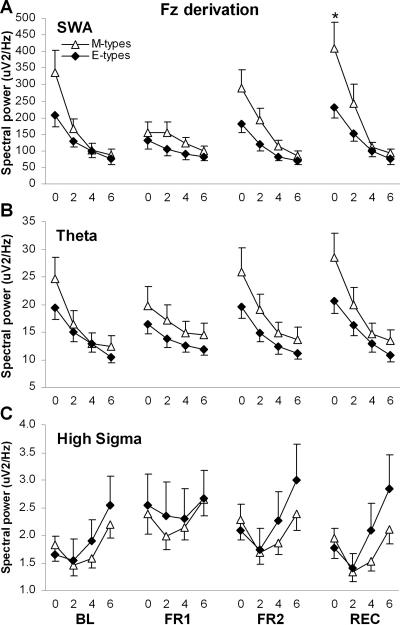
NREM spectral power averaged within the four 2-h periods was compared using Group-by-Night-by-Period ANOVAs for each EEG frequency band. Significant results are summarized in Table 2 and illustrated in Figures 2, 3, and 4, for FZ, CZ, and PZ derivations respectively. In FZ and CZ, significant Group-by-Night-by-Period interactions were found for SWA, theta, and high sigma. The SWA decrease in the course of the night was steeper in M-types than E-types during both BL and FR2 (Group-by-Period contrasts: F3,66 ≥3.2, P ≤0.03). The dynamics of SWA was similar in both diurnal types during FR1. The decrease in theta activity over the course of the night was steeper in M-types than E-types during BL (F3,66 ≥3.3, P <0.03), but there was no difference between diurnal types during FR1 and FR2. For high sigma activity, a significant Group-by-Period interaction was found only for FR2 in FZ (F3,66 = 3.5, P = 0.02), with a larger activity increase in the course of the night in E-types than in M-types. A significant Group-by-Night-by-Period interaction was also found in the alpha band for the PZ derivation (Figure 4A): the decreasing trend of alpha activity between 2-h periods was significant in M-types for BL and FR2 (P <0.05) whereas it was significant only during FR1 for E-types (P <0.01). Also in PZ, a significant Group-by-Period interaction was found in low sigma and is illustrated in Figure 4B. The increase in low sigma activity during the first three 2-h periods and the decrease between the two last 2-h periods were larger in M-types than E-types (P <0.05). A main group effect was found specifically in CZ for the low sigma band, with M-types having higher activity than E-types.
Table 2.
F-values of Significant Effects of Group-by-Night-by-Period ANOVAs of NREM Sleep Spectral Activity for the 6 EEG Frequency Bands Analyzed in FZ, CZ, and PZ Derivations.
| Group effect | Night effect | Period effect | Group × Night interaction | Group × Period interaction | Group × Night × Period interaction | |
|---|---|---|---|---|---|---|
| (df=1,22) | (df=3,66) | (df=3,66) | (df=3,66) | (df=3,66) | (df=9,198) | |
| SWA (1–5 Hz) | ||||||
| FZ | ns | 30.4c | 39.1c | 4.5a | 3.7a | 4.4b |
| CZ | ns | 33.0c | 51.1c | 3.5a | 3.8a | 3.1a |
| PZ | ns | 33.6c | 47.5c | ns | ns | ns |
| Theta (4–8 Hz) | ||||||
| FZ | ns | 18.9c | 58.2c | 4.2b | ns | 2.9a |
| CZ | ns | 12.4c | 55.2c | 5.7b | ns | 2.9a |
| Pz | ns | 11.5c | 46.3c | ns | ns | ns |
| Alpha (8–12 Hz) | ||||||
| FZ | ns | 7.5b | 11.8c | ns | ns | ns |
| Cz | ns | 3.8a | 9.3b | ns | ns | ns |
| Pz | ns | 5.7b | 9.7b | ns | ns | 2.3a |
| Sigma1 (12–14 Hz) | ||||||
| FZ | ns | 10.0c | ns | ns | ns | ns |
| Cz | 4.4a | 9.8c | 6.8c | ns | ns | ns |
| Pz | 8.0b | 9.8c | 19.6c | ns | 5.3b | ns |
| Sigma2 (14–16 Hz) | ||||||
| FZ | ns | 24.3c | 21.0c | ns | ns | 3.2a |
| CZ | ns | 29.0c | 31.3c | ns | ns | 2.4a |
| PZ | ns | 22.8c | 14.4c | ns | ns | ns |
| Beta (16–24 Hz) | ||||||
| FZ | ns | 13.9c | 10.0c | ns | ns | ns |
| CZ | ns | 18.0c | 12.5c | ns | ns | ns |
| PZ | ns | 14.6c | 10.5c | ns | ns | ns |
aP ≤0.05; bP ≤0.01; cP ≤0.001. Probabilities adjusted for repeated measures.
Degrees of freedom are indicated for FZ and CZ (see text).
Figure 3.
NREM sleep spectral activity in the Cz derivation, averaged by 2-h periods during baseline night (BL), first and second nights of sleep fragmentation (FR1 and FR2), and recovery night (REC), for morning types (M-types) and evening types (E-types). The 2-h periods are identified by their lower boundary value. A. Time course of SWA (1–5 Hz). The star indicates a significant between-group difference. B. Time course of theta activity (4–8 Hz). C. Time course of high sigma activity (14–16 Hz).
Figure 4.
NREM sleep spectral activity in the Pz derivation for morning types (M-types) and evening types (E-types), averaged by 2-h periods during baseline night (BL), first and second nights of sleep fragmentation (FR1 and FR2), and recovery night (REC). The 2-h periods are identified by their lower boundary value. A. Time course of alpha activity (8–12 Hz). B. Time course of low sigma activity (12–14 Hz).
2. Recovery from increased sleep pressure (BL vs. REC)
Sleep Architecture
Only duration and percentage of slow wave sleep showed a significant increase between BL and REC (P <0.001), and only percentage of stage 2 sleep showed a significant decrease between BL and REC (P = 0.02). There was no group difference and no Group-by-Night interaction.
All-night 1-Hz EEG Power Spectra
For FZ, CZ, and PZ derivations, spectral activity increased between BL and REC for Hz-Bins 1 to 6 Hz (P ≤0.06; Figure 1A, 1B, and 1C). In Fz, significant Group-by-Night interaction in Hzbins 2 to 5 showed that M-types had a larger activity increase between BL and REC than E-types (P <0.01). In CZ, M-types also showed a larger activity increase between BL and REC than E-types for Hz-bins 2, 3, 5, 6, and 7 (P <0.05). In CZ and PZ derivations, M-types showed a larger decrease between BL and REC for Hz bin 14 (P <0.05).
Time Course of Spectral Activity in EEG Frequency Bands
During REC, SWA was higher in M-types than in E-types during the first 2-h period in FZ and CZ (P <0.05). Also in FZ and CZ, the SWA decrease in the course of the REC night was steeper in M-types than in E-types (Group-by-Period contrasts: F3,66 ≥4.3, P <0.01; Figures 2 and 3). A similar result was found for the time course of theta activity (F3,66 ≥3.4, P = 0.02). For high sigma activity, significant Group-by-Period contrasts were found during REC only in FZ (F3,66 = 3.5, P = 0.02), showing a larger activity increase in the course of the night in E-types than in M-types. Finally, in BL, the decreasing trend of alpha activity between 2-h periods in PZ was significant only in M-types (P <0.05), but no difference was found between the two groups for alpha activity time course during REC (Figure 4A).
Figure 2.
NREM sleep spectral activity in the Fz derivation, averaged by 2-h periods during baseline night (BL), first and second nights of sleep fragmentation (FR1 and FR2), and recovery night (REC), for morning types (M-types) and evening types (E-types). The 2-h periods are identified by their lower boundary value. A. Time course of SWA (1–5 Hz). The star indicates a significant between-group difference. B. Time course of theta activity (4–8 Hz). C. Time course of high sigma activity (14–16 Hz).
Nonlinear Regression Analysis of SWA During REC
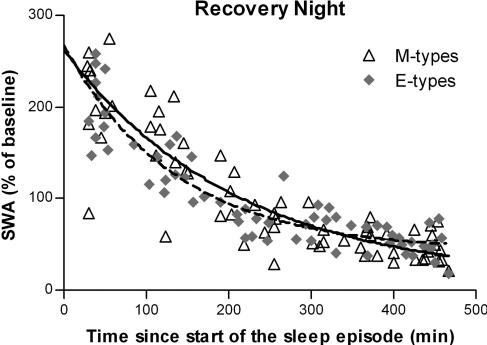
None of the estimates of SWA decay in FZ differed between the two groups of chronotypes during the REC night (Figure 5). Initial relative level of SWA (SWA0) was 251.6 ± 20.9% in M-types and 223.6 ± 14.2% in E-types (F1,122 = 1.3, P = 0.3); decay rate (r) was 0.0048 ± 0.0014 min-1 in M-types and 0.0073 ± 0.0014 min-1 in E-types (F1,122 = 1.6, P = 0.2); and horizontal asymptote (SWA∞) was 11.0 ± 26.3% in M-types and 43.3% ± 10.4% in E-types (F1,122 = 1.9, P = 0.2).
Figure 5.
Exponential decay function adjusted on relative SWA in NREM sleep for all-night EEG in the Fz derivation. Exponential decay fits: SWAt = SWA∞ + SWA0* e−rt. Solid line, morning types; dashed line, evening types. In morning types, regression was computed on 65 cycles (open triangels) and goodness of fit coefficient (R2) was 0.76. In evening types, regression was computed on 63 cycles (grey diamonds) and R2 was 0.82.
DISCUSSION
These results add further support to the hypothesis of a difference in homeostatic sleep regulation between circadian types. The procedure of behavioral sleep fragmentation increased sleep pressure, as shown by increased activity in low EEG frequencies during FR2, as well as during REC compared to baseline. As predicted, the increase in homeostatic sleep pressure produced a larger increase in SWA in M-type individuals than in E-types, for both FR2 and REC compared to BL. This difference between circadian types was observed in all-night activities and was more pronounced in the first 2 hours of the sleep episode, especially during REC. The larger response of M-types to the increased duration of time awake covered a wide range of slow EEG frequencies, including the theta band. Moreover, this response was observed not only in the frontal derivation, considered to be the most sensitive brain area for sleep homeostasis,9,12,18,19 but also in the central site.
The procedure of sleep fragmentation produced a relatively large homeostatic response as indicated by the increase in low frequency activity during recovery sleep. Since the behavioral sleep disruption was maintained over the entire night, very little recuperation could occur during the nights of fragmentation, especially during the first night (FR1), when the subjects had not yet adapted to the procedure and took more time to go back to sleep. In addition to producing a modest increase in time awake (about 2 hours in FR1 and 1.3 h in FR2), the procedure also decreased SWS and low-frequency EEG activity during the nights of sleep fragmentation. Even in the absence of increased wake time, decreased SWS and SWA caused by acoustic stimulations during sleep have been shown to increase both SWS and SWA during recuperation.32,33 Therefore, the large homeostatic response observed during the recovery night probably resulted from the combination of the decreased SWS due to sleep fragmentation (slowing the recuperative process) and the increased time awake (increasing the sleep pressure) accumulated over the 2 nights of sleep fragmentation. Of primary importance in the context of this study is that the changes to sleep architecture caused by sleep fragmentation, including the increase in time awake and decrease in SWS, were similar in the 2 groups of chronotypes.
Like others,13,21 we previously reported that M-types and E-types displayed no difference in sleep architecture during a normal sleep episode.34 The results reported here indicate that sleep architecture remains similar in the 2 groups even during and after experimental disruption of the sleep episode. When quantitative EEG of the baseline night was analyzed, the only significant difference in spectral power found between the 2 groups was in the low sigma frequency band (12–14 Hz), with more activity in M-types than in E-types in all derivations except FZ.12,34 A similar difference was also observed during the nights of sleep fragmentation and during the night of recuperation. However, there was no Group-by-Night interaction, showing that the response of this frequency band to the increase in sleep pressure was similar in the 2 groups of subjects. Thus even if this frequency range has been associated with the homeostatic response to sleep deprivation,9,17 the difference between the 2 groups does not seem to depend on the previous duration of time awake, at least in the context of this protocol. At present, we do not have an explanation for this group difference, but it may relate to differences in the occurrence and/or amplitude of sleep spindles which may derive from variations in thalamo-cortical circuitry, the neuronal correlate of spindles frequency activity.35
Sleep fragmentation increased high sigma activity, which subsequently decreased during recovery. These effects are consistent with the known repercussion of increased sleep pressure on NREM sleep EEG activity.8,9 In all-night analysis, M-types showed a larger decrease than E-types in centro-parietal 14-Hz activity during FR2 and REC compared to BL. This observation is also consistent with an increased homeostatic response in M-types. As observed in our data, activity in high spindle frequencies, as well as its diminution following sleep deprivation, generally dominates in the centro-parietal area.9,36,37 Conversely, in the present study, E-types showed a fronto-central increase in high sigma activity in FR2 and REC compared to BL in the second half of the nights. A frontal increase in high sigma activity following sleep deprivation has also been observed previously.19 Sleep spindles have been hypothesized to preserve sleep as inhibitors of information processing,35,38 and sigma activity has been associated with the inhibition of thalamus activity.39 Since E-types do not show a considerable increase in SWA in response to sleep fragmentation, an increase in sleep spindles may help to consolidate their sleep and explain how they could display a sleep architecture comparable to that of M-types. More direct measures of sleep spindles would be necessary in order to confirm this hypothesis.
Contrary to the results of analyses of 2-h periods, the modelization using nonlinear regression did not show any significant difference between M-types and E-types for the dynamics of SWA decay during REC. Two reasons may explain this discrepancy. First, the nonlinear regression computes the initial level of SWA on the first NREM/REM cycle, which usually lasts <2 hours. It is therefore possible that the higher SWA level found for the first 2 hours of sleep included accumulated SWA on more than one sleep cycle. Second, nonlinear regression is applied on every individual cycle, whereas analysis of variance computes the group mean of 4 time points per night. The increased variability associated with the regression methods may have contributed to the absence of between-group difference.
In conclusion, our results show that when the influence of the circadian phase is kept constant, M-types have a higher response than E-types to an increase in homeostatic sleep pressure. Compared to BL, low-frequency activities (<8 Hz) showed a higher increase during FR2 and REC in M-types than in E-types for both frontal and central brain areas; this response was predominant in the first part of the sleep episode, particularly for SWA. This result points toward a higher homeostatic response in M-types for a similar increase in time awake. A higher homeostatic response may be the result of a faster rate of accumulation of sleep pressure during wakefulness, as proposed by Taillard et al,13 or it may be caused by a higher capacity to generate slow waves as observed in older adolescents.15 It is not known whether individual variations in homeostatic response could lead to a “neither type” classification in subjects with early or late endogenous circadian phases, or result in short or long durations of the sleep episode. Volunteers with those characteristics did not meet our selection criteria but would be interesting subjects for future studies. Finally, dose-response curves with varying amounts of time awake will be needed to determine more precisely the nature of the homeostatic differences associated with morningness-eveningness.
ACKNOWLEDGMENTS
This study was supported by a grant from the Canadian Institutes of Health Research (MD) and by a graduate fellowship from the Natural Sciences and Engineering Research Council of Canada (VM). We thank the anonymous reviewers for their helpful suggestions. We are grateful to Sonia Frenette, Hélène Blais, and Jean Paquet for their invaluable technical assistance. We thank all volunteers and research staff.
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Mongrain and Dumont have indicated no financial conflicts of interest.
REFERENCES
- 1.Kerkhof GA, Van Dongen H. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996;218:153–6. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–50. [PMC free article] [PubMed] [Google Scholar]
- 3.Baehr EK, Revelle W, Eastman CI. Individual difference in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–27. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 4.Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J Biol Rhythms. 2004;19:248–57. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- 5.Borbély AA. A two process model of sleep regulation. Human Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 6.Beersma DGM. Models of human sleep regulation. Sleep Med Rev. 1998;2:31–43. doi: 10.1016/s1087-0792(98)90052-1. [DOI] [PubMed] [Google Scholar]
- 7.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 8.Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of the circadian melatonin rhythm in humans. J Physiol. 1997;(505.3):851–8. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoblauch V, Kräuchi K, Renz C, Wirz-Justice A, Cajochen C. Homeostatic control of slow-wave and spindle frequency activity during human sleep: effect of differential sleep pressure and brain topography. Cereb Cortex. 2002;12:1092–110. doi: 10.1093/cercor/12.10.1092. [DOI] [PubMed] [Google Scholar]
- 10.Cajochen C, Brunner DP, Kraüchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–4. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 11.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–4. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 12.Mongrain V, Carrier J, Dumont M. Difference in sleep regulation between morning and evening circadian types as indexed by antero-posterior analysis of the sleep EEG. Eur J Neurosci. 2006;23:497–504. doi: 10.1111/j.1460-9568.2005.04561.x. [DOI] [PubMed] [Google Scholar]
- 13.Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J Sleep Res. 2003;12:275–82. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 14.Aeschbach D, Cajochen C, Landolt H, Borbély AA. Homeostatic sleep regulation in habitual short sleepers and long sleepers. Am J Physiol. 1996;270:R41–53. doi: 10.1152/ajpregu.1996.270.1.R41. [DOI] [PubMed] [Google Scholar]
- 15.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 16.Dijk DJ, Brunner DP, Beersma DGM, Borbély AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 18.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta activity after sleep loss in humans. Sleep Res Online. 1999;2:65–9. [PubMed] [Google Scholar]
- 19.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neurosci. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 20.Breithaupt H, Hildebrandt G, Döhre D, Josch R, Sieber U, Werner M. Tolerance to shift of sleep, as related to the individual's circadian phase position. Ergonomics. 1978;21:767–74. doi: 10.1080/00140137808931780. [DOI] [PubMed] [Google Scholar]
- 21.Foret J, Touron N, Benoit O, Bouard G. Sleep and body temperature in “morning” and “evening” people. Sleep. 1985;8:311–18. doi: 10.1093/sleep/8.4.311. [DOI] [PubMed] [Google Scholar]
- 22.Lancel M, Kerkhof GA. Sleep structure and EEG power density in morning types and evening types during a simulated day and night shift. Physiol Behav. 1991;49:1195–201. doi: 10.1016/0031-9384(91)90351-n. [DOI] [PubMed] [Google Scholar]
- 23.Gillberg M, Akerstedt T. Body temperature and sleep at different times of day. Sleep. 1982;5:378–88. doi: 10.1093/sleep/5.4.378. [DOI] [PubMed] [Google Scholar]
- 24.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glovinsky PB, Spielman AJ, Carroll P, Weinstein L, Ellman SJ. Sleepiness and REM sleep recurrence: The effects of stage 2 and REM sleep awakenings. Psychophysiology. 1990;27:552–9. doi: 10.1111/j.1469-8986.1990.tb01974.x. [DOI] [PubMed] [Google Scholar]
- 26.Dumont M, Paquet J, Bergevin A, et al. Manipulation of the recuperative value of sleep with behavioral sleep fragmentation. Sleep. 2000;23:A71–2. [Google Scholar]
- 27.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 29.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1976;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 30.Feinberg I, Campbell IG. Kinetics of non-rapid eye movement delta production across sleep and waking in young and elderly normal subjects: Theoretical implications. Sleep. 2003;26:192–200. doi: 10.1093/sleep/26.2.192. [DOI] [PubMed] [Google Scholar]
- 31.Brunner DP, Vasko RC, Detka CS, Monahan JP, Reynolds CF, 3rd, Kupfer DJ. Muscle artifacts in the sleep EEG: Automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5:155–64. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- 32.Dijk DJ, Beersma DGM. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalogr Clin Neurophysiol. 1989;72:312–20. doi: 10.1016/0013-4694(89)90067-9. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara M, De Gennaro L, Bertini M. Selective slow-wave sleep (SWS) deprivation and SWS rebound: do we need a fixed SWS amount per night? Sleep Res Online. 1999;2:15–19. [PubMed] [Google Scholar]
- 34.Mongrain V, Carrier J, Dumont M. Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep. 2005;28:819–27. doi: 10.1093/sleep/28.7.819. [DOI] [PubMed] [Google Scholar]
- 35.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 36.Jobert M, Poiseau E, Jähnig P, Schulz H, Kubicki S. Topographical analysis of sleep spindle activity. Pharmacoelectroencephalography. 1992;26:210–17. doi: 10.1159/000118923. [DOI] [PubMed] [Google Scholar]
- 37.Werth E, Achermann P, Dijk DJ, Borbély AA. Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalogr clin Neurophysiol. 1997;103:535–42. doi: 10.1016/s0013-4694(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 38.Yamadori A. Role of the spindles in the onset of sleep. Kobe J Med Sci. 1971;17:97–111. [PubMed] [Google Scholar]
- 39.Hofle N, Paus T, Reutens D, et al. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17:4800–08. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]